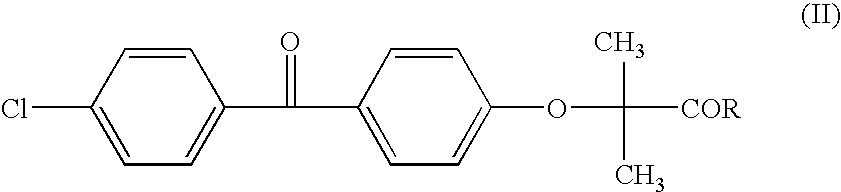
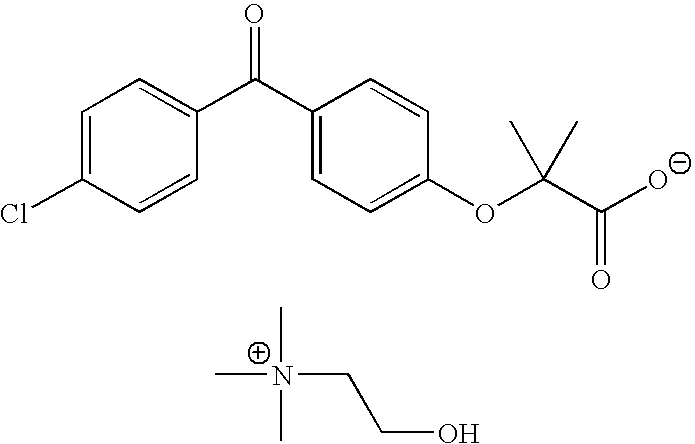
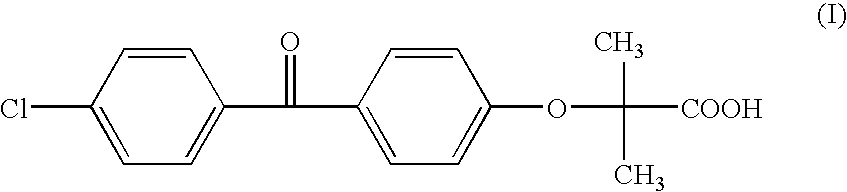
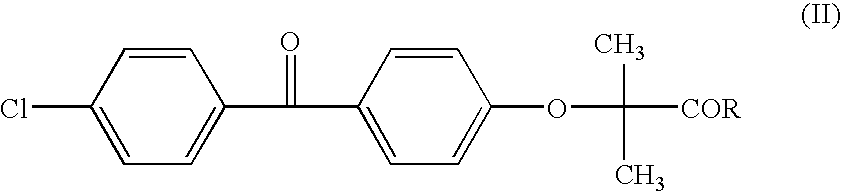
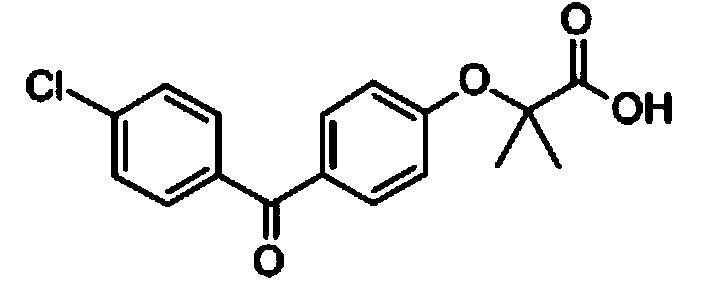
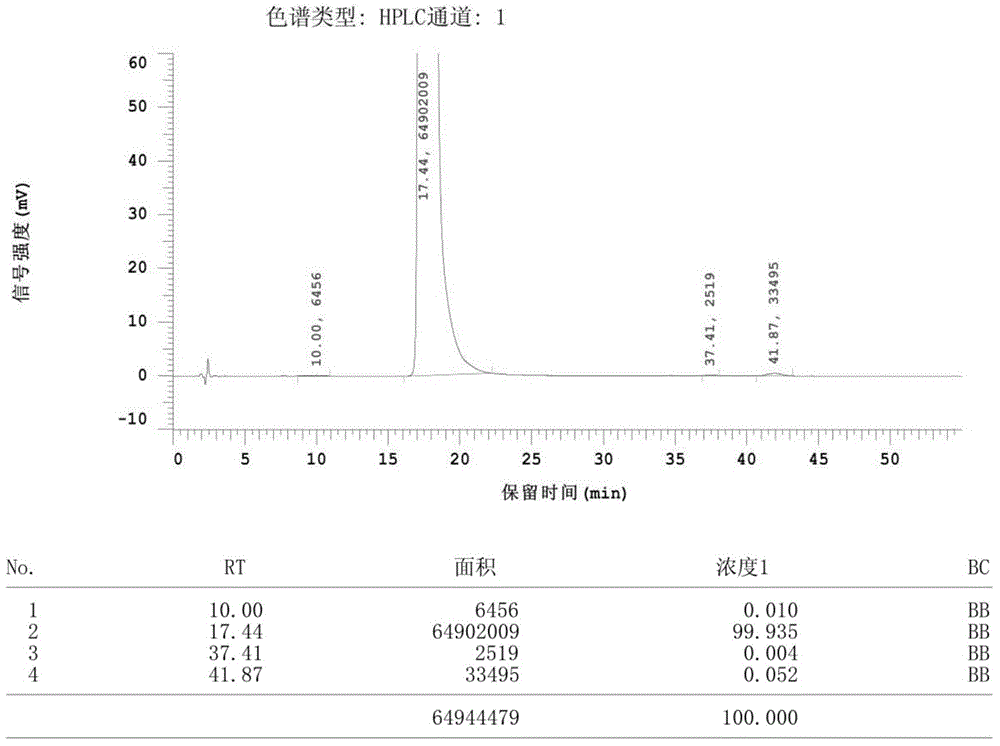
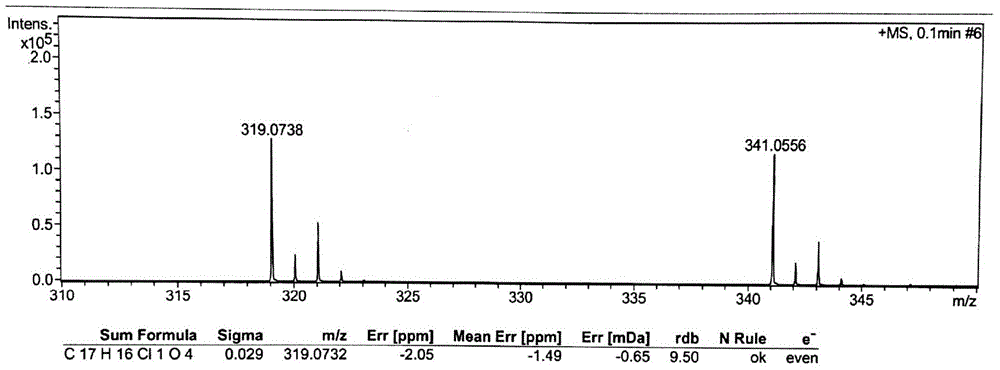
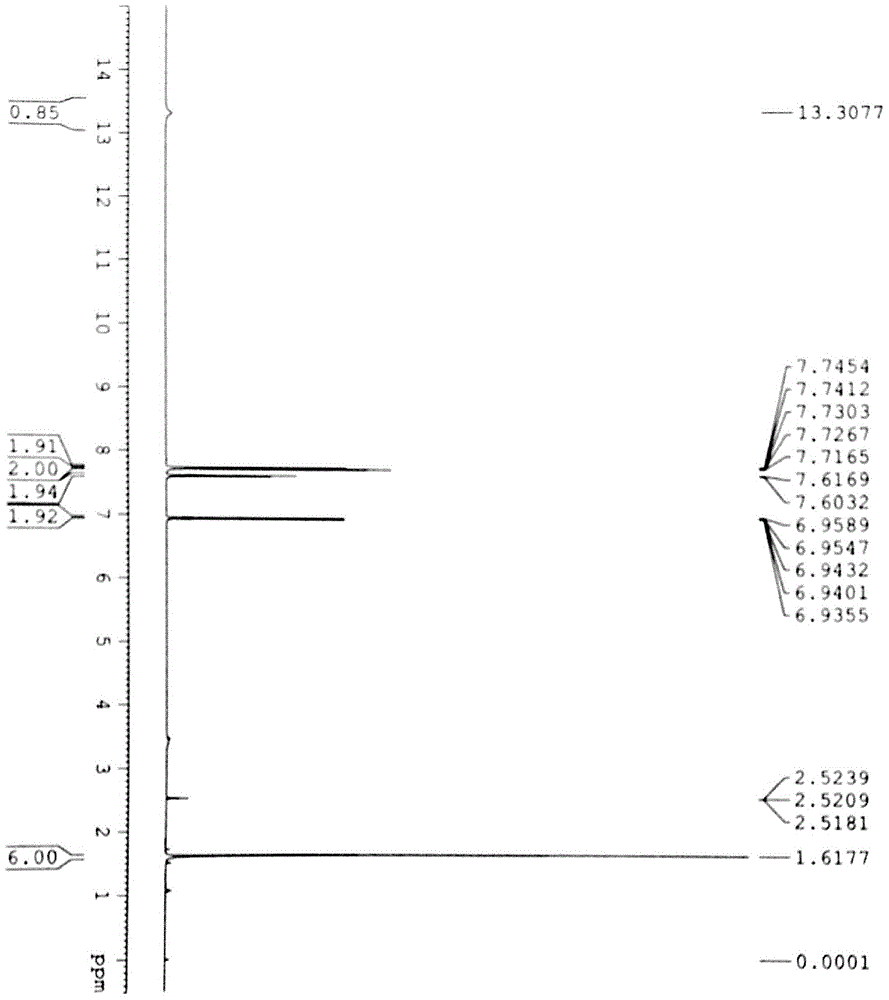
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
66 results about "FENOFIBRIC ACID" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
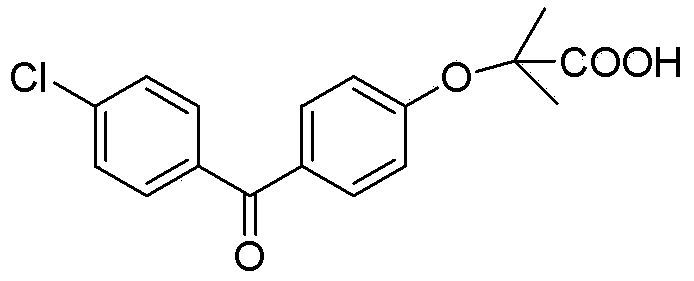
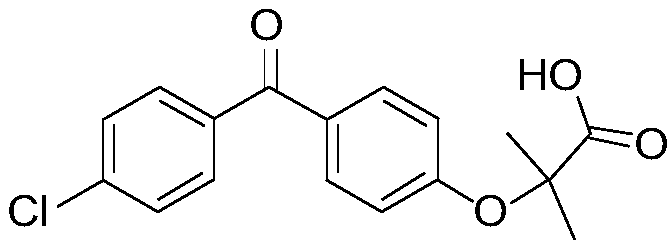
Fenofibric acid helps reduce cholesterol and triglycerides (fatty acids) in the blood. High levels of these types of fat in the blood are associated with an increased risk of atherosclerosis (clogged arteries).. Fenofibric acid is sometimes given together with other cholesterol-lowering medications.
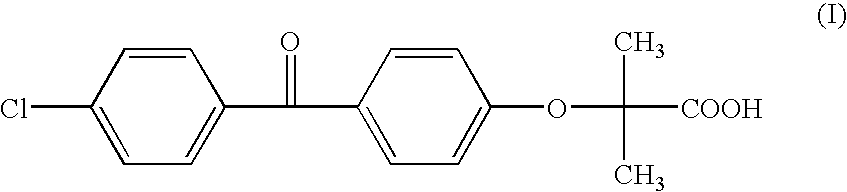
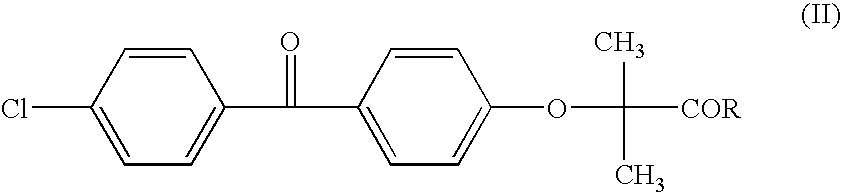
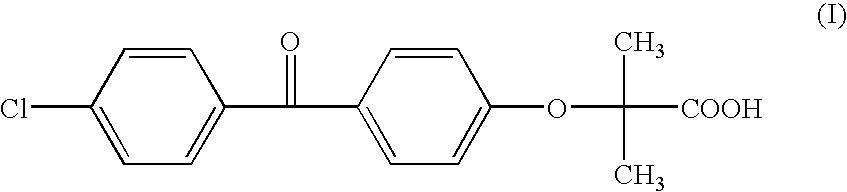
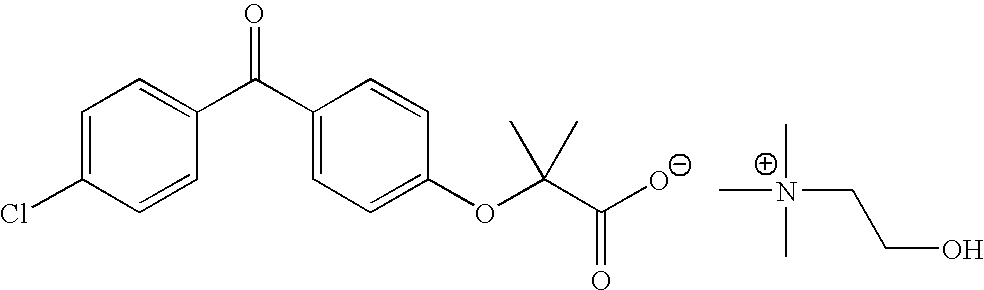
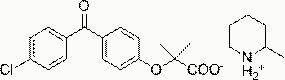
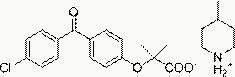
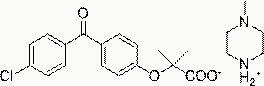
Salts of fenofibric acid and pharmaceutical formulations thereof
In one aspect, the present invention relates to a formulation in the form of molecular dispersion comprising i) fenofibric acid, a physiologically acceptable salt or derivative thereof and optionally other active substances, ii) a binder component comprising at least one enteric binder, and optionally iii) other physiologically acceptable excipients. In a second aspect, the present invention relates to novel salts of fenofibric acid that are photostable when compared to other salts of fenofibric acid.
