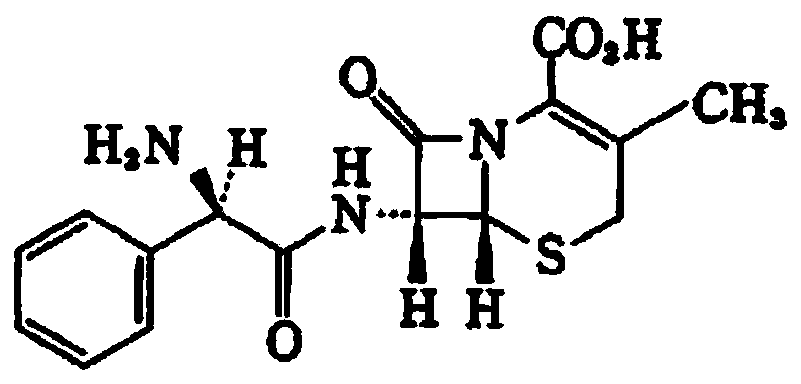
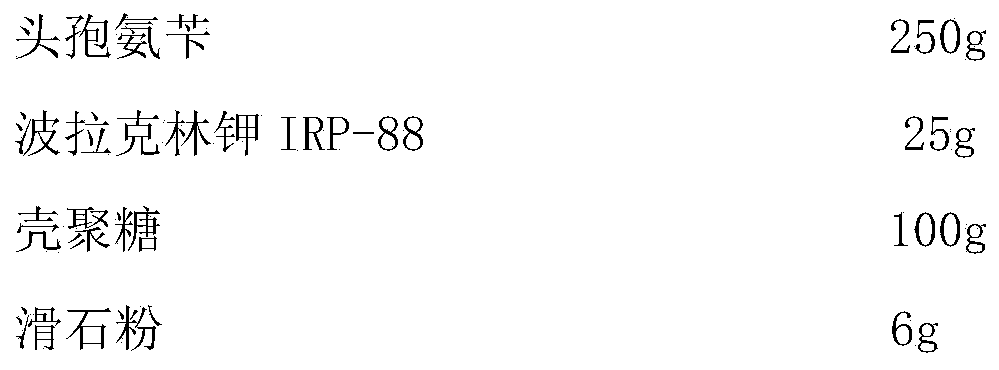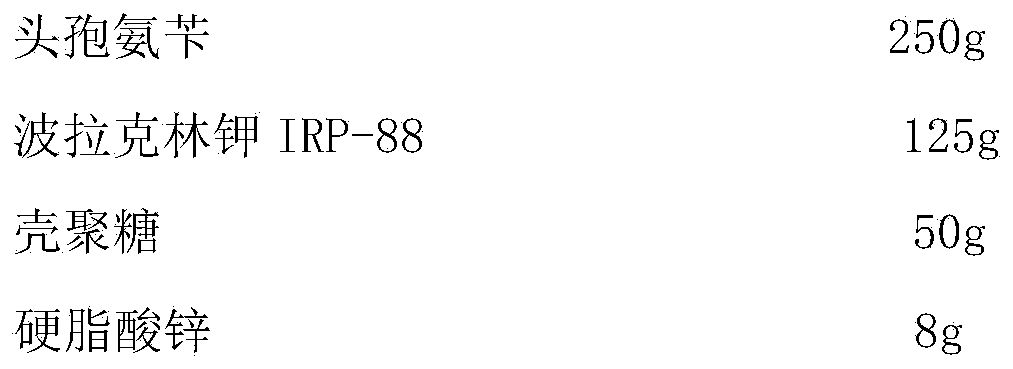Cefalexin capsule and preparation process thereof
The technology of cephalexin capsules and cephalexin is applied in the directions of capsule delivery, medical preparations without active ingredients, medical preparations containing active ingredients, etc. and other problems, to achieve the effects of less toxic and side effects, low polymer content, and good formulation stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
[0029] Preparation process: cephalexin, polacrilin potassium IRP-88, and chitosan are passed through 80-mesh sieve respectively, and the prescription quantity is weighed to take cephalexin, polacrilin potassium IRP-88, and chitosan, add talcum powder and mix evenly, and fill the capsule , that is.
Embodiment 2
[0031]
[0032] Preparation process: cephalexin, polacrilin potassium IRP-88, and chitosan are respectively passed through a 80-mesh sieve, and the prescription amount is weighed to take cephalexin, polacrilin potassium IRP-88, and chitosan, and zinc stearate is added to mix evenly. Fill capsules and get ready.
Embodiment 3
[0034]
[0035] Preparation process: cephalexin, polacrilin potassium IRP-88, and chitosan are respectively passed through a 80-mesh sieve, and the prescription amount is weighed to take cephalexin, polacrilin potassium IRP-88, and chitosan, and zinc stearate is added to mix evenly. Fill capsules and get ready.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com