Pharmaceutical formulation containing candida urate oxidase
A technology of uric acid oxidase and Candida, which is applied to medical preparations containing active ingredients, medical preparations without active ingredients, drug combinations, etc., can solve the problem of protein having no general value, unusable, molecular stability and properties Big difference and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1 Preparation of stable Candida urate oxidase solution
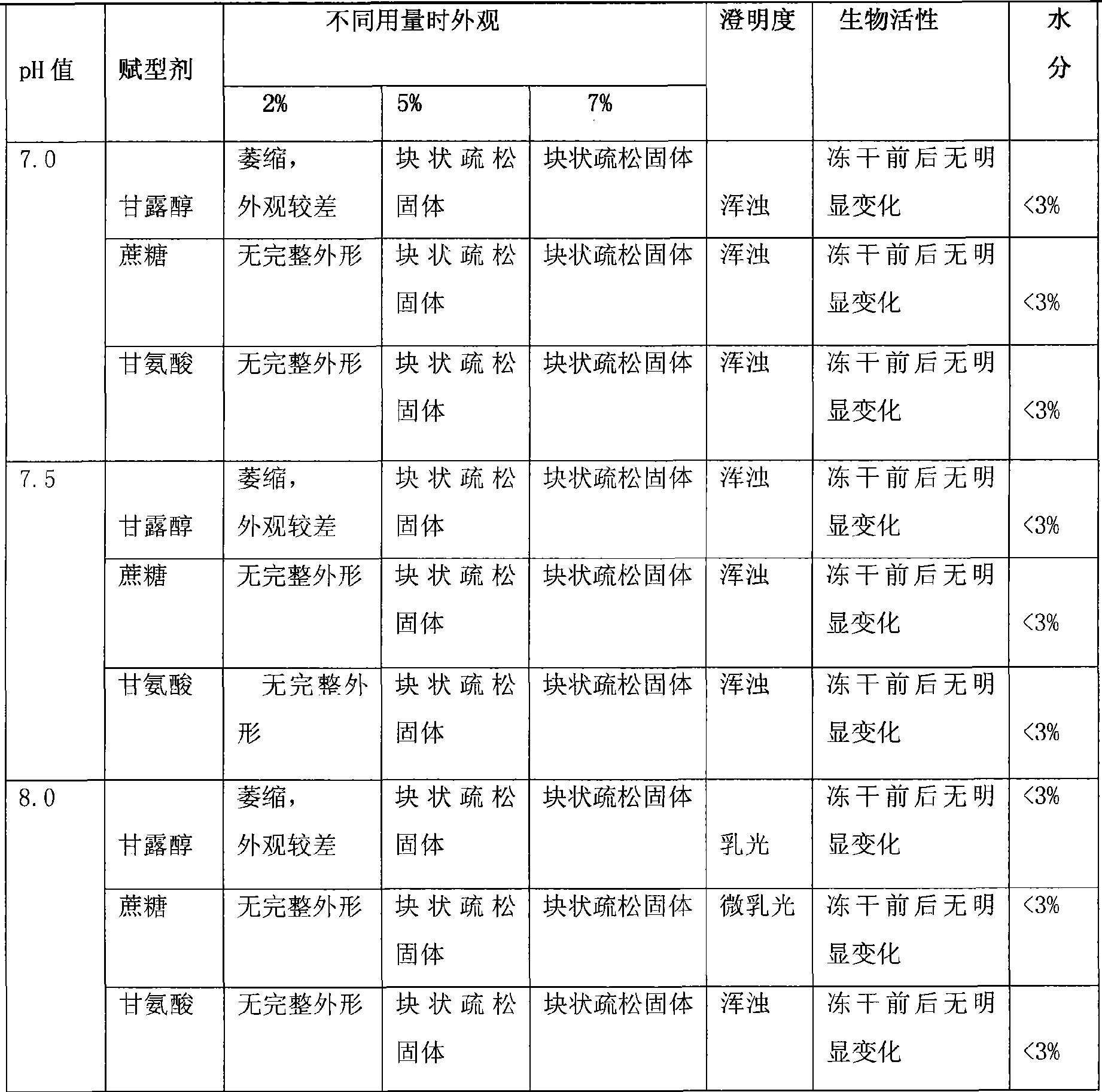
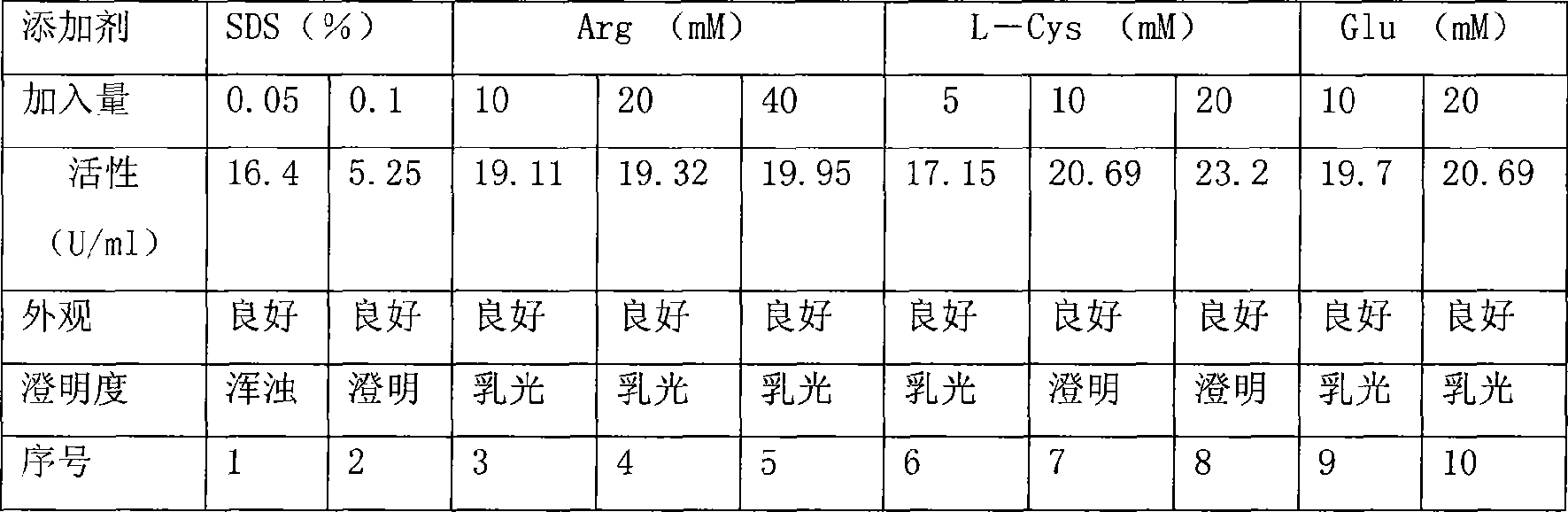
[0065] Stock solution preparation: the recombinant Candida urate oxidase prepared according to the method of Chinese patent 031091504 was replaced by ultrafiltration into the following buffer solution: glycine 100mM / L, Tris-HCl (pH9.0 / 100mM / L), L-Cys ( 20mM / L), prepared into a stock solution whose concentration of recombinant Candida urate oxidase is 3.6mg / ml;
[0066] Dosing: take 500ml of the stock solution, add 25% mannitol 200ml, add water for injection to make up to 1000ml;
[0067] Sterilization by filtration: use 0.22 micron filter membrane to filter;
[0068] Subpackaging: vials are used to subpackage into 1ml preparations each;
[0069] Freeze-drying: Freeze-drying to make the final preparation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com