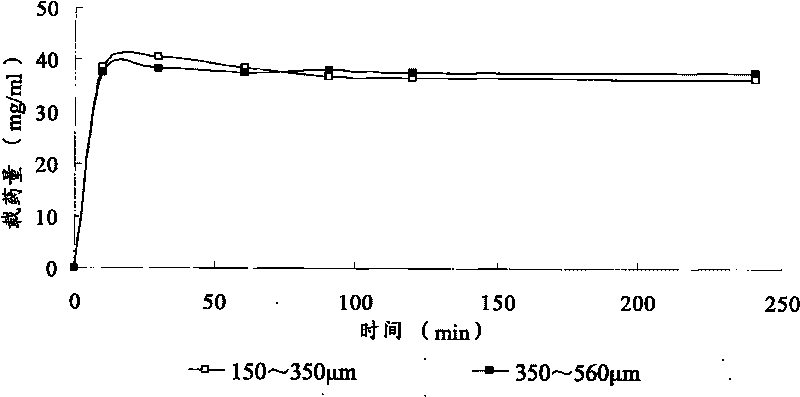
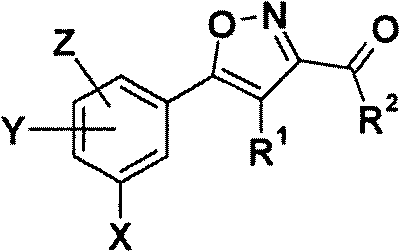
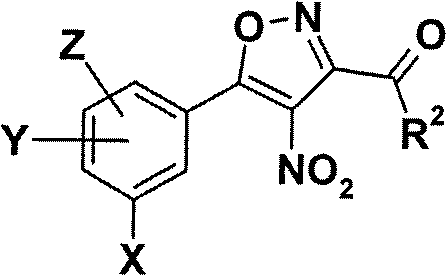
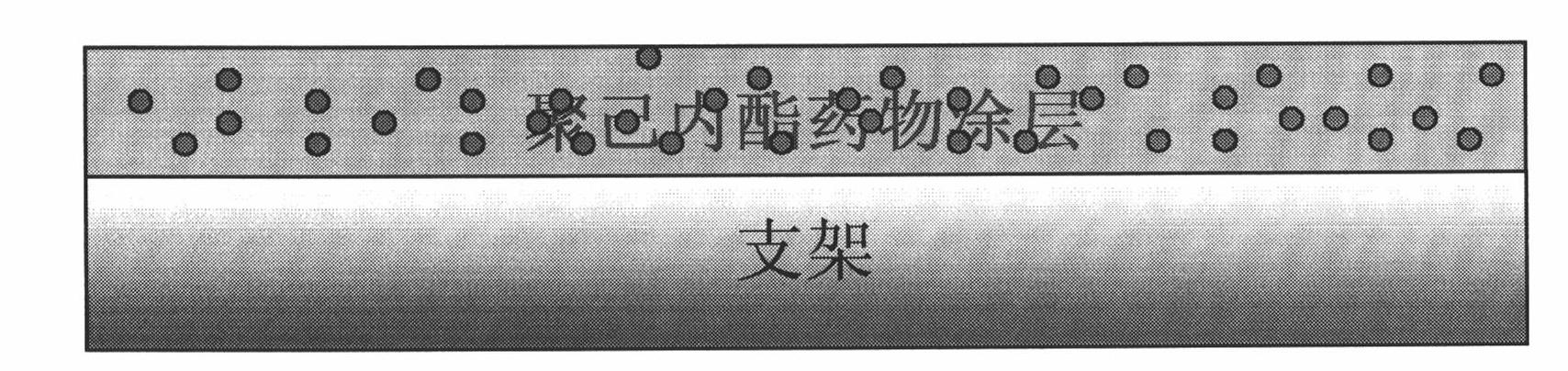
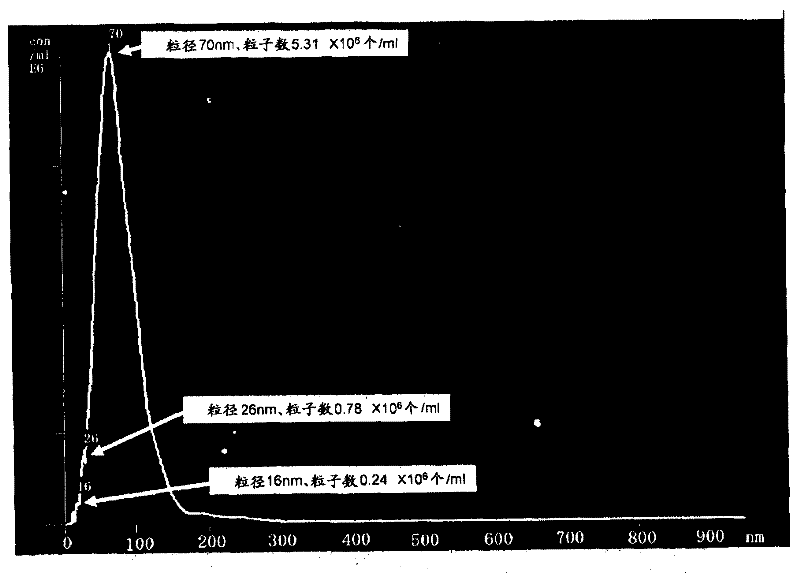
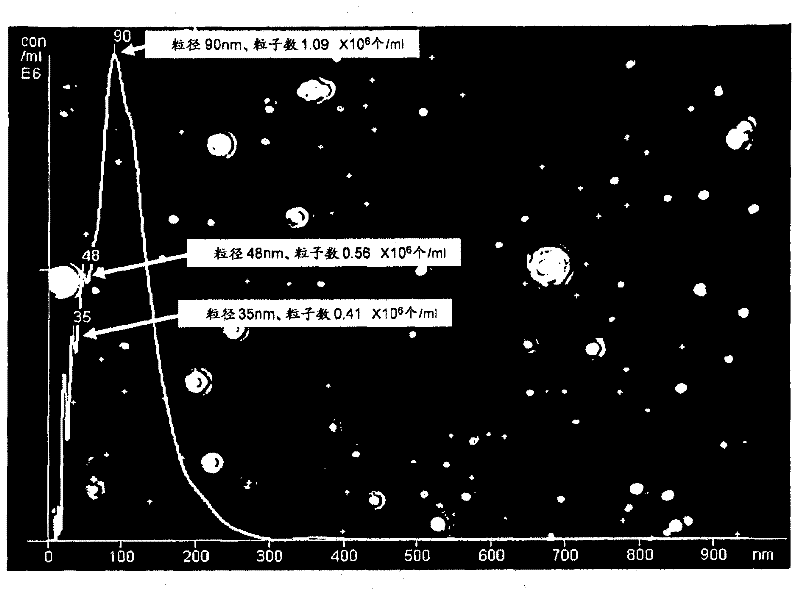
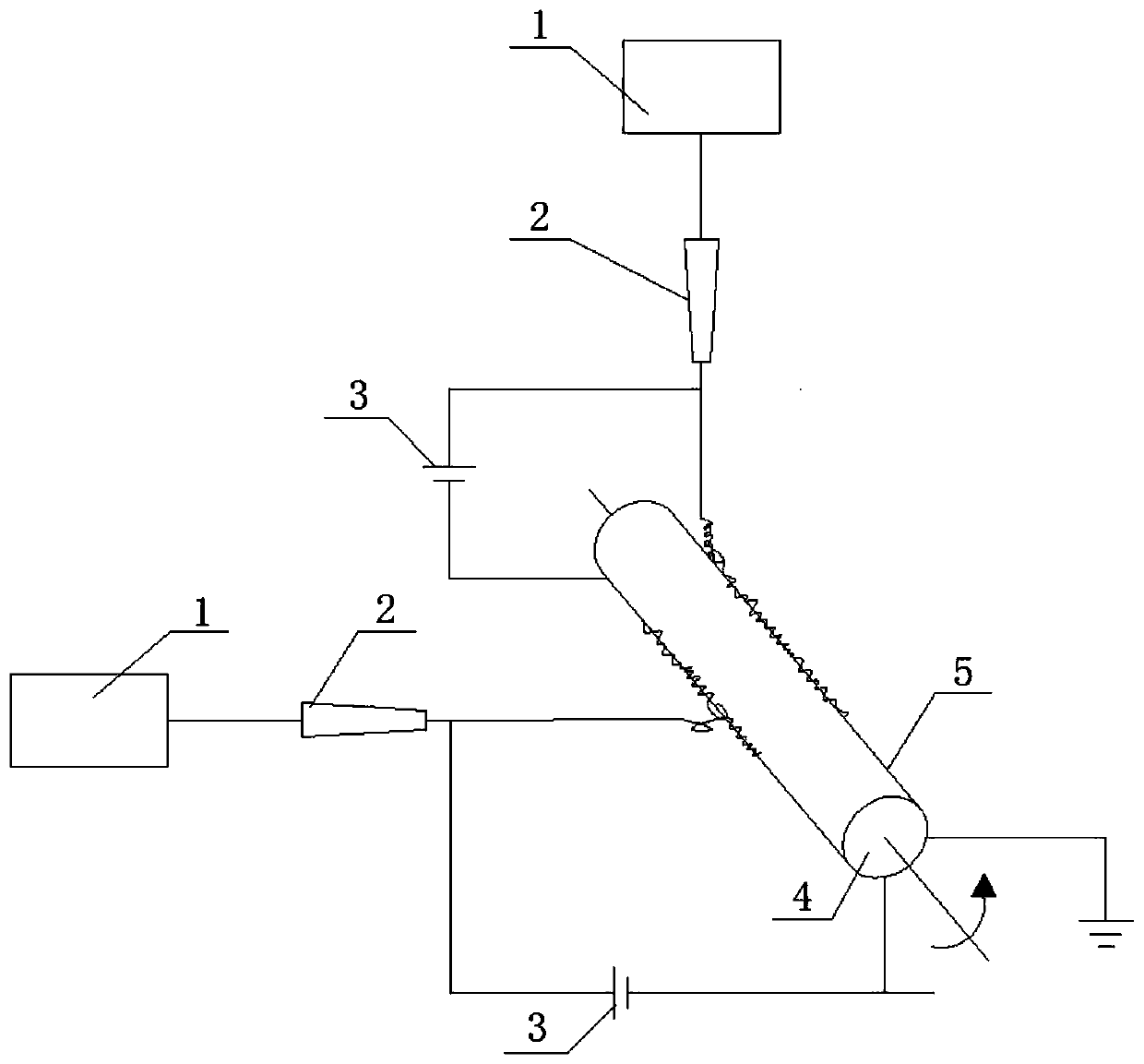
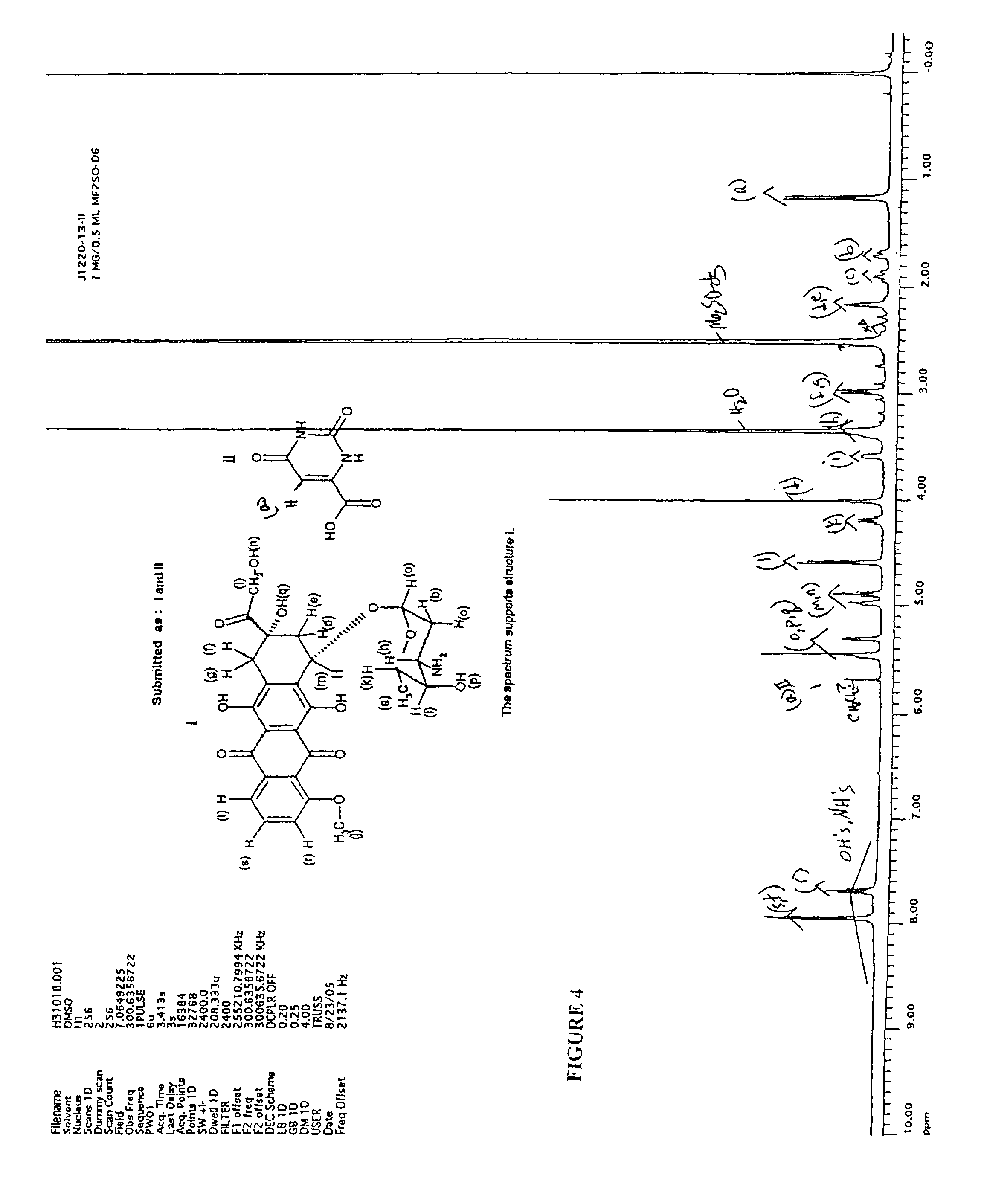
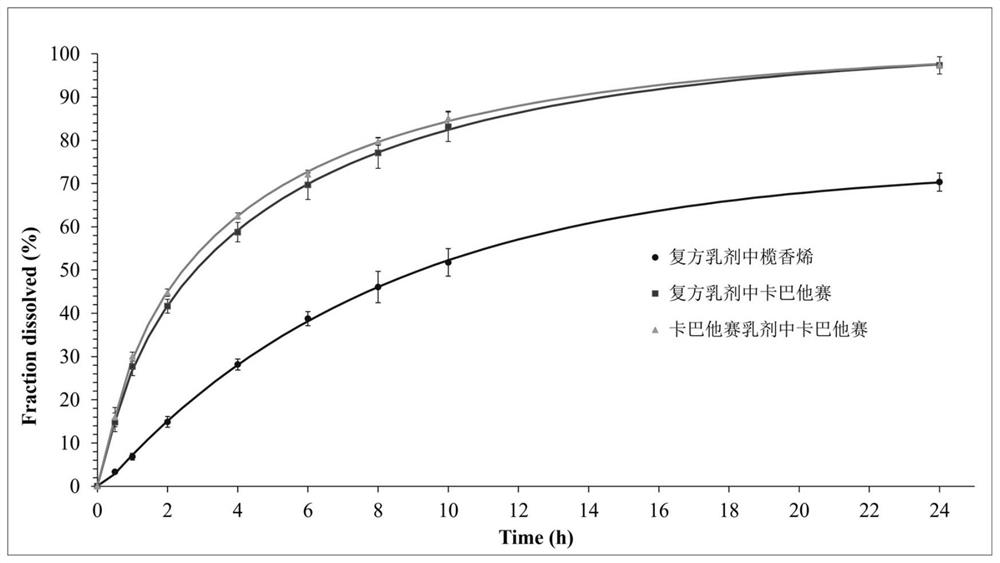
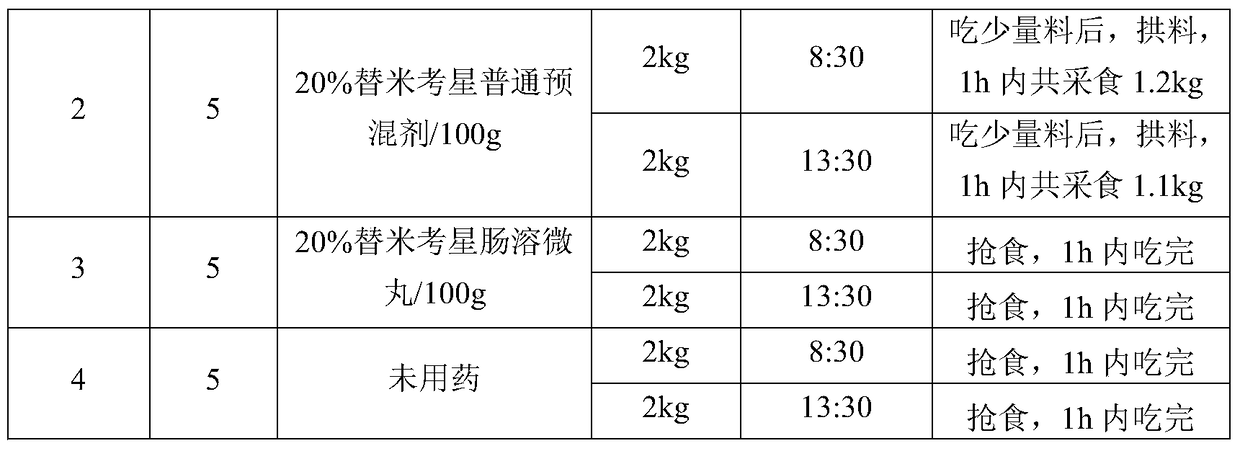
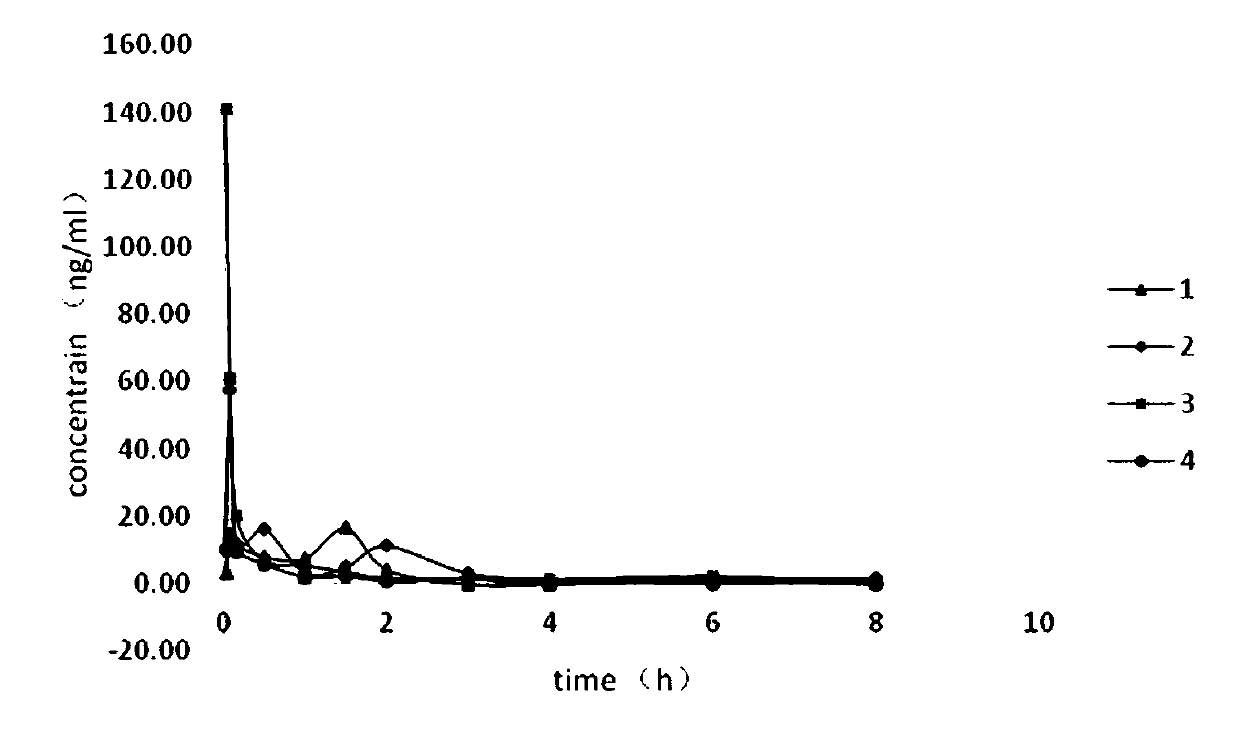
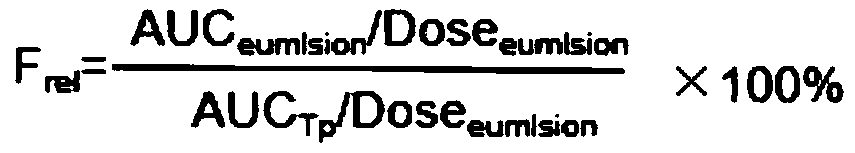Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
54results about How to "Lower drug concentration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
