Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
49 results about "Ubenimex" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
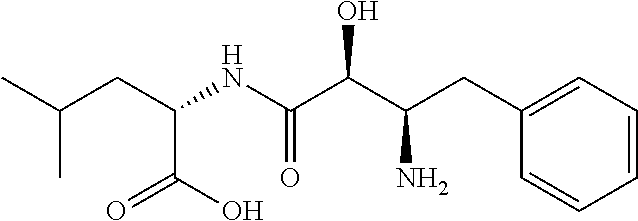
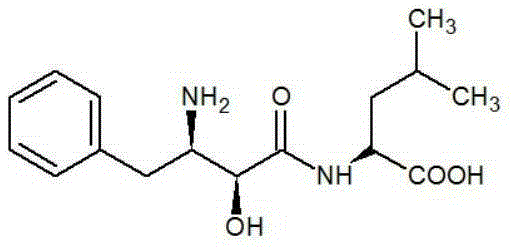
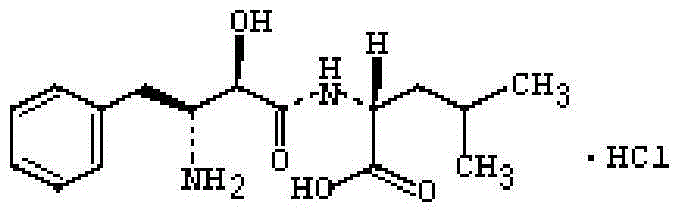
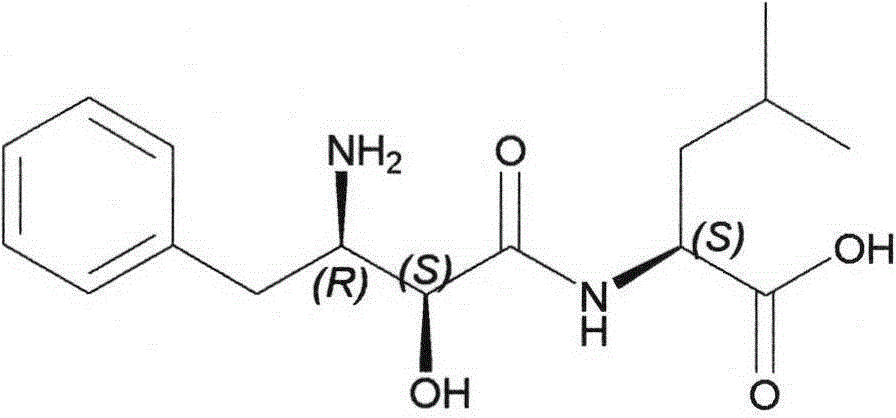
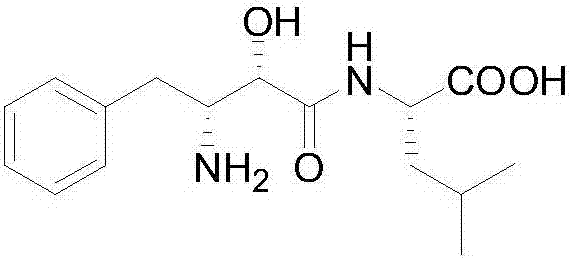
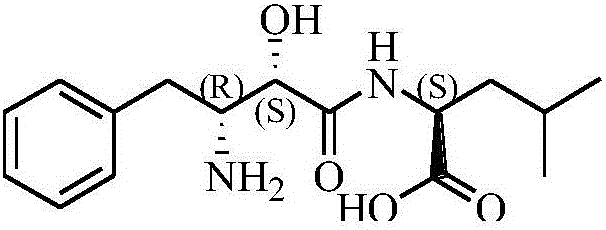
Ubenimex (INN), also known more commonly as bestatin, is a competitive, reversible protease inhibitor. It is an inhibitor of arginyl aminopeptidase (aminopeptidase B), leukotriene A₄ hydrolase (a zinc metalloprotease that displays both epoxide hydrolase and aminopeptidase activities), alanyl aminopeptidase (aminopeptidase M/N), leucyl/cystinyl aminopeptidase (oxytocinase/vasopressinase), and membrane dipeptidase (leukotriene D₄ hydrolase). It is being studied for use in the treatment of acute myelocytic leukemia and lymphedema. It is derived from Streptomyces olivoreticuli. Ubenimex has been found to inhibit the enzymatic degradation of oxytocin, vasopressin, enkephalins, and various other peptides and compounds.
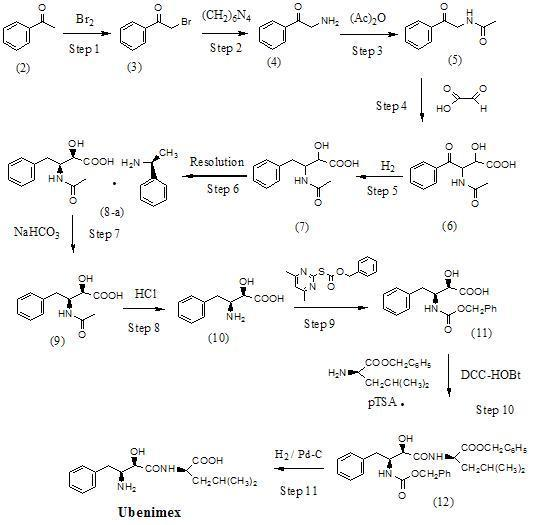
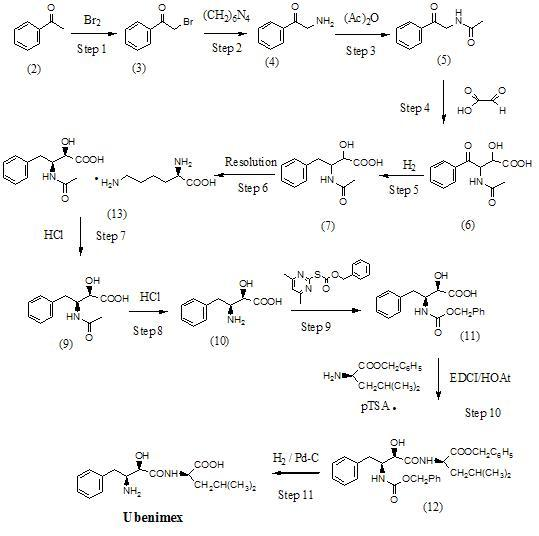
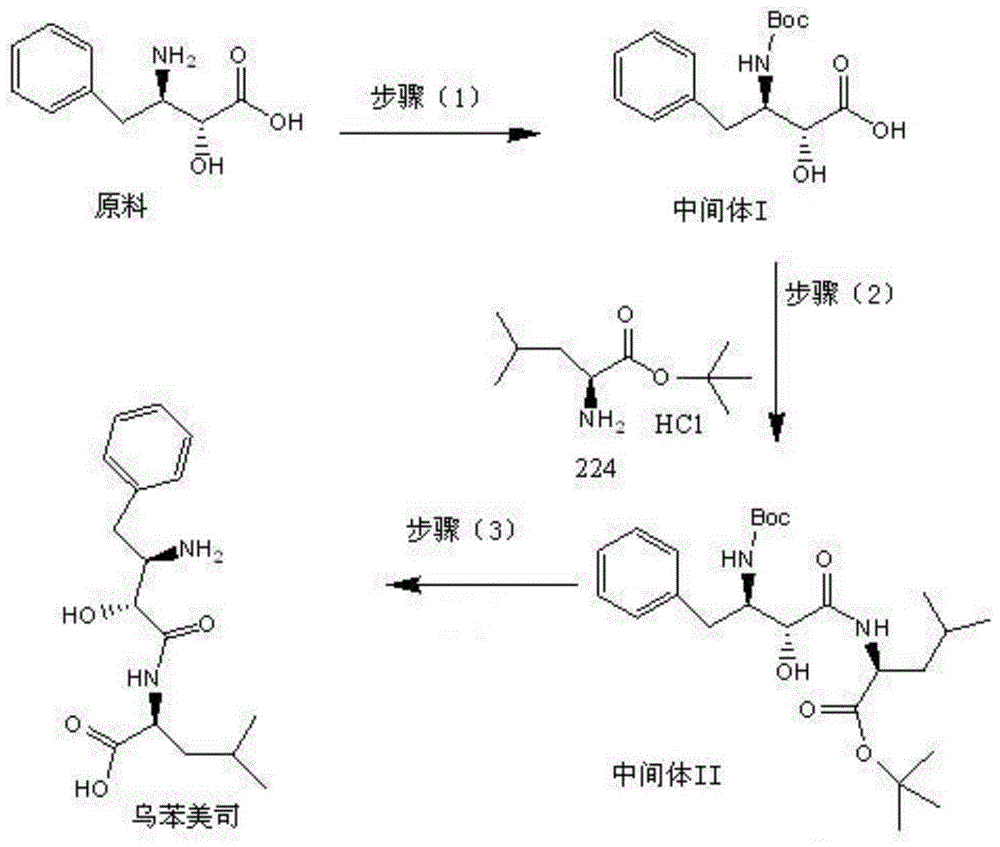
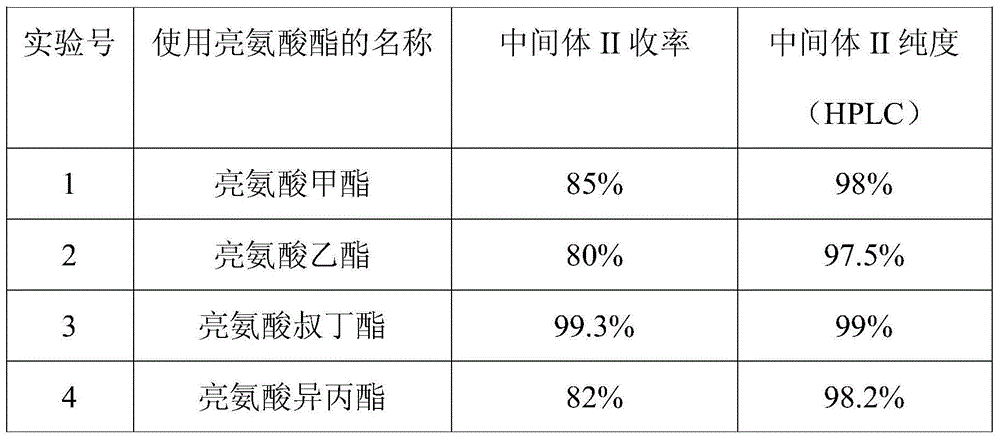
Preparation method for ubenimex
ActiveCN101891647AOrganic compound preparationCarboxylic acid amides optical isomer preparationArginine3-amino-2-hydroxy-4-phenylbutyric acid
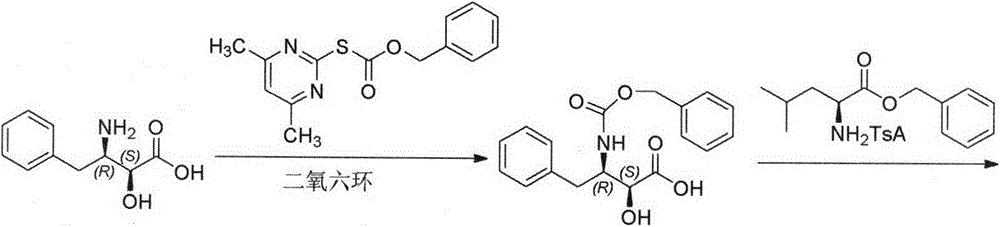
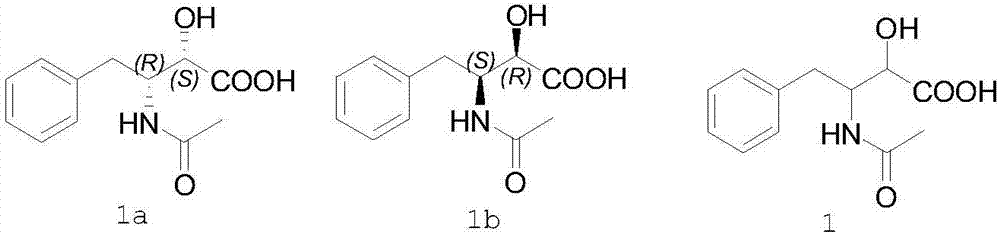
The invention belongs to the field of antineoplastic agent preparation and provides a preparation method for ubenimex. The preparation method comprises the following steps of: preparing high-purity key intermediate (2S,3R)-3-amino-2-hydroxy-4-phenyl butyric acid by taking L-lysine, L-arginine or L-histidine as a resolving reagent; and preserving the chirality of C-5 in forming a peptide chain by taking EDCI / HOAt as a condensing agent. Due to the adoption of the method, the problem that the (2S,3R)-3-amino-2-hydroxy-4-phenyl butyric acid and the (2S,3R)-3-acetamino-2-hydroxy-4-phenyl butyric acid cannot be completely separated by the conventional resolving agent and the racemization problem in the condensation of amide are effectively solved; and the purity of the ubenimex prepared by the method can reach over 99.5 percent.
Owner:ZHEJIANG APELOA KANGYU PHARMA +1
Ubenimex dispersion tablet and its preparing method
InactiveCN1943562AEasy to takeDisintegrates quicklyOrganic active ingredientsPill deliverySuspending AgentsGlidant
The invention relates to ubenimex disket and its process, a composition disket comprising such as active ubenimex, disintegrating agent, filler, suspending agent bond, glidant, corrigent. Said ubennimex disket is convenient for medication, suitable for the old and small children and patient with swallowing difficulties.
