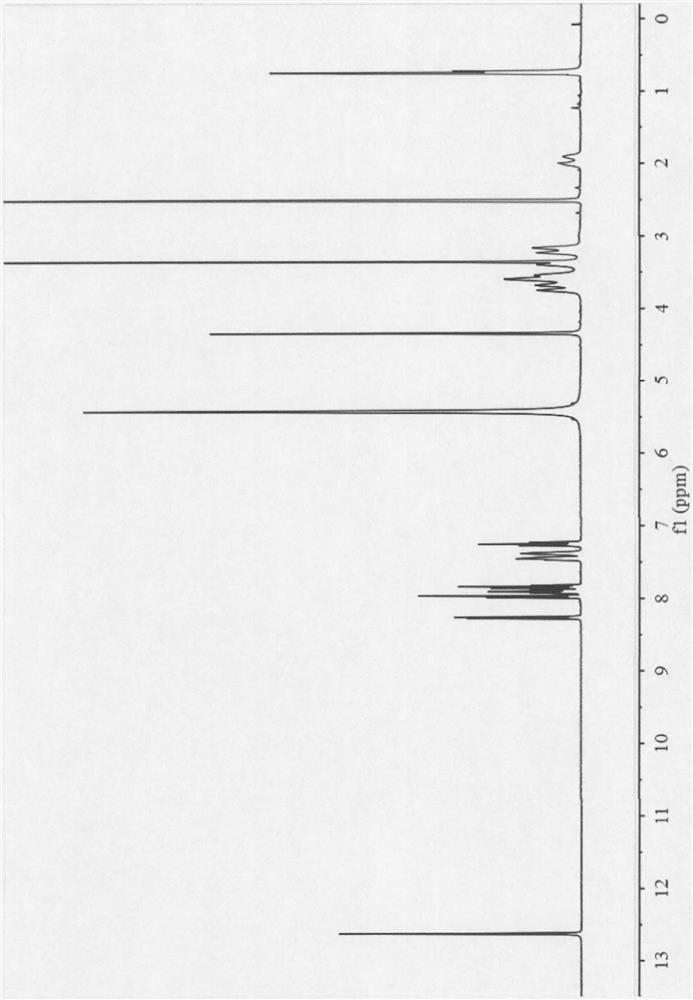
Olaparib-urea eutectic crystal and preparation method thereof
A technology of urea and solvent, which is applied in the field of medicinal chemistry, can solve the problems of limiting the oral absorption efficiency of drugs, low solubility, failure to obtain the co-crystal form A of olaparib and urea, etc., and achieves easy control of the crystallization process and heavy weight. The effect of good presentability and high apparent solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Weigh 1000 mg of olaparib and 276 mg of urea, add 15 mL of ethyl acetate to obtain a suspension, stir the suspension at room temperature for 1 h, filter, and dry the obtained white solid at 40 ° C to obtain olaparib and urea Eutectic solid sample in 84% yield.
Embodiment 2
[0040] Weigh 60 mg of olaparib and 16.5 mg of urea, add 1 mL of isopropyl acetate to obtain a suspension, stir the suspension at room temperature for 12 hours, filter, and dry the obtained white solid at 40°C to obtain olaparib Solid sample co-crystallized with urea.
Embodiment 3
[0042] Weigh 60 mg of olaparib and 16.5 mg of urea, add them to a ball mill jar, then add 20 μL of ethanol, grind at a frequency of 20 Hz for 30 min, and dry the resulting white solid at 40°C to obtain a solid sample of eutectic olaparib and urea.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com