A kind of preparation method of ammonium chromate crystal
An ammonium chromate and crystal technology, which is applied in the field of preparation of ammonium chromate crystals, can solve the problems such as difficulty in obtaining potassium chromate raw materials, inability to obtain ammonium chromate crystals, long preparation process, etc. The effect of decomposing and shortening the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] As an aspect of the technical solution of the present invention, what it relates to is a kind of preparation method of ammonium chromate crystal, and it comprises:
[0048]Using ferrochrome carbon as the working electrode, and constructing an electrochemical reaction system with at least a counter electrode and an electrolyte, wherein the electrolyte includes ammonia water (below the saturation concentration) or a mixed solution of ammonia water and ammonium chromate;
[0049] Electrically connecting the working electrode and the counter electrode to the positive pole and the negative pole of the power supply, so that an electrochemical reaction occurs in the electrochemical reaction system, and a mixed slurry is obtained;
[0050] Separating the mixed slurry from solid to liquid, and then freezing the separated liquid phase system to obtain a solid;
[0051] The solid was sublimed and dried to obtain ammonium chromate crystals.
[0052] In some preferred embodiments, ...
Embodiment 1
[0080] Weigh 500g of carbon ferrochrome and put it into a titanium anode frame, place the nickel mesh as the cathode on both sides of the anode, add 750mL of pure ammonia water into the electrolytic cell with a volume of 1L, and use a peristaltic pump to circulate the electrolyte. The water bath will control the temperature of the electrolyte to be 50°C, and control the circulation flow of the electrolyte to be 72L / h. Then turn on the power supply, stop the power supply after 36 hours of electrolysis, wash the ferrochrome, and mix the electrolyte and washing water to filter. The filtrate was frozen at -20°C for 5 hours, and then vacuum-dried at -50°C under a pressure of 5 Pa for 12 hours after freezing to obtain bright yellow crystalline ammonium chromate powder.
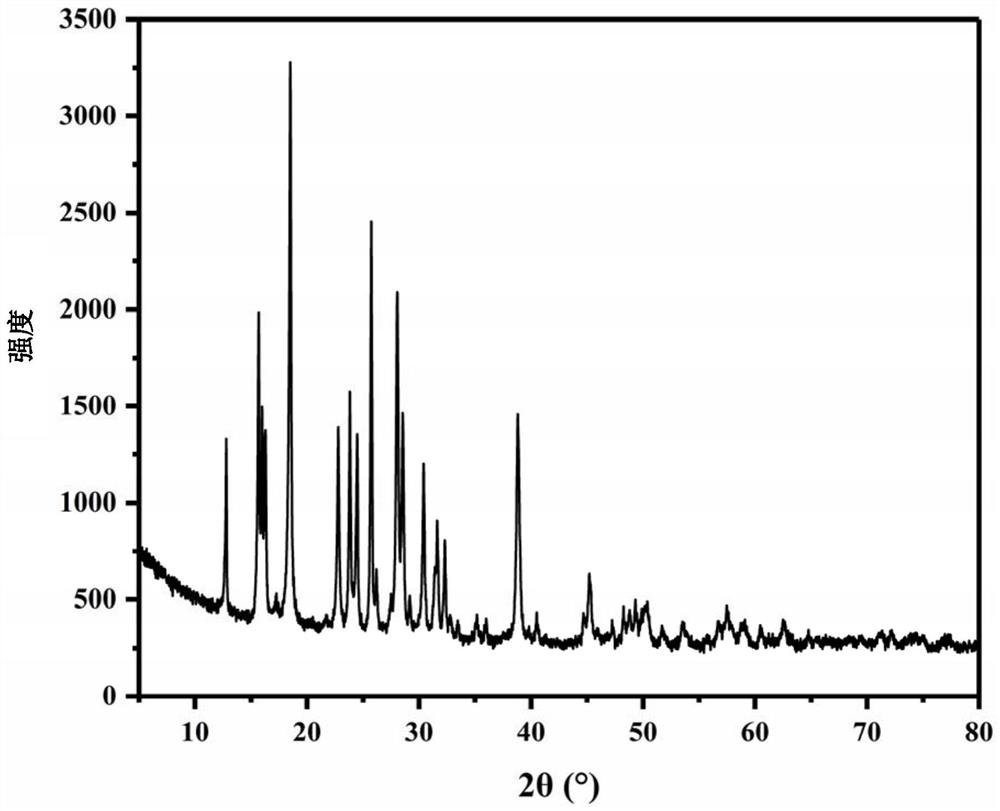
[0081] For the XRD figure of the ammonium chromate crystal prepared in this embodiment, please refer to figure 1 shown, SEM image please refer to figure 2 Shown, please refer to the physical picture image 3 As ...
Embodiment 2
[0085] Weigh 550 g of carbon ferrochrome and put it into a nickel anode frame, place a rod-shaped hollow stainless steel cathode in the anode frame, and separate the cathode and anode with an insulating net. Add 800mL of a mixed solution of ammonia water and ammonium chromate at a ratio of 1:10 to the electrolytic cell, control the temperature of the electrolyte to 30°C through a constant temperature water bath, and control the circulation flow rate of the electrolyte to 0. Turn on the power, after 6 hours of electrolysis, stop the power supply. Use a centrifuge to separate the electrolyte from solid to liquid, measure the supernatant and place it at -15°C for freezing for 9 hours. After freezing, dry it in vacuum at -45°C under a pressure of 10 Pa for 18 hours to obtain bright yellow crystalline ammonium chromate powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com