Owner:宛六一 +1
Ubenimex fat emulsion injection and preparation method thereof
InactiveCN101601649ANo side effectsHigh clinical application valueOrganic active ingredientsEmulsion deliveryFat emulsionUbenimex
The invention discloses a ubenimex fat emulsion injection, comprising ubenimex, oil for injection, emulsifier, stabilizer, isoosmotic adjusting agent and water, wherein, the concentration of the ubenimex is 0.05-10g / ml, the concentration of the oil for injection is 5-30g / ml, the concentration of the emulsifier is 0.8-3.0g / ml, the concentration of the stabilizer is 0.1-2.0g / ml, and the concentration of the isoosmotic adjusting agent is 2-3g / ml. Through measuring, the quality and the appearance of the fat emulsion injection of the invention are uniform, the average particle diameter is about 300nm, and the pH value is 5.0-9.0, thus, the fat emulsion injection can be used for injection. The ubenimex fat emulsion injection has obvious anti-tumor function. According to the characteristics of common fat emulsion injection, the ubenimex fat emulsion injection as an anti-tumor medicine also has the functions of slow releasing, targeting, providing organisms with energy and the like.
Owner:SHANDONG UNIV
Method for recrystallizing ubenimex
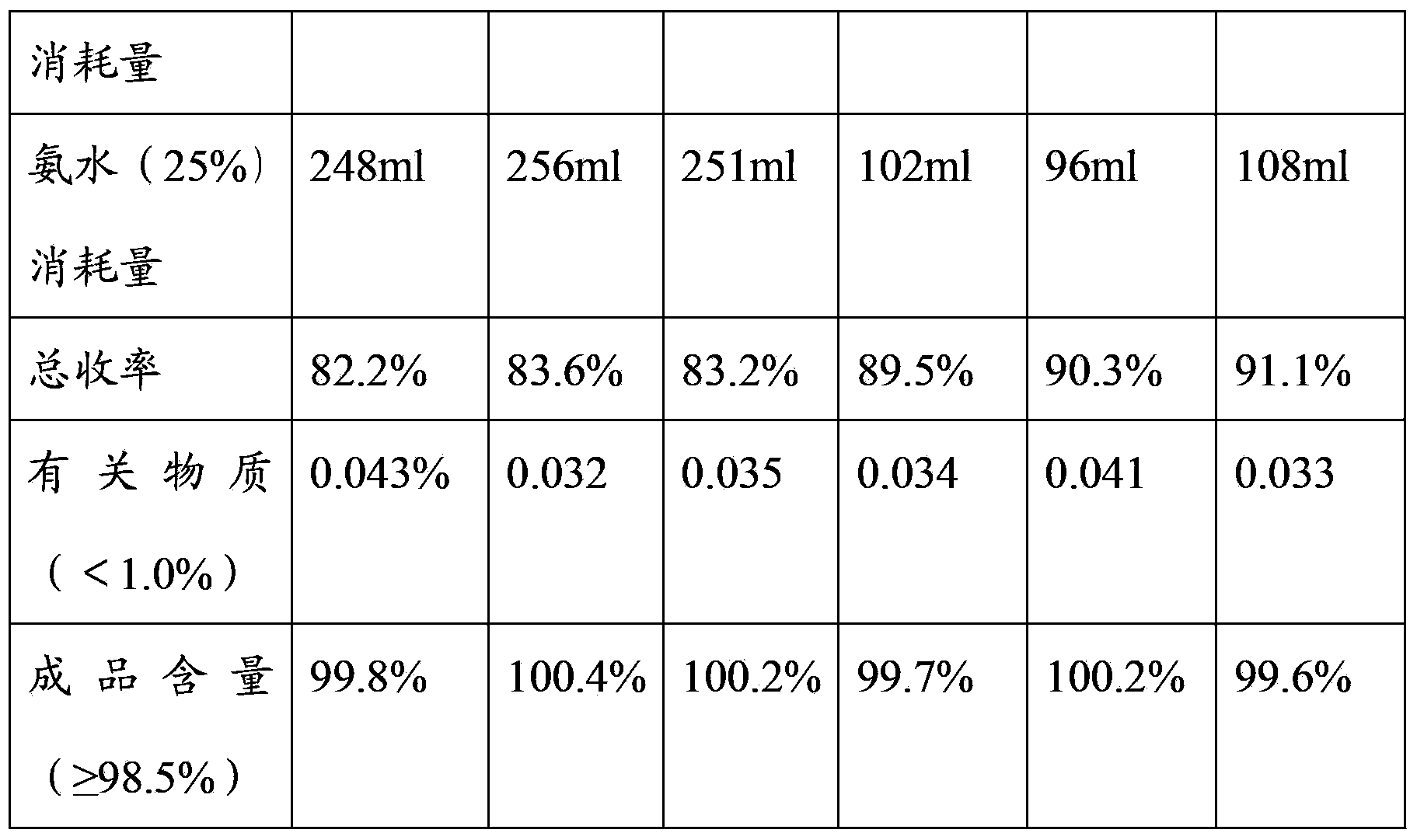
InactiveCN103360277AAvoid temperature riseCarboxylic acid amide separation/purificationChemistryUbenimex
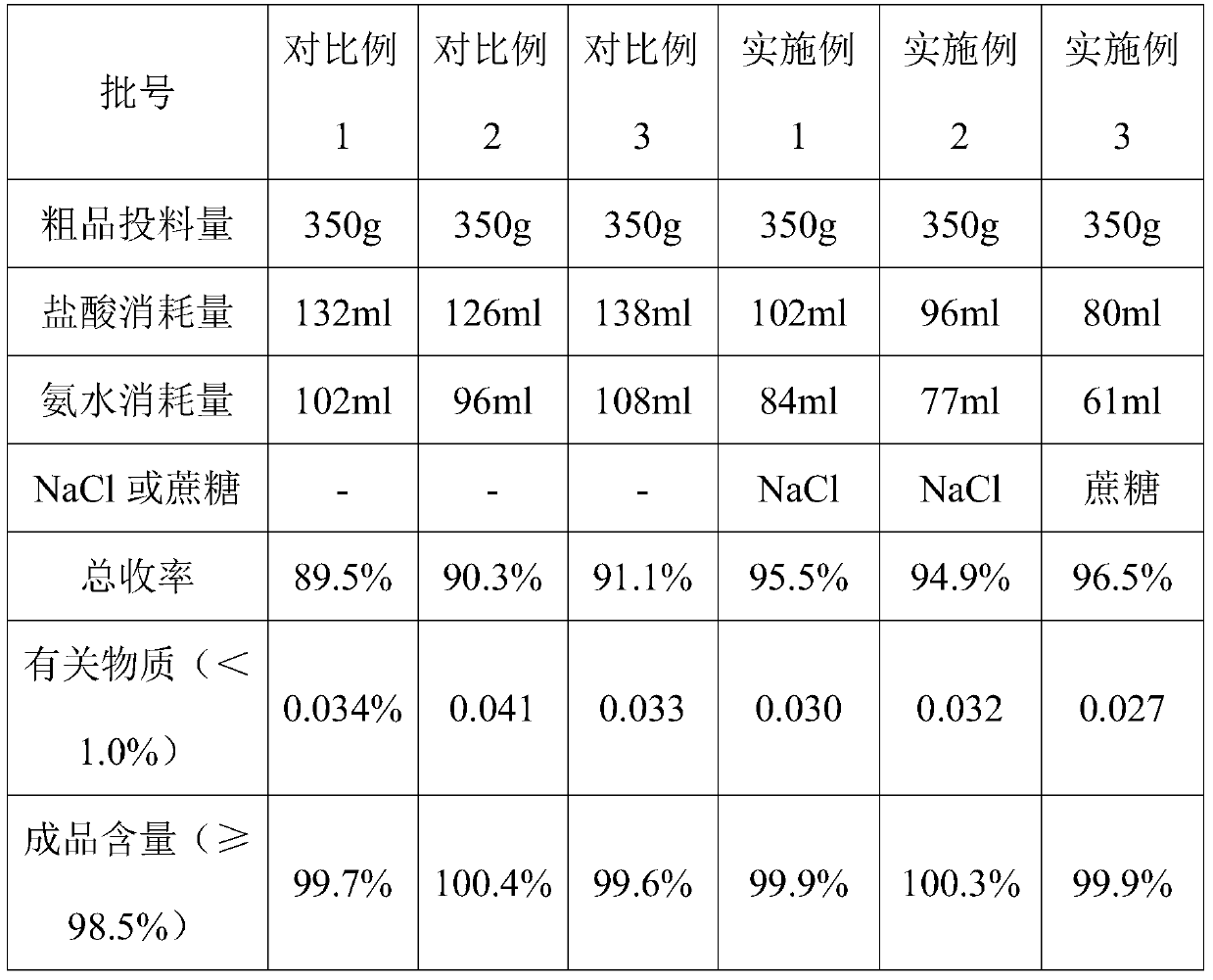
The invention belongs to the technical field of medicines and in particular relates to a method for recrystallizing ubenimex. By adopting the method for recrystallizing ubenimex, the phenomenon that materials can be instantly caked in the existing ubenimex crude product recrystallization process is avoided, hydraulic acid and ammonia water are greatly saved, and the total yield is increased by about 7-8 percent points.
Owner:SHANGHAI SINE WANXIANG PHARMA +1
Ubenimex hydrochloride compound
ActiveCN103910648AGood chemical stabilityImprove securityOrganic active ingredientsCarboxylic acid amide separation/purificationSolubilityHydrochloride
The invention provides an ubenimex hydrochloride crystal-form compound. The ubenimex hydrochloride crystal-form compound has good dissolvability and stability. The invention also provides a preparation method of the ubenimex hydrochloride crystal-form compound and a pharmaceutical composition containing the ubenimex hydrochloride crystal-form compound. The ubenimex hydrochloride crystal-form compound can be processed to form any dosage forms such as tablets, capsules, an injection, pills, particles and drop pills. The invention also provides a use of the ubenimex hydrochloride crystal-form compound in preparation of drugs for treating cancers.
Owner:西安万隆制药股份有限公司
Coated ubenimex table
ActiveCN101066255AOrganic active ingredientsPharmaceutical delivery mechanismWater solubleDissolution
The present invention discloses one kind of coated Ubenimex tablet, which consists of Ubenimex 10-30 weight portions, disintegrant 3-20 weight portions, diluent 50-300 weight portions, lubricant 1-5 weight portions and water soluble coating material 2-20 weight portions. Compared with marketable Ubenimex capsule, the coated Ubenimex tablet has the same dissolution and bioavailability, but simple preparation process and low production cost.
Owner:深圳万乐药业有限公司
Method for synthesizing Ubenimex
ActiveCN105646273AHigh reaction yieldHigh chemical purityOrganic compound preparationCarboxylic acid amides preparation3-amino-2-hydroxy-4-phenylbutyric acidSolvent
The invention belongs to the field of medicine preparation and provides a completely novel method for synthesizing Ubenimex. The method comprises the steps of firstly, protecting amino of a starting raw material, i.e., (2S,3R)-3-amino-2-hydroxyl-4-phenylbutyric acid (AHPA for short) by using di-tert-butyl dicarbonate so as to form a Boc protective product, then, carrying out condensation with L-leucine tert-butyl ester, and finally, removing the protective group in an organic solution of hydrogen chloride, thereby obtaining Ubenimex. According to the method, reagents used by the adopted synthesis route are all commonly-used reagents, produced industrially and low in cost; reaction solvents are all ordinary solvents, are low in toxicity and little in environmental pollution; and each reaction step is mild in conditions and simple and convenient in operation and does not need special equipment, so that the method is suitable for carrying out industrial production. The purity of the prepared product reaches 99.5% or more.
Owner:SUZHOU SIXTH PHARMA PLANT OF JIANGSU WUZHONG PHARMA GROUP
Preparation method of ubenimex intermediate (2S,3R)-3-acetamido-2-hydroxyl-4-phenylbutyric acid
InactiveCN107098827AHigh purityIncrease production capacityCarboxylic acid amides optical isomer preparationPhenylbutyrateEthylamine
The present invention provides a method for preparing an ubenimex intermediate (2S,3R)-3-acetamido-2-hydroxyl-4-phenylbutyric acid by using an organic alkali as a resolving agent, wherein the organic alkali is selected from S-1-naphthalene ethylamine, (S)-1-(2-naphthyl)ethylamine, L-phenylglycinol, and L-phenylalaninol. According to the present invention, the preparation method has advantages of good ubenimex intermediate purity, high yield, simple operation and good reproducibility, and is suitable for industrial production.
Owner:CHANGZHOU AINUOXINRUI PHARMA LTD
Novel synthesis process of ubenimex
ActiveCN104447394AMild responseEfficient responseOrganic compound preparationCarboxylic acid amides preparation3-amino-2-hydroxy-4-phenylbutyric acidTert-leucine
The invention discloses a novel synthesis process of ubenimex. The synthesis process comprises the following steps: enabling a raw material, namely (2S,3R)-3-amino-2-hydroxy-4-phenyl butyric acid, to react with di-tert-butyl dicarbonate ester so as to prepare an intermediate I, wherein di-tert-butyl dicarbonate ester is added in multiple batches; reacting the intermediate I with L-leucine tert-butyl ester hydrochloride so as to obtain an intermediate II, wherein L-leucine tert-butyl ester hydrochloride is preferred and is conducive to the improvement of yield and purity of the intermediate II; and respectively removing protecting groups, namely tert-butyloxy carbonyl and tert-butyl ester, of the intermediate II in an acid-base system, wherein through the operation, the protecting groups are effectively removed by regulating pH value by virtue of the acid-base system, so that the yield and purity of the finished ubenimex are improved.
