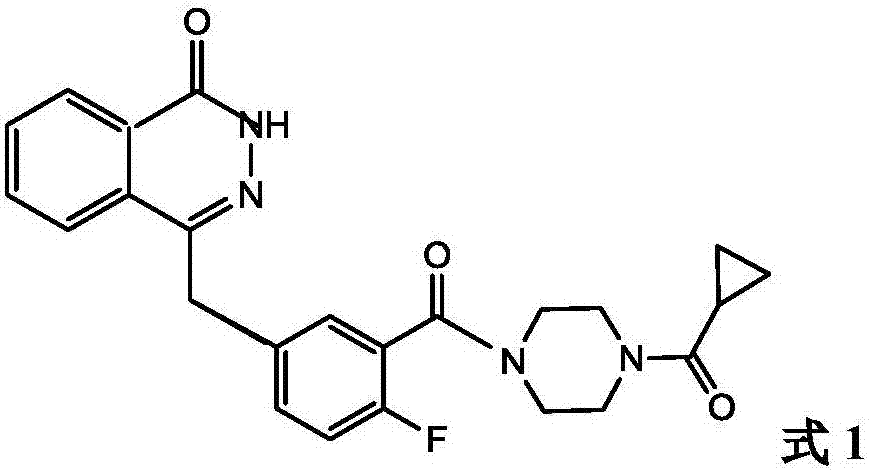
Olaparib compound refining method
A refining method, the technology of olaparib, applied in the direction of organic chemistry, etc., can solve the problem of no scavenging effect of double-substituted products, and achieve the effect of fast reaction rate, high yield, and reduction of product impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
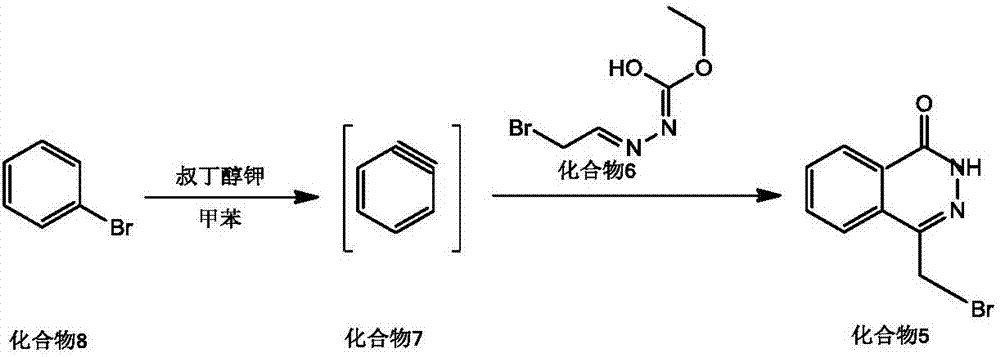
[0042] Dissolve bromobenzene (0.05mol) and potassium tert-butoxide (0.06mol) in 100mL of toluene, heat up to 90°C, react for 3h, add 20mL of toluene dissolved in compound 6 (0.06mol) dropwise, and continue at 110°C Stir for 24h, cool to room temperature, wash with water (3*50mL), wash the organic layer with anhydrous Na 2 SO 4 Dry, concentrate under reduced pressure to remove toluene, and the residue is analyzed by column chromatography (CH 2 Cl 2 as eluent) to obtain compound 5 (11.25 g) with a yield of 94.17%.
Embodiment 2
[0044] Add 0.06mol methyl o-fluorobenzoate, 0.022mol DIC, and 6.6mmol DMAP into 100mL of dichloromethane, stir to dissolve, continue to stir at room temperature for 30min, gradually raise the temperature to 45-50°C, and then add 1- Cyclopropamoylpiperazine (0.08mol), continue to stir and react for 9-10h. After the reaction was completed, the temperature was lowered to 0°C and stirred for 45 minutes, filtered, the filtrate was washed with water (3*50mL), dried over anhydrous sodium sulfate, dichloromethane was distilled off under reduced pressure to obtain a solid, namely compound 2 (15.27g), the yield was 92.57%.
Embodiment 3
[0046] Preparation of ionic liquids
[0047] Install a stirrer on the reaction kettle, add 0.1mol [Emim]Cl under nitrogen protection, and slowly add 0.26mol AlCl in batches 3 , stirred at about 40°C for 3 hours to ensure that the reaction was complete, and a colorless and transparent [Emim]Cl-AlCl was obtained 3 ionic liquid.
[0048] Preparation of crude olaparib
[0049] Compound 5 (0.03mol) and [Emim]Cl-AlCl 3 (0.03mol) ionic liquid was mixed in the bottle, stirred, and the temperature was raised to 50-55°C, and compound 2 (0.02mol) was slowly added dropwise within 30 minutes, and the stirring was stopped after 3-4 hours of reaction. Cool down to room temperature, the product and the ionic liquid are separated automatically, the product layer is poured into chloroform for extraction, the organic phase is retained, washed with toluene, suction filtered, and dried under reduced pressure to obtain olaparib (compound 1) 8.15g, the yield 93.79%.
[0050] Part 2: Refining me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com