Owner:FOURNIER LAB IRELAND +1
Salts of fenofibric acid and pharmaceutical formulations thereof
In one aspect, the present invention relates to a formulation in the form of molecular dispersion comprising i) fenofibric acid, a physiologically acceptable salt or derivative thereof and optionally other active substances, ii) a binder component comprising at least one enteric binder, and optionally iii) other physiologically acceptable excipients.In a second aspect, the present invention relates to novel salts of fenofibric acid that are photostable when compared to other salts of fenofibric acid.
Owner:FOURNIER LAB IRELAND +1
Salts of Fenofibric Acid and Pharmaceutical Formulations Thereof
In one aspect, the present invention relates to a formulation in the form of molecular dispersion comprising i) fenofibric acid, a physiologically acceptable salt or derivative thereof and optionally other active substances, ii) a binder component comprising at least one enteric binder, and optionally iii) other physiologically acceptable excipients. In a second aspect, the present invention relates to novel salts of fenofibric acid that are photostable when compared to other salts of fenofibric acid.
Owner:FOURNIER LAB IRELAND +1
Fenofibrate acid salt, preparation method, pharmaceutical composition and application
InactiveCN102304103AImprove solubilityImprove bioavailabilityOrganic active ingredientsMetabolism disorderSolubilityFENOFIBRIC ACID
The invention discloses fenofibrate acid salt, a preparation method, a pharmaceutical composition and application. The structural general formula of the fenofibrate acid salt is shown as an equation or an equation in the specification, wherein X represents methylene, O, CH3-N or CH3-CH; R1 represents methyl; m represents 0, 1, 2, 3 or 4; R2 represents H or methyl; and R3 represents HOCH2CH2OCH2CH2-, HOCH2CH2CH2-, HOCH2CH(OH)CH2-, HOCH2CH2-, HOCH2C(CH3)2-, (HOCH2)2CCH3- or HOCH2CH(CH3CH2)-. Compared with the current fenofibrate, the solubility of the fenofibrate acid salt provided by the invention in water is obviously increased; the solubility in 1 ml of pure water is more than 1 mg; the highest solubility is above 100 mg, therefore, the fenofibrate acid salt is easily absorbed by human bodies; and the bioavailability is also relatively and obviously increased.
Owner:TETRANOV PHARMA CO LTD
Diisopropylamine fenofibrate, preparation method of same, medicinal composition and use of same
InactiveCN101823956AReduce oxidationReduce storageOrganic active ingredientsMetabolism disorderSolubilitySide effect
