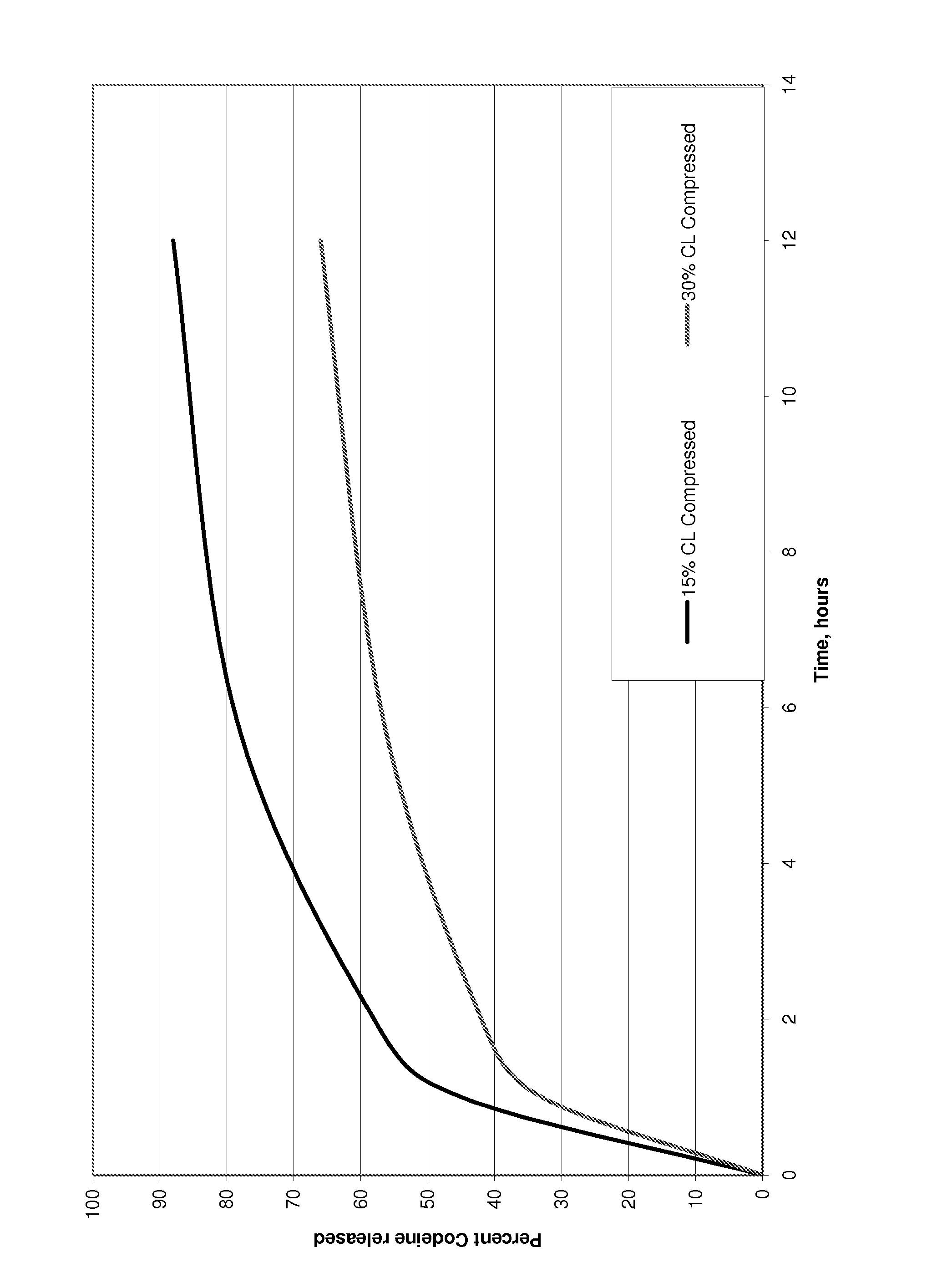
Pharmaceutical Formulation
a technology of pharmaceutical formulation and formulation, applied in the direction of drug composition, microcapsule, capsule delivery, etc., can solve the problems of less therapeutically effective formulation towards the end of the twelve-hour period, concomitant increase in side effects, and little benefit in combining a drug such as codeine with a drug, so as to reduce the observed food
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0047]To facilitate an understanding of the principles and features of the various embodiments of the invention, various illustrative embodiments are explained below. Although preferred embodiments of the invention are explained in detail, it is to be understood that other embodiments are contemplated. Accordingly, it is not intended that the invention is limited in its scope to the details of ingredients and arrangement of components set forth in the following description or illustrated in the drawing. The invention is capable of other embodiments and of being practiced or carried out in various ways. Also, in describing the preferred embodiments, specific terminology will be resorted to for the sake of clarity.
[0048]It must also be noted that, as used in the specification and the appended claims, the singular forms “a,”“an” and “the” include plural references unless the context clearly dictates otherwise. For example, reference to an ingredient is intended also to include composit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com