Co-crystal of tofu glucoside and L-proline and preparation method thereof
A technology of proline and co-crystal crystal form, which is applied to the co-crystal of tofufuside and L-proline and the field of preparation thereof, can solve problems such as few reports, and achieves convenient long-term storage, improved absorption efficiency, stable good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The preparation method of the co-crystal form A of the compound of formula (I) and L-proline:
[0063] Add 185.2 mg of the compound of formula (I) to 10.0 mL of 1,4-dioxane to obtain a suspension, add 76.6 mg of L-proline and place it in a constant temperature incubator at 50°C for 24 hours, then centrifuge to remove the lower layer The solid was dried at a constant temperature at 25°C overnight, and the obtained solid was co-crystal form A.
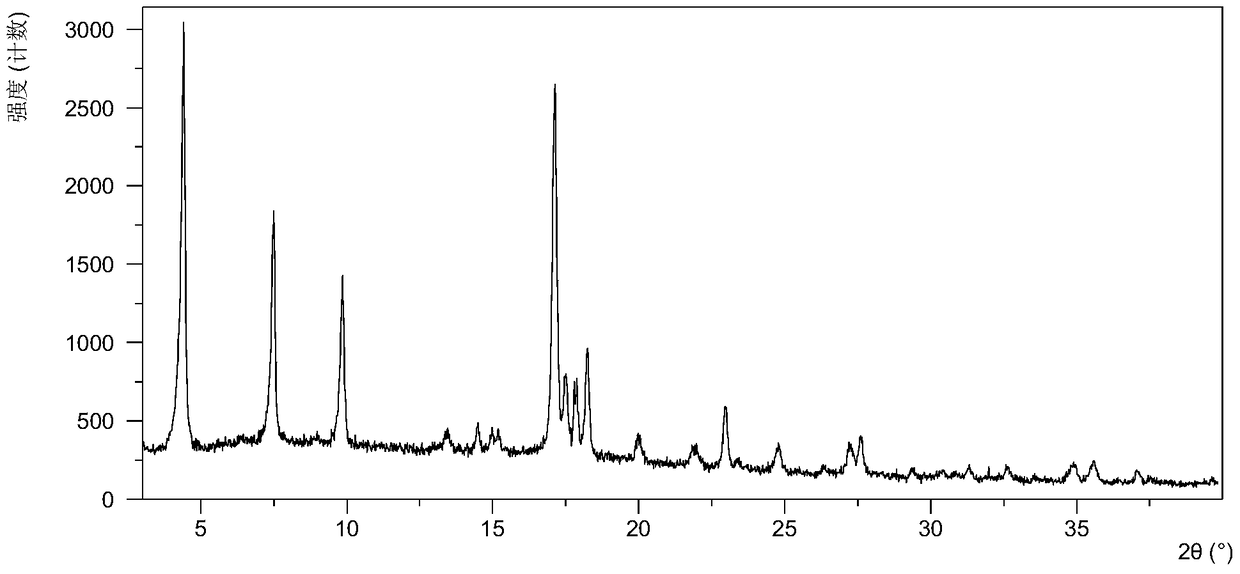
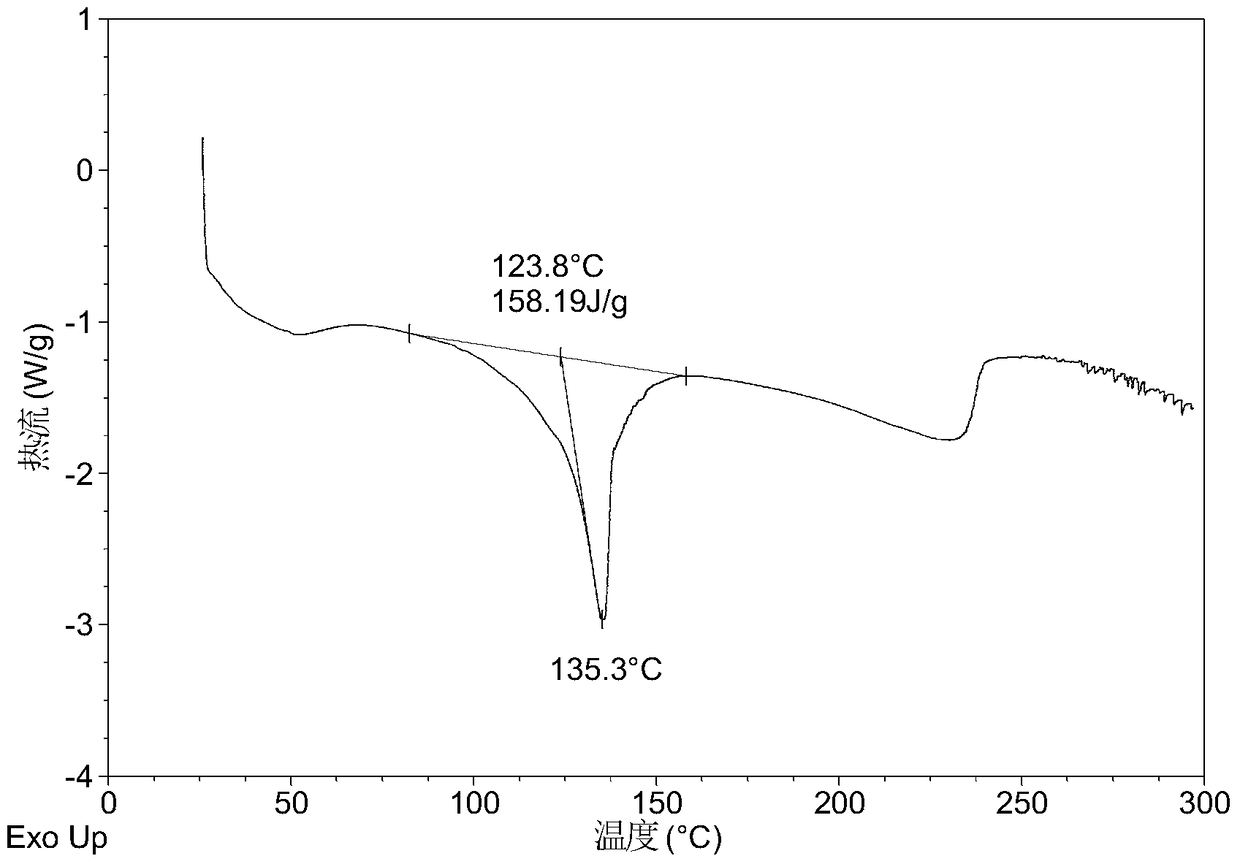
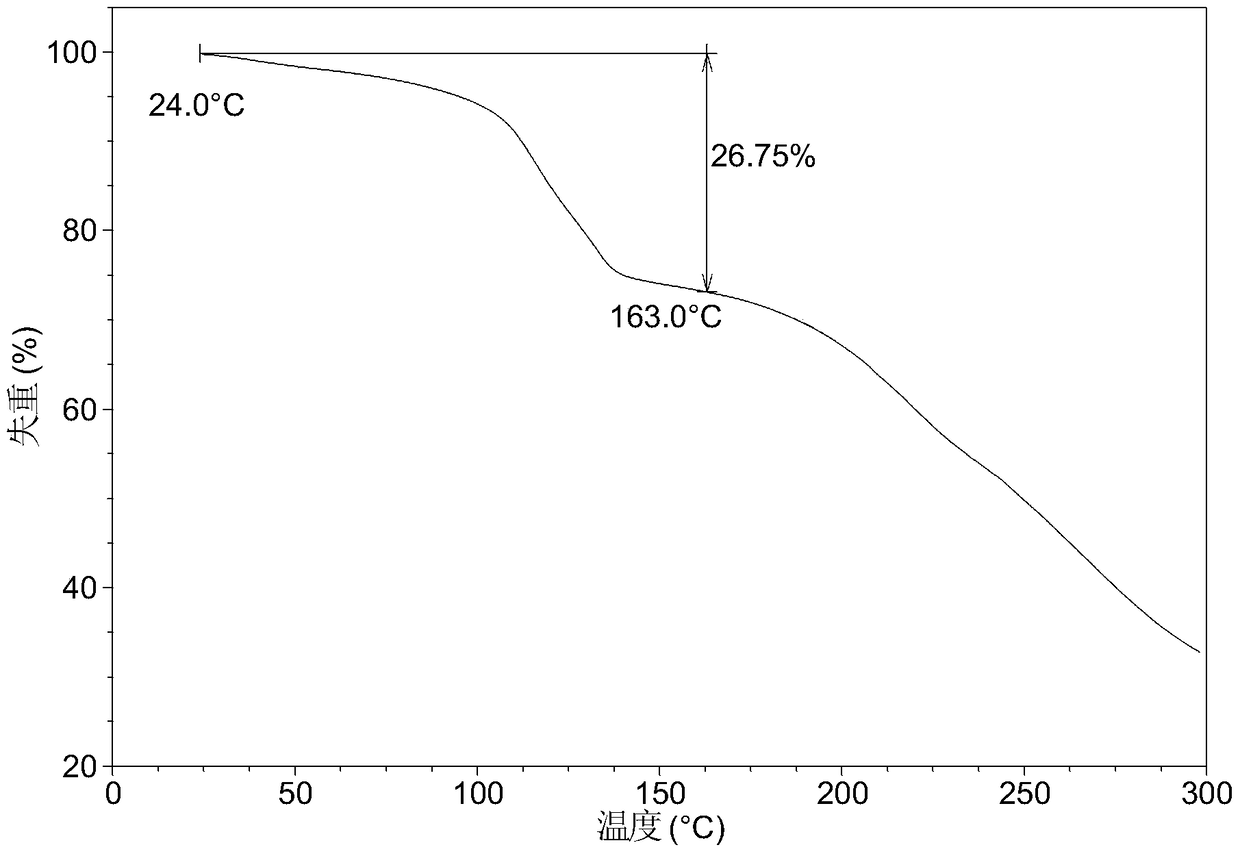