The invention discloses a diisopropylamine fenofibrate, a preparation method of the same, a medicinal composition and use of the same, relating to the field of medicaments for lowering blood fat and treating fatty liver. The problems that fenofibric acid products is poor in water solubility, has toxic and side effects and the like in the prior art are solved. A product of the invention is obtained through the following steps of: adding a fenofibric acid and diisopropylamine into a solvent, heating to 35-70 DEG C, cooling, separating out a solid, pumping a filtrate, washing the solid with ether, and drying to obtain a white solid which is the diisopropylamine fenofibrate. A diisopropylamine part in the invention can overcome the side effect that the fenofibric acid is heated to be converted into ammonia enzyme and blood sugar and exerts a synergetic effect of lowering the blood fat together with fenofibrate.
Owner:曹桂英
Pharmaceutical Formulations
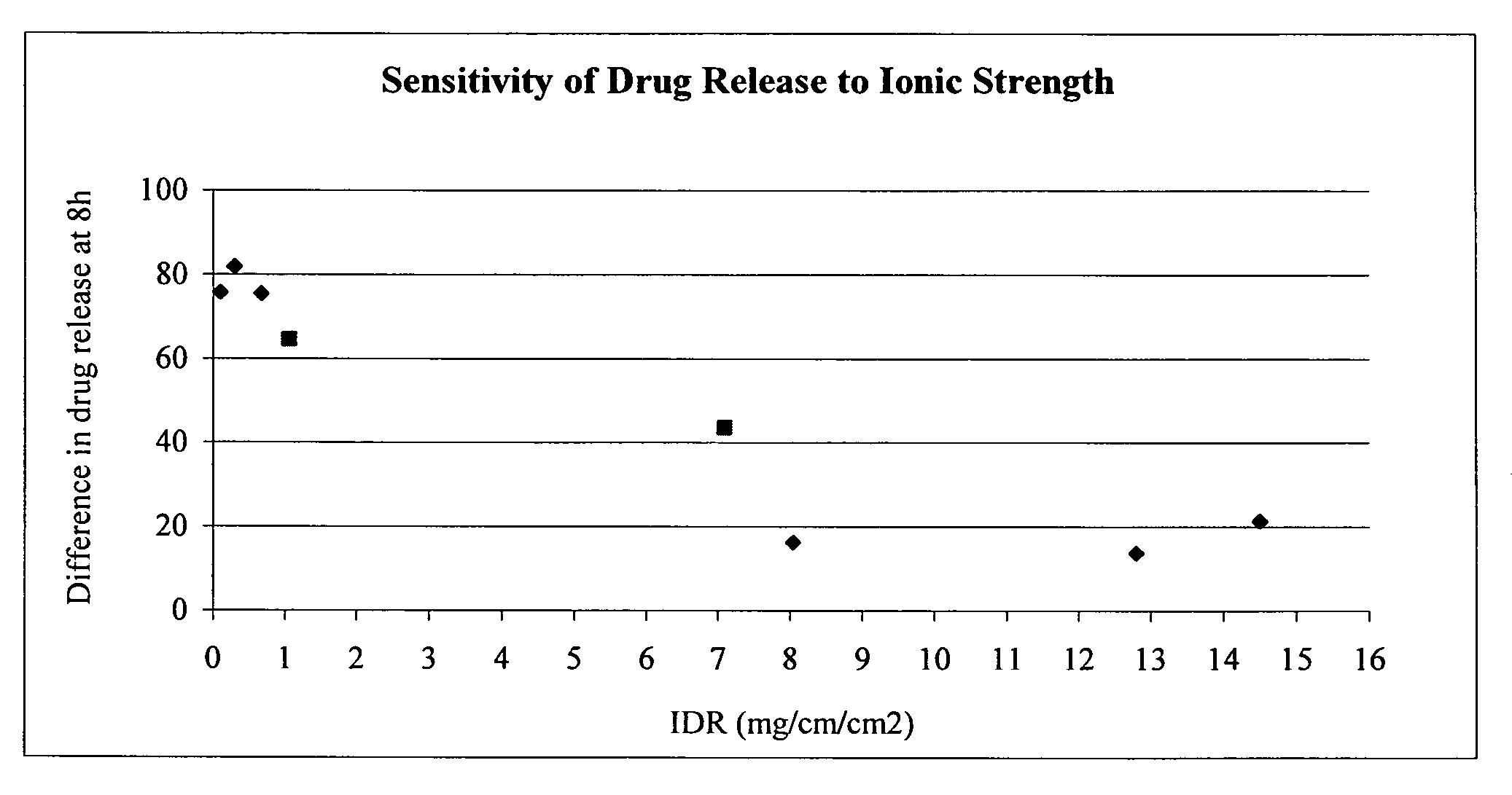
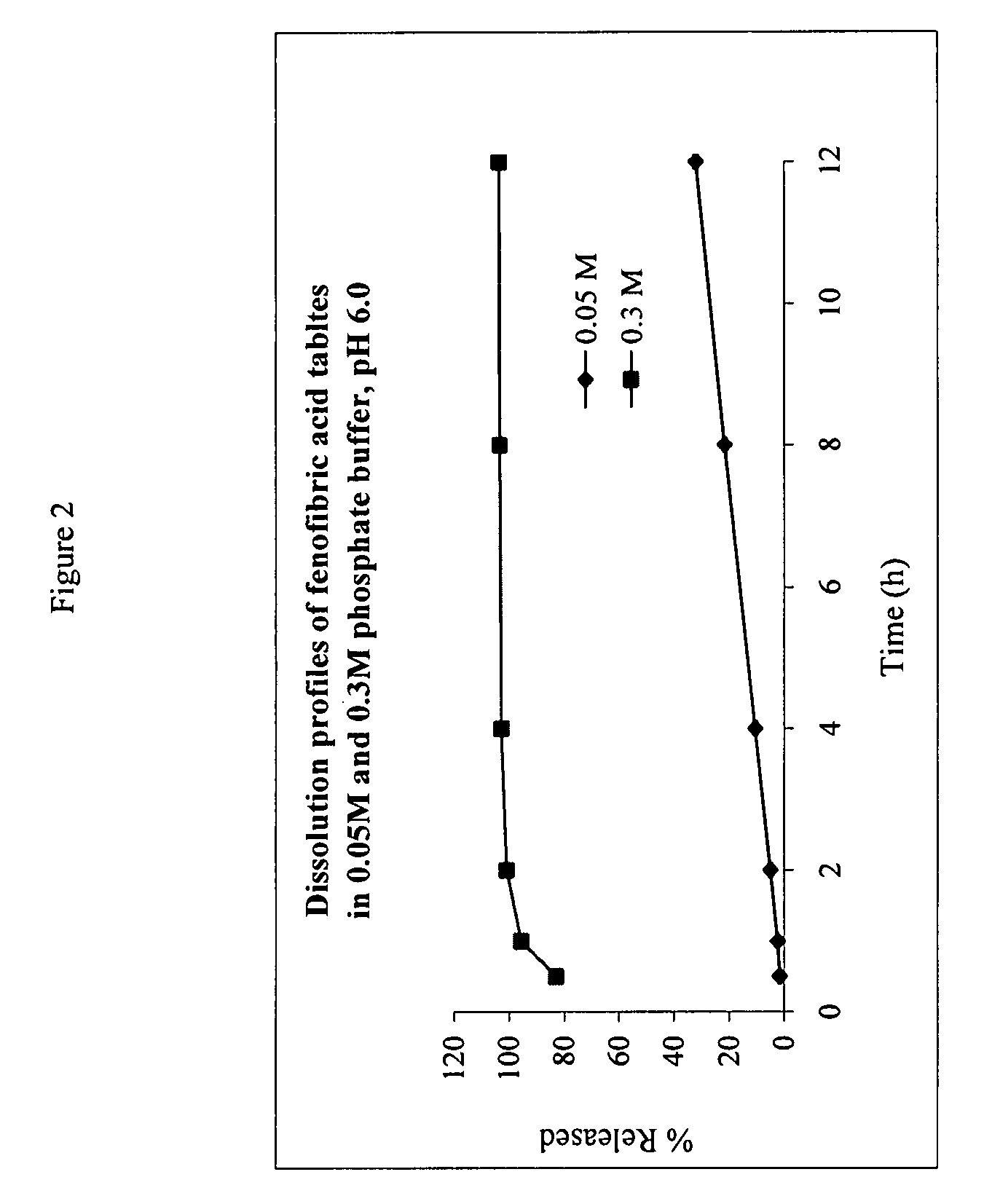
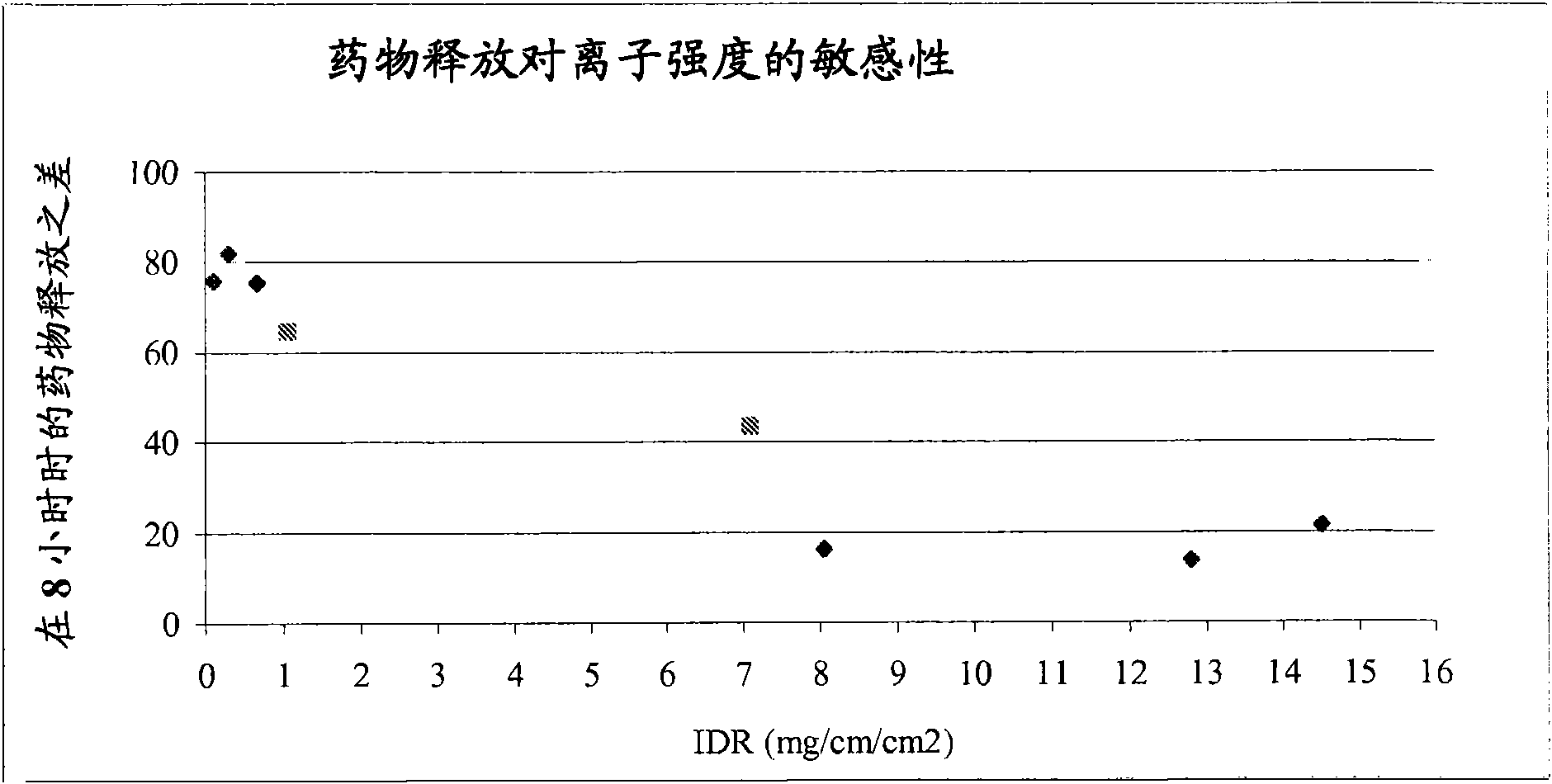
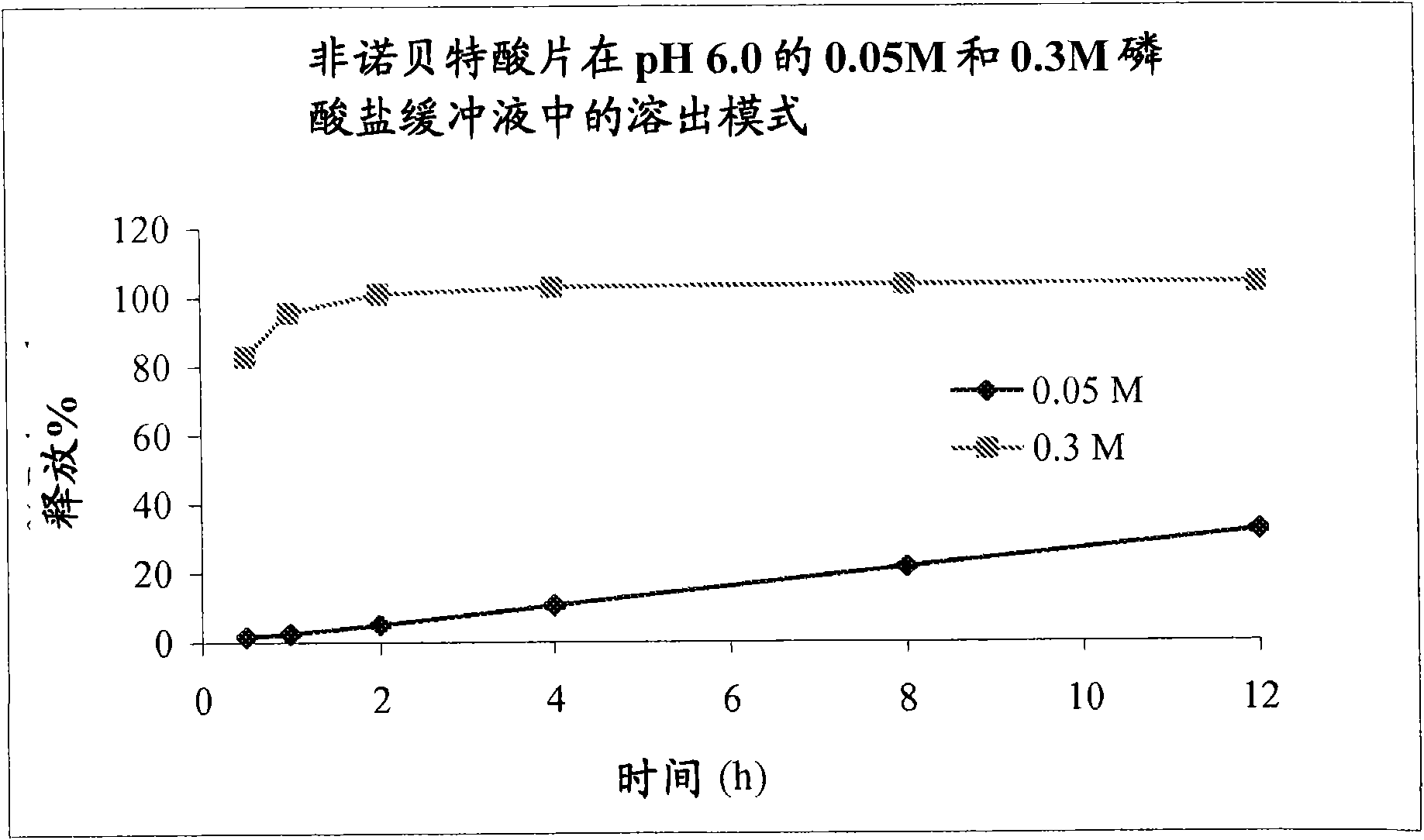
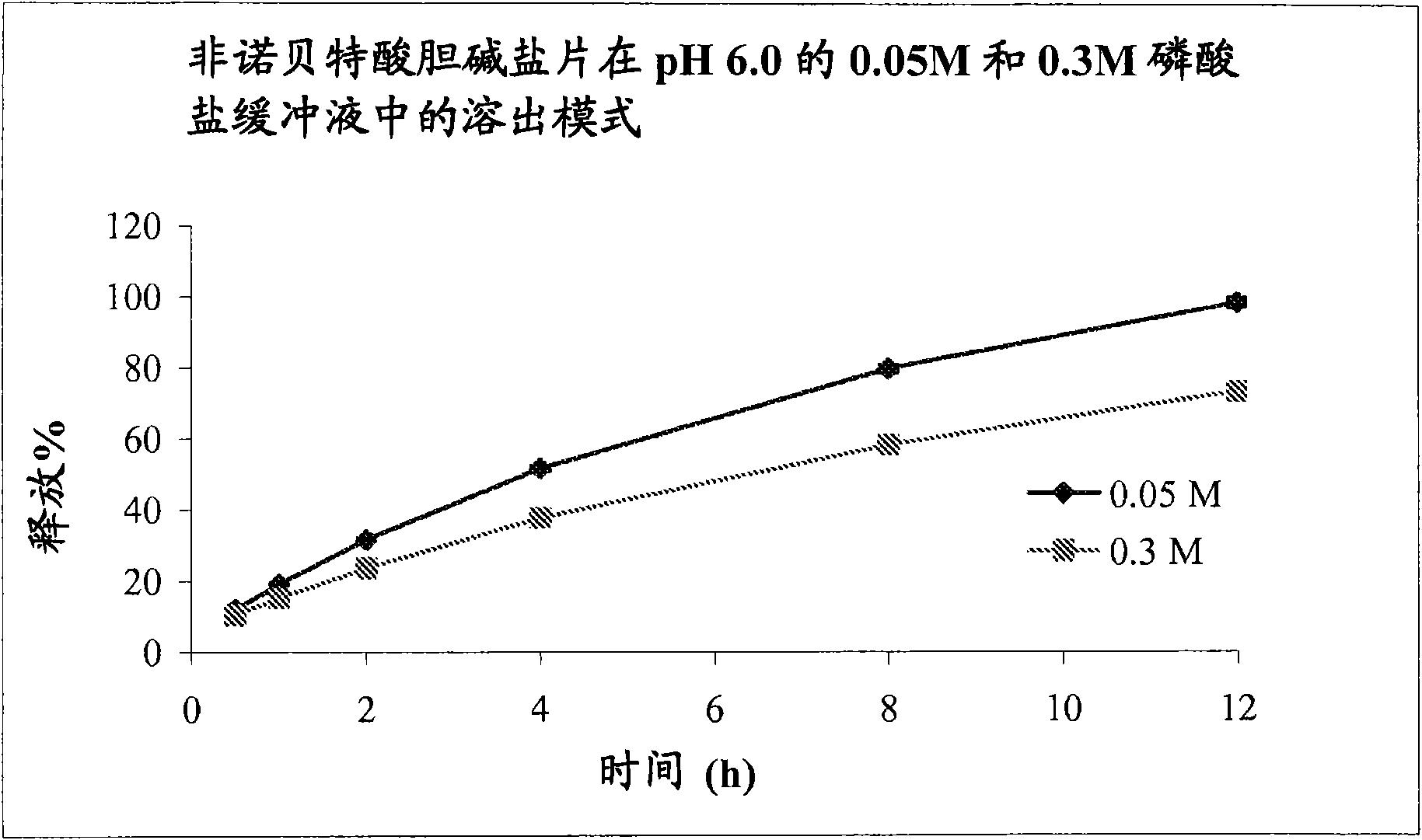
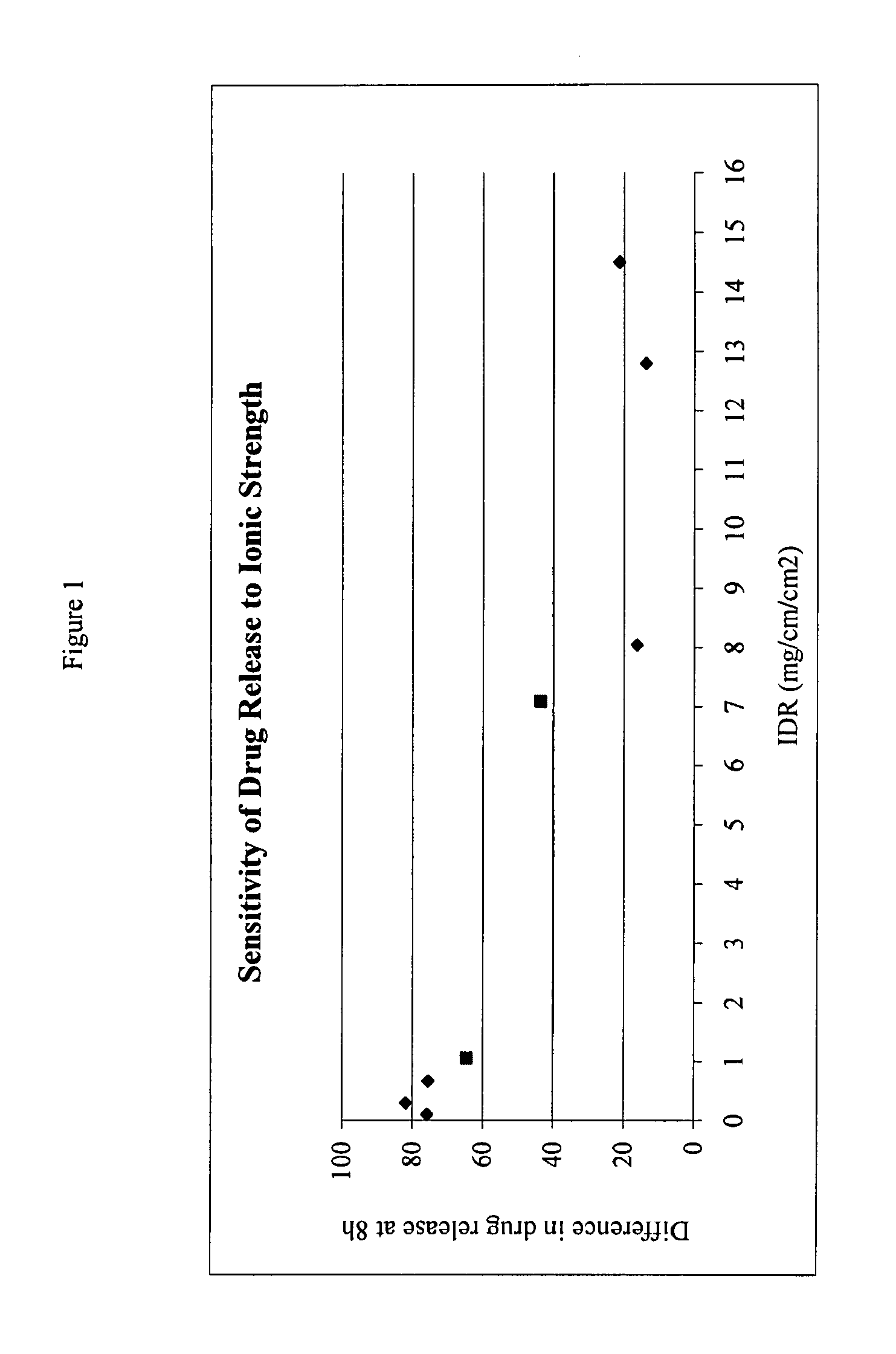
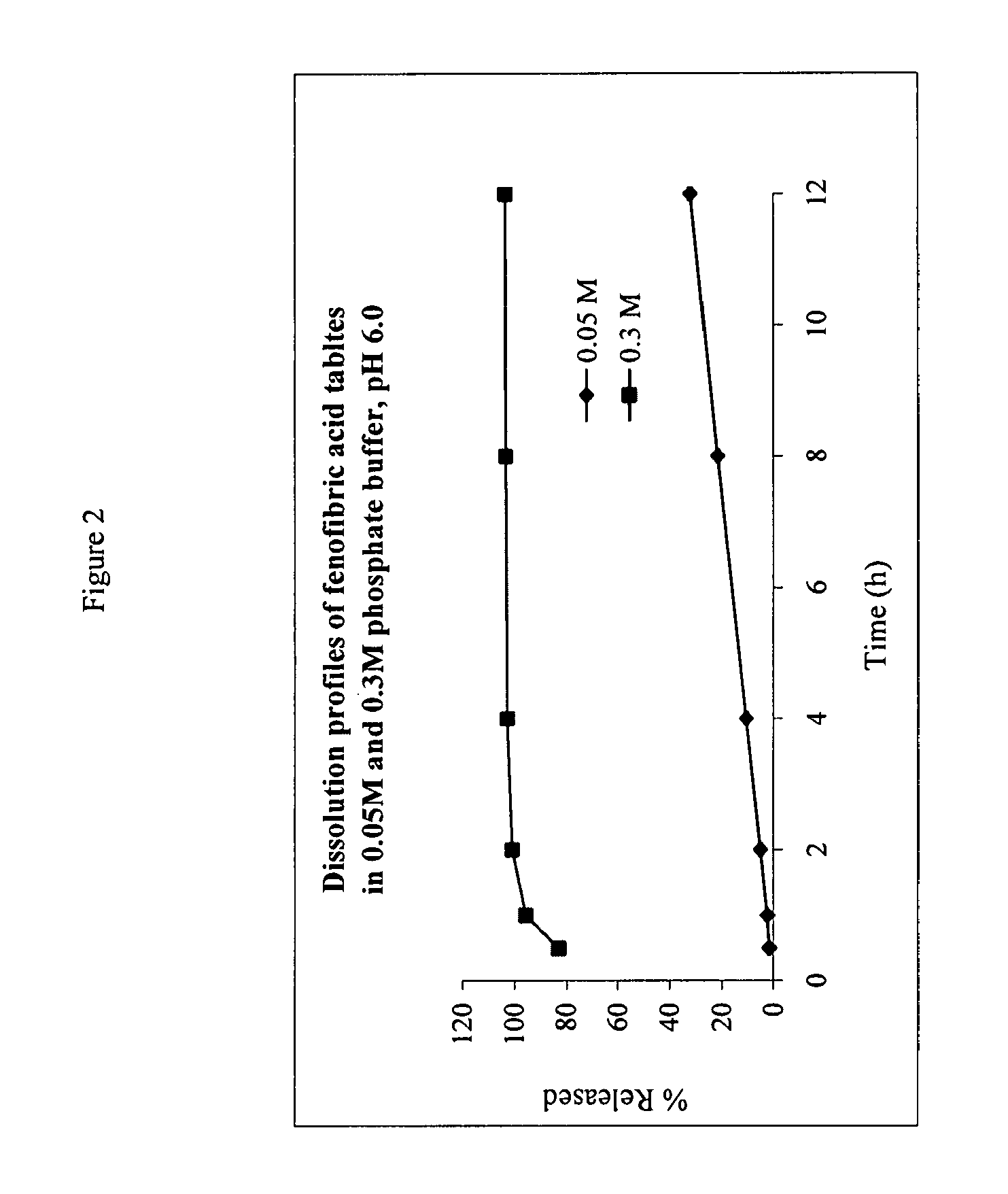
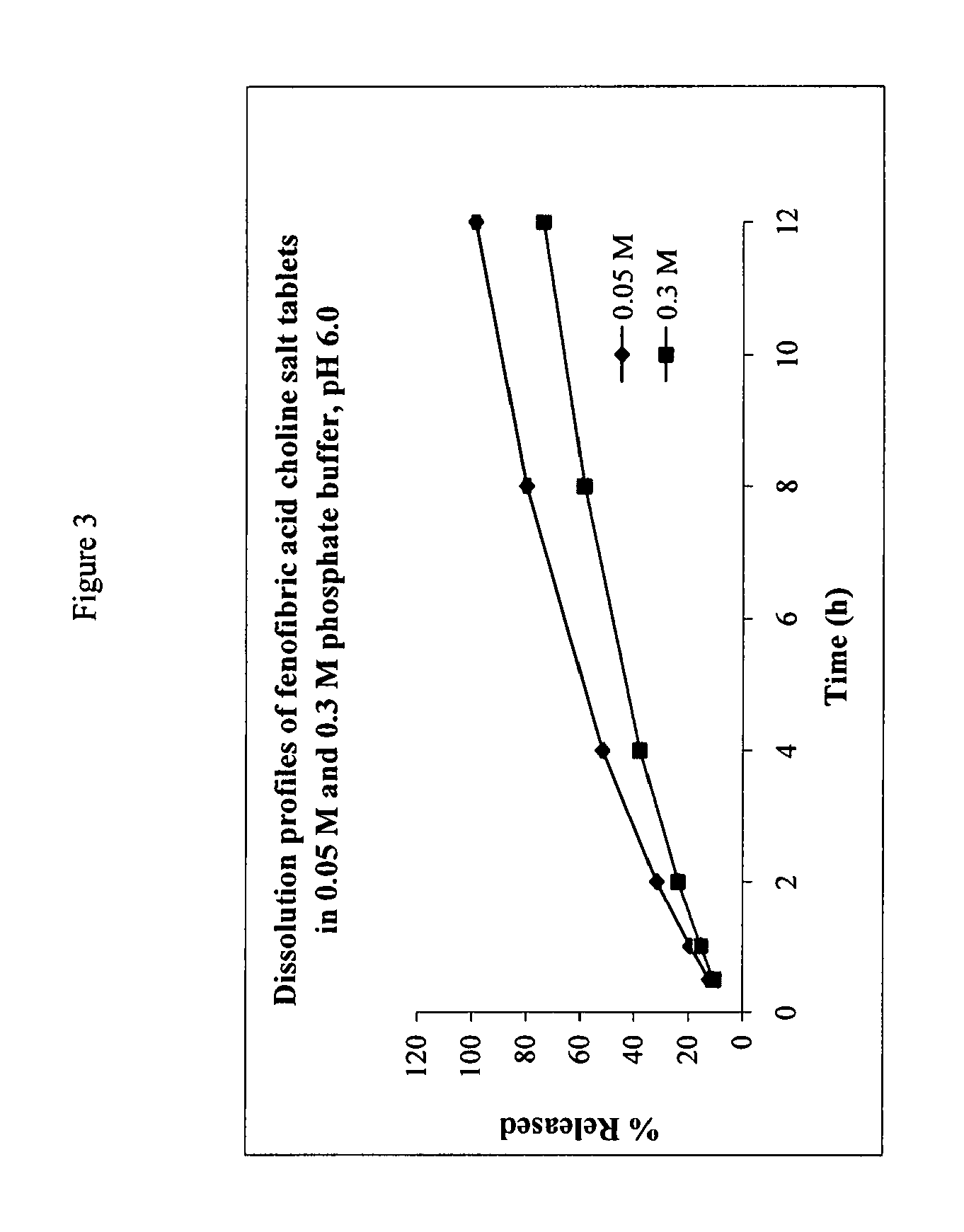
The present invention provides a modified release formulation comprising an active agent in a hydrophilic polymer matrix wherein the active agent is a salt of fenofibric acid wherein the release rate of the formulation in an in vitro dissolution is substantially independent of the ionic strength of the dissolution media.
Owner:FOURNIER LAB IRELAND
Formulation comprising fenofibric acid, a physiologically acceptable salt or derivative thereof
In one aspect, the present invention relates to a formulation in the form of molecular dispersion comprising i) fenofibric acid, a physiologically acceptable salt or derivative thereof and optionally other active substances, ii) a binder component comprising at least one enteric binder, and optionally iii) other physiologically acceptable excipients. In a second aspect, the present invention relates to novel salts of fenofibric acid that are photostable when compared to other salts of fenofibric acid.
Owner:ABBOTT GMBH & CO KG
Preparation method of high purity fenofibric acid
ActiveCN103360240AHigh purityLow costOrganic active ingredientsOrganic compound preparationFENOFIBRIC ACIDMedicinal chemistry
The invention provides a preparation method of high purity fenofibric acid. The preparation method successively comprises the following steps: reacting to produce fenofibric acid; crudely purifying of the fenofibric acid to obtain a fenofibric acid crude product; and refining the fenofibric acid to obtain the high purity fenofibric acid. The high purity fenofibric acid prepared by the preparation method has a purity of more than 99.5%, and is safe and reliable.
