Pharmaceutical composition for treating breast cancer and preparation method of pharmaceutical composition
A composition and technology for breast cancer, which are applied in directions such as drug combinations, pharmaceutical formulations, and non-active ingredients medical preparations, can solve the problems of low dissolution rate, poor dissolution rate, large difference in tablet weight, etc. The method is simple and easy to control, the product has good stability, and the effect of small difference in tablet weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
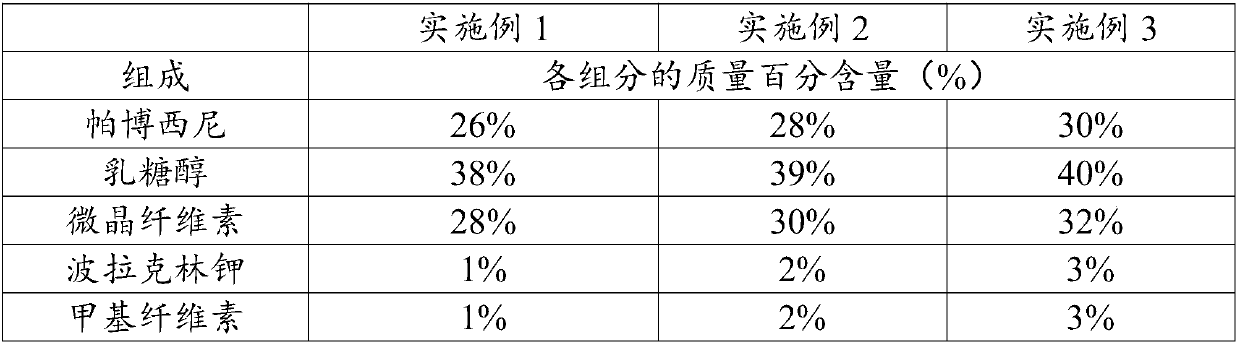
Embodiment 1-3
[0040]
[0041] Preparation:
[0042] (1) Weighing according to the prescription quantity;
[0043] (2) Adhesive preparation: add methyl cellulose to purified water at 80-90 ° C, the weight percentage of methyl cellulose and purified water is 1:19, stir until completely dissolved, and wait until the temperature is naturally cooled to 30 ° C use;
[0044] (3) Add palbociclib, lactitol, microcrystalline cellulose, and polacrilin potassium to the wet mixing granulator and mix, then add the prepared binder, stir for 150-180S, and the stirring frequency is 40Hz , to prepare soft material;
[0045] (4) Place the granulated soft material in a fluidized bed dryer, dry at 60-65°C for 3-4 hours, granulate, and control the particle size to 280 μm-320 μm and moisture content to 0.55%-0.65%;
[0046] (5) adopt high-speed tablet press machine to carry out tabletting;
[0047] (6) Packaging.
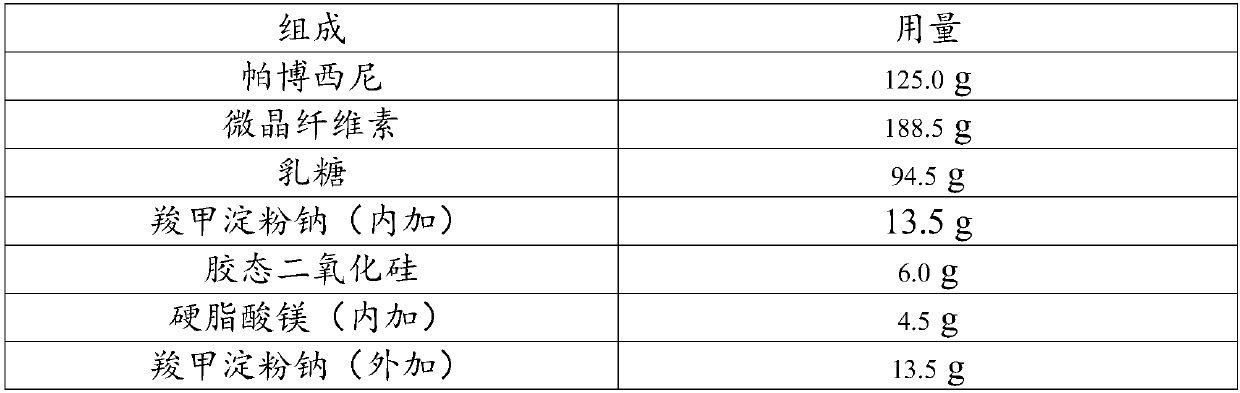
Embodiment 4
[0049] composition
[0050] Palbociclib, lactitol, microcrystalline cellulose, and polacrilin potassium were added into a mixer and mixed for 4 minutes, and directly compressed into tablets.
Embodiment 5
2%
[0053] (1) Take each component by weight percentage of the component;
[0054] (2) Pulverize the weighed palbociclib through a 100-mesh sieve, respectively pass through a 60-mesh sieve for lactitol and polacrilin potassium, then mix them uniformly, and dry granulate;
[0055] (3) Mix the granules obtained above with microcrystalline cellulose, and press into tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com