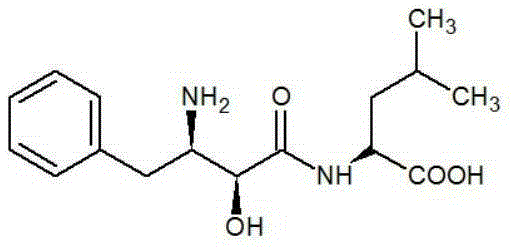
A method for asymmetrically synthesizing ubenimex
An asymmetric technology of ubenimex, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems such as the lack of asymmetric synthesis of ubenimex, the use of dangerous reagents, many steps, and the like. The effect of high atom utilization, low production cost and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
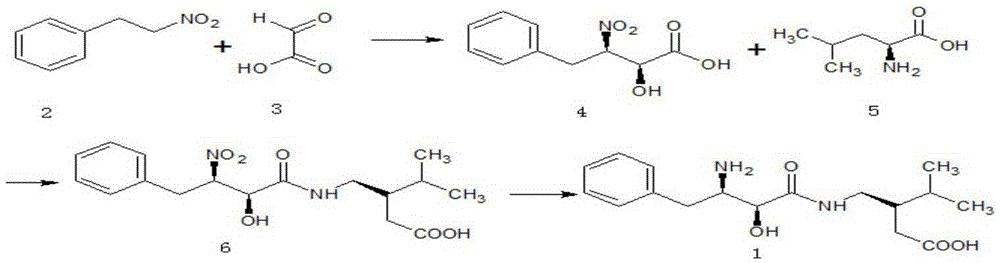
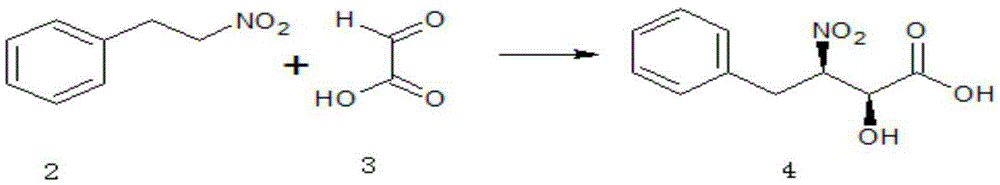
[0056] (1) Synthesis of (2S, 3R)-2-hydroxyl-3-nitro-4-phenylbutyric acid (4)
[0057]
[0058] In a 500ml three-necked flask equipped with a thermometer and mechanical stirring, add 50g N, N-dimethylformamide, 15.1g (0.1mol) nitrophenylethane, 8.1g (0.11mol) glyoxylic acid and 2g (0.2 mol) triethylamine, and after 30 min, 1.8 g (0.005 mol) of trifluoromethanesulfonic acid ketone and 1.1 g (0.005 mol) of ferrocenemethanol were added. Then react at 20-60°C for 3-7 hours.
[0059] After the reaction is complete, add 200ml of water, and use 2mol / L hydrochloric acid to adjust the pH to 2-3, then extract with 50ml and 30ml of ethyl acetate respectively, combine the organic phases, wash with 30ml of water and 30ml of saturated brine in sequence, After drying over sodium sulfate, ethyl acetate was distilled off under reduced pressure at 30-50°C, and dried at 50°C to obtain (2S,3R) 2-hydroxy-3-nitro-4-phenylbutyric acid 19.1g, molar yield 84.9%, melting point 82.1~82.5℃, [α] D 2...
Embodiment 2
[0073] (1) Synthesis of (2S, 3R)-2-hydroxyl-3-nitro-4-phenylbutyric acid (4)
[0074] In a 500ml three-necked flask equipped with a thermometer and mechanical stirring, add 100g N, N-diethylformamide, 30.2g (0.2mol) nitrophenylethane, 16.2g (0.22mol) glyoxylic acid, 8.1g ( 0.15mol) sodium methoxide, after 30min, add 3.3g (0.018mol) copper acetate and 2.6g (0.012mol) ferrocenemethanol. Then react at 20-60° C. for 3-7 hours until liquid chromatography shows that the reaction of nitrophenylethane is complete.
[0075] After the reaction was completed, 500ml of water was added, and the pH was adjusted to 2-3 with 2mol / L hydrochloric acid, and then extracted with 100ml, 60ml, and 30ml of ethyl acetate, respectively. The organic phases were combined, washed twice with 50ml of water and 50ml of saturated brine, dried over anhydrous sodium sulfate, distilled off under reduced pressure at 30-50°C to remove ethyl acetate, and dried at 50°C to obtain (2S,3R)2 -Hydroxy-3-nitro4-phenylbu...
Embodiment 3
[0087] (1) Synthesis of (2S, 3R)-2-hydroxyl-3-nitro-4-phenylbutyric acid (4)
[0088] Into a 500ml three-neck flask equipped with a thermometer and mechanical stirring, add 100g tetrahydrofuran, 30.2g (0.2mol) nitrophenylethane, 22.1g (0.3mol) glyoxylic acid, 23.7g (0.34mol) pyridine in sequence, and after 30min , Then add 5.3g (0.04mol) copper chloride, 3.4g (0.016mol) ferrocenemethanol. Then react at 20-60° C. for 3-7 hours until liquid chromatography shows that the reaction of nitrophenylethane is complete.
[0089] After the reaction was completed, 300ml of water was added, and the pH was adjusted to 2-3 with 2mol / L hydrochloric acid, and then extracted with 100ml and 50ml of ethyl acetate, respectively. The organic phases were combined, washed twice with 100ml of water, washed with 50ml of saturated brine, dried over anhydrous sodium sulfate, and ethyl acetate was distilled off under reduced pressure at 30-50°C to obtain (2S, 3R) 2-hydroxy-3-nitrate 36.1 g of 4-phenylbu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com