Owner:SHANDONG DANHONG PHARMA
Microemulsion Topical Delivery Platform
Provided are pharmaceutical carriers suitable based on oil-in-water microemulsions and methods of making same. Also provided are pharmaceutical compositions comprising a carrier of the invention and a lipophilic active pharmaceutical ingredient (API), as well as methods for making same. The pharmaceutical compositions are particularly suitable for use in formulating lipophilic APIs for topical administration to the eye. Specifically included are pharmaceutical compositions comprising fenofibrate or fenofibric acid as API. Also provided is a method of treating a disease of the posterior segment of the eye. Also provided is a pharmaceutical composition comprising a compound represented byformulated for topical administration to the eye.
Owner:EYECRO
Salts of fenofibric acid and pharmaceutical formulations thereof
In one aspect, the present invention relates to a formulation in the form of molecular dispersion comprising i) fenofibric acid, a physiologically acceptable salt or derivative thereof and optionally other active substances, ii) a binder component comprising at least one enteric binder, and optionally iii) other physiologically acceptable excipients.In a second aspect, the present invention relates to novel salts of fenofibric acid that are photostable when compared to other salts of fenofibric acid.
Owner:ABBOTT LAB INC
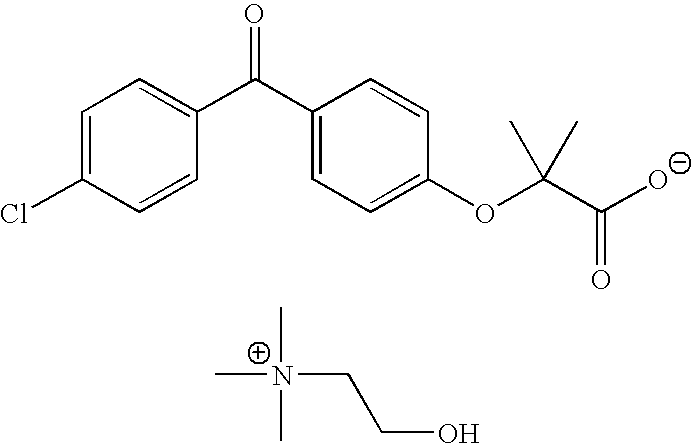
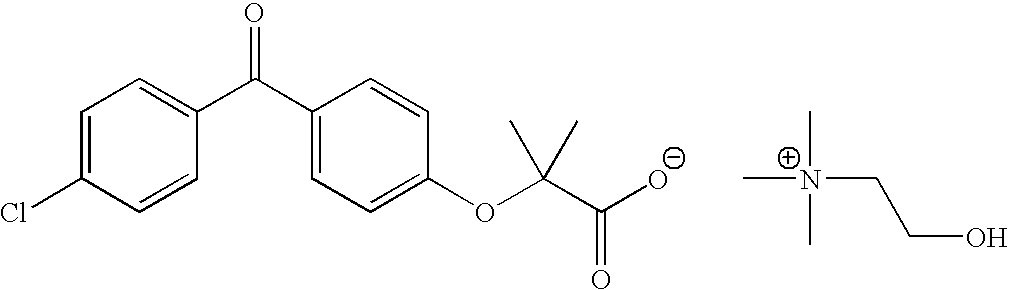
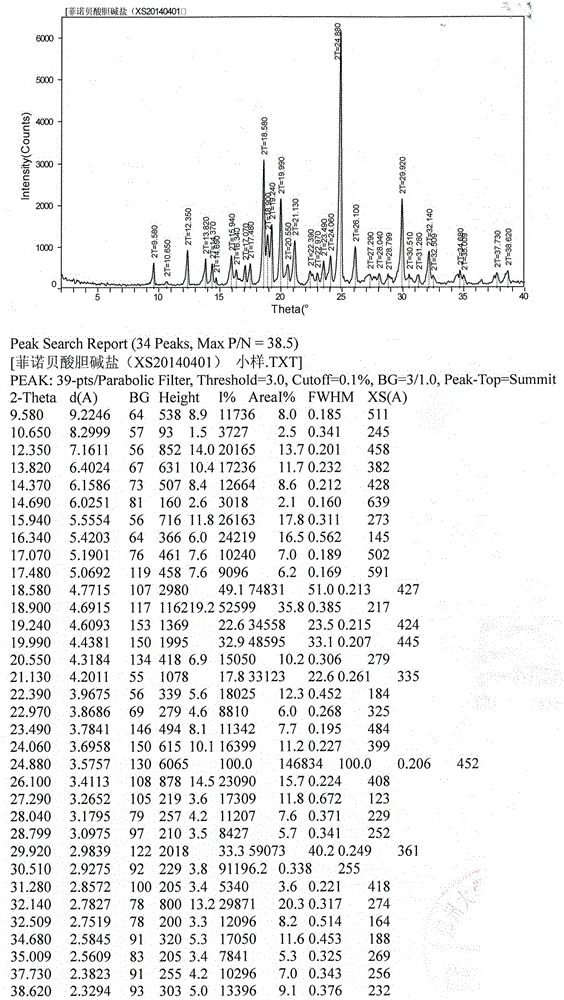
Fenofibric acid choline salt crystal form and preparation method thereof
InactiveCN104628564AImprove stabilityImprove bioavailabilityOrganic compound preparationCarboxylic acid esters preparationMedicineFENOFIBRIC ACID
The invention belongs to the field of pharmacy and in particular relates to a novel crystal form of a fenofibric acid choline salt and a preparation method thereof. The X-ray powder diffraction spectrum of the fenofibric acid choline salt crystal form has characteristic peaks at the following d values: 7.161, 5.555, 4.772, 4.692, 4.609, 4.438, 4.201, 3.696, 3.576, 3.411, 2.984 and 2.783. The method for preparing the fenofibric acid choline salt crystal form comprises the following steps: dissolving a crude product or a crystal form of the fenofibric acid choline salt in a mixed solvent of isopropanol and water, and crystallizing, thereby obtaining the crystal form. The fenofibric acid choline salt crystal form is high in stability, and the preparation method is high in controllability and high in reproducibility and is suitable for large-scale pharmaceutical production.
