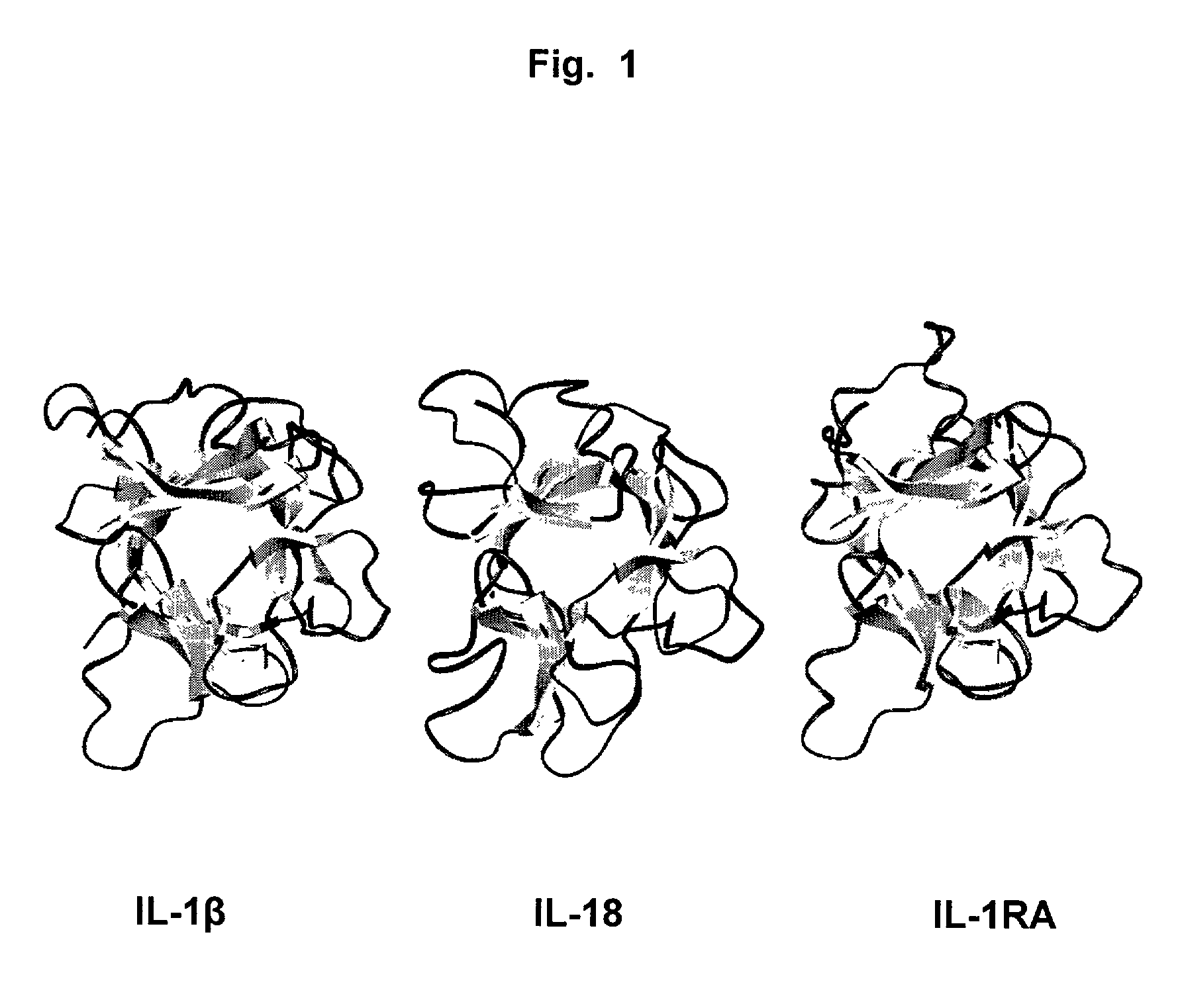
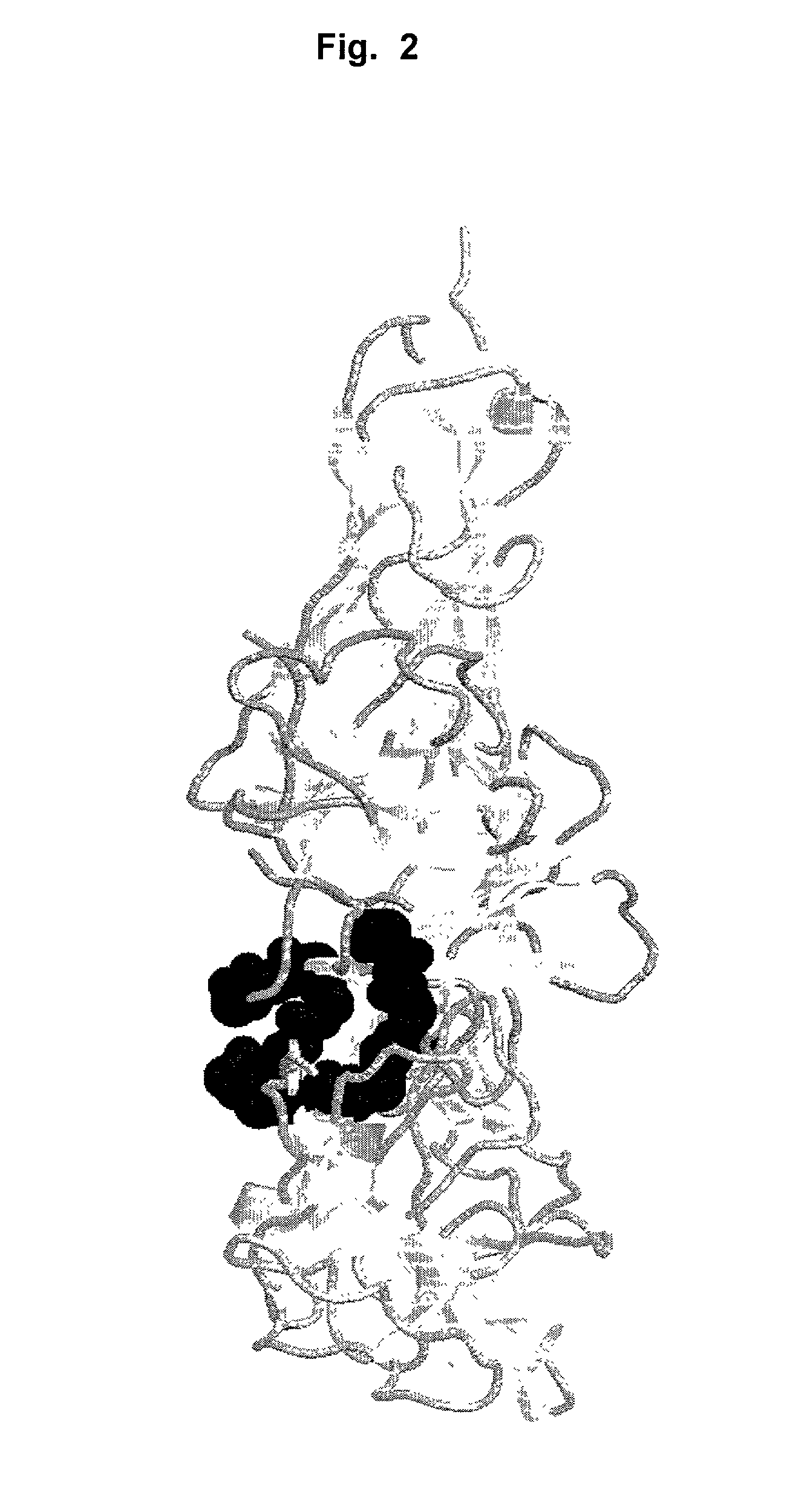
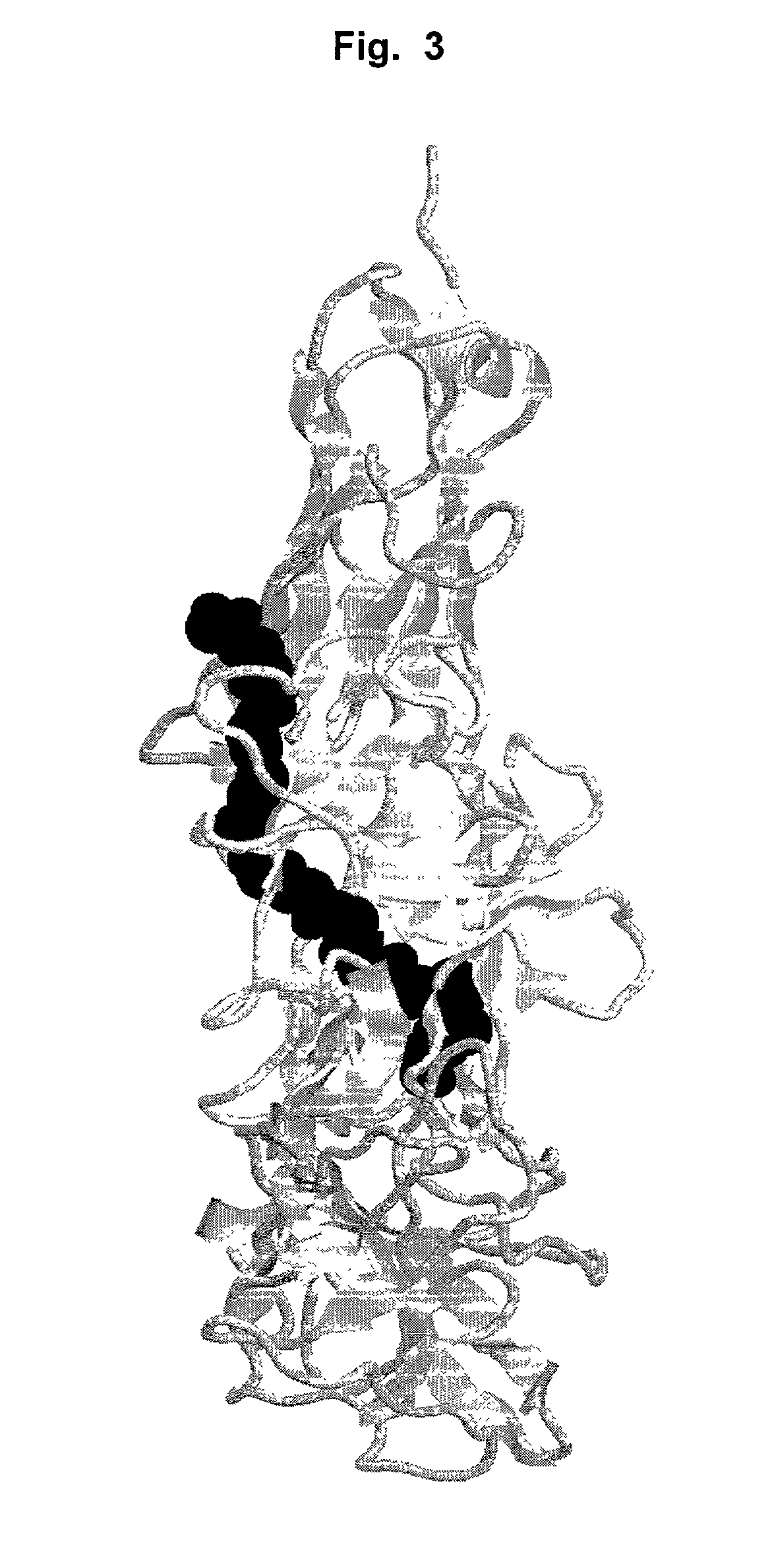
Antibodies that bind IL-18 and methods of inhibiting IL-18 activity
a technology of antibody and il-18, which is applied in the field of antibodies that bind il-18 and methods of inhibiting il-18 activity, can solve the problems of antibody not being described, eliciting unwanted immune reactions, and limited use of murine il-18 antibodies in vivo
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Isolation of Anti-IL-18 Antibodies
[0100]Antibodies to ML-18 were isolated by screening separate scFv phage display libraries prepared using human VL and VH cDNAs from mRNA derived from human B cells (e.g., tonsils and spleen). Construction of the library and methods for selection are described in Vaughan et al. (1996) Nature Biotech. 14: 309-314.
