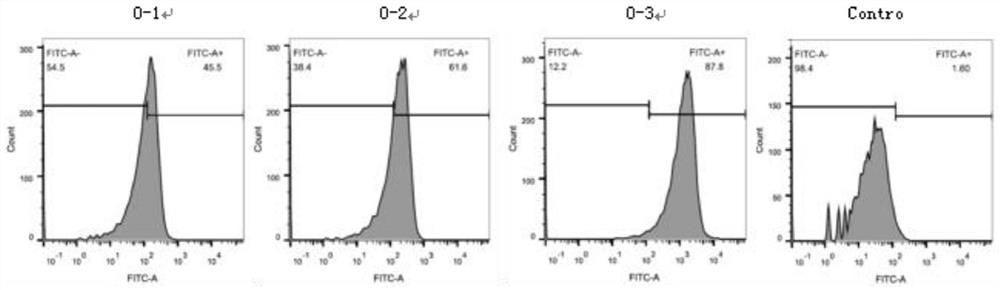
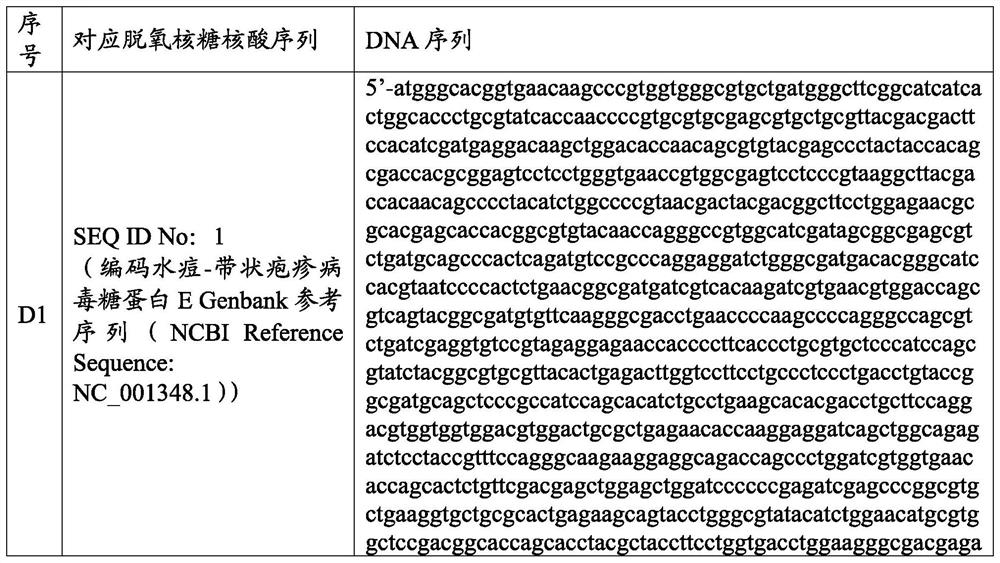
Varicella-zoster virus mRNA vaccine as well as preparation method and application thereof
A technology of herpes zoster virus and chickenpox, which is applied in the field of varicella-zoster virus mRNA vaccine and its preparation, and can solve problems such as unstable glycosylation products, difficult quality control, and no herpes zoster vaccine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0109] In some embodiments, the preparation method of the varicella-zoster virus nucleic acid vaccine comprises:
[0110] (a) Dissolving the optimized varicella-zoster virus glycoprotein E mRNA in an aqueous solution of auxiliary materials to obtain an aqueous phase; the aqueous solutions of the auxiliary materials include but are not limited to citrate buffer, sodium acetate solution or phosphate buffer, preferably is sodium acetate solution;
[0111] (b) dissolving protonatable cationic lipids, structured lipids, auxiliary lipids and surfactants in an organic solution according to the formula ratio to obtain an organic phase; the organic solution includes absolute ethanol, isopropanol or acetone, preferably is anhydrous ethanol;
[0112] (c) the aqueous phase obtained by (a) and the organic phase obtained by (b) are mixed to obtain a mixed solution, and the solvent in the replacement mixed solution is a buffer to obtain a varicella-zoster virus nucleic acid vaccine; The aq...
Embodiment 1
[0119] Example 1 UTR sequence optimization
[0120] The UTR sequence optimization scheme in the present embodiment is shown in Table 1, and the details are as follows:
[0121] The key elements of the F-1 mRNA sequence are ARCA, X-GLOBIN 5'-UTR, varicella-zoster virus glycoprotein E Genbank reference sequence (NCBI Reference Sequence: NC_001348.1), X-GLOBIN 3'-UTR and polyA sequences and subsequent components.
[0122] The key elements of the F-2 mRNA sequence are ARCA, A-GLOBIN 5'-UTR, varicella-zoster virus glycoprotein E Genbank reference sequence (NCBI Reference Sequence: NC_001348.1), A-GLOBIN 3'-UTR and polyA sequences and subsequent components.
[0123] The key elements of the F-3 mRNA sequence are ARCA, X-GLOBIN 5'-UTR, varicella-zoster virus glycoprotein E Genbank reference sequence (NCBI Reference Sequence: NC_001348.1), lipoxygenase gene 3'-UTR and polyA sequences and subsequent elements.
[0124] The key elements of the F-4 mRNA sequence are ARCA, NCA-7d 5'-UTR...
Embodiment 2
[0129] Example 2 T7 promoter sequence optimization
[0130] The T7 promoter sequence optimization scheme in the present embodiment is shown in Table 2, and the details are as follows:
[0131] pcDNA3.1(+)(Ampicillin) was used as the expression vector, and the T7 promoter sequence in the vector was transformed to construct T1, T2, and T3 expression vectors respectively. The T7 promoter sequence in the T1 expression vector was 5'-taatacgactcactataggg- 3' (SEQ ID No: 13); the T7 promoter sequence in the T2 expression vector is 5'-taatacgactcactataagg-3' (SEQ ID No: 14); the T7 promoter sequence in the T3 expression vector is 5'-taatacgactcactataag-3' (SEQ ID No: 15).
[0132] The key elements of the glycoprotein E DNA sequence are X-GLOBIN 5'-UTR, varicella-zoster virus glycoprotein EGenbank reference sequence (NCBI Reference Sequence: NC_001348.1), X-GLOBIN 3'-UTR and polyA (80bp) Sequence and subsequent elements (F-1 in Example 1). The above genes were cloned into modified T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com