Owner:ZHONGSHUAI PHARMA SCI & TECH CO LTD
Pharmaceutical formulations
InactiveCN101677981AEasy to understandOrganic active ingredientsMetabolism disorderIonic strengthHydrophilic polymers
The present invention provides a modified release formulation comprising an active agent in a hydrophilic polymer matrix wherein the active agent is a salt of fenofibric acid wherein the release rateof the formulation in an in vitro dissolution is substantially independent of the ionic strength of the dissolution media.
Owner:ABBOTT LAB INC +1
Method for preparing fenofibric acid by using inorganic alkali as catalyst
ActiveCN103951557AReduce dosageGuaranteed yieldPreparation from carboxylic acid saltsOrganic compound preparationFENOFIBRIC ACIDDistillation
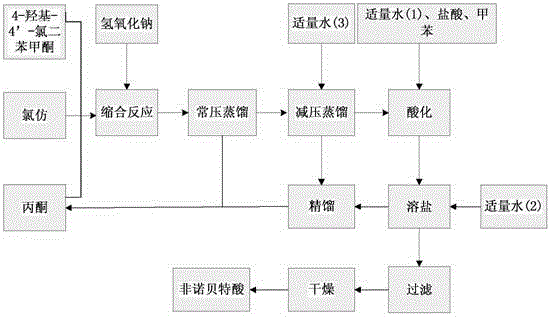
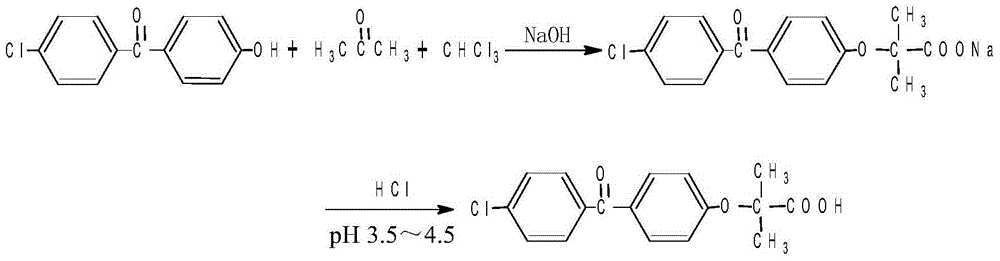
The invention discloses a method for preparing fenofibric acid by using inorganic alkali as a catalyst, belonging to the field of fenofibric acid catalytic synthesis processes. The method comprises the following steps: by using 4-hydroxyl-4'-chlorobenzophenone, acetone and chloroform as initial raw materials, using sodium hydroxide as a catalyst, and using acetone as a solvent at the same time, performing condensation reaction, and then performing atmospheric distillation, reduced pressure distillation, acidification, filtration and drying to obtain fenofibric acid. The method can be used for greatly reducing the consumption of main raw materials, significantly reducing the production cost of products, and obviously reducing three wastes, so that the method is an efficient and environment-friendly method for synthesizing fenofibric acid, and is suitable for large-scale industrial popularization and application.
Owner:XUZHOU CURRENCY MAGNETOELECTRICITY
Microemulsion topical delivery platform
Owner:EYECRO
Refining method for high-purity clofibric acid blood-lipid regulating drug--fenofibric acid
InactiveCN103965038AHigh purityResponse equipment requirements are lowCarboxylic compound separation/purificationOrganic solventFENOFIBRIC ACID
The invention provides a refining method for a high-purity clofibric acid blood-lipid regulating drug--fenofibric acid and specifically relates to a method of three-step recrystallization of a crude fenofibric acid product for preparation of high-purity fenofibric acid, belonging to refining methods in the field of physics. According to the invention, the crude fenofibric acid product is subjected to organic solvent recrystallization, mixed solvent recrystallization and methanol recrystallization so as to prepare a finished fenofibric acid product. Solvents used in the method are frequently used solvents and are cheap and easily available, requirements on reaction equipment are low, so transformation production is facilitated. With the method, the finished fenofibric acid product with purity of no less than 99.9% can be obtained.
Owner:肖广常
Preparation method for high-purity fenofibric acid crude drugs
InactiveCN105130795ALess impuritiesHigh purityOrganic compound preparationCarbonyl compound preparation by condensationFENOFIBRIC ACIDEthyl Chloride
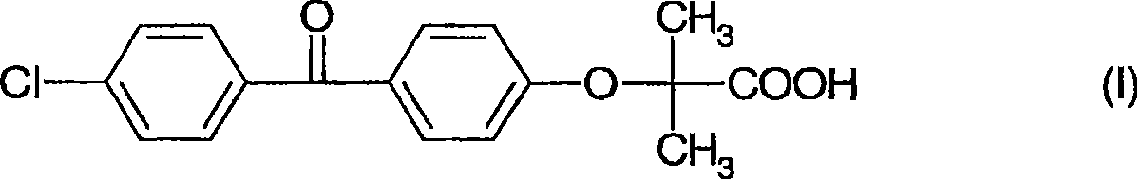
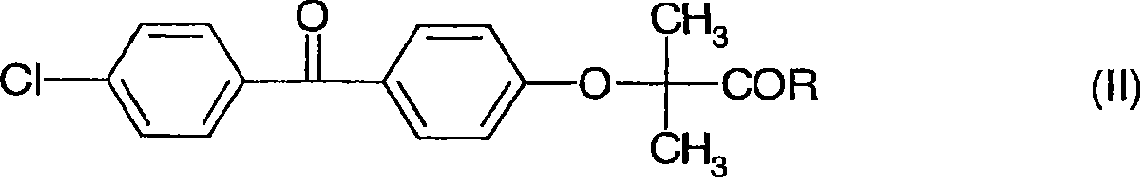
The invention discloses a preparation method for fenofibric acid. According to the preparation method, with a compound expressed by the structural formula (I) being a raw material, the compound, acetone and chloroform are subjected to condensation reaction under the condition that sodium hydroxide serves as a catalyst to obtain fenofibric acid, and the structural formula (I) is shown as in the specification. In the structural formula (I), the expressed compound is 4'-chlorine-4-hydroxybenzophenone. The compound in the structural formula (I), acetone and chloroform are subjected to condensation reaction under catalyzing of sodium hydroxide, hydrolysis is performed through a sodium hydroxide solution, the pH value is adjusted two times, and then a fenofibric acid crude product is obtained; the crude product is recrystallized through isopropanol to obtain the high-purity fenofibric acid. The fenofibric acid product obtained through synthesis by the adoption of the method is high in yield and low in cost, and the preparation method is easy to implement and suitable for industrialized production.
Owner:KANGYA OF NINGXIA PHARMA
Novel method of synthesizing fenofibrate
InactiveUS20120065421A1High chemical yieldImprove qualityMetabolism disorderOrganic compound preparationFENOFIBRIC ACIDIndustrial scale
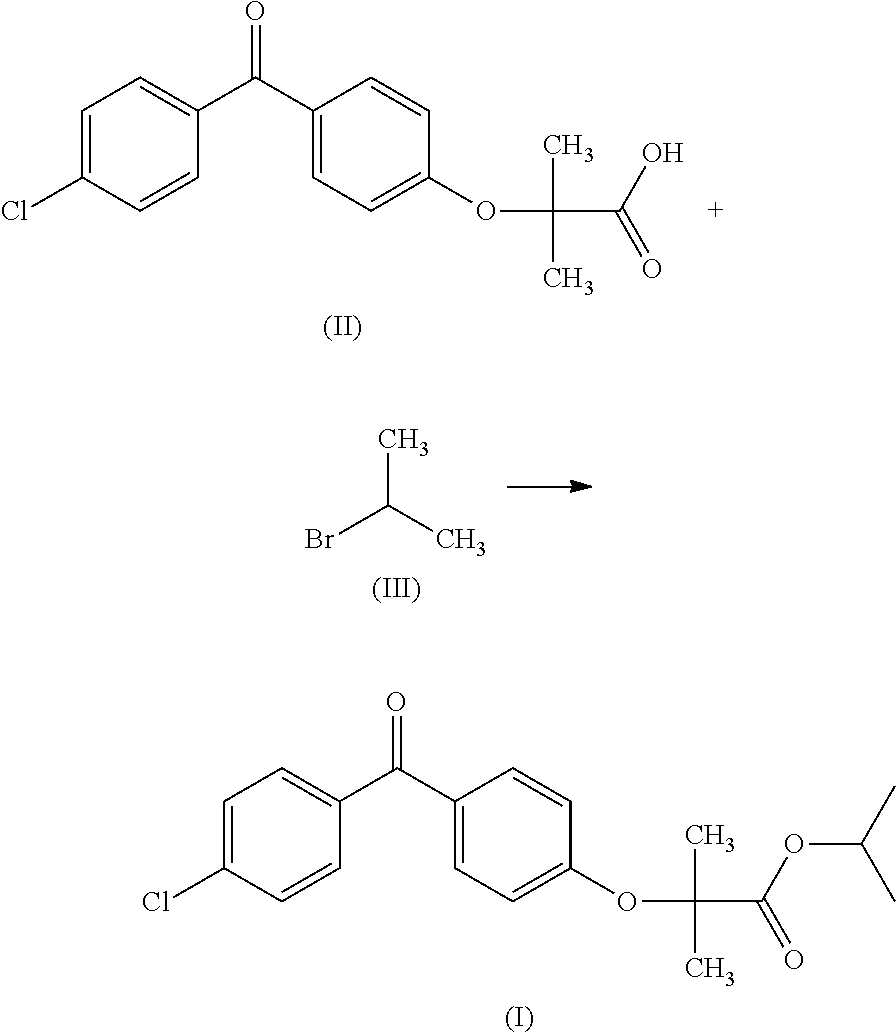
A novel process for the synthesis of fenofibrate, includes reacting a metal salt of fenofibric acid with an isopropyl halide, in a solvent system composed of a mixture of dimethyl sulfoxide and a C2-C4 alkyl acetate. The process can be used on an industrial scale and makes it possible to obtain a fenofibrate of a quality in accordance with the Pharmacopoeia without the need for purification by recrystallization.
Owner:SYNKEM
Production technology of Fenofibric acid
ActiveCN103922925AHigh yieldHigh purityOrganic compound preparationCarboxylic compound separation/purificationFENOFIBRIC ACIDBenzyl cyanide
The invention belongs to the technical field of medicine and discloses a production technology of Fenofibric acid. The production technology mainly comprises the following steps that (1) under an alkaline condition, a condensation reaction is conducted through 4-chlorine-4'-hydroxybenzophenone, trichloromethane and acetone so that the Fenofibric acid can be generated and after separation and purification are conducted on reaction liquid, the crude Fenofibric acid is obtained; (2) a crystal solution is added to the crude Fenofibric acid obtained in the step (1) and dissolution and crystallization are conducted to obtain the refined Fenofibric acid; the crystal solution is a mixed solution composed of one or more of methyl alcohol, alcohol, acetone, toluene, acetonitrile and ethyl acetate. According to the production technology of the Fenofibric acid, solvent for separating and purifying the crude product and technological parameters are optimized and the recovery rate and the purity of the crude product are greatly improved. According to the production technology, great optimization and adjustment are conducted on crystallization solvent and a crystallization condition in the following refined purification process. Compared with the prior art, the recovery rate of the medicine-level pure Fenofibric acid is increased by 10% to 20%.
Owner:CHENGDU YILUKANG MEDICAL TECH & SERVICE
Choline fenofibric acid sustained release pellets and preparation method thereof
InactiveCN104434847AIncreased surface distribution areaReduce absorption rateOrganic active ingredientsMetabolism disorderSustained release pelletsFENOFIBRIC ACID
The invention provides choline fenofibric acid sustained release pellets. The choline fenofibric acid sustained release pellets comprise medicine-containing pellets and coating layers, wherein the medicine-containing pellets are coated with the coating layers; the medicine-containing pellets comprise 45mg of choline fenofibric acid, 100-200mg of hollow cores, 100-200mg of filling agents, 25-125mg of lubricating agents and 5-50mg of bonding agents; the coating layers comprise 35-175mg of Eudragit NE30D and 5-52mg of talcum powder. A preparation method of the choline fenofibric acid sustained release pellets comprises the following processes: 1. material preparation; 2. mixing; 3. preparation of the bonding agents; 4. preparation of the pellets; 5. preparation of coating agents; 6. coating; 7. filling; 8. aluminium-plastic packaging and preparation of finished products. The choline fenofibric acid sustained release pellets used for reducing blood lipid have the beneficial effects that as the two kinds of advanced technologies, namely novel sustained release preparations and pellet preparations, are adopted, the choline fenofibric acid sustained release pellets have stable treatment effects and higher bioavailability and have the advantages of good medicine stability, convenience in packaging, transportation and storage, and the like. The preparation method of the choline fenofibric acid sustained release pellets is simple and practicable and is suitable for industrial production.
Owner:HARBIN SHENGJI PHARMA
Enteric coated tablet of fenofibric acid and physiologically acceptable salts and preparation method of enteric coated tablet
ActiveCN103083275ASimple processImprove stabilityOrganic active ingredientsMetabolism disorderSustained Release TabletPlasticizer
The invention discloses an enteric-coated sustained release tablet of fenofibric acid or physiologically acceptable salts and a preparation method of the enteric-coated sustained release tablet. The enteric-coated sustained release tablet is prepared form the following components: the fenofibric acid or physiologically acceptable salts, a sustained-release material, a filling agent, a lubricant, an enteric coating material and alternatively plasticizer and / or an anti-sticking agent. The enteric-coated sustained release tablet of the fenofibric acid or the physiologically acceptable salts and the preparation method of the enteric-coated sustained release tablet have the advantages of a simple process, taking convenience and the like.
