Reagent for determining content of human cholyglycine by using latex immunoturbidimetry technology
A technology of latex immunity and glycocholic acid, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of poor stability of labeled enzymes and cannot meet clinical use, and achieve the advantages of reagent stability and increase sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1 Prepare the reagent of mensuration human serum glycocholic acid content by the method of the present invention
[0026] 1. Preparation of reagents 1
[0027] 1. Preparation of latex microspheres-BSA-glycocholic acid conjugates
[0028] 1) Activation of Glycocholic Acid
[0029] Accurately weigh 400mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDC), dissolve it in 100mL MES buffer (20mmol / L, pH=6.0) to obtain EDC solution; Accurately weigh 200mg glycocholic acid and 400mg Sulfo-NHS, dissolve in 100mL MES buffer (20mmol / L, pH=6.0) to obtain a glycocholic acid solution; slowly add the EDC solution to the glycocholic acid solution while stirring After the addition, the stirring reaction was continued for 30 minutes to obtain an activated glycocholic acid solution.
[0030] 2) Coupling of Glycocholic Acid and BSA
[0031] Accurately weigh 100mg of BSA and dissolve it in 100mL MES buffer (20mmol / L, pH=6.0) to obtain the BSA solution; while stirring, sl...
Embodiment 2
[0040] Embodiment 2 detection method and equipment
[0041] The detection of glycocholic acid in the present invention requires fresh serum or plasma without hemolysis. Samples that interfere with the reaction absorbance, such as hemolysis or lipid turbidity, may affect the final result. It is recommended to re-collect blood.
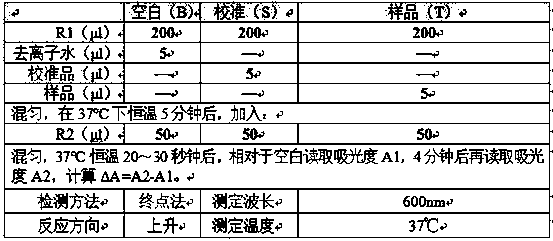
[0042] Reagent of the present invention is applied to various types of biochemical instruments, such as Hitachi Hitachi7080 / 7600P, Olympus Olympus AU400, Beckman Coulter LX20 / AU680 / AU5800, Toshiba Toshiba 120FR, Abbott C8000, Siemens SIEMENS ADVIA1800 / ADVIA2400 / Dimension RxL Max biochemical analyzer, etc.
[0043] The specific detection parameter settings are shown in the table below, and the reading time of A1 and A2 is set according to different instruments:
[0044]
Embodiment 3
[0045] Embodiment 3 correlation comparison
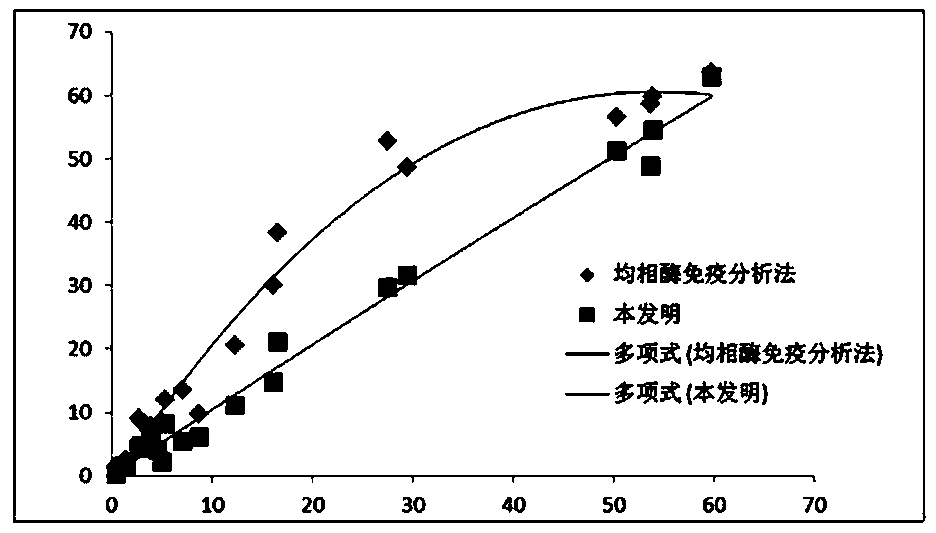
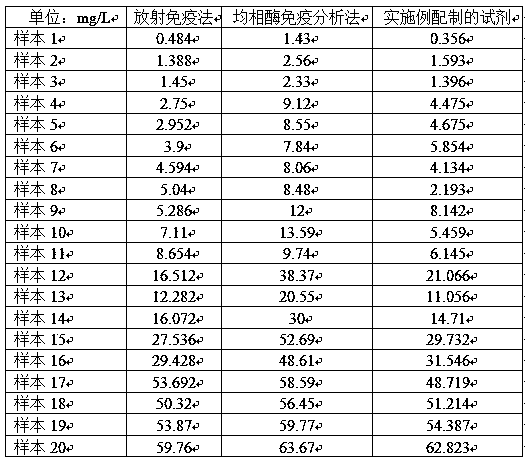
[0046] With the reagent prepared in Example 1, the same sample is detected with the routinely used radioimmunoassay and homogeneous enzyme immunoassay, and the correlation data obtained are shown in the following table 1 and figure 1 :
[0047]
[0048] We can find out from Table 1 that the measured data of the present invention is close to the result obtained by radioimmunoassay, and the measured result of homogeneous enzyme immunoassay is better correlated with the result obtained by radioimmunoassay in the low value stage, but the high value stage is significantly On the low side, so its linearity is lower than that of the present invention and the radioimmunoassay reagent.
[0049] figure 1 Then relatively intuitively embodies the comparative relationship of the three reagents, the trend line of the measured data of the present invention and the result obtained by radioimmunoassay is close to a straight line, showing that b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com