Owner:西安万隆制药股份有限公司
Preparation method of high-purity Ubenimex
ActiveCN105968026AHigh yieldHigh purityOrganic chemistry methodsCarboxylic acid amide separation/purificationOrganic solventChemical preparation
The invention belongs to the field of medicinal chemical preparation, and particularly relates to a preparation method of high-purity Ubenimex. The method includes the following steps of adding a Ubenimex crude product to a mixed solution of water and other organic solvent to be heated, stirred and dissolved, after dissolving, conducting cooling, crystallizing and suction filtration, and conducting vacuum drying on obtained filter cakes to obtain the final Ubenimex product. By controlling parameters of the preparation method, the yield and purity of Ubenimex can be remarkably improved, product purity is high, and product quality is better ensured.
Owner:SICHUAN QINGMU PHARMA CO LTD
Preparation method of ubenimex
InactiveCN107141232AAvoid using bothAvoid safety hazardsCarbamic acid derivatives preparationOrganic compound preparationTert-leucineEthyl acetate
The invention provides a preparation method of ubenimex. The preparation method comprises that L-leucine benzyl ester p-toluenesulfonate and HOBt undergo a condensation reaction to produce an activated ester solution, (2S, 3R)-3-benzyloxyformamido-2-hydroxy-4- phenylbutyric acid and a weak acid strong alkali inorganic salt are mixed, the activated ester solution is added into the mixture drop by drop and undergoes a reaction to produce N-[(2S, 3R)-3-benzoylformamido-2-hydroxy-4-phenylbutyryl]-L-leucine, and N-[(2S, 3R)-3-benzoylformamido-2-hydroxy-4-phenylbutyryl]-L-leucine is reduced into ubenimex through hydrogen gas. The preparation method utilizes ethyl acetate as an organic solvent, saves tetrahydrofuran-caused potential safety hazard, utilizes the weak acid strong alkali inorganic salt as a base catalyst, saves a cost, is free of an organic solvent and is easy to operate.
Owner:SINOPHARM CHUANKANG PHARMACEUTICAL CO LTD
Orally disintegrated Ubenimex tablet and its prepn process
InactiveCN1706373ADisintegrates quicklyGood compressibilityOrganic active ingredientsCross-linkOrally disintegrating tablet
The orally disintegrated Ubenimex tablet consists of Ubenimex as active component and supplementary material including disintegrating agent, soluble diluent, adhesive and lubricant. The disintegrating agent is mixture of 2 or 3 of low substituted hydroxypropyl cellulose, cross-linked carboxymethyl cellulose, cross-linked sodium carboxymethyl starch, cross-linked polyvidon and microcrystalline cellulose. The preparation process is the combination of wet pelletizing and direct tabletting. The orally disintegrated Ubenimex tablet has fast disintegration, administration convenience, fast absorption, high bioavailability, and less irritation to gastrointestinal tract.
Owner:JILIN UNIV
Multi-targeted ubenimex prodrug derivative and preparation method and use thereof
ActiveUS20160193347A1Improve anti-tumor activityGood treatment effectBiocideUrea derivatives preparationBiological studiesStructural formula
The present invention relates to the design, synthesis, and biological study of multi-targeted Ubenimex pro-drug derivative. More particularly, provided in the present invention is a compound as shown by general structural formula (I) (wherein the definition of R is shown in the description). The derivative is a multi-targeted compound obtained by binding an aminopeptidase (APN / CD13) inhibitor, Ubenimex, with some anti-tumor drugs already on the market through an ester bond or amide bond, and is suitable for use as an anti-tumor drug for the treating various malignant tumors, and is especially suitable for treating various solid tumors.
Owner:WEIFANG BOCHUANG INT ACAD OF BIOTECH & MEDICINE
Ubenimex drop pill and preparing method thereof