Owner:YANGTZE RIVER PHARM GRP CO LTD
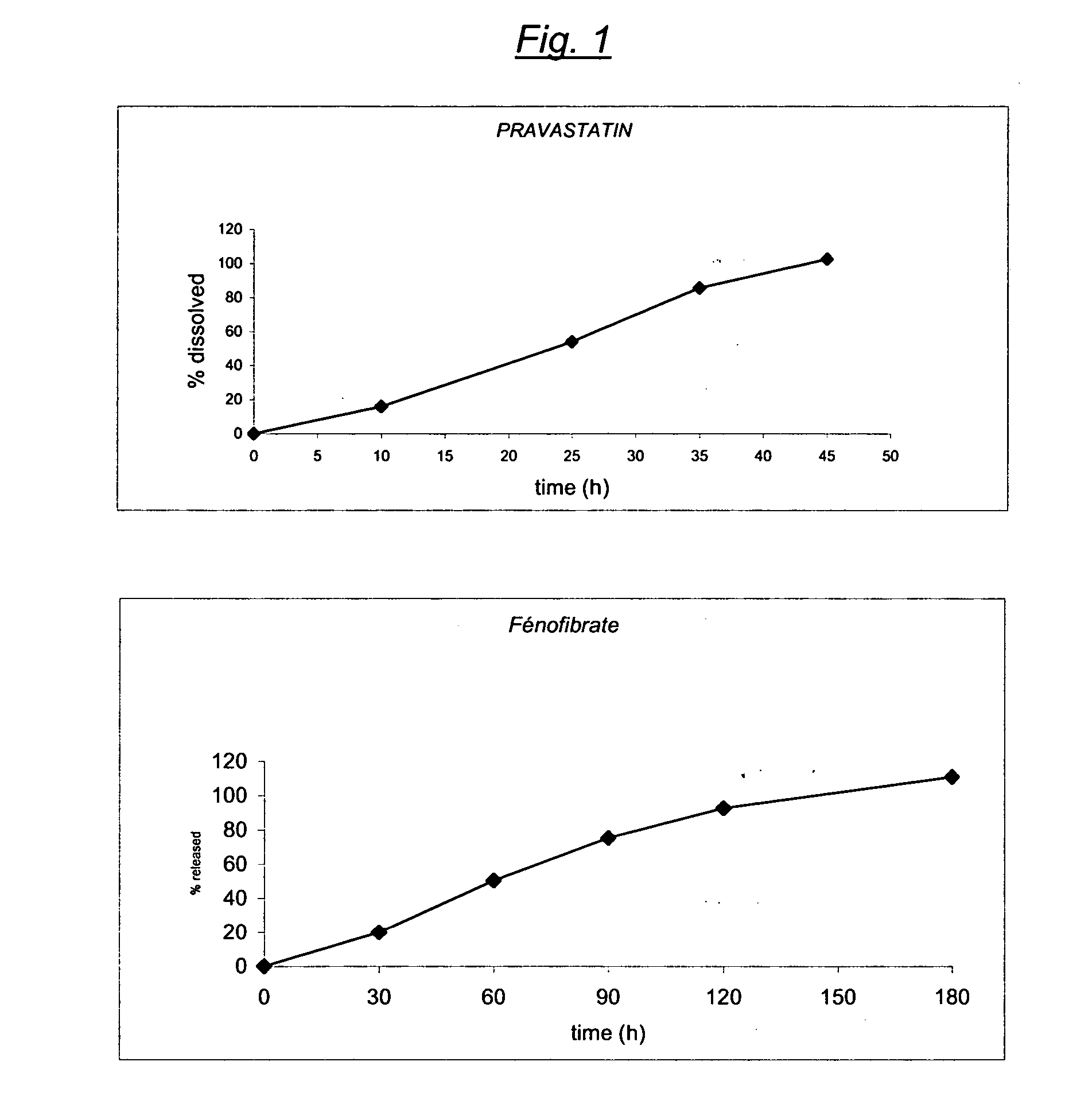
Stable controlled release pharmaceutical compositions containing fenofibrate and pravastatin
InactiveUS20070092567A1Eliminate side effectsReduced bioavailabilityBiocideMetabolism disorderControlled releaseFENOFIBRIC ACID
A controlled Release Pharmaceutical composition comprising an effective amount of Pravastatin and Fenofibrate, characterised in that the difference, in absolute value, between the times of maximal concentration (Tmax) of Pravastatin and Fenofibric acid is not less than 1.5 hours upon administration with food to humans.
Owner:GALEPHAR PHARMA RES
Calcium rosuvastatin and fenofibric acid choline salt time-selecting osmotic pump controlled release tablet and preparation method thereof
InactiveCN103356500ASmall toxicityImprove complianceOrganic active ingredientsMetabolism disorderFENOFIBRIC ACIDControlled Release Tablet
The invention provides a compound preparation consisting of calcium rosuvastatin and a fenofibric acid choline salt time-selecting osmotic pump controlled release tablet and a preparation method thereof. The compound preparation structurally comprises the following components from inside to outside in sequence: a tablet chip consisting of a medicine containing layer and a boosting layer, a controlled release coating for a medicine release hole, a calcium rosuvastatin quick release layer and a protective gastric soluble thin film coating. The preparation method comprises the following steps of: (1) preparing the medicine containing layer; (2) preparing the boosting layer; (3) pressing the tablet chip; (5) coating a control release coating; (6) punching the coating tablet; (6) coating the calcium rosuvastatin quick release layer; and (7) coating the protective gastric soluble thin film coating.
Owner:肖广常
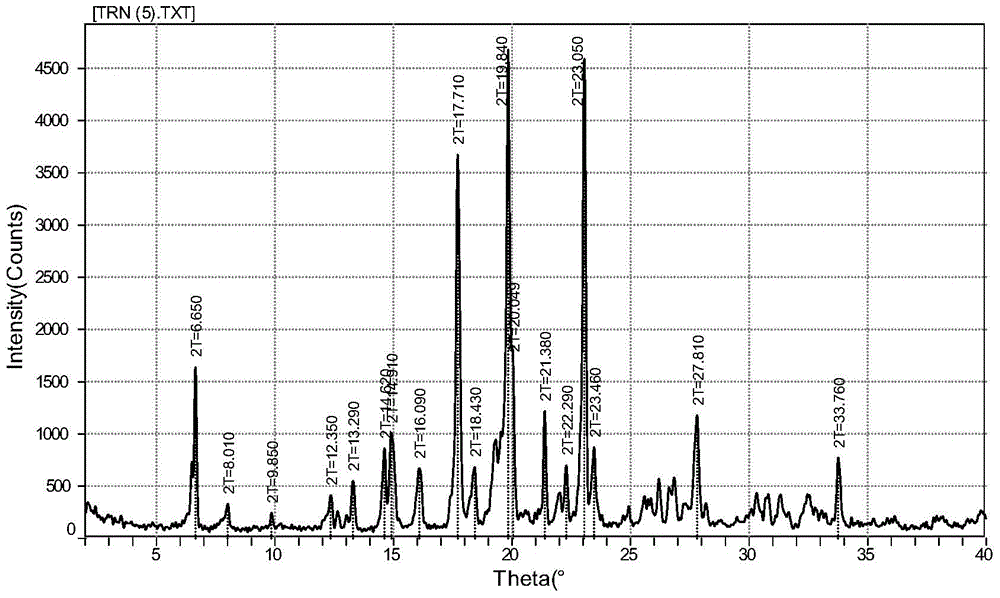
Fenofibric acid glycine ethyl ester crystal form and preparation method thereof
InactiveCN104803842AImprove stabilityImprove bioavailabilityMetabolism disorderOrganic compound preparationSecondary hyperlipidemiaFENOFIBRIC ACID
The invention discloses a fenofibric acid glycine ethyl ester crystal form and a preparation method thereof. The crystal form is the fenofibric acid glycine ethyl ester crystal form shown in a structural formula (I) and is represented through a 2theta angle in an X-ray powder diffraction map, and X-ray powder diffraction peaks exist at the angles of 6.650 degrees, 8.010 degrees, 9.850 degrees, 12.350 degrees, 13.290 degrees, 14.620 degrees, 14.910 degrees, 16.090 degrees, 17.710 degrees, 18.430 degrees, 19.840 degrees, 20.049 degrees, 21.380 degrees, 22.290 degrees, 23.050 degrees, 23.460 degrees, 27.810 degrees and 33.760 degrees. The fenofibric acid glycine ethyl ester crystal form has high stability, the stability and the bioavailability of fenofibric acid glycine ethyl ester are greatly improved, and the fenofibric acid glycine ethyl ester has a broad application prospect in the hyperlipidemia treatment process.
Owner:TETRANOV PHARMA CO LTD
Method of synthesizing fenofibrate
InactiveUS8445715B2High chemical yieldImprove qualityMetabolism disorderOrganic compound preparationFENOFIBRIC ACIDIndustrial scale
A novel process for the synthesis of fenofibrate, includes reacting a metal salt of fenofibric acid with an isopropyl halide, in a solvent system composed of a mixture of dimethyl sulfoxide and a C2-C4 alkyl acetate. The process can be used on an industrial scale and makes it possible to obtain a fenofibrate of a quality in accordance with the Pharmacopoeia without the need for purification by recrystallization.
Owner:SYNKEM
Method for preparing fenofibrate by virtue of intelligent temperature control and remote monitoring
ActiveCN104276950AMild reaction conditionsShort reaction timeOrganic compound preparationCarboxylic acid esters preparationTemperature controlAfter treatment
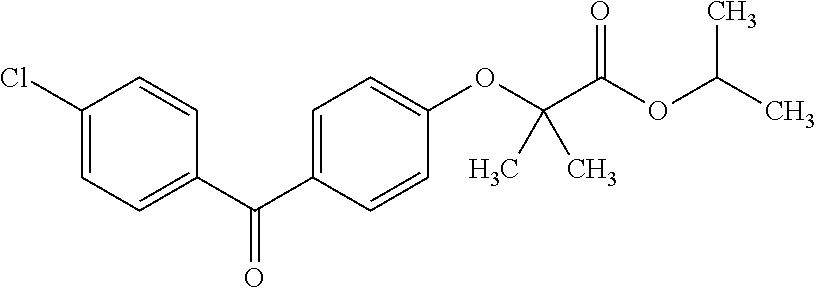
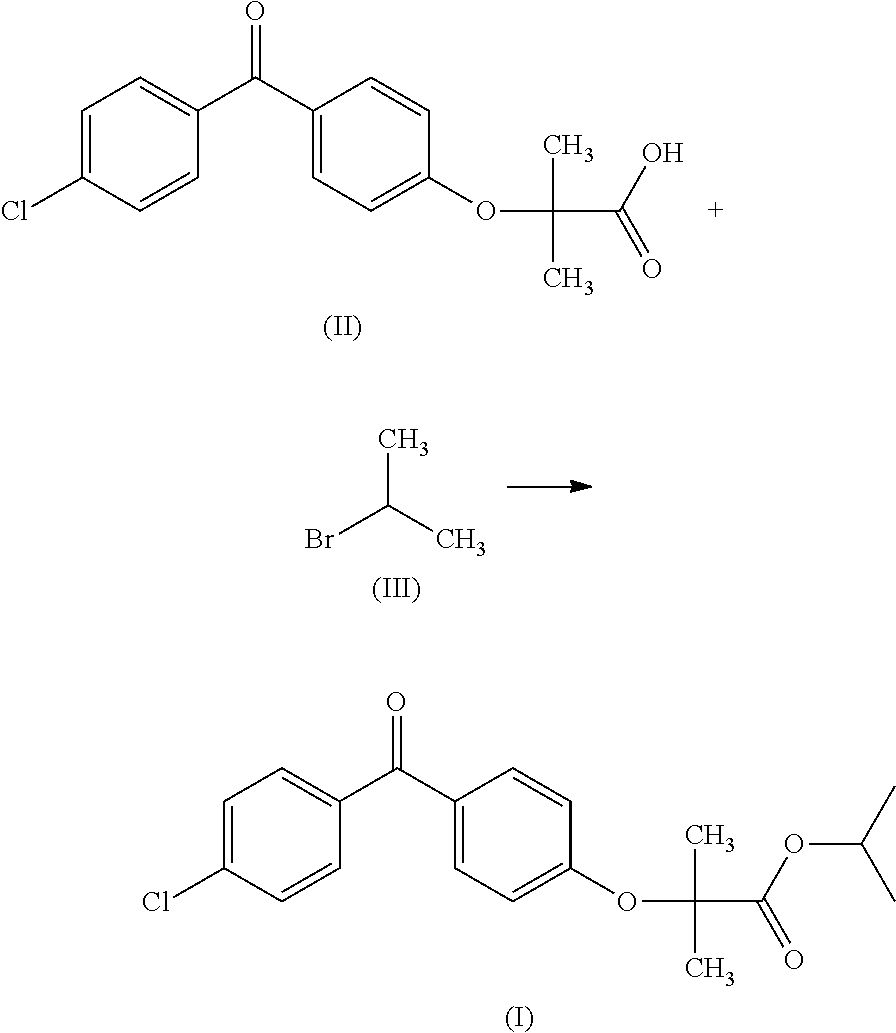
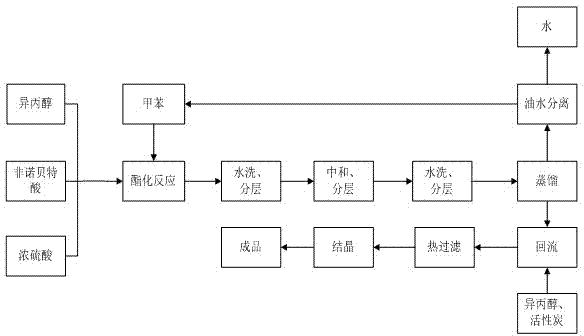
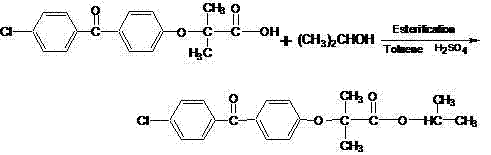
The invention discloses a method for preparing fenofibrate and specifically relates to a method for preparing fenofibrate by virtue of intelligent temperature control and remote monitoring. The method comprises the following steps: reacting by taking fenofibric acid and isopropanol as raw materials and concentrated sulfuric acid as a catalyst, and after the reaction of the raw materials in the presence of the catalyst is finished, performing water washing, neutralizing, water washing, distilling and purifying operations on the product to obtain the fenofibrate. Besides, toluene is taken as a water-carrying agent, the method has the advantages of mild reaction conditions, short reaction time, convenient after-treatment, low dosage of acid and alkali, few impurities, low environmental pollution, recycling of non-reacting fenofibric acid and the like; the product yield is above 85% and the production cost is low; as a result, the method for synthesizing the fenofibrate is efficient and environment-friendly, and is advantageous to large-scale industrial production.