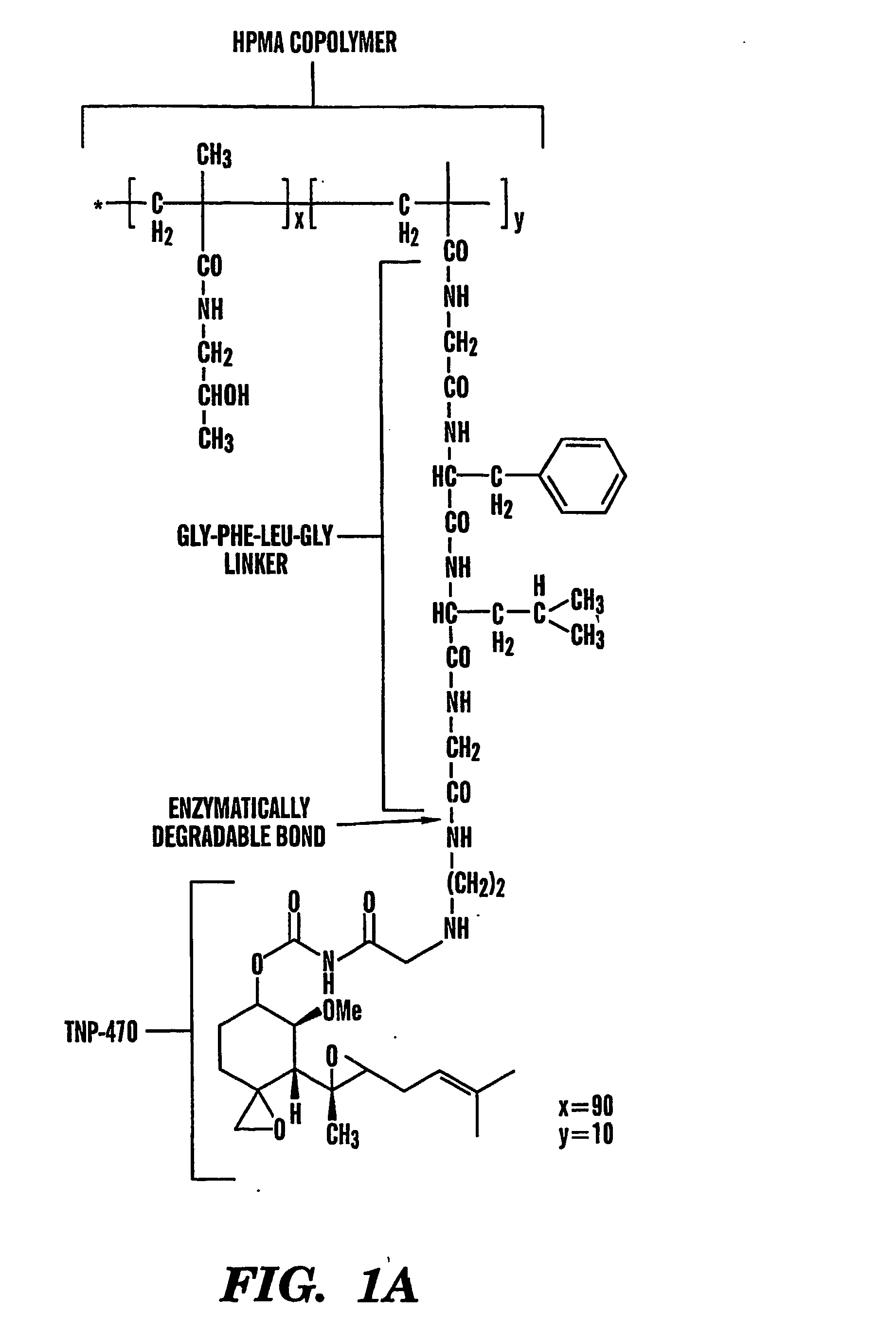
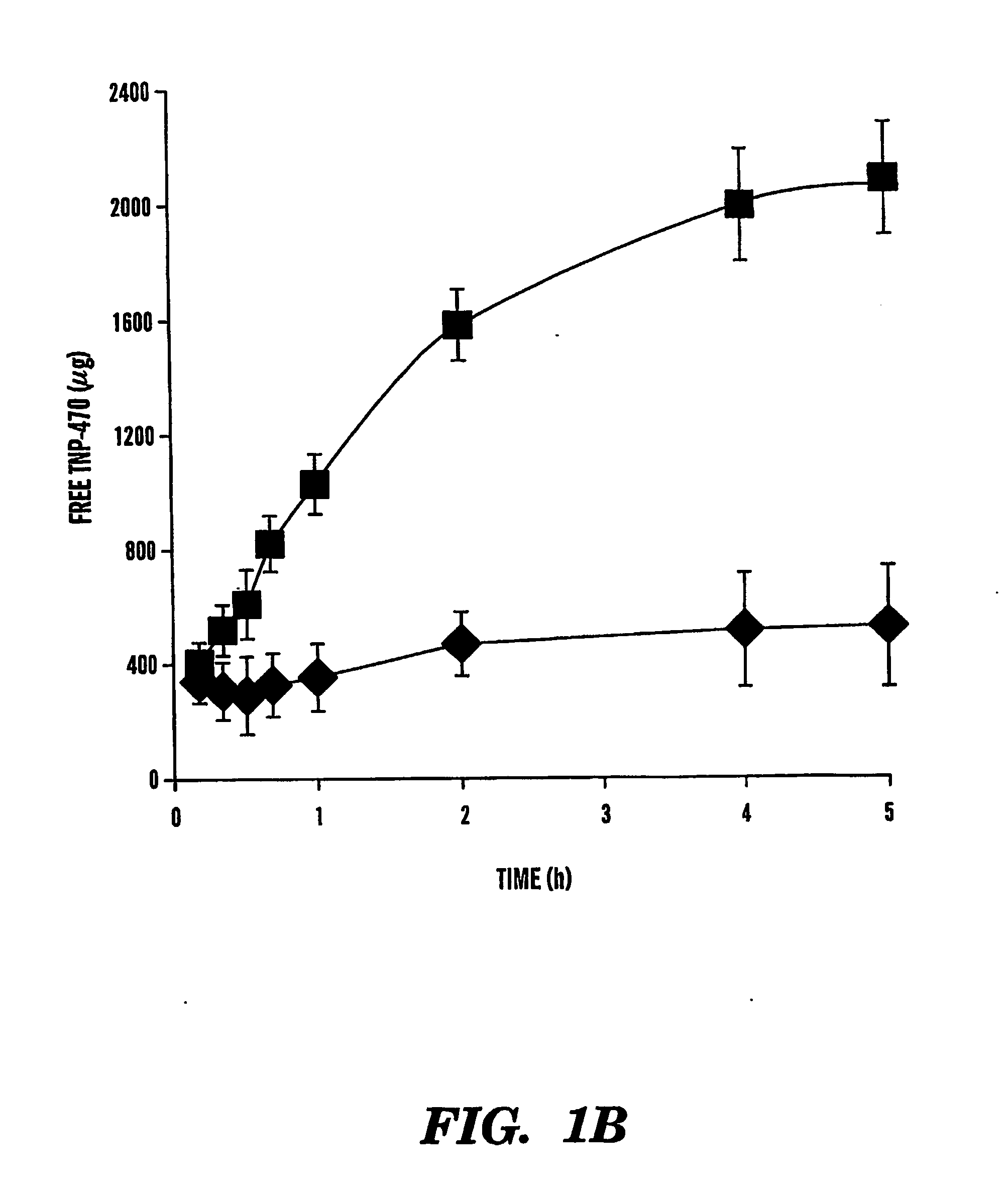
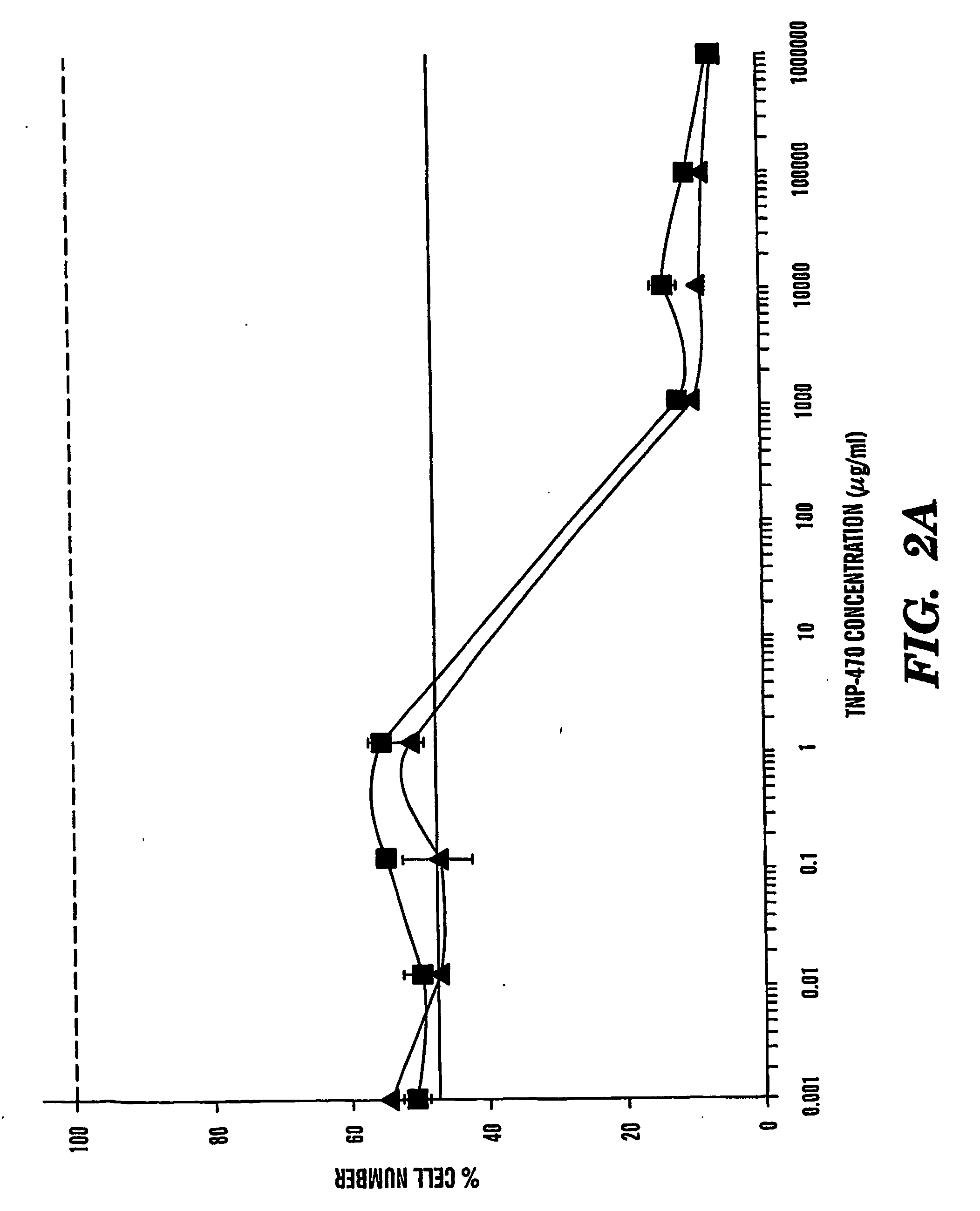
TNP-470 species, polymer conjugates and use thereof
InactiveUS6949584B2Reduce neurotoxicityAvoid problemsBiocideOrganic active ingredientsTumor vesselWater soluble
The present invention relates to conjugates of water-soluble polymers and o-(chloracetyl-carbamoyl) fumagillol (TNP-470) and use of those conjugates as specific intracellular carriers of the TNP-470 into tumor vessels. The present invention further relates to use of those conjugates to lower the neurotoxicity of TNP-470. Preferably, the polymer has