InactiveCN1528277ADisintegration and dissolution fastHigh dissolution rateOrganic active ingredientsPill deliveryMedicineReduced dose
The present invention utilizes ultramicropulverization and dripping pill preparation production process to make ubenimex dripping pills, and can attain the goal of raising disintegration and dissolution speed, quickly obtaining therapeutic effect, raising stability of medicine, reducing dose of auxiliary material, reducing production cost and convenient administration. Said pill not only can be sucked, and can be swallowed, and its compliance property is good.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Dropping pills containing ubenimex and method for preparing the same
InactiveCN101194902ASmall molecular weightFast dissolutionOrganic active ingredientsPharmaceutical product form changeChemical synthesisFood additive
The invention provides a medicament which can enhance immune function. The invention overcomes the shortcomings of the prior dripping pills that pure natural degrees of findings which are used by the prior dripping pills are not high, and chemosynthesis findings which are commonly used are not in food additives catalogs of some countries, and tastes of the dripping pills are worse. The invention is a pharmaceutical preparation whose natural degree is higher, safety is stronger, and side effect is lower.
Owner:TIANJIN TASLY PHARMA CO LTD
A kind of ubenimex hydrochloride compound
ActiveCN103910648BGood chemical stabilityImprove securityOrganic active ingredientsCarboxylic acid amide separation/purificationSolubilityHydrochloride
The invention provides an ubenimex hydrochloride crystal-form compound. The ubenimex hydrochloride crystal-form compound has good dissolvability and stability. The invention also provides a preparation method of the ubenimex hydrochloride crystal-form compound and a pharmaceutical composition containing the ubenimex hydrochloride crystal-form compound. The ubenimex hydrochloride crystal-form compound can be processed to form any dosage forms such as tablets, capsules, an injection, pills, particles and drop pills. The invention also provides a use of the ubenimex hydrochloride crystal-form compound in preparation of drugs for treating cancers.
Owner:西安万隆制药股份有限公司
Ubenimex fat emulsion injection and preparation method thereof
InactiveCN101601649BUniform quality and appearanceImprove anti-tumor effectOrganic active ingredientsEmulsion deliveryFat emulsionUbenimex
Owner:SHANDONG UNIV
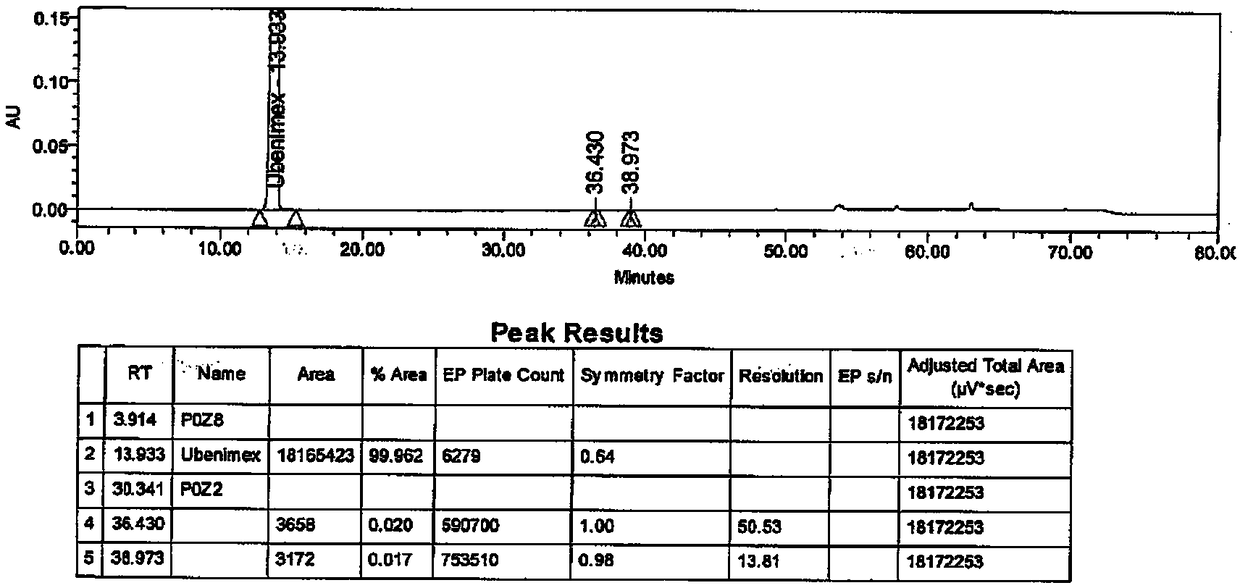
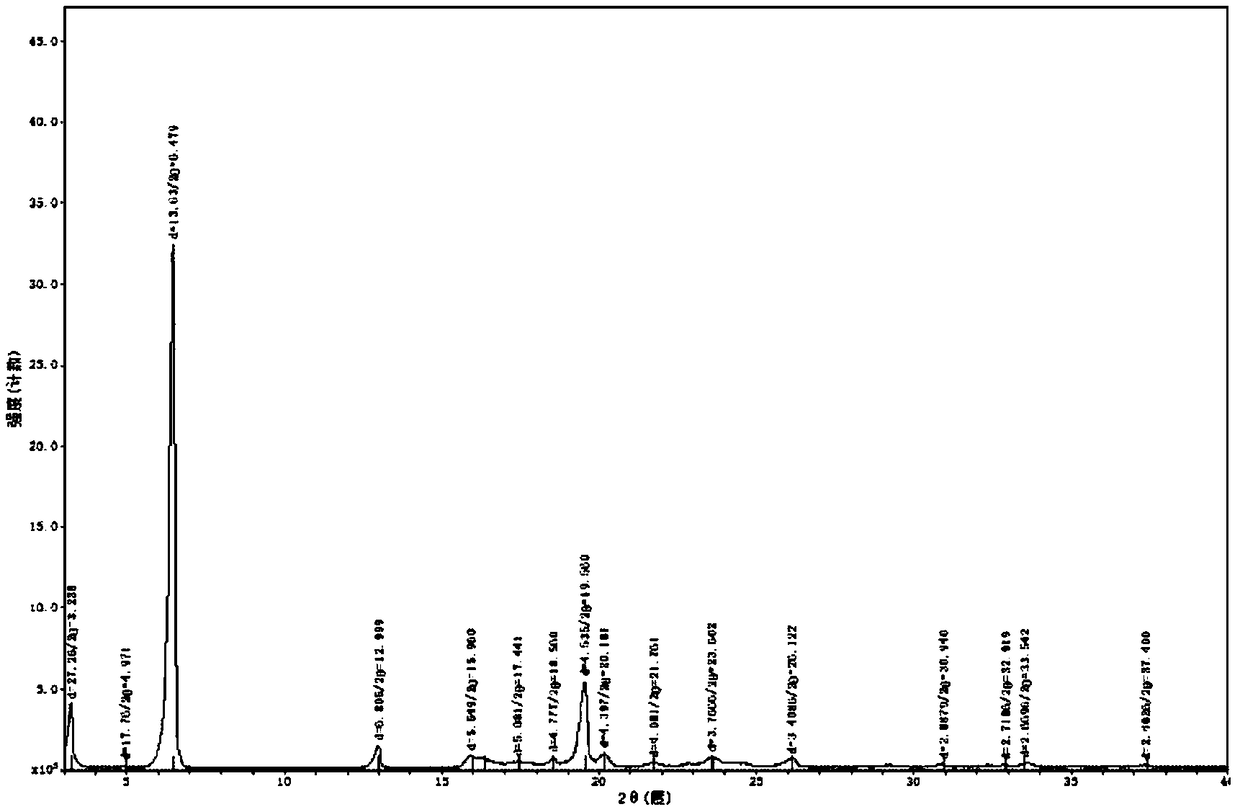
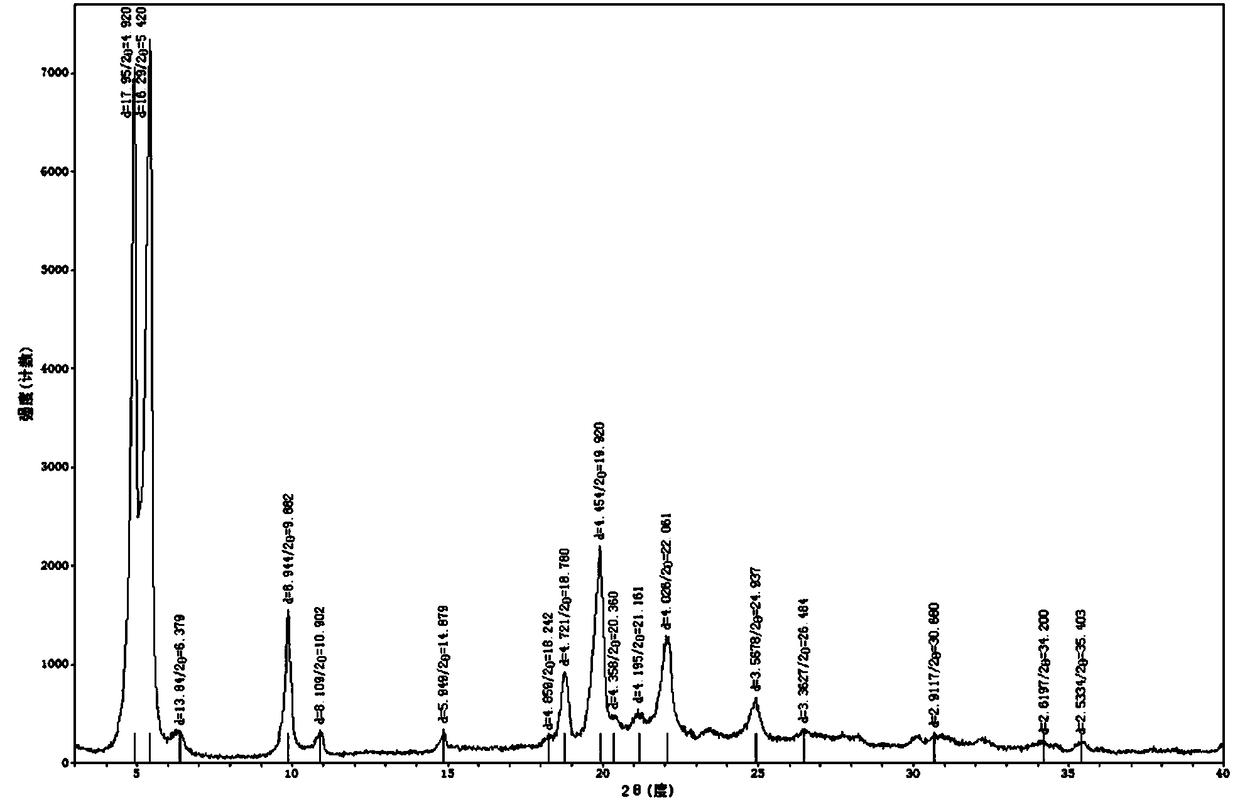
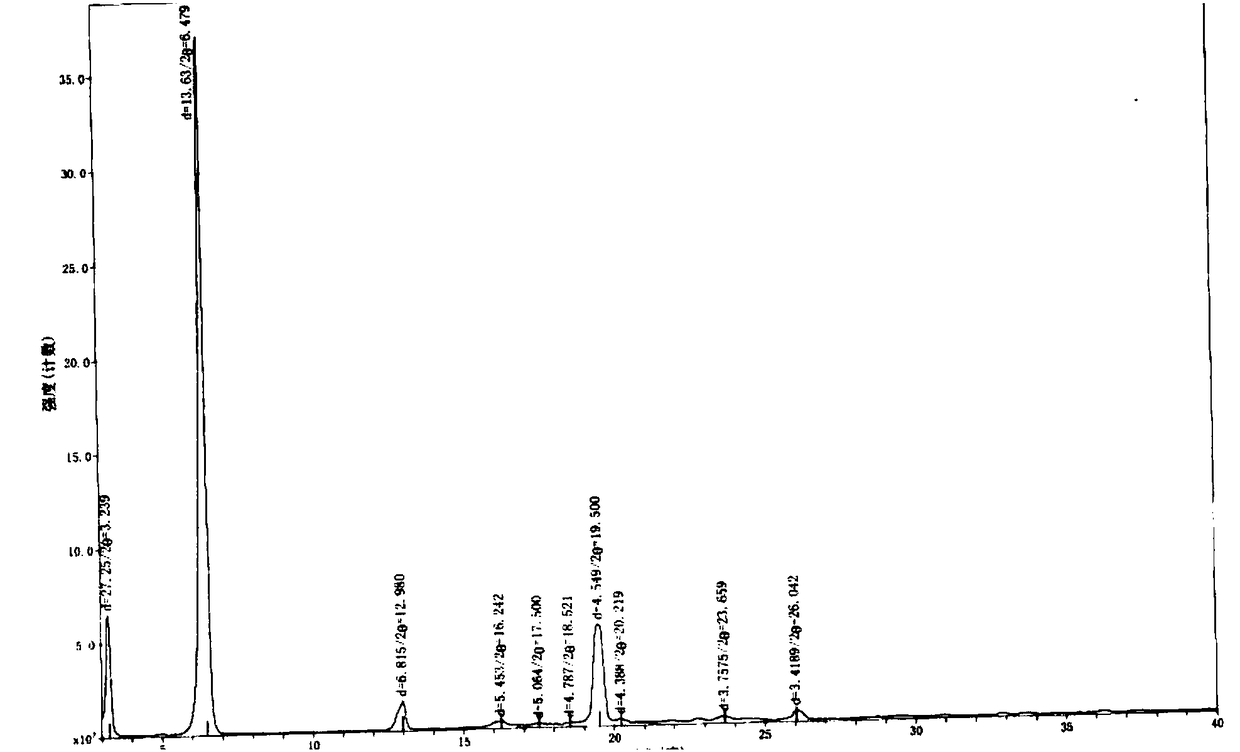
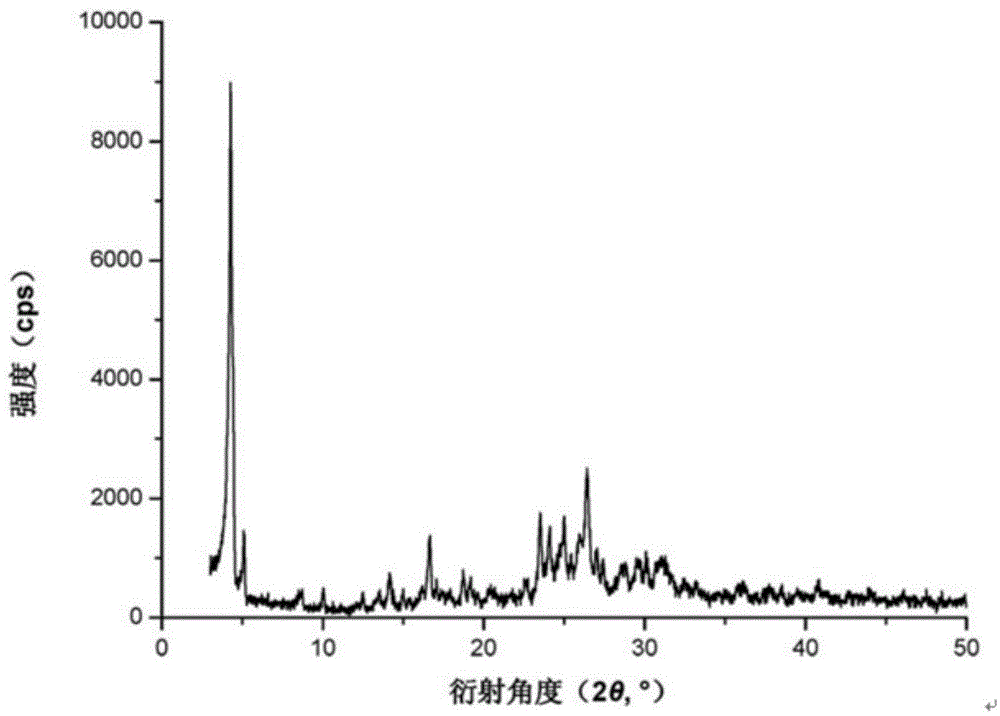
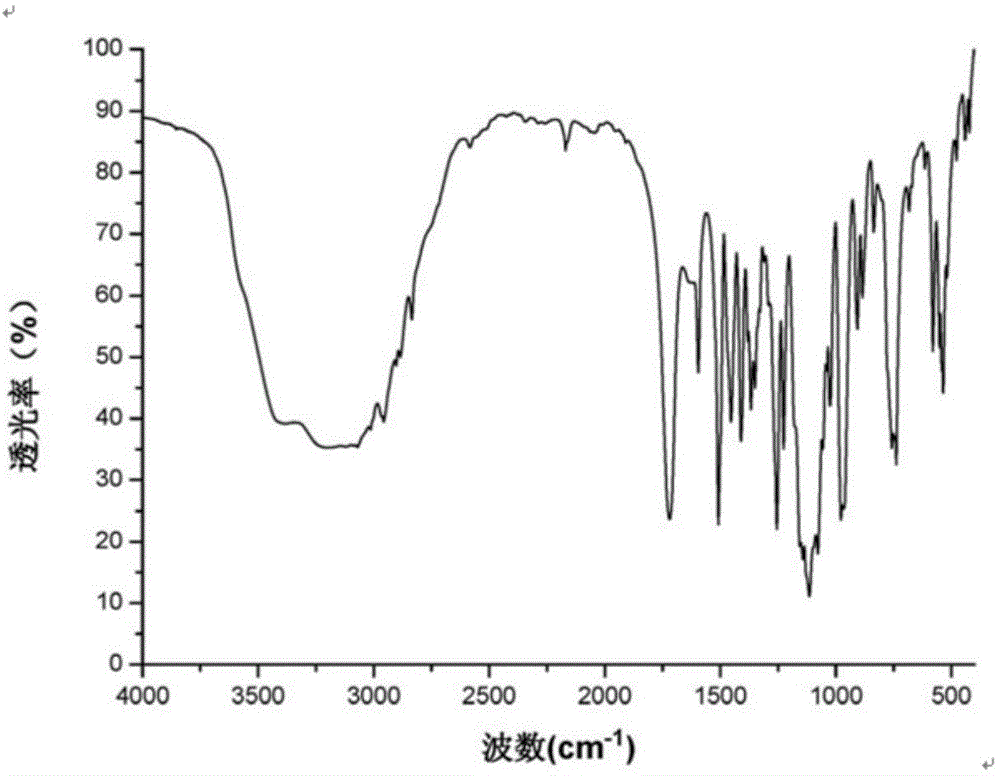
Crystal form of N-[(2S, 3R)-4-phenyl-3-benzyl-3-formylaminobenzoxy-2-hydroxybutyryl]-L-benzyl leucine and preparation method thereof
InactiveCN103333089ACarbamic acid derivatives preparationOrganic compound preparationOrganic solventSolvent
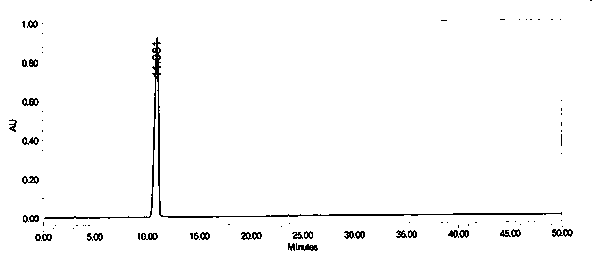
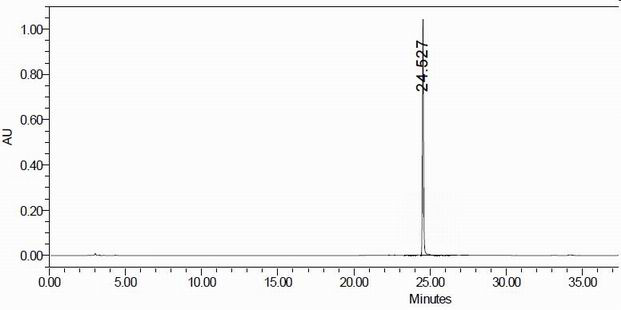
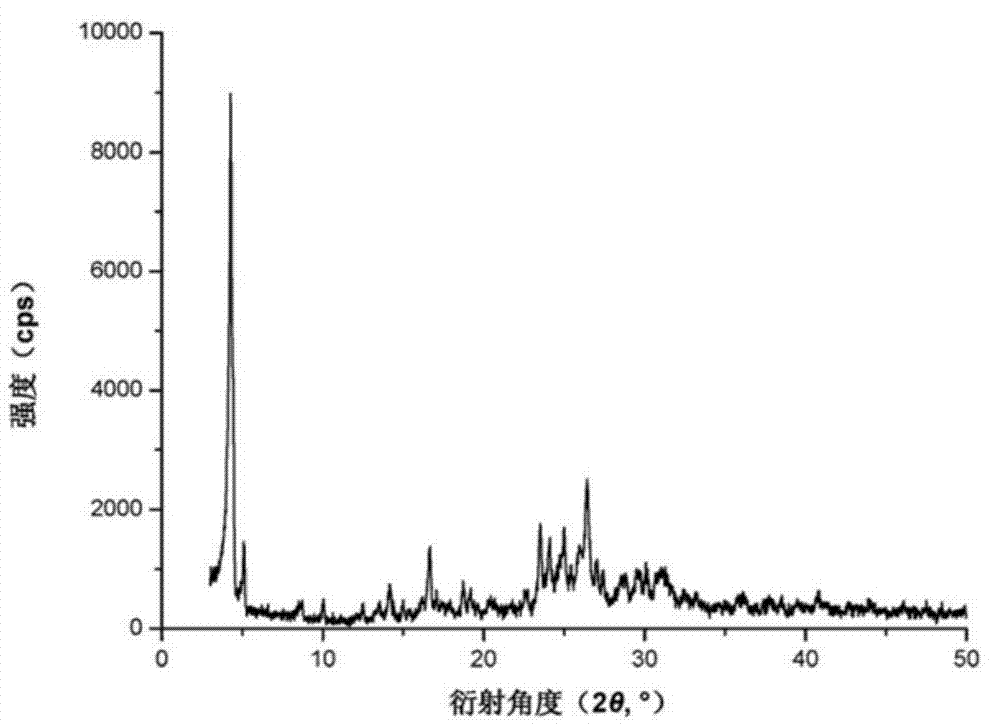
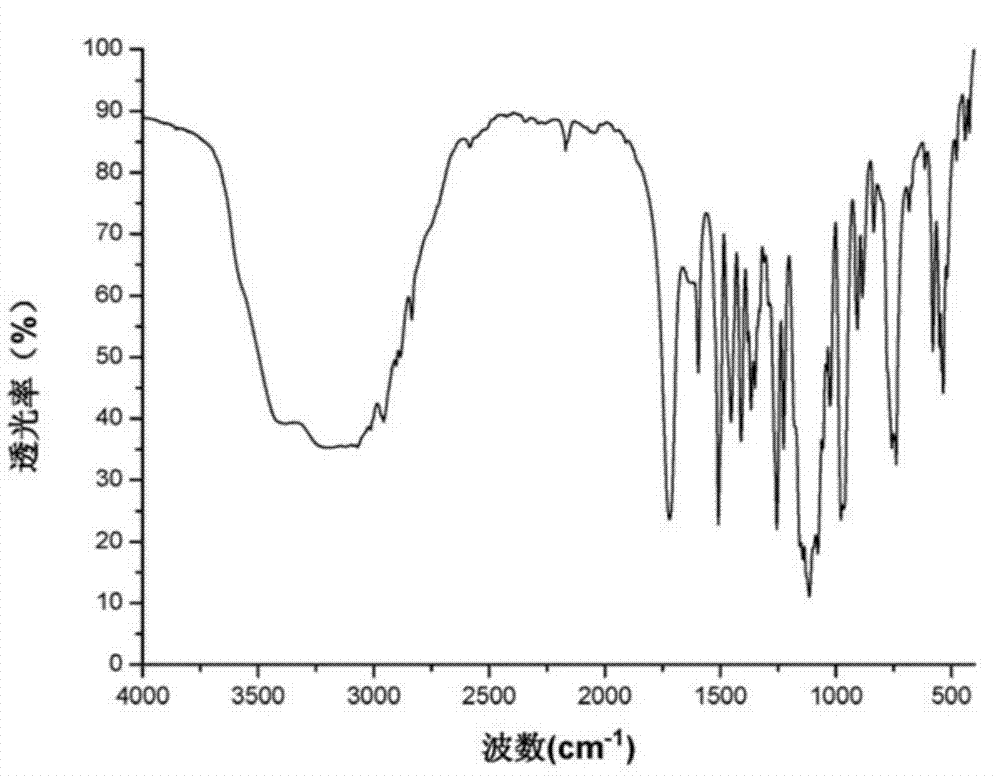
The invention provides a crystal form of an ubenimex intermediate N-[(2S, 3R)-4-phenyl-3-benzyl-3-formylaminobenzoxy-2-hydroxybutyryl]-L-benzyl leucine. The ubenimex intermediate with the crystal form has high purity and good stability and is suitable for further preparing high-purity ubenimex active pharmaceutical ingredients. The invention also provides a preparation method of the crystal form, wherein according to the method, recrystallization is used for preparation; through HPLC (High Performance Liquid Chromatography), the purity of the obtained crystal is over 98.5%; and moreover, the adopted organic solvent is a common solvent with low price, a single-solvent recrystallization is more convenient for recovery, and the method is suitable for industrial production.
