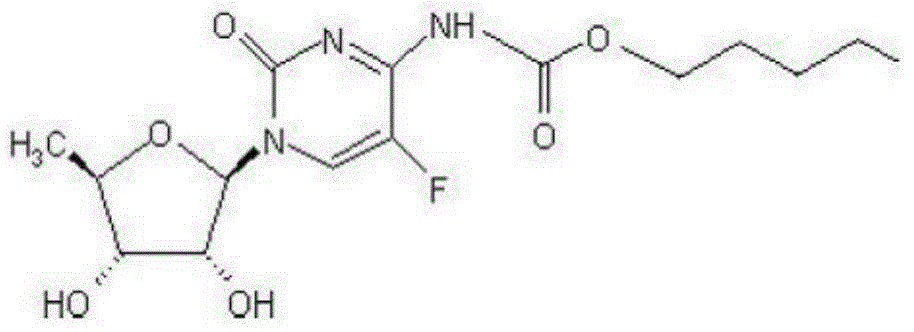
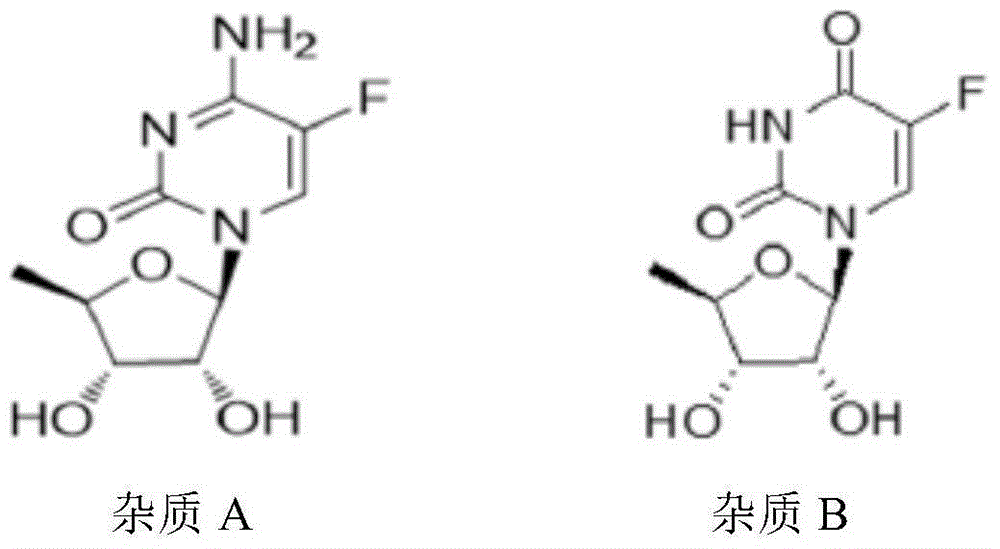
High-stability capecitabine tablets and preparation method thereof
A high-stability capecitabine technology, applied in the field of high-stability capecitabine tablets and its preparation, can solve the problems of poor drug stability, cumbersome preparation process, failure to provide stability, etc., and achieve The effect of rapid dissolution and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]
[0038]
[0039] Preparation Process:
[0040] (1) Add L-methionine to the purified water by weighing the prescription amount, stir until completely dissolved, and set aside;
[0041] (2) Capecitabine is pulverized and passed through an 80 mesh sieve, and the prescription amount is weighed to mix capecitabine, polycridine potassium, lactose, and copovidone, add L-methionine aqueous solution to granulate, pass through a 20 mesh sieve, Dry at 50°C, pass through a 20-mesh sieve for granulation, and dry the granules for later use;
[0042](3) Take the dry granules and mix them with sodium stearate fumarate evenly, punch the tablets with a Φ12mm inclined plane, and control the hardness to 80N-120N to obtain the product.
Embodiment 2
[0044]
[0045] Preparation Process:
[0046] (1) Add L-methionine to the purified water by weighing the prescription amount, stir until completely dissolved, and set aside;
[0047] (2) Pulverize capecitabine and pass through an 80-mesh sieve, weigh capecitabine, pocridine potassium, mannitol, and copovidone in the prescribed amount, mix evenly, add L-methionine aqueous solution to granulate, and pass through a 20-mesh sieve , dried at 50°C, passed through a 20-mesh sieve for granulation, and the dry granules are ready for use;
[0048] (3) Take the dry granules and mix them with magnesium stearate evenly, punch the tablets with a Φ12mm inclined plane, and control the hardness to 80N-120N to obtain the product.
Embodiment 3
[0050]
[0051]
[0052] Preparation Process:
[0053] (1) Add L-methionine to the purified water by weighing the prescription amount, stir until completely dissolved, and set aside;
[0054] (2) Pulverize capecitabine and pass through an 80-mesh sieve, weigh capecitabine, pocridine potassium, sorbitol, and copovidone in the prescribed amount, mix evenly, add L-methionine aqueous solution to granulate, and pass through a 20-mesh sieve , dried at 50°C, passed through a 20-mesh sieve for granulation, and the dry granules are ready for use;
[0055] (3) Take the dry granules and mix them evenly with calcium stearate, punch the tablets with a Φ12mm inclined plane, and control the hardness to 80N-120N.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com