Cocrystal of olaparib and urea and preparation method thereof
A technology of eutectic crystal form and constant temperature, which is applied in the preparation of organic compounds, preparation of urea derivatives, chemical instruments and methods, etc., can solve the problem of low solubility of free base crystal form, simplify the preparation and post-treatment process, facilitate Long-term storage, the effect of improving drug absorption efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Preparation of Olaparib and Urea Cocrystal Form A:
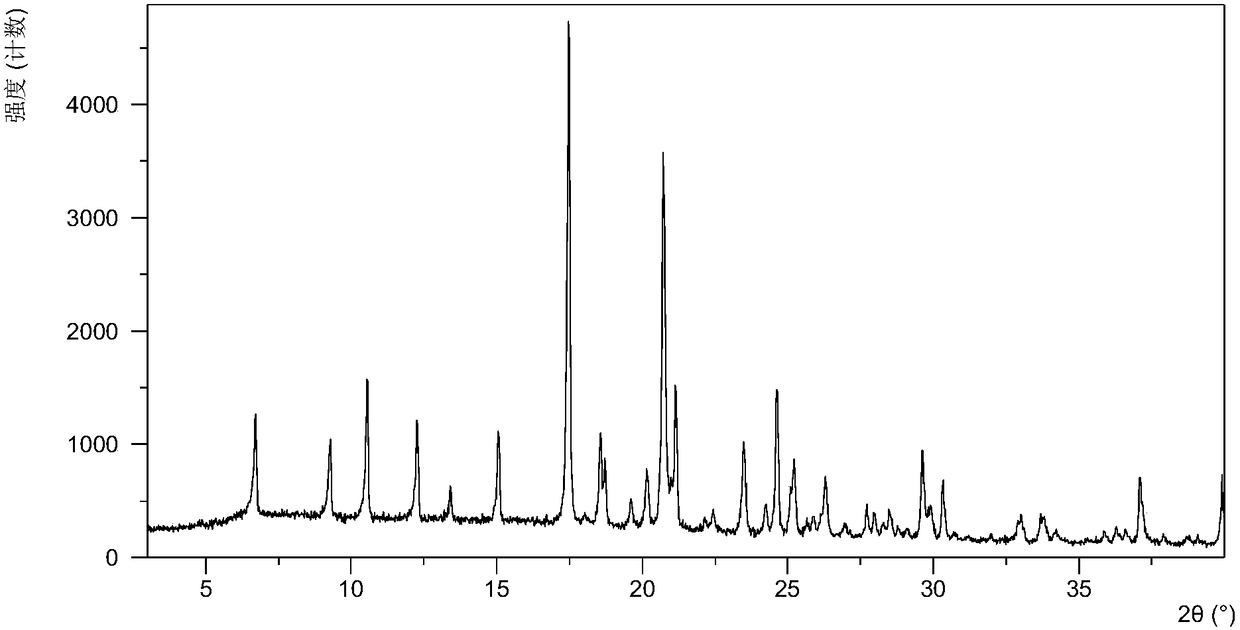
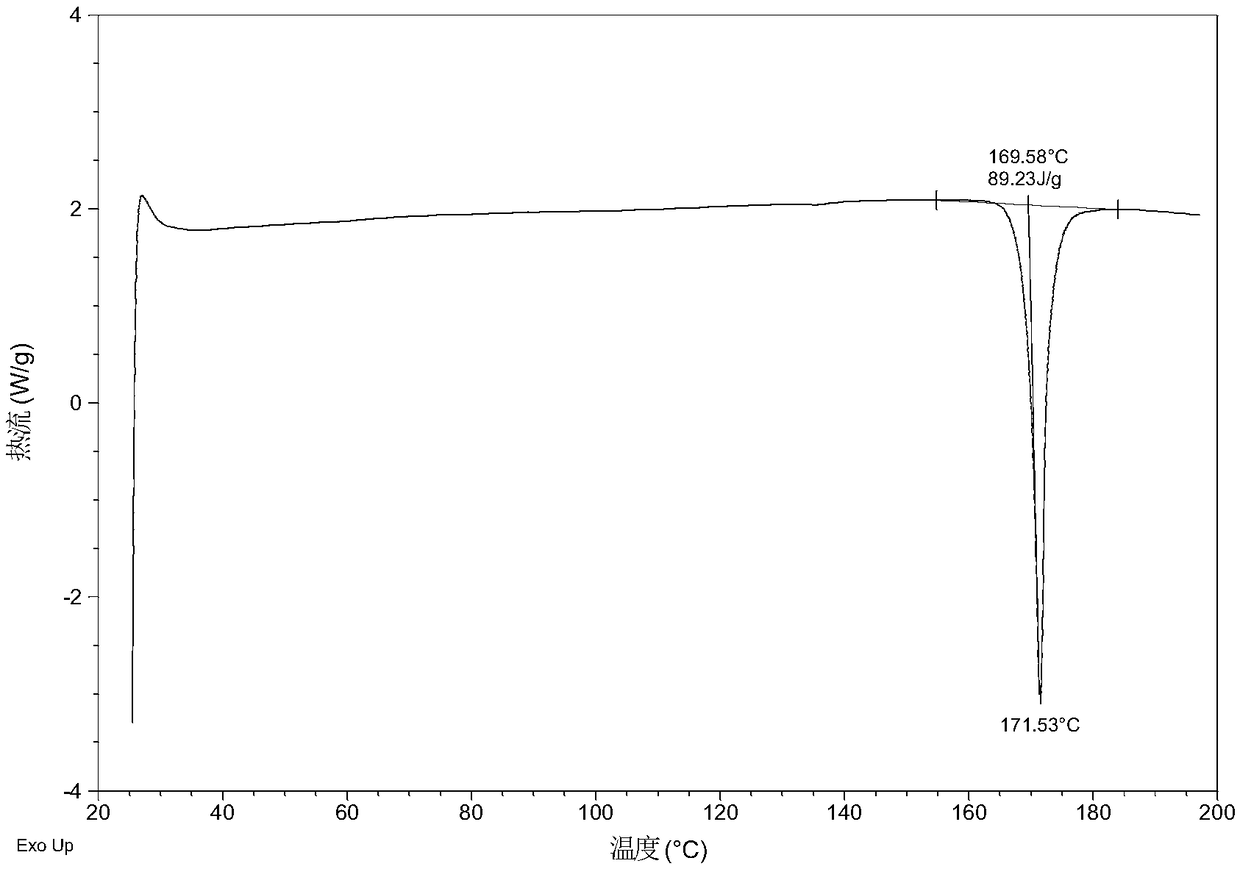
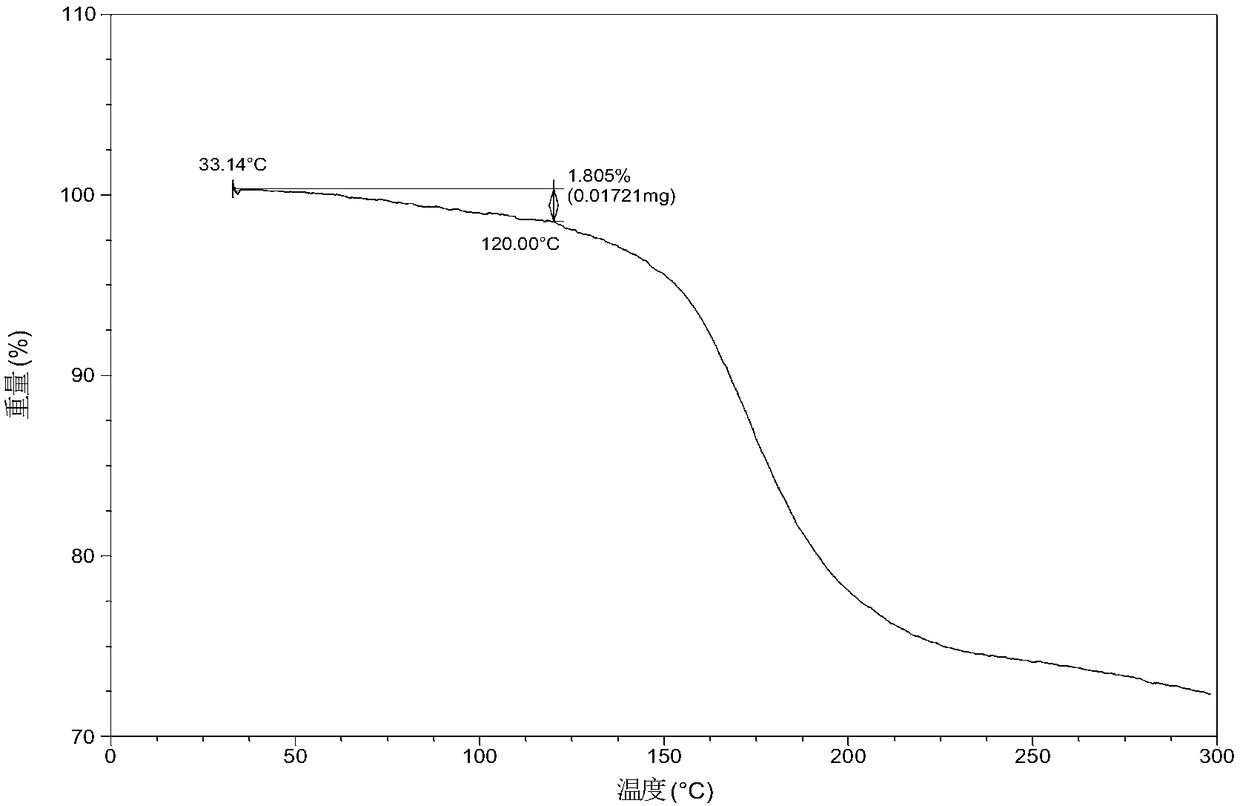
[0056] Dissolve 10.0 mg of olaparib free base (amorphous) in 0.6 mL of ethanol, add 11.8 mg of urea, heat to 60 °C at a heating rate of 1.0 °C / min, stir at 60 °C for 600 min, and then heat at 0.37 °C / min The temperature was lowered to 5° C. at a cooling rate of min, and the above heating and cooling process was repeated three