Owner:深圳万乐药业有限公司
Preparation method for ubenimex gamma crystal form
InactiveCN108892625AIncrease generation costLow costOrganic chemistry methodsCarboxylic acid amide separation/purificationSingle crystalUbenimex
The invention relates to a preparation method for a ubenimex gamma crystal form. Through controlling key parameters in the preparation method, the preparation method provided by the invention is capable of obviously increasing the yield of the gamma crystal form and acquiring a single crystal form, has high technological repeatability, is capable of achieving controllable industrial production conditions and is beneficial to large-scale industrialization.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD +1
A kind of ubenimex delta crystal form and preparation method thereof
ActiveCN106631880BImprove solubilityImprove stabilityCarboxylic acid amide separation/purificationChemical recyclingSolubilityCombinatorial chemistry
This application relates to the field of pharmaceutical crystal forms, in particular to a δ crystal form of Ubenimex and its preparation method; the δ crystal form prepared by the method of this application has better solubility and stability, and better fluidity, compared with The existing crystal form has more preparation advantages; and the preparation reaction conditions are mild and the operation is simple. The solvents used are only ethanol and water, which is conducive to environmental protection, convenient recycling, reduces reagent costs and energy consumption, and realizes controllable industrial production conditions. large-scale industrialization.
Owner:SICHUAN QINGMU PHARMA CO LTD
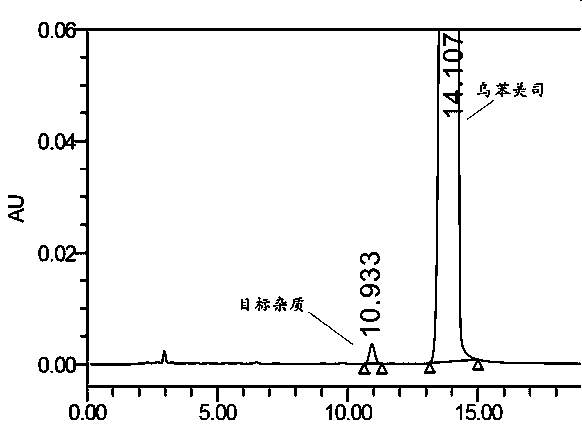
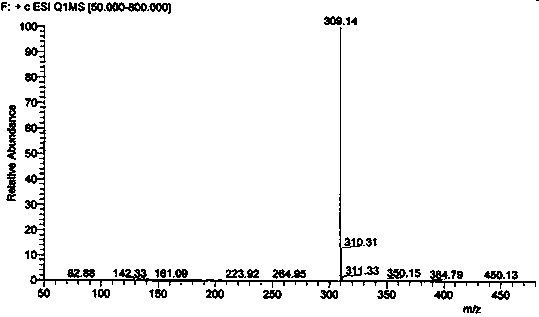
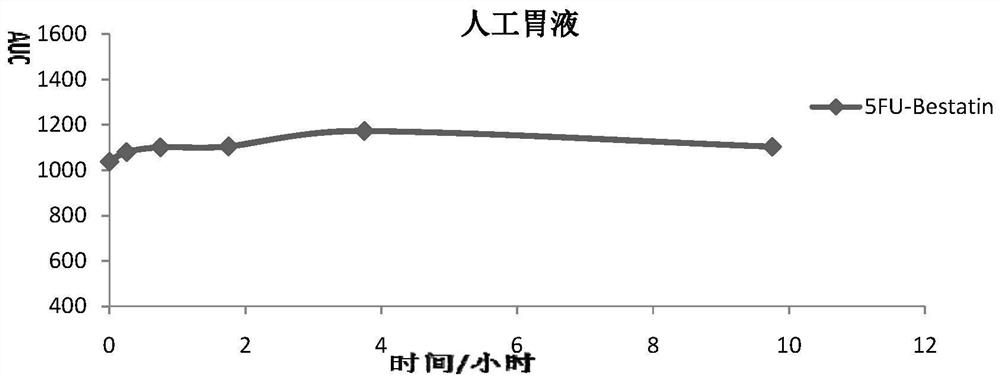
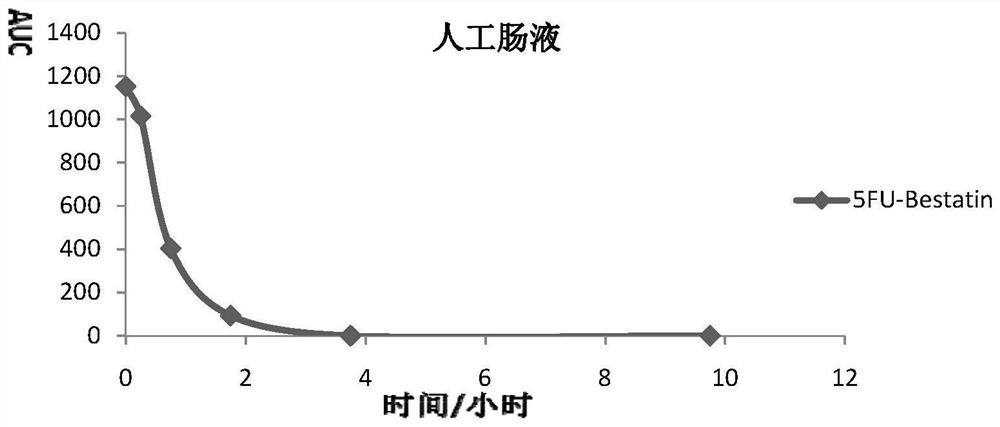
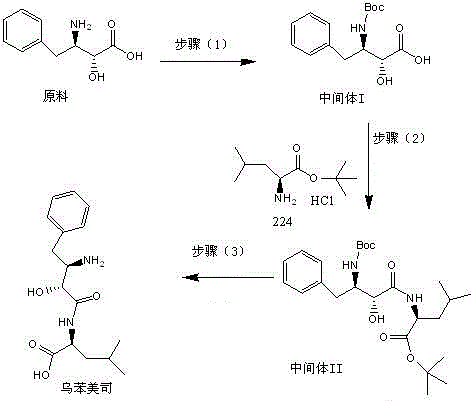
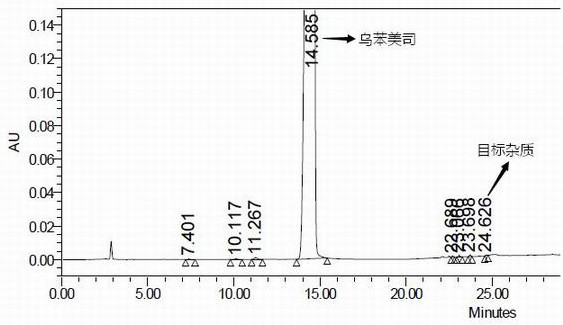
Impurity in ubenimex synthesis and production method thereof
The invention provides an impurity in a ubenimex crude drug. Identification of a structure of the impurity benefits quality control of ubenimex so that the high-quality ubenimex crude drug can be obtained. The invention furthermore provides a production method of the impurity; and a product obtained through the method is high in purity, and can be used as an impurity reference substance of a ubenimex crude drug quality studying process.