[0101]The libraries were screened using either full length human IL-18 (SEQ ID NO: 61), a peptide epitope of IL-18 (SEQ ID NOS: 1-3), or a panel of overlapping 15 amino acid peptides representing IL-18 (the epitope sequence of which is presented in Table 5; SEQ ID NOS: 31-60). IL-18 specific antibodies were selected by coating the antigen onto immunotubes using standard procedures (Marks et al., (1991) J. Mol. Biol. 222: 581-597). The scFv libraries were screened using either IL-18, a peptide epitope of IL-18, or an IL-18 peptide panel to generate a significant number of IL-18 specific binders. Several different clonotypes were selected, det...
example 2
Affinity Maturation of an Anti-18 Antibodies
[0104]A single chain Fv version of antibody 2E1 having an identified IL-18 binding activity and the heavy chain and light chain sequence shown in Table 6 was further modified for improved neutralization of IL-18 activity.
[0105]
TABLE 6Sequence of Single-Chain Anti-IL-18 Antibody 2E12E1 Heavy Chain(SEQ ID NO: 18) CDR1 (SEQ ID NO: 9)QVQLVQSGAEVKKPGASMKVSCKTSGYTFTGYYIHWVRQAHGQGFEWI CDR2 (SEQ ID NO: 10) CDR3 (SEQ ID NO: 11)GRLNPTTGDANFAEKFQGRVALTRDTSISTAYLQLDSLKSDDTAVYYCAGKEGAWGQGTLVTVSS2E1 Light Chain(SEQ ID NO: 19) CDR1 (SEQ ID NO: 12) CDR2 (SEQ ID NO: 13)SSELTQDPAVSVALGQTVRITCQGDSLRHFYPNWYQQKPGQAPVLVIYGKNNRPS CDR3 (SEQ ID NO: 14)GIPDRFSGSGSGNTGSLTITGAQAEDEADYYCGSRDSSGIHVVFGGGTKVTVLG
[0106]The anti-IL-18 antibody 2E1 was independently selected using an IL-18 peptide and sequential, overlapping, peptide panel representative o...
example 3
Binding Activity of Human Antibodies to IL-18
[0120]Real-time binding interactions between ligand (biotinylated recombinant human IL-18 (rhIL-18) immobilized on a biosensor matrix) and analyte (antibodies in solution) were measured by surface plasmon resonance (SPR) using the BIAcore system (Pharmacia Biosensor, Piscataway, N.J.). The system utilizes the optical properties of SPR to detect alterations in protein concentrations within a dextran biosensor matrix. Proteins are covalently bound to the dextran matrix at known concentrations. Antibodies are injected through the dextran matrix and specific binding between injected antibodies and immobilized ligand results in an increased matrix protein concentration and resultant change in the SPR signal. These changes in SPR signal are recorded as resonance units (RU) and are displayed with respect to time along the y-axis of a sensorgram.
[0121]To facilitate immobilization of biotinylated rhIL-18 on the biosensor matrix, streptavidin is co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
| structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com