Aclatacit pharmaceutical co-crystal and preparation method thereof
A technology for actarid and medicine, which is applied in the field of actali drug co-crystal and preparation thereof, can solve problems such as decreased solubility, and achieve the effects of low cost, improved oral absorption efficiency, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
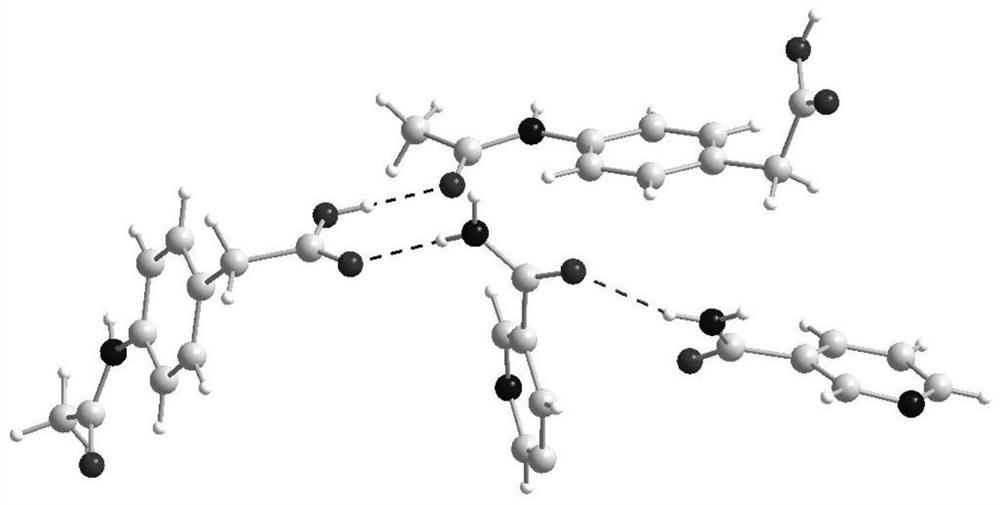
[0036] Weigh 193.2mg of actarit and 122.1mg of nicotinamide, dissolve in 10mL of acetone and cyclohexane mixed solvent with a volume ratio of 1:1, ultrasonically react for 50min, filter and place in a constant temperature incubator at 35°C After cultivating at a medium constant temperature for 8 days, the drug co-crystal of actaritide-nicotinamide was obtained. Using SXRD to characterize and analyze the crystal, the obtained actarit-nicotinamide crystal structure is as follows figure 1 As shown, its crystal structure belongs to the monoclinic crystal system, and the space group is P2 1 / n, axis length: Angle: α / °=90, β / °=98.9990(10), γ / °=90, Z=4.
Embodiment 2
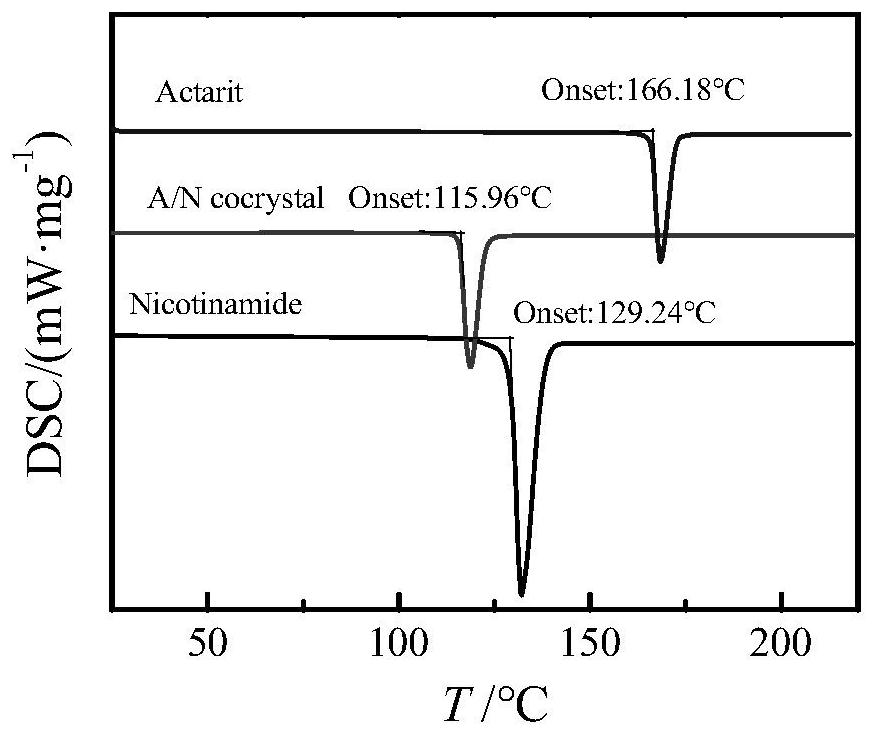