[0064] Table 1 shows the X-ray powder diffraction data of the crystal forms obtained in this example. Its XRPD pattern is as follows figure 1 , and its DSC graph is shown in figure 2 , and its TGA figure is shown in image 3 ,That 1 H NMR picture as Figure 7 .
[0065] The product of the eutectic crystal form A prepared by the above method, which 1 H NMR identification data are as follows:
[0066] 1 H-NMR (400 MHz, DMSO-d 6 )δ9.89(s,1H),7.87(d,J=8.7Hz,2H),7.18(d,J=8.7Hz,2H),5.25(d,J=7.9Hz,1H),4.90-5.20( m, 2H), 4.38-...
Embodiment 2
[0070] The preparation method of the co-crystal form A of the compound of formula (I) and L-proline:
[0071] Add 15.0 mg of the compound of formula (I) to 1.0 mL of 1,4-dioxane to obtain a suspension, add 6.0 mg of L-proline, place in a constant temperature incubator at 50°C and stir for 24 hours, centrifuge to take the lower layer of solid , 25 ° C constant temperature drying overnight, the resulting solid is the eutectic crystal form A.
[0072] The X-ray powder diffraction data of the crystal forms obtained in this example are shown in Table 2.
[0073] Table 2
[0074] 2theta
Embodiment 3
[0076] The preparation method of the co-crystal form B of the compound of formula (I) and L-proline:
[0077] Add 99.6 mg of co-crystal form A to 4.0 mL of tetrahydrofuran to obtain a suspension, place the suspension in a constant temperature incubator at 50°C and stir for 24 hours, centrifuge to remove the lower layer of solid, and dry at a constant temperature of 25°C overnight, the obtained solid is Eutectic Form B.
[0078] The X-ray powder diffraction data of the crystal forms obtained in this example are shown in Table 3. Its XRPD pattern is as follows Figure 4 , and its DSC graph is shown in Figure 5 , and its TGA figure is shown in Figure 6 , whose H 1 -NMR picture as Figure 8 .
[0079] The product of the eutectic crystal form B prepared by the above method, which 1 H NMR identification data are as follows:
[0080] 1 H-NMR (400 MHz, DMSO-d 6 )δ9.90(s,1H),7.88(d,J=8.7Hz,2H),7.19(d,J=8.7Hz,2H),5.26(d,J=7.9Hz,1H),4.90-5.21( m, 2H), 4.43-4.78(m, 2H), 3.95(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com