Owner:NANJING GWDR POWER TECH
Pharmaceutical formulations
The present invention provides a modified release formulation comprising an active agent in a hydrophilic polymer matrix wherein the active agent is a salt of fenofibric acid wherein the release rate of the formulation in an in vitro dissolution is substantially independent of the ionic strength of the dissolution media.
Owner:ABBVIE INC
Reduced dose oral pharmaceutical compositions of fenofibrate
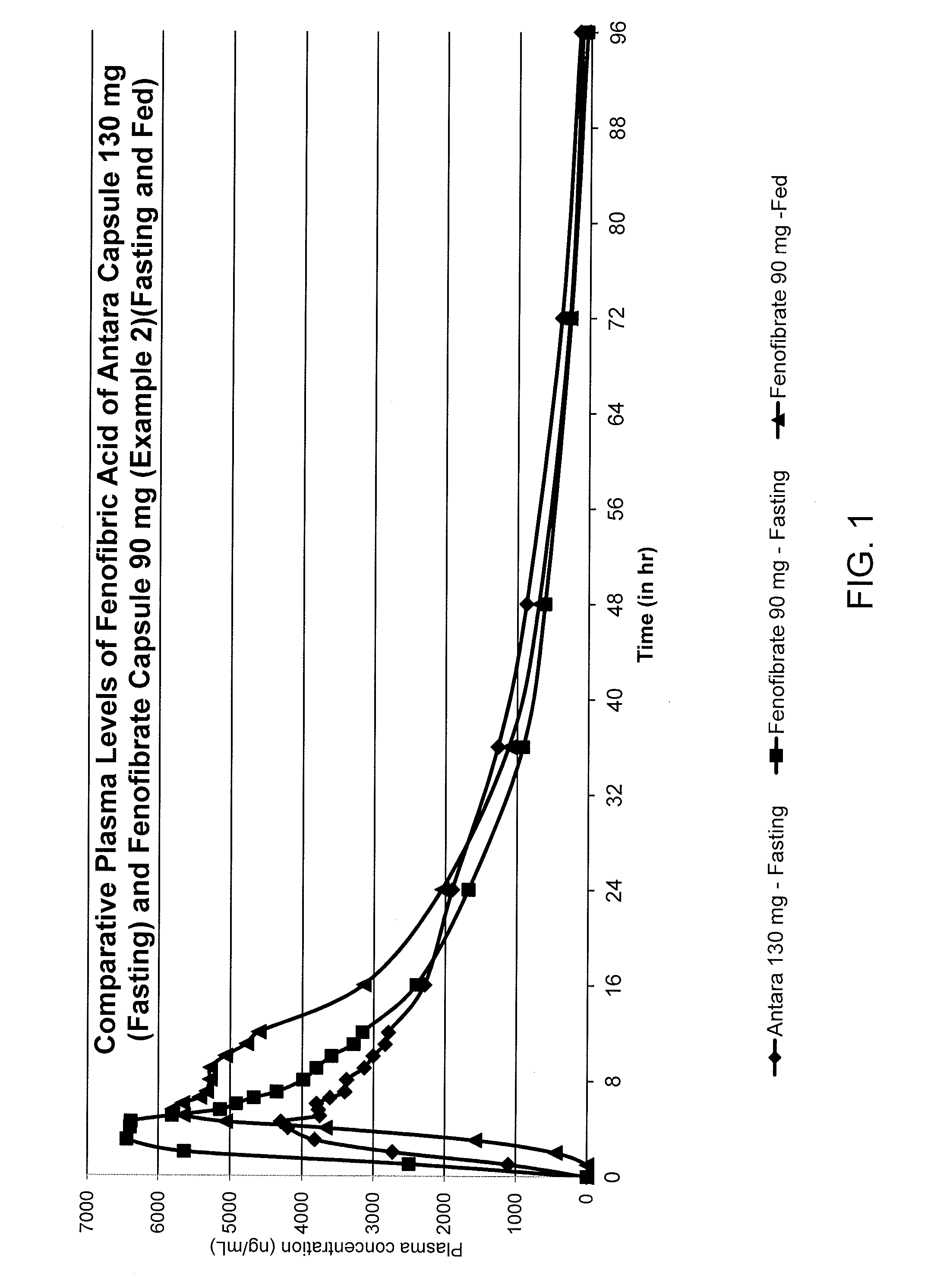
The invention relates to reduced dose oral pharmaceutical composition of fenofibrate which exhibits substantial bioequivalence to Antara® Capsules under fasting condition and also capable of reducing the food effect on bioavailability of fenofibrate. Provided is a pharmaceutical composition comprising about 90 mg of fenofibrate particles having a D90 particle size of less than about 600 nm and a pharmaceutically acceptable carrier, wherein the pharmaceutical composition is a solid dosage form suitable for oral administration and is substantially free of food effect such that when administered orally to a human provides an AUC0-t value for fenofibric acid in the blood plasma of the human under a fed state which is higher than the AUC0-t value under a fasted state by up to 12%, wherein t is 96 hours from the administration of the pharmaceutical composition.
Owner:LUPIN ATLANTIS HLDG
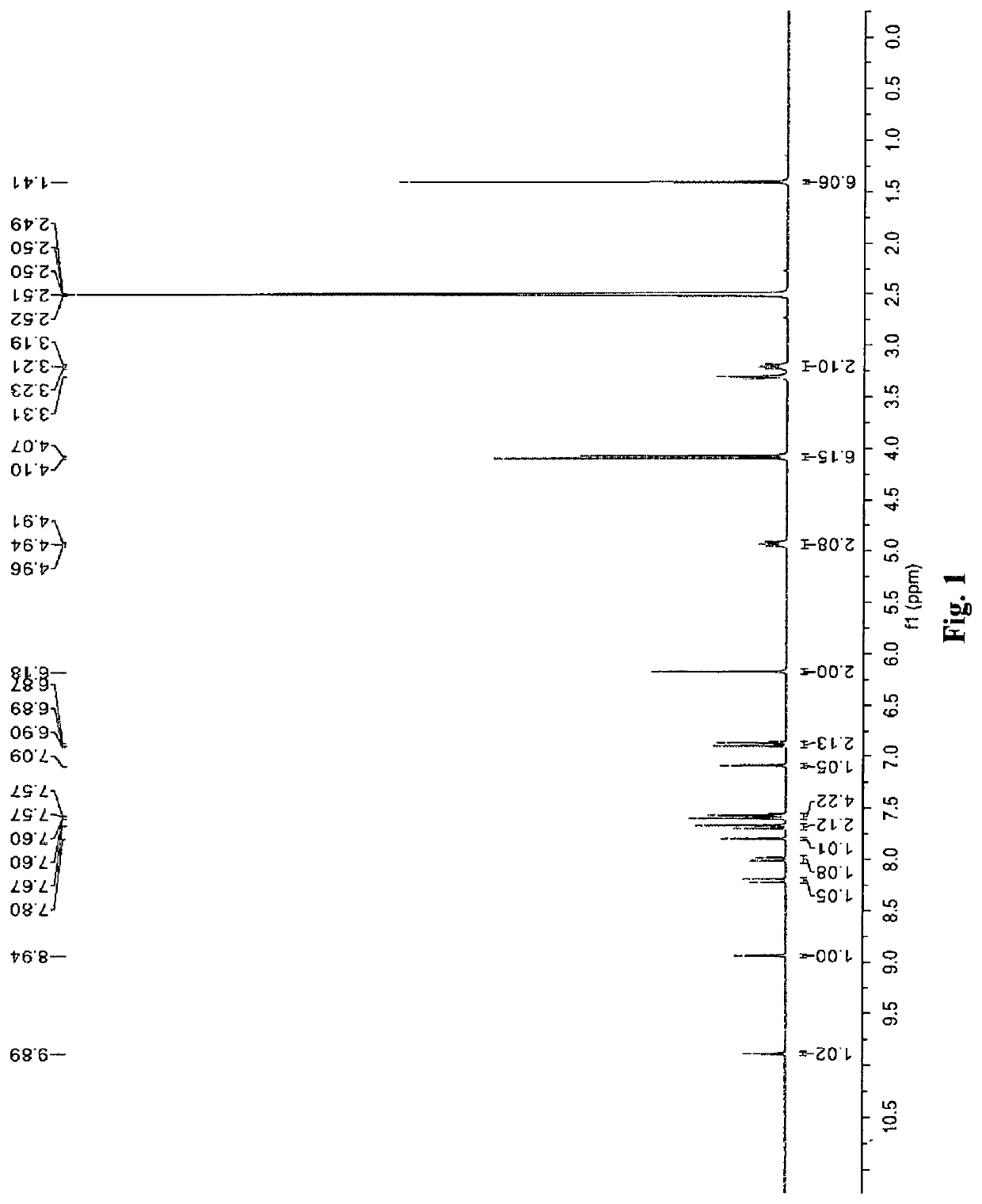
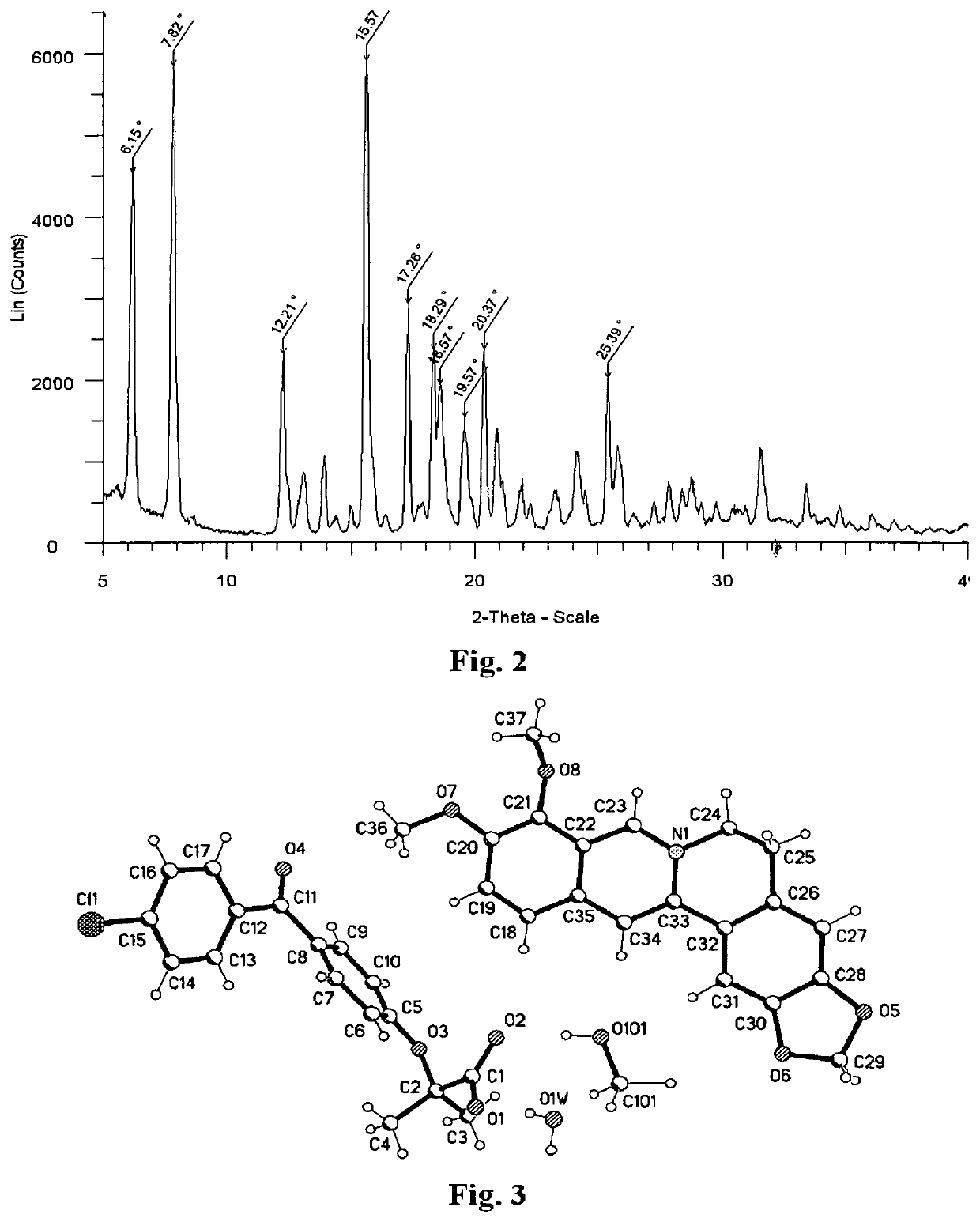
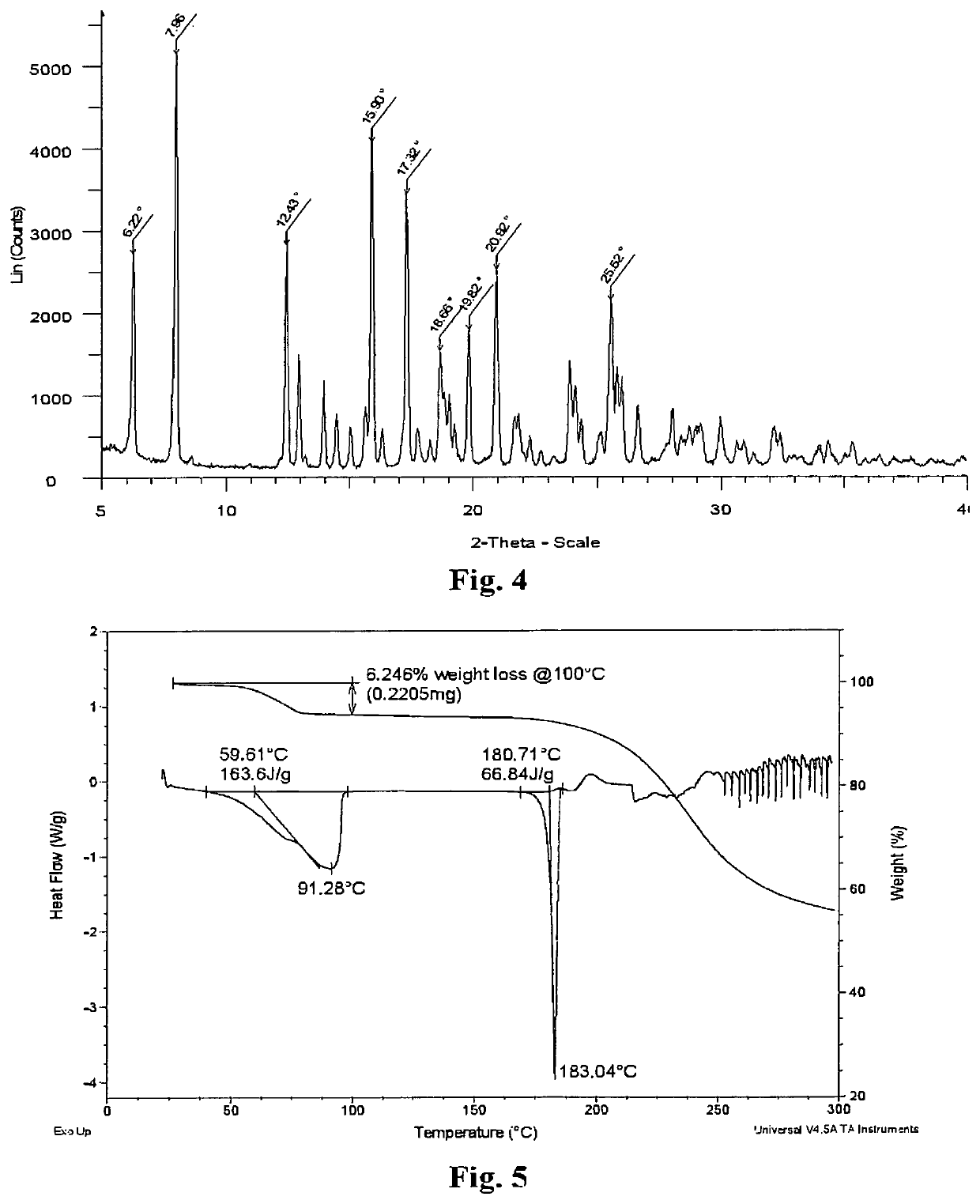
Fenofibric acid salt with berberine or its analogues, crystalline forms, methods of preparation, and applications thereof
ActiveUS10577379B1Eliminate side effectsOrganic active ingredientsOrganic compound preparationBerberineFENOFIBRIC ACID
The present disclosure relates salts of fenofibric acid with berberine or its analogues, their crystalline forms, their preparation methods, and their uses, wherein the salts comprise fenofibric acid as anion and berberine or its analogues as cation. The disclosed salts of fenofibric acid with berberine or its analogues are free of unnecessary cation or anion, such as choline cation in a fenofibric acid salt with choline and chloride ion in a chloride salt of berberine or its analogs, and thus free of side effects caused by such cation or anion, including instability caused by choline and digesting system irritation caused by high acidity caused by the chloride salt of berberine analogs.