[0038] Weigh 193.2mg of actarit and 183.2mg of nicotinamide, dissolve in 20mL of acetonitrile and chloroform mixed solvent with a volume ratio of 2:1, react with ultrasound for 70min, filter and place in a constant temperature incubator at 30°C After culturing for 10 days, the drug co-crystal of Actarit-Nicotinamide was obtained. The drug co-crystal was characterized by differential scanning calorimetry, and the actaritide-nicotinamide co-crystal had an absorption peak at 115.96°C, and the DSC figure was as follows figure 2 shown.
Embodiment 3
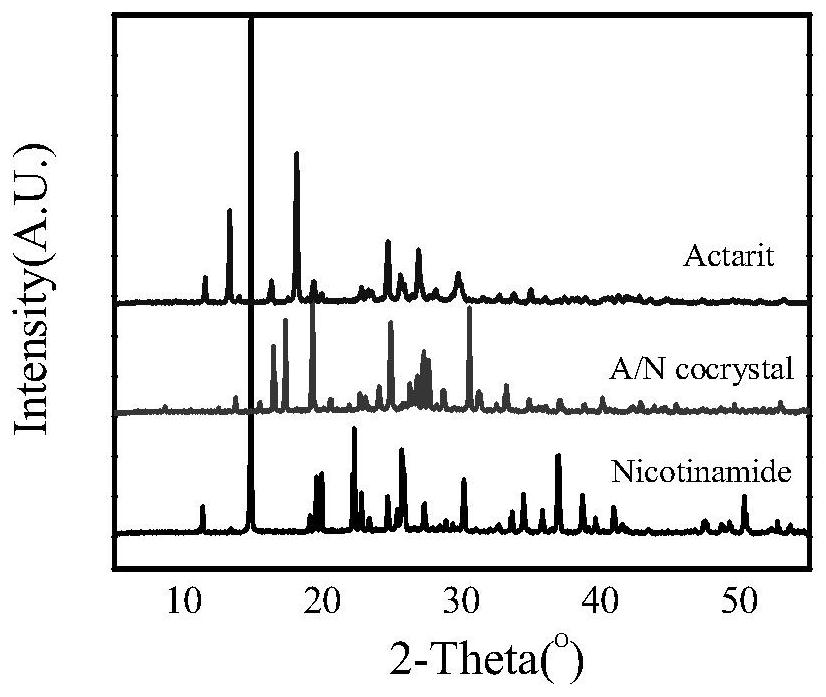
[0040] Weigh 96.6mg of actarit and 122.1mg of nicotinamide, dissolve in 15mL of a mixed solvent of methanol and chloroform with a volume ratio of 1:6, react with ultrasound for 40min, filter, and place in a constant temperature incubator at 40°C for constant temperature After culturing for 14 days, the drug co-crystal of Actarit-Nicotinamide was obtained. Using PXRD to characterize the obtained crystals, the actarityl-nicotinamide co-crystals were at 2θ=8.6°, 13.7°, 15.4°, 16.4°, 17.3°, 19.3°, 20.5°, 22.6°, 24.1°, 24.9° , 26.2°, 26.8°, 27.3°, 27.6°, 28.6°, 30.5°, 31.3°, 33.2°, 34.8°, 36.9°, 38.7° and 40.1° have characteristic peaks, and the characteristic curves are as follows image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com