times. After the reaction was completed, it was filtered, and the obtained solid was washed with ethanol, dried, and collected. After testing, the solid obtained in this example is eutectic crystal form A, and its X-ray powder diffraction data are shown in Table 1. Its DSC diagram is as follows figure 2 , and its TGA figure is shown in image 3 ,That 1 H NMR picture as Image 6 .
[0057] The eutectic product of olaparib and urea prepared by the above method, its 1H NMR identification data are as follows:
[0058] 1 H NMR (400MHz, DMSO) δ12.57(s, 1H), 8.26(d, J=7.8Hz, 1H), 7.97(d, J=7.9Hz...
Embodiment 2
[0063] Preparation method of co-crystal of olaparib and urea: 101.9 mg of olaparib free base was dissolved in 3.0 mL of ethanol, 98.7 mg of urea was added, heated to 60 °C at a heating rate of 1.0 °C / min, and then Stir at 60°C for 600min, then cool down to 5°C at a cooling rate of 0.37°C / min, repeat the above heating and cooling process three times, filter after the reaction, and wash the obtained solid with ethanol and dry.
[0064] After testing, the solid obtained in this embodiment is crystal form A, and its X-ray powder diffraction data are shown in Table 2, and its XRPD pattern is as follows figure 1 .
[0065] Table 2
[0066] 2theta
Embodiment 3
[0068] Preparation of co-crystals of olaparib and urea:
[0069] Dissolve 10.0 mg of olaparib free base (amorphous) in 0.6 mL of acetone, add 10.8 mg of urea, heat to 60 °C at a heating rate of 1.0 °C / min, stir at 60 °C for 600 min, and cool down (cooling rate 0.37 °C / min) to 5 °C, repeat the above heating and cooling process three times, filter after the reaction, and wash the obtained solid with ethanol, dry, cool to room temperature, and collect the solid.
[0070] After testing, the solid obtained in this example is consistent with the crystal form obtained in Example 1, which is the eutectic crystal form A, and its X-ray powder diffraction data are shown in Table 3.
[0071] table 3
[0072]
[0073]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com