Owner:深圳万乐药业有限公司
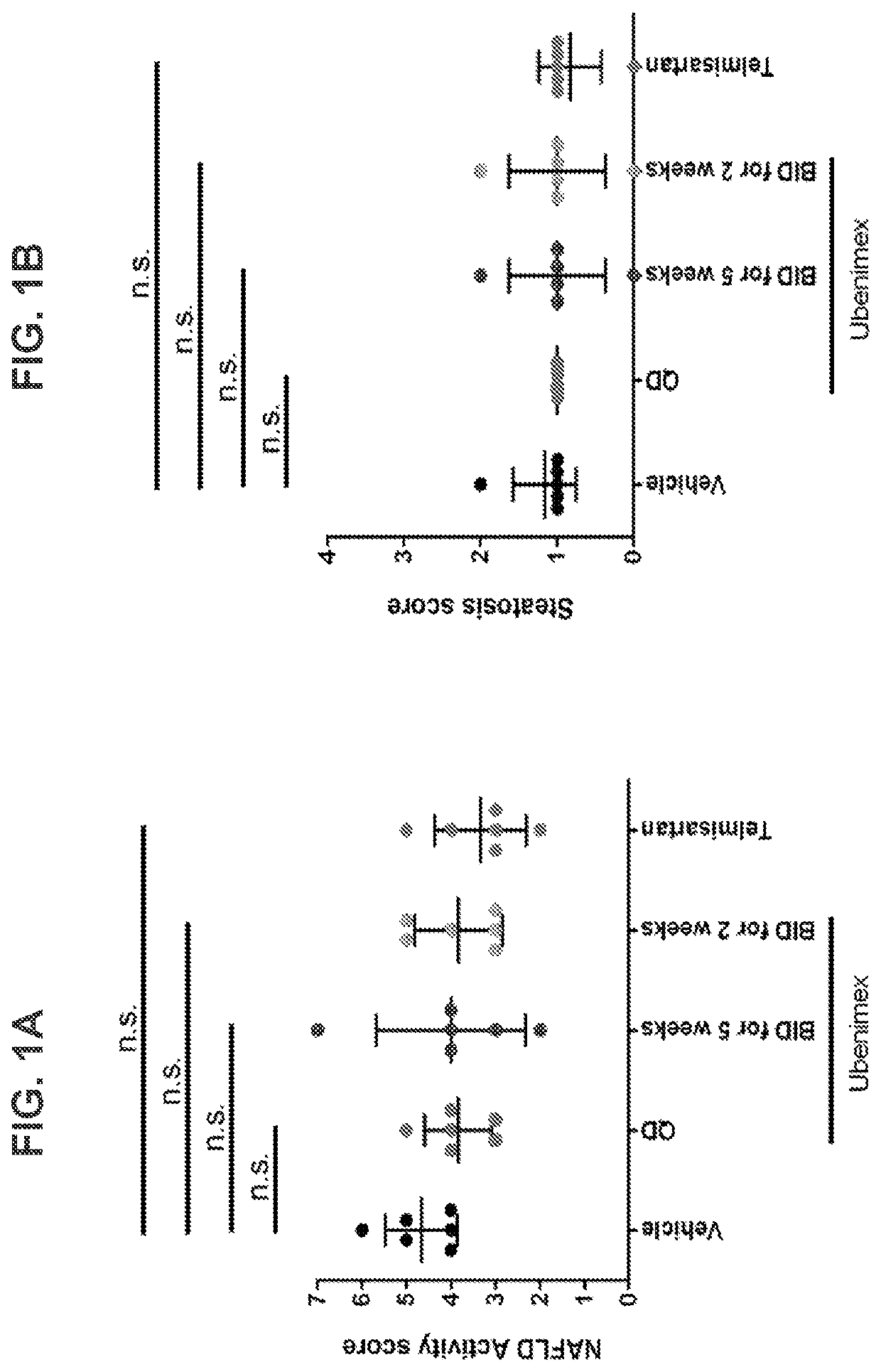
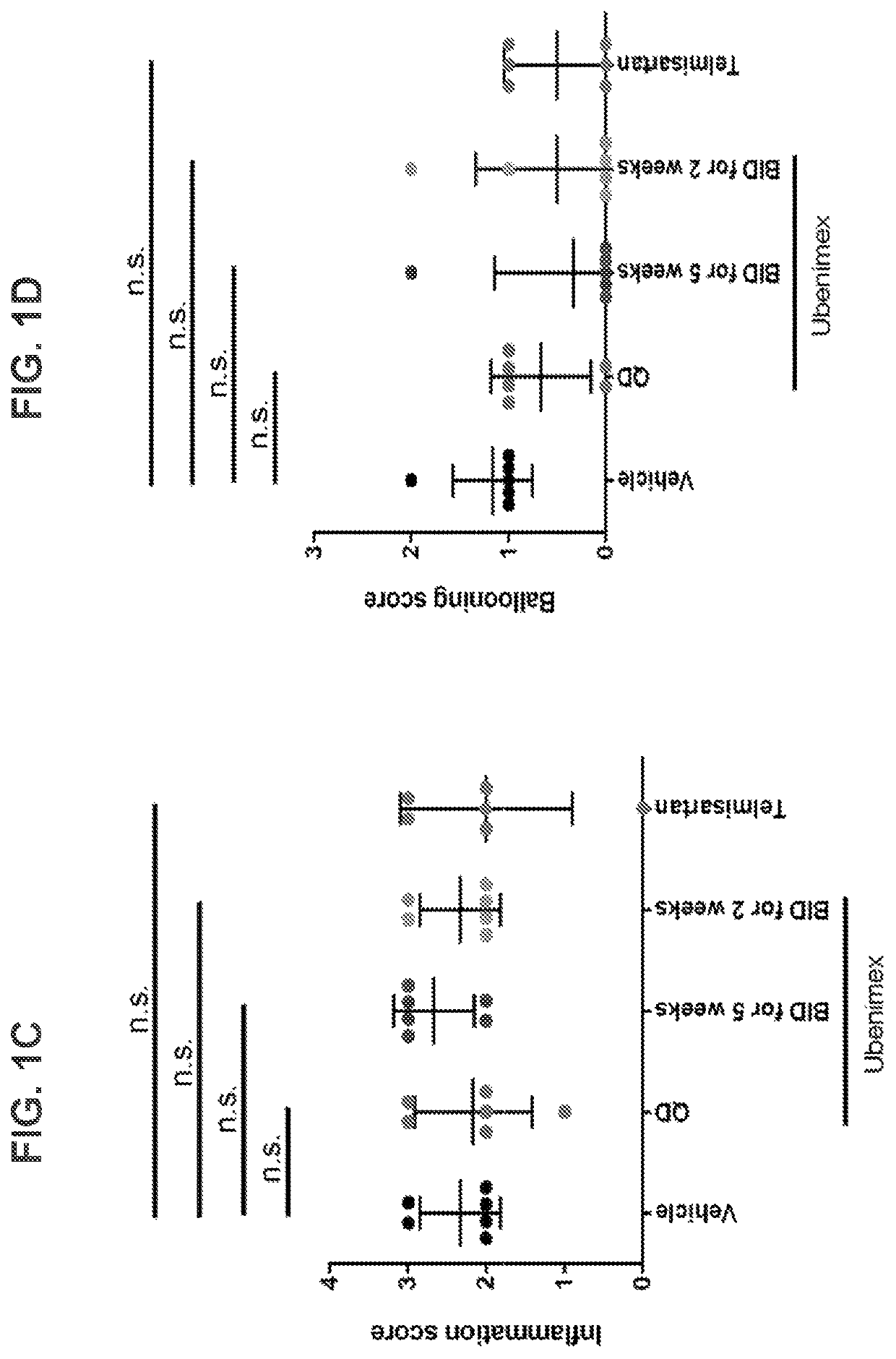
Methods and pharmaceutical compositions for the treatment of non-alcoholic steatohepatitis
ActiveUS10588880B2Slow and prevent progression of diseaseEasy to packOrganic active ingredientsPeptide/protein ingredientsPharmaceutical drugPharmacology
Owner:EIGER BIOPHARMLS
Ubenimex recrystallization device and method thereof
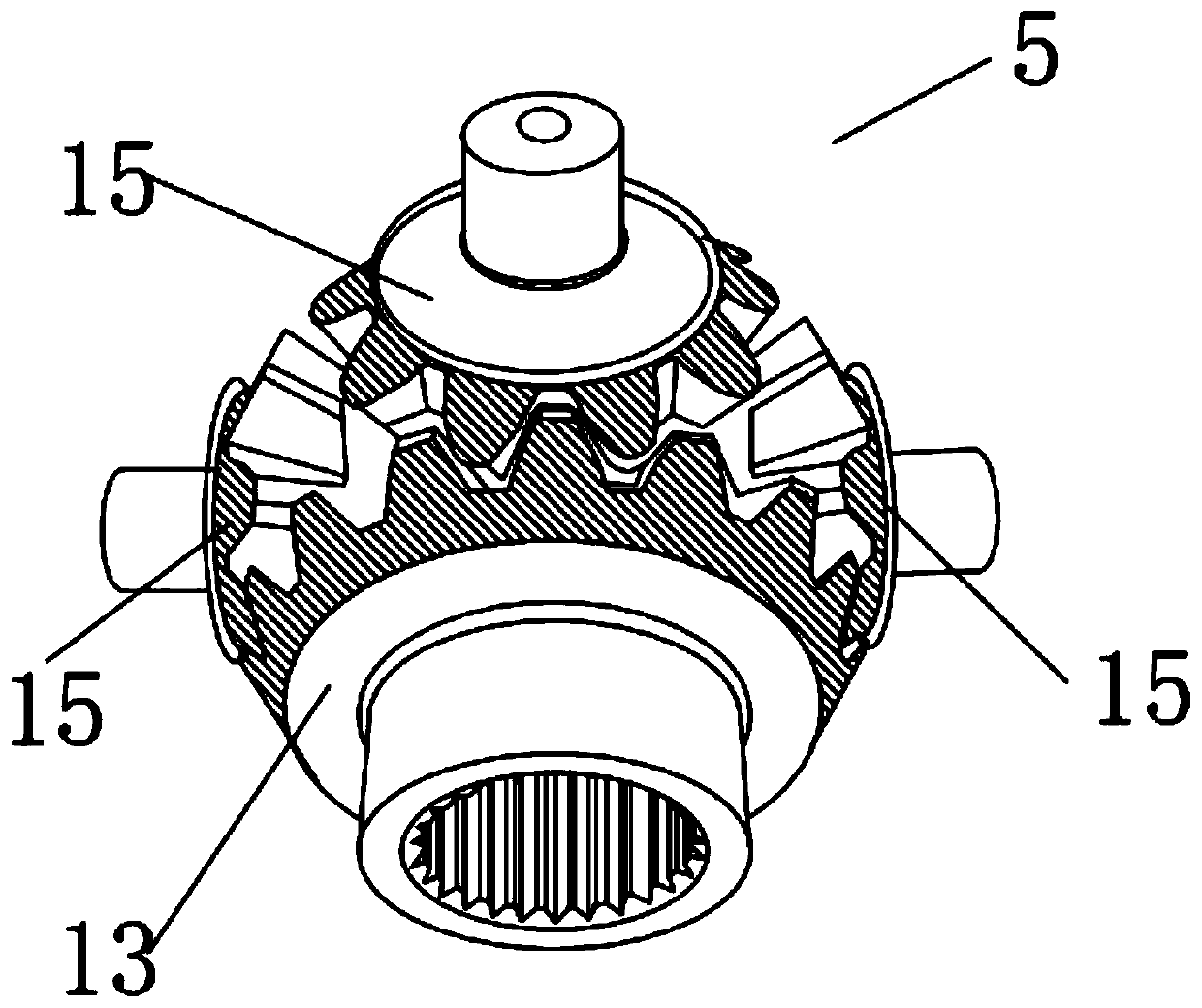
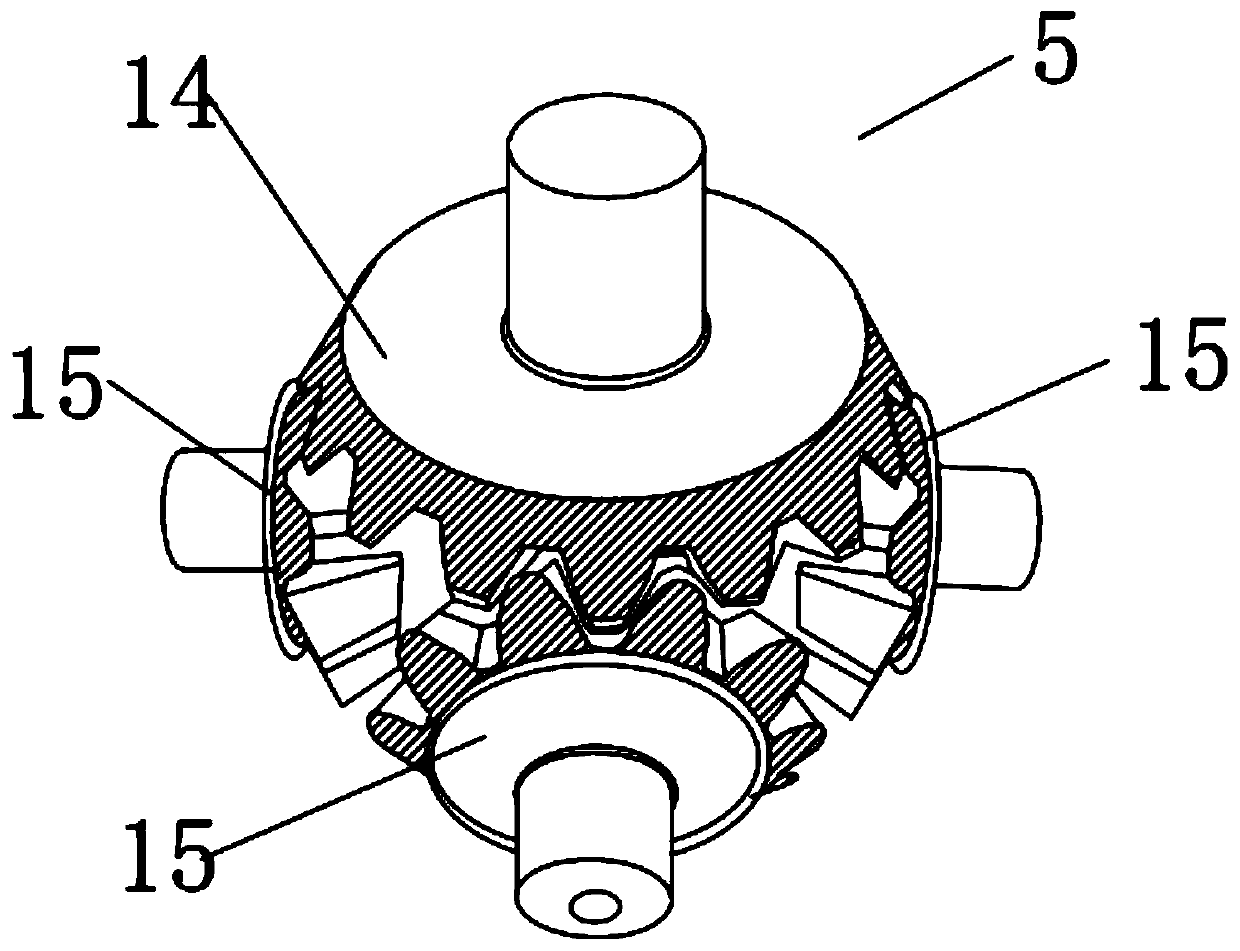
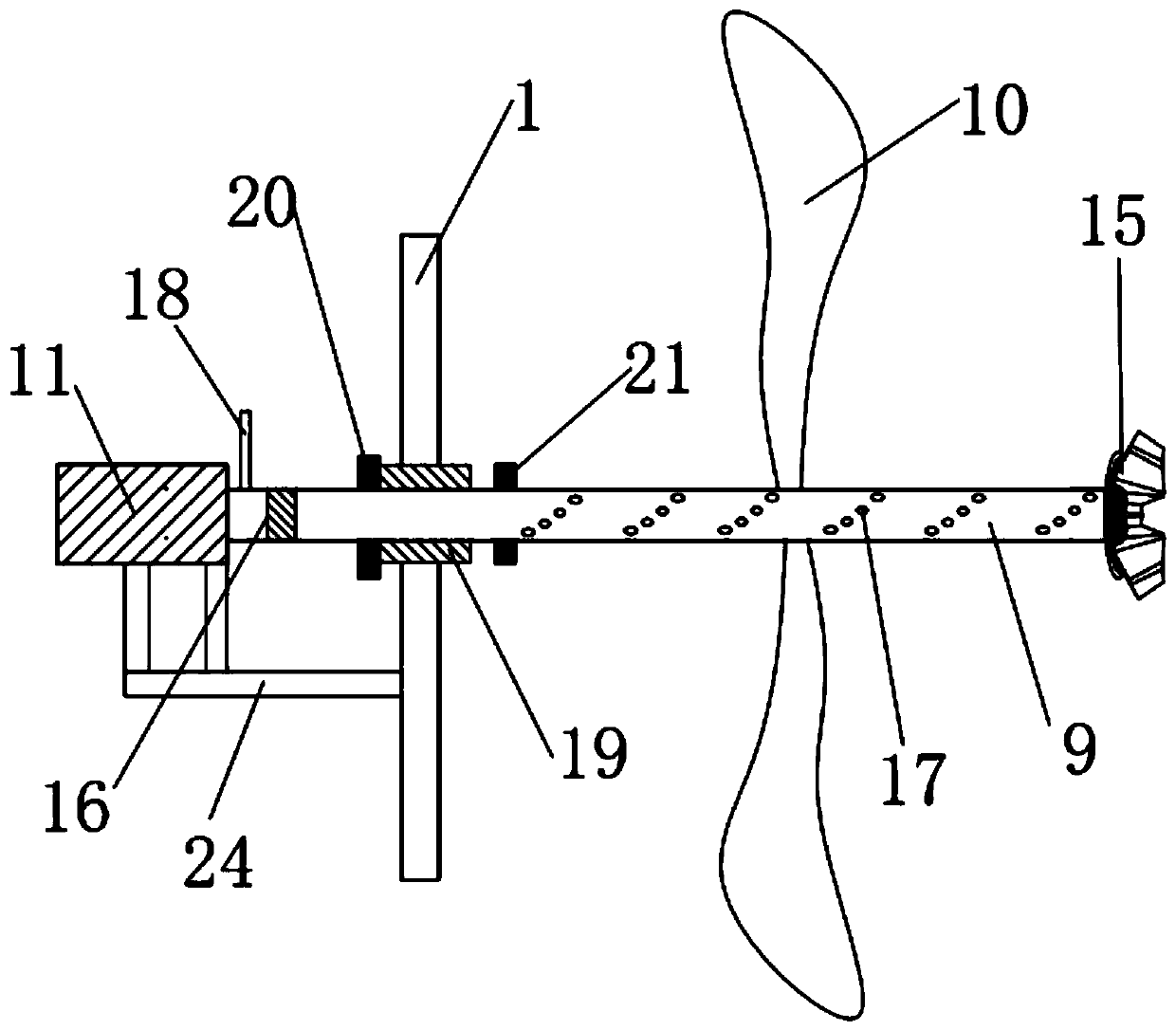
PendingCN111450568AReduce generationReduce dead angleCrystallization preventionSolution crystallizationGear wheelElectric machinery
The invention discloses an ubenimex recrystallization device and a method thereof. The recrystallization device comprises a reaction kettle and a driving motor, and the driving motor is connected witha bevel gear transmission mechanism arranged in the reaction kettle through a main stirring shaft; the bevel gear transmission mechanism is composed of a main planet gear, at least one side planet gear and an auxiliary planet gear which are arranged from bottom to top, the side planet gear is connected with the main planet gear and the auxiliary planet gear respectively, and the main planet gearis connected with the main stirring shaft through a shaft; the auxiliary planetary gear is connected with the top end of the reaction kettle through a vertically-arranged auxiliary connecting shaft; and the side planetary gear is connected with the side wall of the reaction kettle through a horizontally-arranged side connecting shaft. According to the ubenimex recrystallization device, six-direction stirring can be achieved, the effect of dispersing gel is achieved, dead corners are greatly reduced, the problem that in the prior art, caking is likely to happen is solved, the production processis easier to control, and the quality is more stable; the product purity is improved, and the yield is also increased.