a molecular weight in the range of 100 Da to 800 kDa. More preferably, the polymer has a molecular weight no greater than 60 kDa. Most preferably, the polymer has a molecular weight in the range of 15 kDa to 40 kDa.
Owner:CHILDRENS MEDICAL CENT CORP
Anticancer sustained-release agent containing epothilone
InactiveCN101433520AIncrease drug concentrationLower drug concentrationOrganic active ingredientsSolution deliverySodium carboxymethylcelluloseCarboxymethyl cellulose
The invention relates to anti-cancer drug slow release agent containing epothilone, which consists of slow release microspheres and solvent, wherein the slow release microspheres comprise anti-cancer effective components and slow release accessories; and the solvent is special solvent containing suspending agent. The anti-cancer effective components are combinations of epothilone, epothilone derivative, epothilone B, epothilone D and anticancer drugs selected from phosphoinositide 3-kinase inhibitor, pyrimidine analog and / or DNA repairase inhibitor, and the like; the slow release accessories are biocompatible high molecules such as polylactic acid and copolymer thereof, polyethylene glycol, terminal carboxyl polylactic acid copolymer, di-fatty acid and sebacic acid copolymer, poly (erucic acid dimmer-sebacic acid), poly (fumaric acid-sebacic acid), polifeprosan, polylactic acid, and the like; and the suspending agent is selected from sodium carboxymethyl cellulose and the like when the viscosity of the suspending agent is 100 to 3,000cp (at a temperature of between 20 and 30 DEG C). The anti-cancer effective components and the slow release microspheres can also be made into slow release implant agents, can effectively inhibit tumor growth through injection or placement and energy removal in tumor or tumor periphery, and can also remarkably enhance curative effect of non-operative treatment such as chemicotherapy and the like.