Owner:JIANGXI FUSHINE PHARMA CO LTD
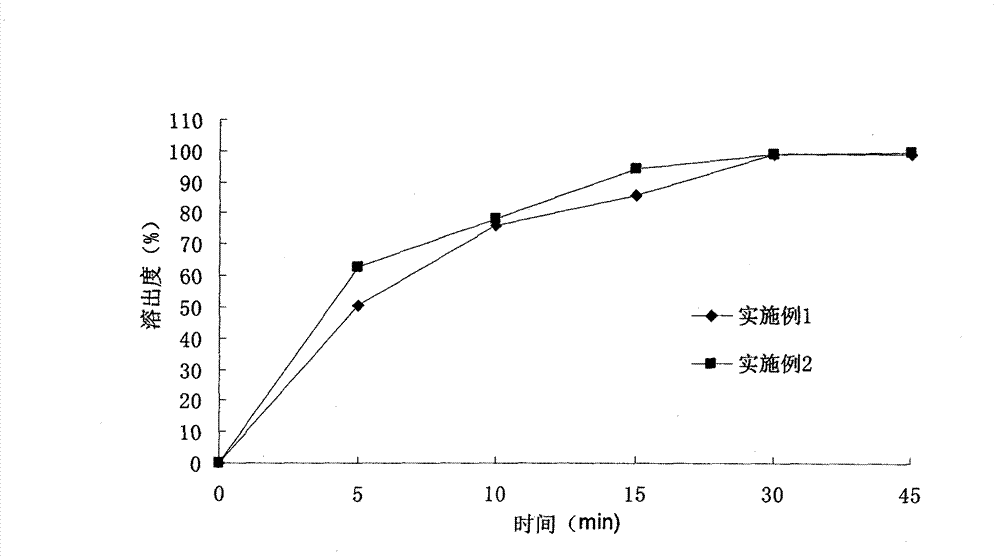
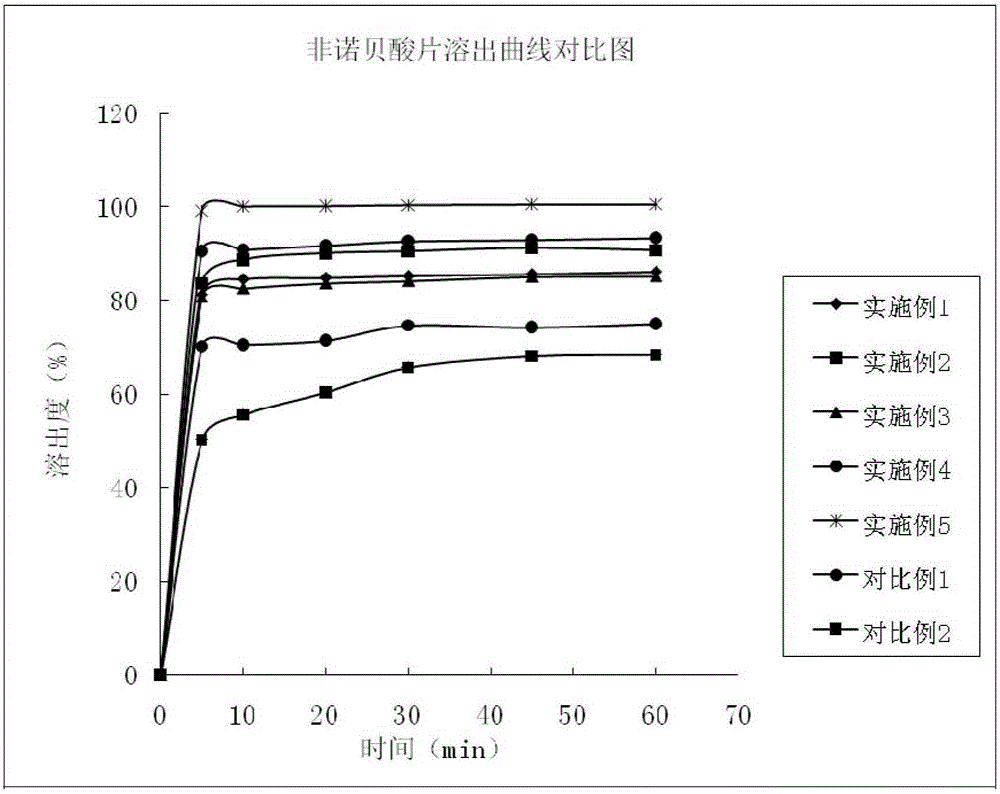
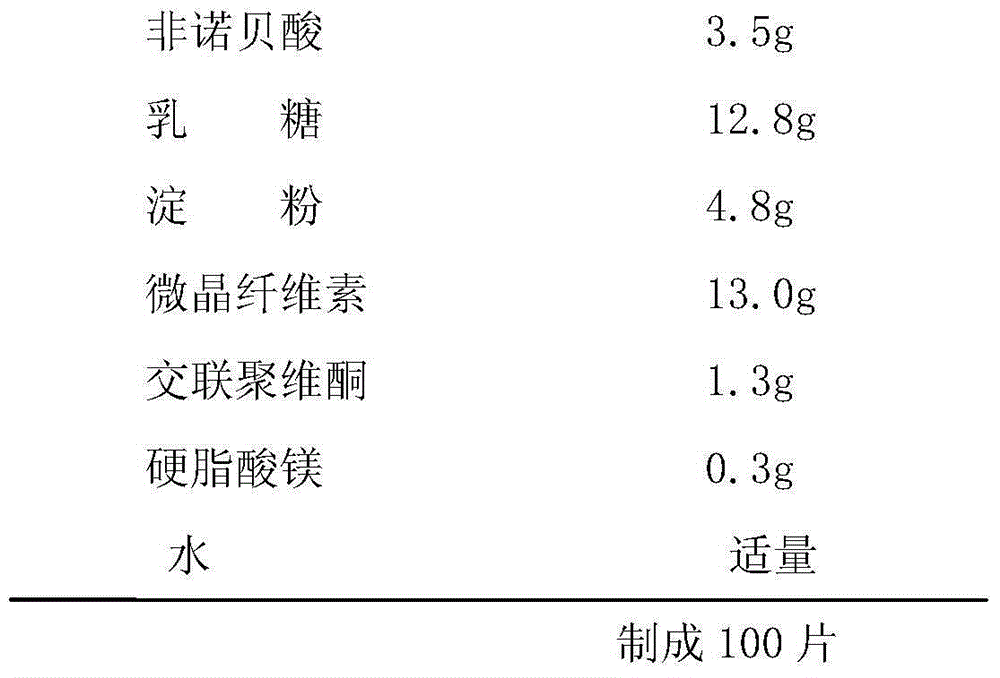
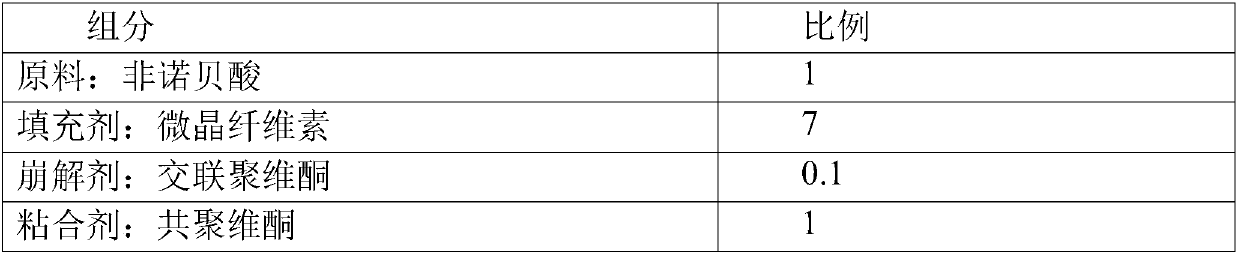
Fenofibric acid tablet and preparing method thereof
InactiveCN105147631AReduce dosageHigh dissolution rateOrganic active ingredientsMetabolism disorderSolubilityClinical efficacy
The invention discloses a fenofibric acid tablet and a preparing method thereof. The fenofibric acid tablet is prepared from fenofibric acid, filler, a disintegrating agent, a lubricating agent and a proper amount of adhesive. According to the fenofibric acid tablet, the dissolution rate of the fenofibric acid is increased, the fenofibric acid has high solubility in the small intestine, the bioavailability of the fenofibric acid is improved, and the clinical effect is improved.
Owner:KANGYA OF NINGXIA PHARMA
Stable fenofibric acid tablet and preparation method thereof
InactiveCN107550875AReduce consumptionEasy to makeOrganic active ingredientsMetabolism disorderFENOFIBRIC ACIDAdhesive
The invention relates to a stable fenofibric acid tablet and a preparation method thereof. The preparation method aims to solve the technical problems that the conventional preparation method of fenofibric acid is complex in technology and high in cost. The stable fenofibric acid tablet adopts the technical scheme that the stable fenofibric acid tablet comprises the following components with the weight ratio: fenofibric acid, a filling age, a disintegrating agent, an adhesive and a lubricant with the weight ratio being 1:(4-7):(0.1-2):(0.1-1):(0.01-0.1). The preparation method adopts a powderdirect tabletting technology, the preparation process is simple, the energy consumption and material consumption are reduced, the production period is shortened, and the production cost is reduced; auxiliary materials of the fenofibric acid tablet are screened and matched, the number of varieties of the auxiliary materials is reduced while the changeless bioavailability is guaranteed, the preparation technology is simplified, and the fenofibric acid tablet which is good in stability, rapid in disintegration and dissolution and simple in technology is formed.
Owner:赵剑锋
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com