Fenofibric acid choline salt crystal form and preparation method thereof
A kind of technology of fenofibrate acid choline salt and crystal form, which is applied in the new crystal form of fenofibrate acid choline salt and its preparation field, and can solve the problem that there is no report on the crystal form of dexmethylphenidate hydrochloride, fenofibrate The problems of stability and poor bioavailability of choline fibrate acid salts can achieve the effect of good controllability, good reproducibility and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
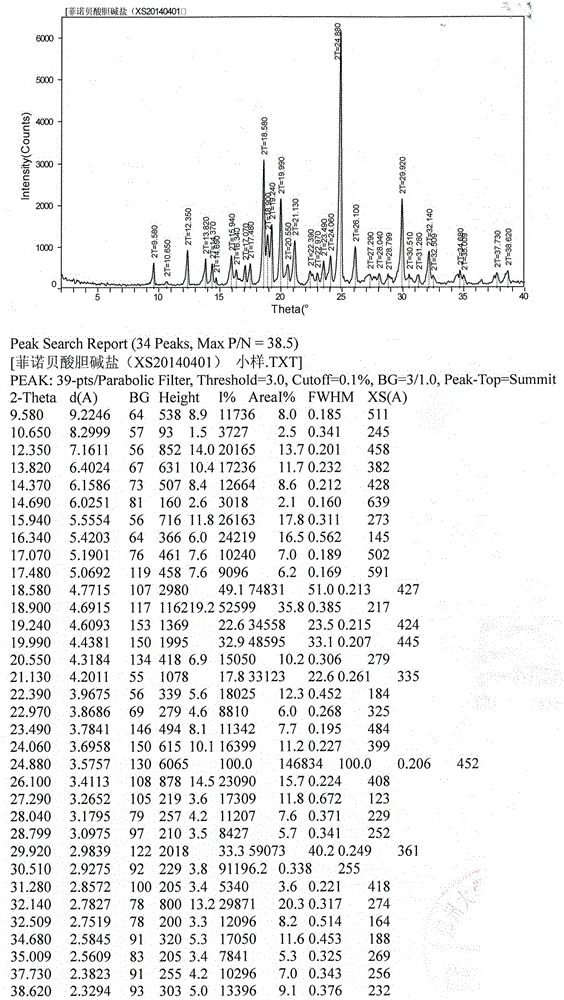
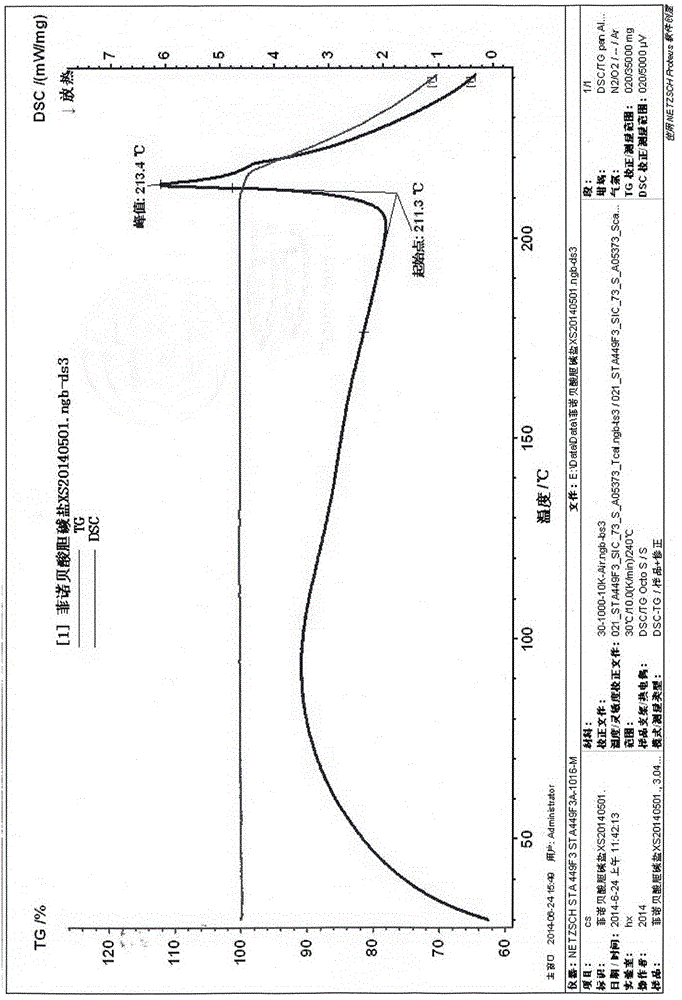
[0023] Preparation of fenofibric acid choline salt crystal form: dissolve the crude product of fenofibric acid choline salt in a mixed solvent of isopropanol and water (85:15 by volume), add it to the reaction bottle, stir and heat up to reflux , keep stirring until completely dissolved, add fenofibric acid choline salt crude product or activated carbon with 1% mass of other crystal forms, keep stirring and decolorize for 30 minutes, filter while hot, cool the filtrate to 25°C, slowly stir and grow the crystal overnight, filter , the filter cake was rinsed with isopropanol, the solid was collected, and vacuum-dried at 50°C for 24 hours to obtain the fine product of fenofibric acid choline salt - white crystals; its X-ray powder diffraction (XRPD) pattern was as follows figure 1 As shown, its differential thermal / thermogravimetric analysis (DSC / TG) spectrum is as follows figure 2 as shown, 1 H-NMR (D 2 O, 400MHz) δ: 7.386 (d, J=8.8Hz, 2H), 7.292 (d, J=8.4Hz, 2H), 7.112 (d, J...
Embodiment 2
[0028] Preparation of fenofibric acid choline salt crystal form: dissolve the crude fenofibric acid choline salt in a mixed solvent of isopropanol and water (volume ratio 80:20), add it to the reaction bottle, stir and heat up to reflux , keep stirring until completely dissolved, add fenofibric acid choline salt crude product or activated carbon with 1% mass of other crystal forms, keep stirring and decolorize for 30 minutes, filter while hot, cool the filtrate to 25°C, slowly stir and grow the crystal overnight, filter , the filter cake was rinsed with isopropanol, the solid was collected, and vacuum-dried at 50° C. for 24 hours to obtain the fine product of fenofibric acid choline salt—white crystals, whose XRPD, DSC, and TG patterns were the same as those in Example 1.
Embodiment 3
[0030] Preparation of fenofibric acid choline salt crystal form: dissolve the crude fenofibric acid choline salt in a mixed solvent of isopropanol and water (volume ratio 90:10), add it to the reaction bottle, stir and heat up to reflux , keep stirring until completely dissolved, add fenofibric acid choline salt crude product or activated carbon with 1% mass of other crystal forms, keep stirring and decolorize for 30 minutes, filter while hot, cool the filtrate to 25°C, slowly stir and grow the crystal overnight, filter , the filter cake was rinsed with isopropanol, the solid was collected, and vacuum-dried at 50° C. for 24 hours to obtain the fine product of fenofibric acid choline salt—white crystals, whose XRPD, DSC, and TG patterns were the same as those in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com