Owner:JINAN SHUAIHUA PHARMA TECH
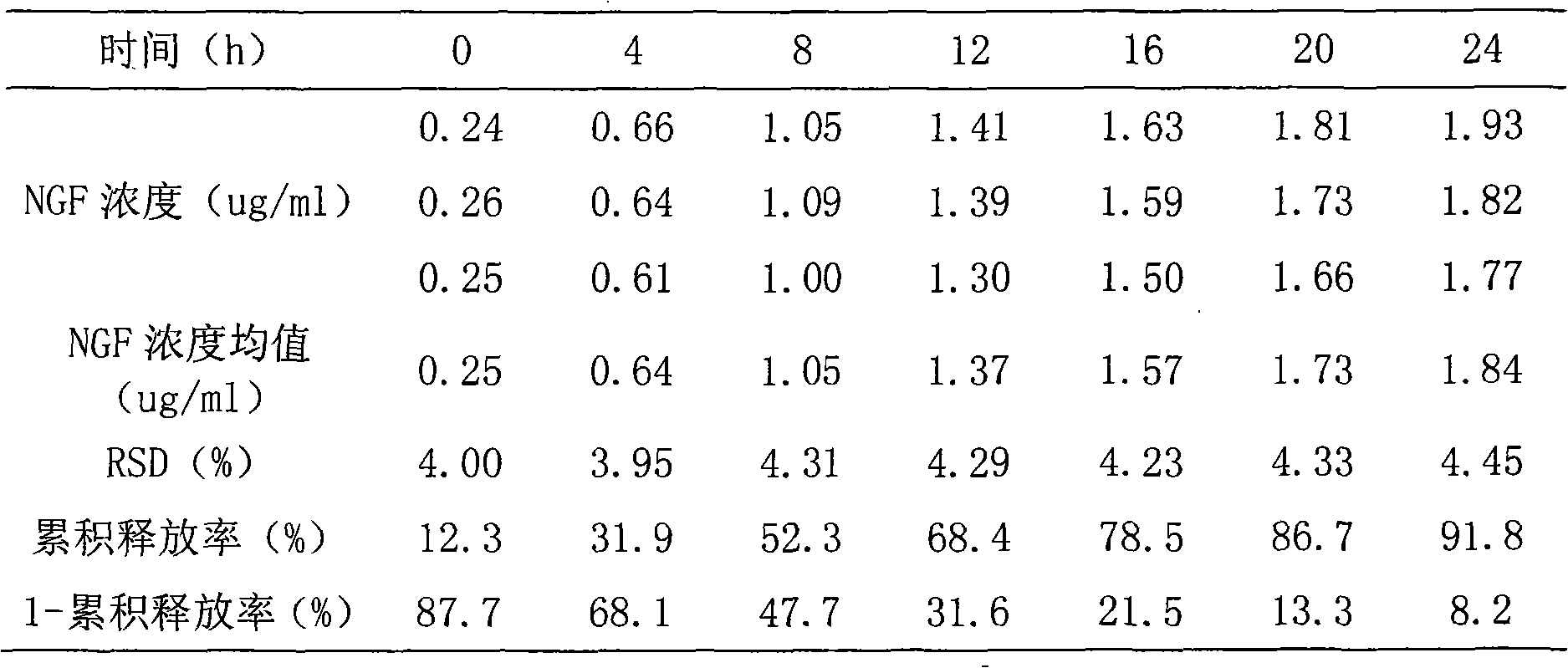
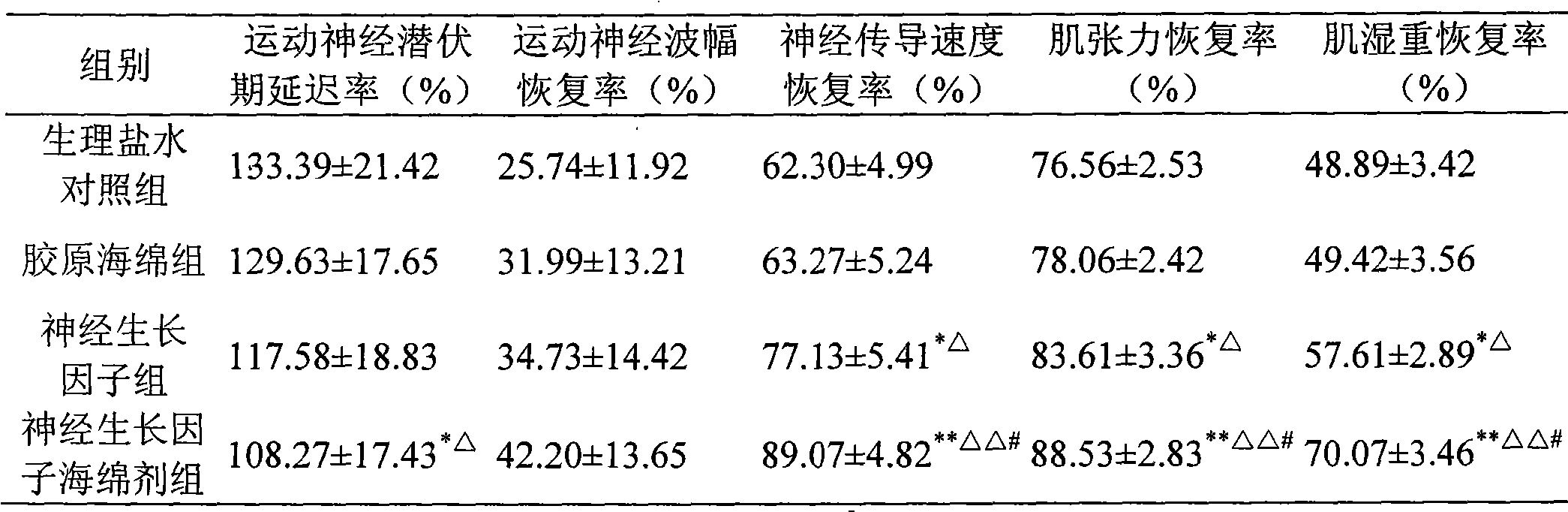
Nerve growth factor sponginum and preparation method thereof
InactiveCN101612113AIncrease concentrationRelieve painConnective tissue peptidesNervous disorderGelatin spongeNerve pathology
The invention relates to a nerve growth factor sponginum and a preparation method thereof, belonging to the technical field of medicine. The sponginum comprises nerve growth factor with weight percent content being 0.01-2% and collagen sponge or gelatin sponge matrix, the nerve growth factor is fixed on the collagen sponge or gelatin sponge matrix in a crosslinking way by an aldehydes crosslinker. The sponginum can cause the nerve growth factor to be released to the nerve pathology or wound tissue partial position continuously for a long time in effective dose, and play a role in effectively curing nerve tissue damage.
