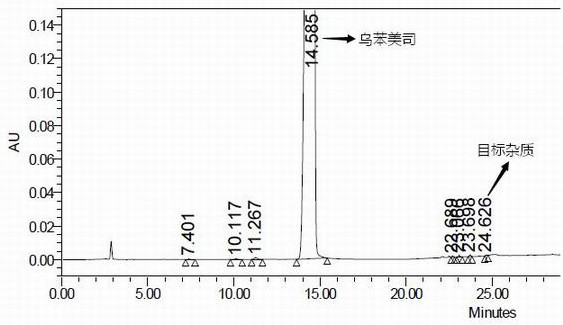
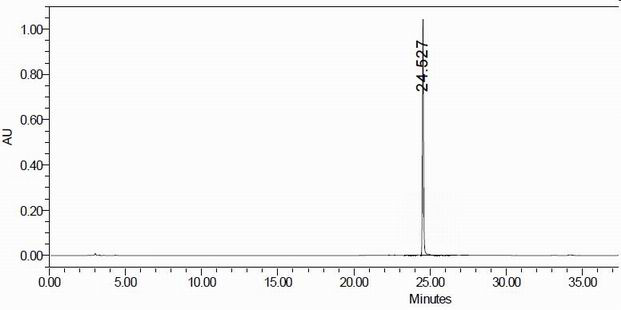
Impurity in ubenimex bulk drug and preparation method of impurity
A technology of Ubenimex and raw material medicine, which is applied in the field of impurity and the preparation of the impurity, and can solve problems such as difficult quality control and difficult structure confirmation of Ubenimex raw material medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Synthesis of Example 1 Ubenimex bulk drug
[0047] Add 1000 mL of tetrahydrofuran into the three-necked flask, start stirring, add 100 g of Z-AHPA, 131.5 g of L-leucine benzyl ester p-toluenesulfonate and 48.5 g of 1-hydroxybenzotriazole (HOBt) in sequence, and then cool down to 0 ±2°C. Add 33.6g of triethylamine and 74.5g of dicyclohexylcarbodiimide (DCC), control the temperature at 12±2°C after the addition, stir and react for 20-24 hours, and filter the reaction solution after the reaction is complete. The filtrate was concentrated under reduced pressure until no distillate was evaporated to obtain an oily substance, and then ethyl acetate was added and stirred until the oily substance was completely dissolved. Wash the above ethyl acetate solution with 650 mL of 0.5 mol / L hydrochloric acid, 650 mL of purified water, 650 mL of saturated sodium bicarbonate solution, and 650 mL of saturated sodium chloride solution, respectively. The organic layer was dried by adding a...
Embodiment 2
[0052] The preparation of embodiment 2 formula I compound
[0053] i Weigh 20 g of Ubenimex and 11.0 g of sodium bicarbonate, add them to a glass reaction bottle, add 195 ml of water, 195 ml of tetrahydrofuran, stir, cool to 0, and add 11 g of benzyl chloroformate dropwise at 0 °C, the reaction temperature is 0- 4. ℃ after the dropwise addition was completed, the temperature was raised to room temperature and the reaction was continued overnight, 162 milliliters of water was added to the reaction solution, extracted twice with 162 milliliters of ethyl acetate, the two organic phases were combined, washed with 162 milliliters of saturated sodium chloride solution, and the organic phase was washed with 162 milliliters of saturated sodium chloride solution. Dry over anhydrous magnesium sulfate, filter, wash the filter cake with ethyl acetate, concentrate the filtrate to dryness under reduced pressure, add 100 ml of ethyl acetate, heat to dissolve, cool down to 0°C for crystalliza...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com