Owner:SHANGHAI SINE WANXIANG PHARMA +1
Novel ubenimex recrystallization method
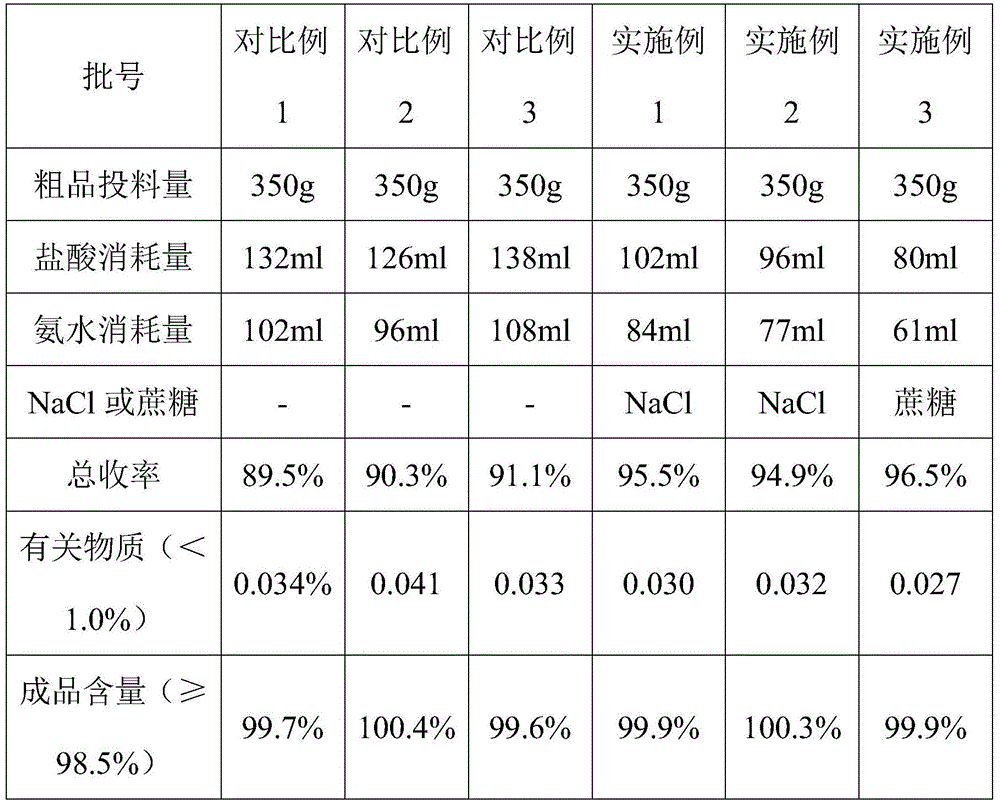
ActiveCN106117075AEasy to controlReduce usageCarboxylic acid amide separation/purificationPh controlAmmonium hydroxide
The invention discloses a novel ubenimex recrystallization method. According to the method, PH is controlled in a manner of bidirectionally, simultaneously and dropwise adding an ubenimex acid solution and an alkali solution, and a PH buffer solution is used as a dispersion system, so that PH control is more accurate, the problem that agglomerates are easy to produce in the prior art is solved, the production process is easy to control, the quality is stable, the yield is also increased, the usage quantity of an ammonia solution and the usage quantity of hydrochloric acid are reduced, the cost is reduced, besides, the generation of three wastes is also reduced, the total yield is increased, and besides, the novel ubenimex recrystallization method is environmentally-friendly.
Owner:SHANGHAI SINE WANXIANG PHARMA +1
Compound preparation having anti-tumor action
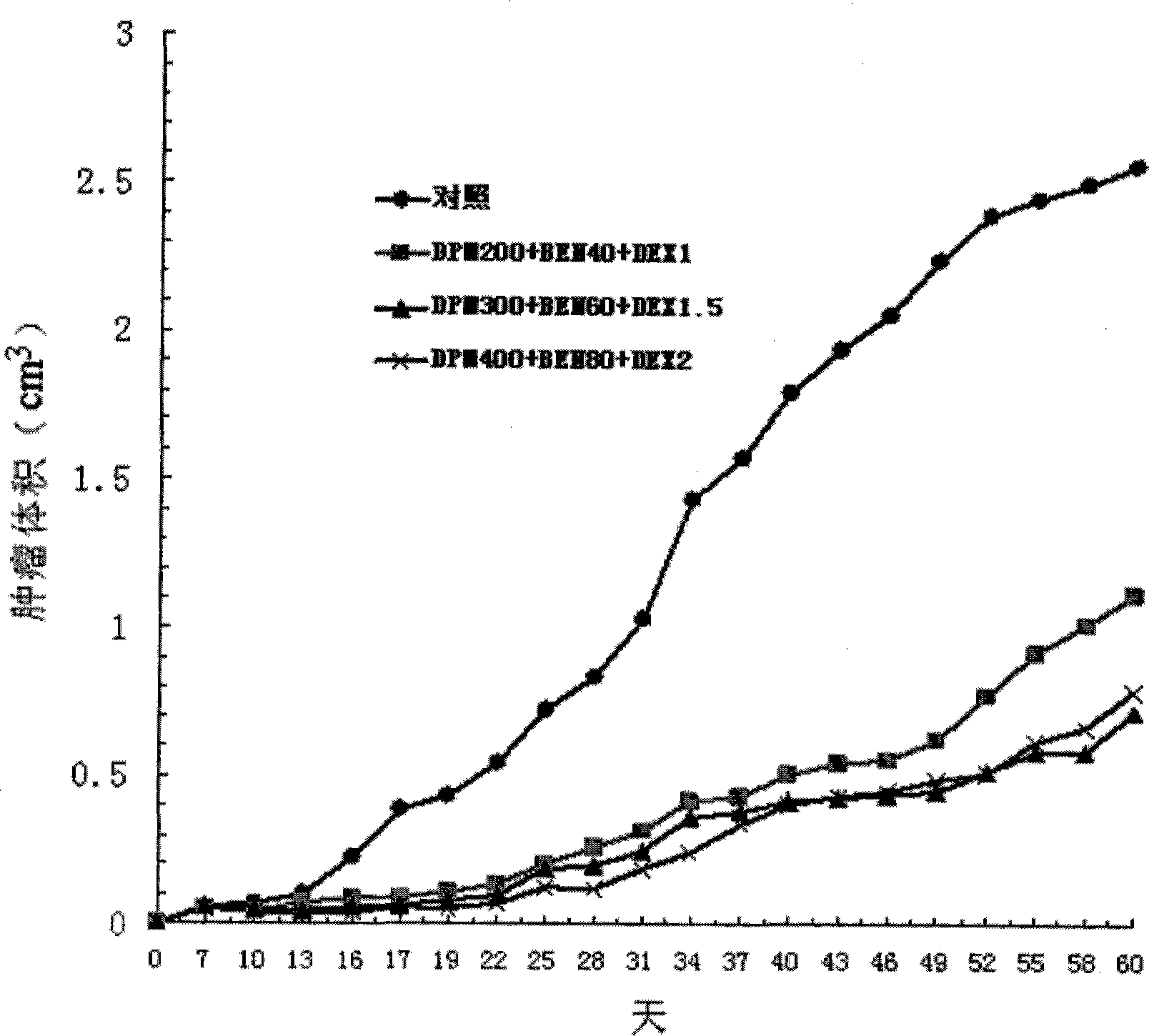
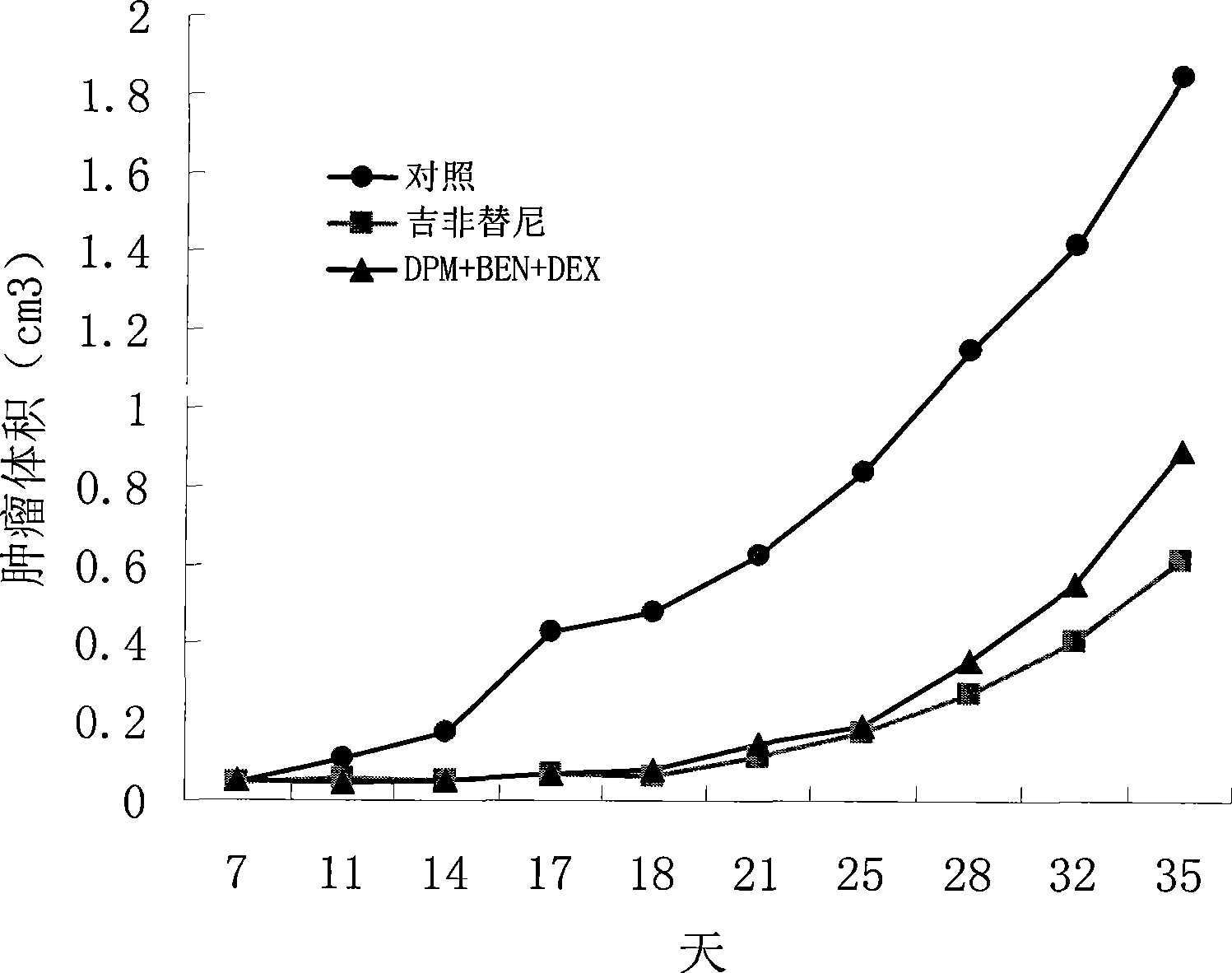
InactiveCN102429914AStrong penetrating powerPromote absorptionOrganic active ingredientsAntineoplastic agentsCelluloseDexamethasone
The invention provides a compound preparation having an anti-tumor action. The compound preparation comprises therapeutically effective amounts of ingredient A, ingredient B, ingredient C and a pharmaceutically acceptable carrier; or mixture of the ingredient A, the ingredient B and the ingredient C; or a capsule taking the mixture of the ingredient B and the ingredient C as restricted substances; the ingredient A is enteric coating particles of dipyridamole and is formed by dipyridamole enteric particles and coating layers for packing the dipyridamole enteric particles; the ingredient B is ubenimex adhesion particles taking insoluble cellulose and biological adhesive materials as carriers; and the ingredient C is dexamethasone with a particle size of 100-200 nm. The compound preparation having the anti-tumor action formed by the three compositions can reach the peak value in blood at a higher speed, and can remain longer effective concentration time respectively, so that the requirement of clinical application can be met.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT +1
A solid dosage form of a prodrug derivative containing ubenimex and a preparation method thereof
ActiveCN109646429BExtended stayGood pharmacokinetic propertiesOrganic active ingredientsPill deliveryPharmaceutical medicineBULK ACTIVE INGREDIENT
The invention relates to the field of medicinal chemistry, and discloses a solid dosage form containing a prodrug derivative of ubenimex and a preparation method thereof. The solid dosage form contains pharmaceutically active components, fillers, disintegrants and lubricants, based on the total weight of the solid dosage form, the content of the pharmaceutically active components is 20-80% by weight, the content of the filler The content of the disintegrant is 10-70% by weight, the content of the disintegrant is 0.5-5% by weight, and the content of the lubricant is 0.5-5% by weight, wherein the active ingredient of the drug is the precursor of Ubenimex Drug derivatives or their optical isomers, diastereoisomers, racemic mixtures and pharmaceutically acceptable salts thereof. The solid dosage form of the invention can prolong the residence time of the drug in the body, improve the pharmacokinetic properties of the drug, and increase the bioavailability of the drug, so as to achieve better anti-tumor effect.