Owner:WUHAN HITECK BIOLOGICAL PHARMA
Medicine composite used for embolotherapy and acesodyne and preparation method thereof
ActiveCN101716349ALower drug concentrationSmall side effectsAntipyreticDigestive systemLidocaine HydrochlorideDouble bond
The invention provides a medicine composite used for embolotherapy and acesodyne and a preparation method thereof. The medicine composite comprises a biocompatibility macromolecular compound containing hydroxy, a monomer containing unsaturated double bond and anion group, a polymer, and local anesthetic containing amino group, wherein the polymer is generated through a polymerization reaction of an optional vinyl monomer and the polymerization reaction is initiated by free radicals, and the local anesthetic is combined to an anion group of the generated polymer. In the invention, lidocaine hydrochloride is combined to a polymer carrier; which can give full play to the acesodyne effect of the local anesthetic in the embolotherapy; the anion part of the polymer can properly combine with the local anesthetic containing the amino group, which can both realize higher medicine loading capacity and enable the medicine in an emboliaztion agent to be exchanged by cations in vivo and then slowly released. Moreover, the polymer emboliaztion carrier has simple technology, low cost, and suitability for large scale industrial production.
Owner:HYGEA MEDICAL TECH CO LTD
5-phenyl-isoxazole-3-carboxamides modulating HSP90 with antitumoral activities
The present invention relates to formula I compounds having antitumoural activities through, as one possible biological target, the molecular chaperone heat shock protein 90 (Hsp90) inhibition. The invention includes the use of such compounds in medicine, in relation to cancer disease as well as other diseases where an inhibition of Hsp90 is responsive, and the pharmaceutical compositions containing such compounds.
Owner:SIGMA TAU RES SWITZERLAND
Fat emulsion of oil of zedoary turmeric and preparation method