Owner:江西润泽药业有限公司
A novel synthesis process of ubenimex
ActiveCN104447394BMild responseEfficient responseOrganic compound preparationCarboxylic acid amides preparationTert-leucineTert butyl
The invention discloses a novel synthesis process of ubenimex. The synthesis process comprises the following steps: enabling a raw material, namely (2S,3R)-3-amino-2-hydroxy-4-phenyl butyric acid, to react with di-tert-butyl dicarbonate ester so as to prepare an intermediate I, wherein di-tert-butyl dicarbonate ester is added in multiple batches; reacting the intermediate I with L-leucine tert-butyl ester hydrochloride so as to obtain an intermediate II, wherein L-leucine tert-butyl ester hydrochloride is preferred and is conducive to the improvement of yield and purity of the intermediate II; and respectively removing protecting groups, namely tert-butyloxy carbonyl and tert-butyl ester, of the intermediate II in an acid-base system, wherein through the operation, the protecting groups are effectively removed by regulating pH value by virtue of the acid-base system, so that the yield and purity of the finished ubenimex are improved.
Owner:西安万隆制药股份有限公司
Impurity in ubenimex bulk drug and preparation method of impurity
The invention provides an impurity generated in the preparation process of ubenimex bulk drug, and also provides a preparation method of the impurity. The purity of the impurity prepared by the methodreaches 99%, and the impurity can be used as a reference substance for the quality control process of the ubenimex bulk drug and preparation products of the ubenimex bulk drug.
Owner:深圳万乐药业有限公司
A novel ubenimex recrystallization method
ActiveCN106117075BEasy to controlReduce usageCarboxylic acid amide separation/purificationPh controlAmmonium hydroxide
Owner:SHANGHAI SINE WANXIANG PHARMA +1
A method for asymmetrically synthesizing ubenimex
InactiveCN104341317BSimple stepsAvoid Subsequent SplitsOrganic compound preparationCarboxylic acid amides preparationGlyoxylic acidEnantioselective synthesis
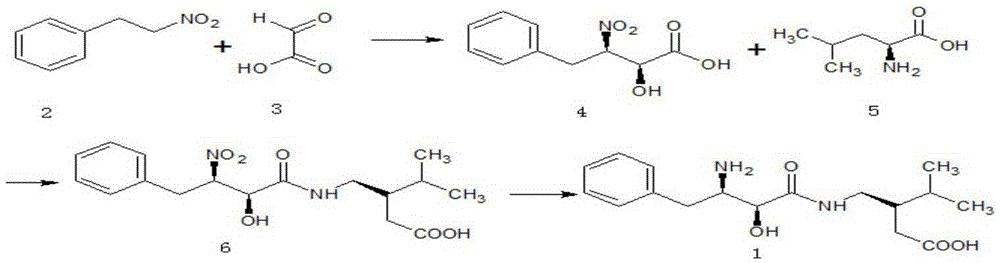
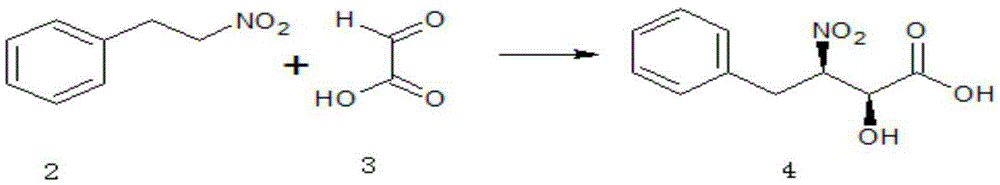
The invention relates to an ubenimex synthesis method which is simple and convenient in operation. The method comprises the steps: (S1) catalyzing nitrobenzene ethane and glyoxylic acid, which serve as raw materials, by a catalyst, so as to obtain (2S,3R)-2-hydroxy-3-nitro-4-phenyl-butyric acid; (S2) catalyzing (2S,3R)-2-hydroxy-3-nitro-4-phenyl-butyric acid and L-leucine, which serve as raw materials, by a condensation agent and an activator, so as to obtain N-[(2S,3R)-4-phenyl-3-nitro-2-hydroxy butyryl]-L-leucine; (S3) reducing nitro of N-[(2S,3R)-4-phenyl-3-nitro-2-hydroxy butyryl]-L-leucine, which serves as a raw material, into amino, thereby obtaining ubenimex. By adopting the method to asymmetrically synthesize ubenimex, the steps are simple, subsequent resolution is avoided, the utilization ratio of atoms is high, and the production cost is low.
Owner:上海博速医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com











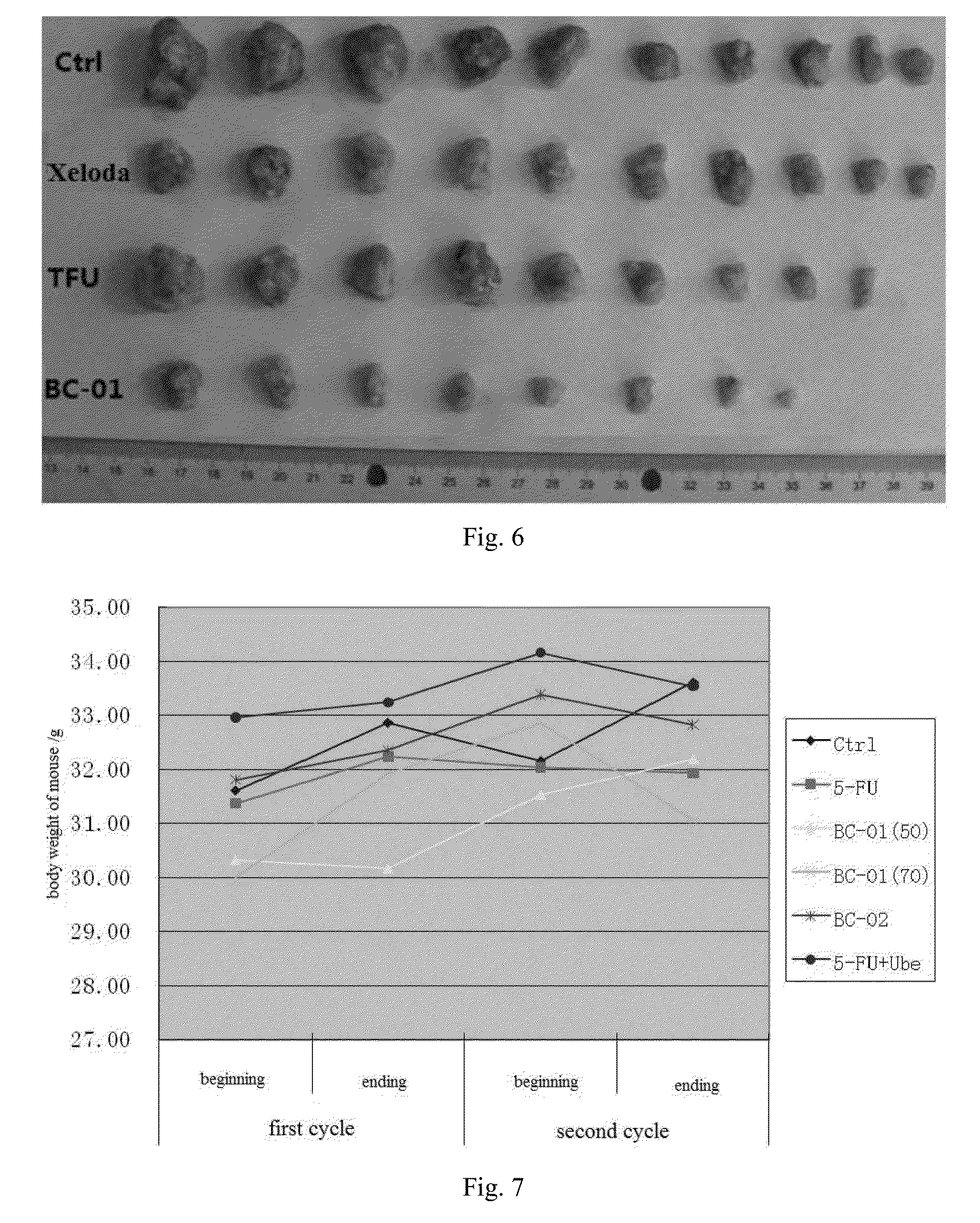




![Crystal form of N-[(2S, 3R)-4-phenyl-3-benzyl-3-formylaminobenzoxy-2-hydroxybutyryl]-L-benzyl leucine and preparation method thereof Crystal form of N-[(2S, 3R)-4-phenyl-3-benzyl-3-formylaminobenzoxy-2-hydroxybutyryl]-L-benzyl leucine and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9e1874ca-8c37-4d0c-ba03-312bfaaad135/HSA00000916726500011.PNG)
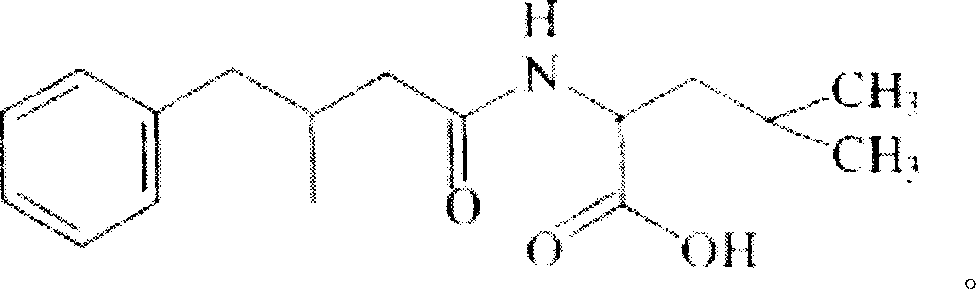
![Crystal form of N-[(2S, 3R)-4-phenyl-3-benzyl-3-formylaminobenzoxy-2-hydroxybutyryl]-L-benzyl leucine and preparation method thereof Crystal form of N-[(2S, 3R)-4-phenyl-3-benzyl-3-formylaminobenzoxy-2-hydroxybutyryl]-L-benzyl leucine and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9e1874ca-8c37-4d0c-ba03-312bfaaad135/HSA00000916726500012.PNG)
![Crystal form of N-[(2S, 3R)-4-phenyl-3-benzyl-3-formylaminobenzoxy-2-hydroxybutyryl]-L-benzyl leucine and preparation method thereof Crystal form of N-[(2S, 3R)-4-phenyl-3-benzyl-3-formylaminobenzoxy-2-hydroxybutyryl]-L-benzyl leucine and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9e1874ca-8c37-4d0c-ba03-312bfaaad135/HSA00000916726500021.PNG)