InactiveCN1795875ALess irritatingImprove complianceSolution deliveryEmulsion deliveryZedoary oilFat emulsion
An oil-in-water zedoary oil-fat emulsion for treating viral disease and cancer by injecting or oral taking is proportionally prepared from zedoary oil, soybean oil, lecithin, glycerin, oleic acid and the water for injection through high-speed stirring or ultrasonic oscillating, and high-pressure homogenizing. Its advantages are low by-effect and high curative effect.
Owner:董英杰
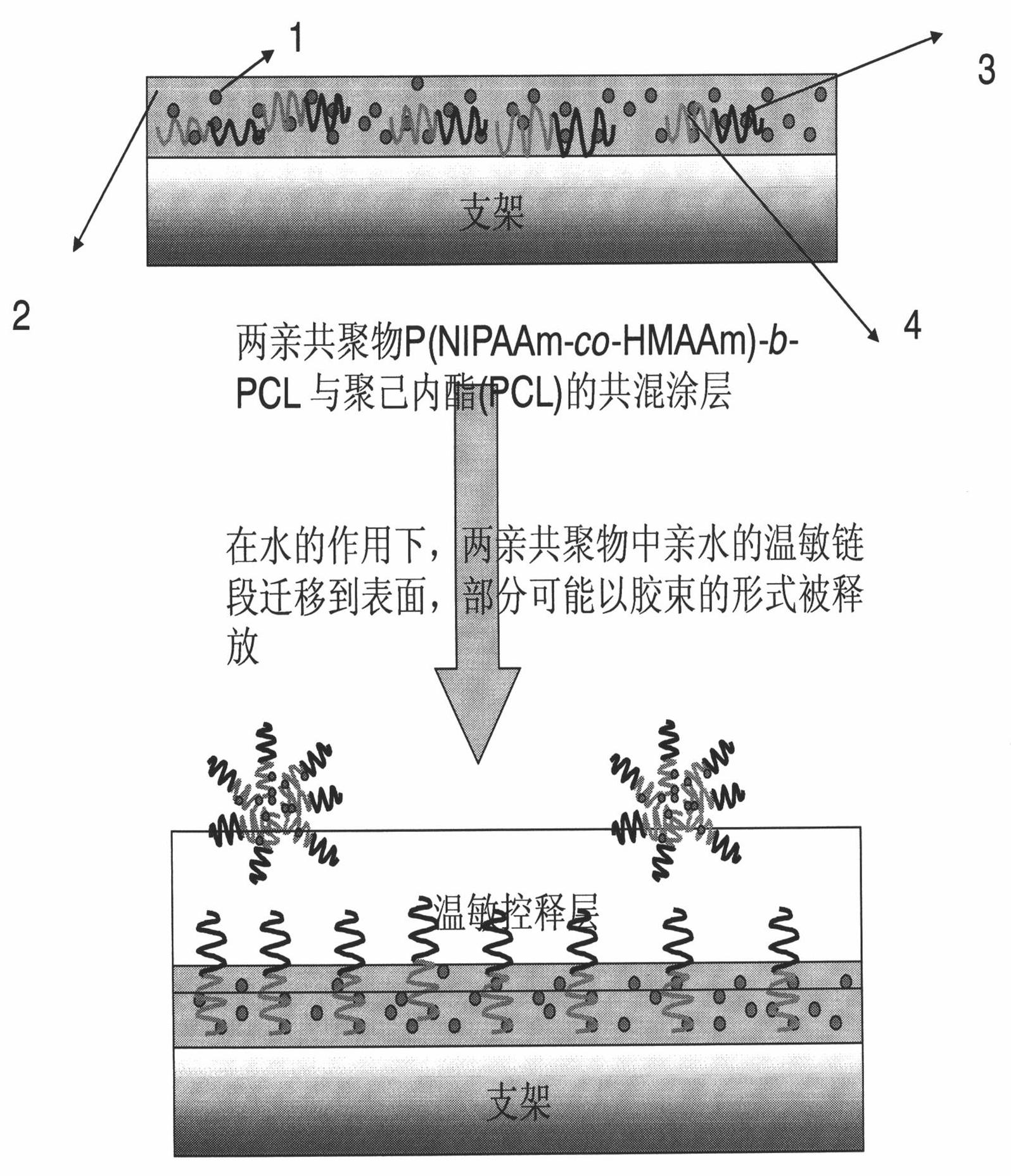
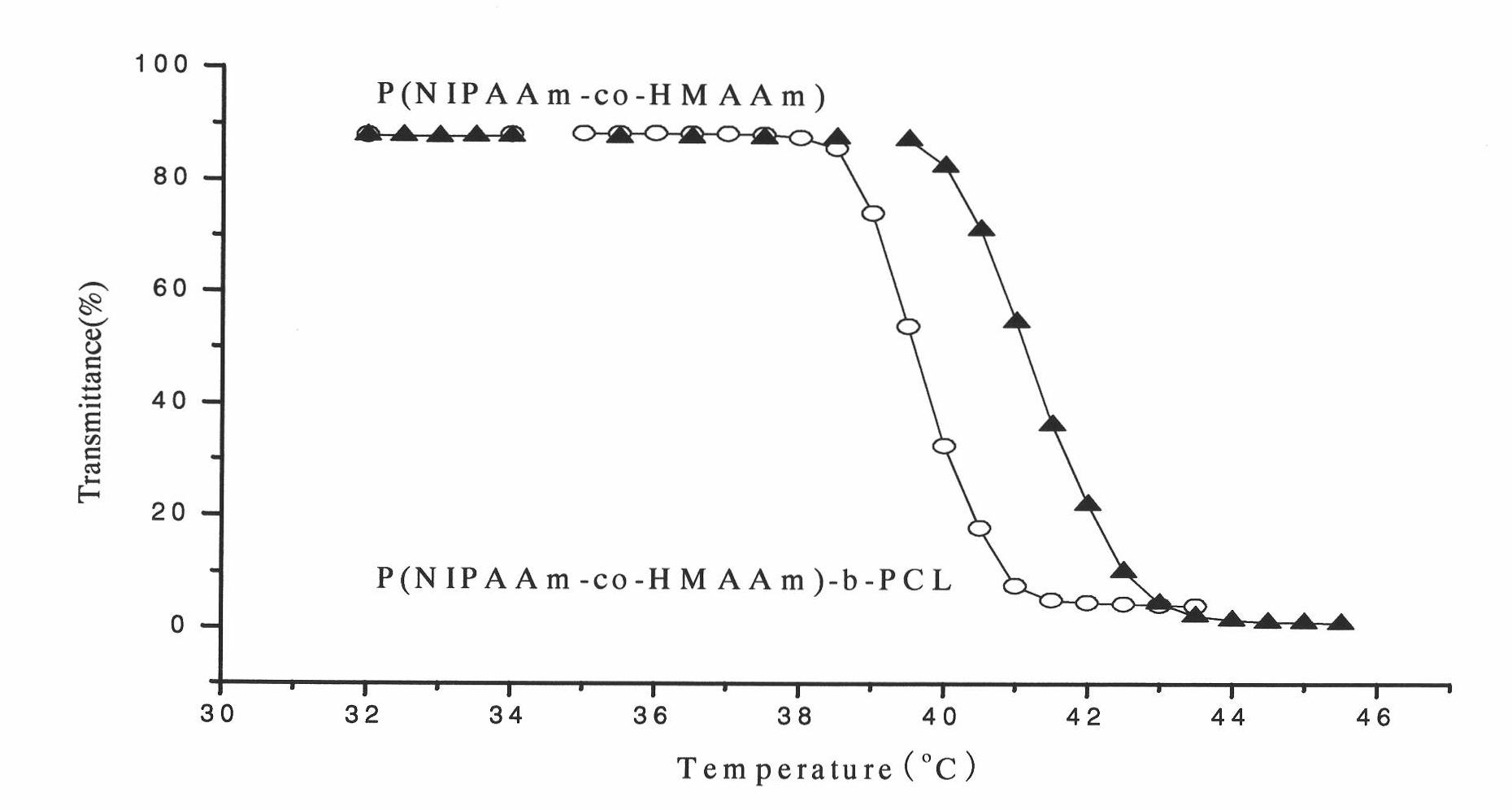
Preparation method of bracket with drug temperature-sensitive controlled-release function
The invention relates to a preparation method of a bracket with a drug temperature-sensitive controlled-release function. By the preparation method of the bracket, the coating with the drug temperature-sensitive controlled-release function is applied on the surface of the bracket in the modes of spin coating, dip coating or spray coating. The invention also provides a preparation method of the coating with the drug temperature-sensitive controlled-release function. The preparation method of the invention is simple and has strong operability. The coating with the drug temperature-sensitive controlled-release function prepared by the method of the invention has good temperature sensibility and drug controlled release function. The bracket with the drug temperature-sensitive controlled-release function prepared by the method can make histocyte drugs around the bracket have high concentration, and the drugs in blood have low concentration. With the existing drug eluting bracket, the histocyte drugs around the bracket have low concentration, and the drugs in the blood have high concentration. Compared with the existing drug eluting bracket, the bracket with the drug temperature-sensitive controlled-release function prepared by the method of the invention has significant advantages.
Owner:XIN HUA HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
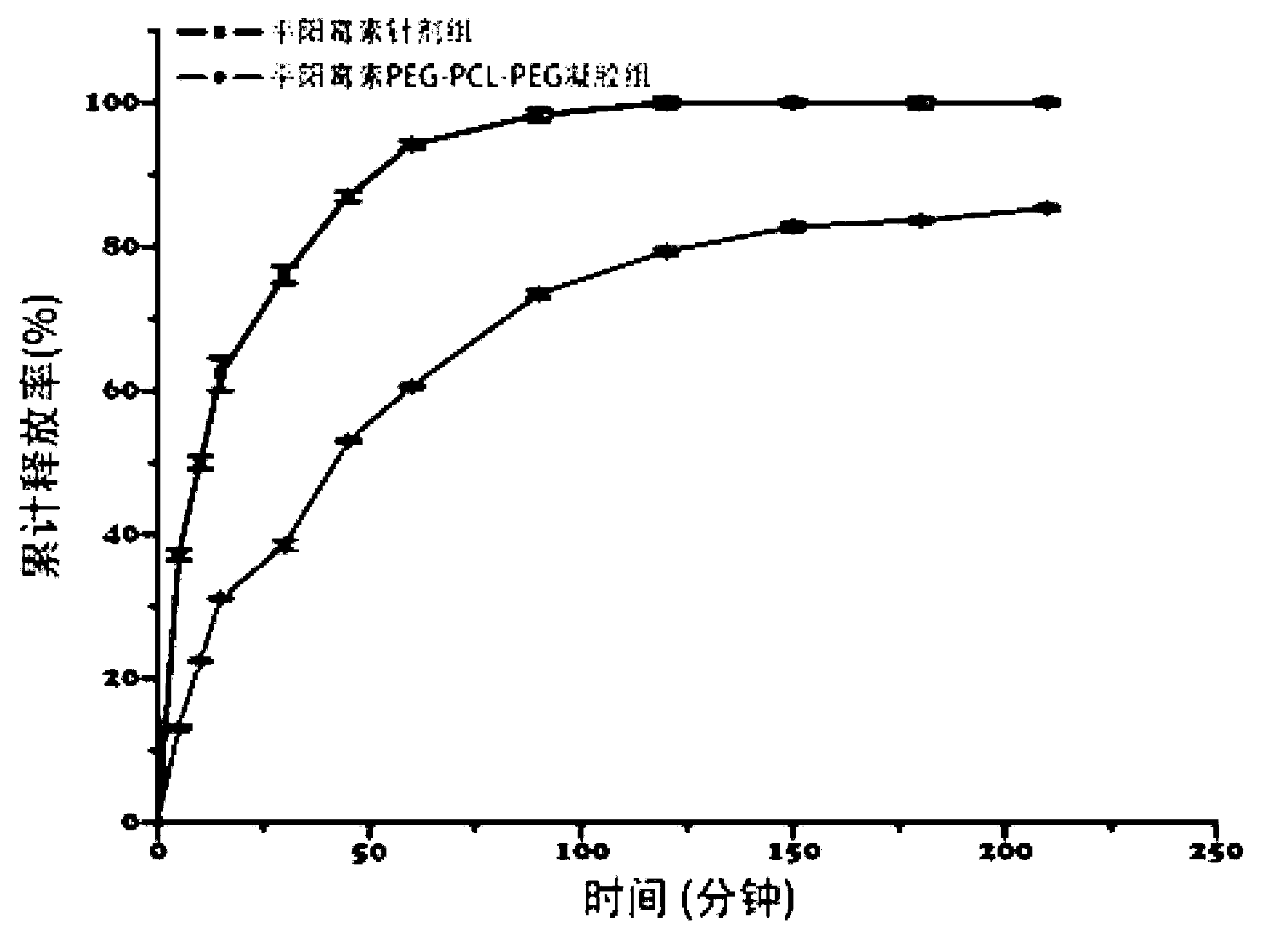
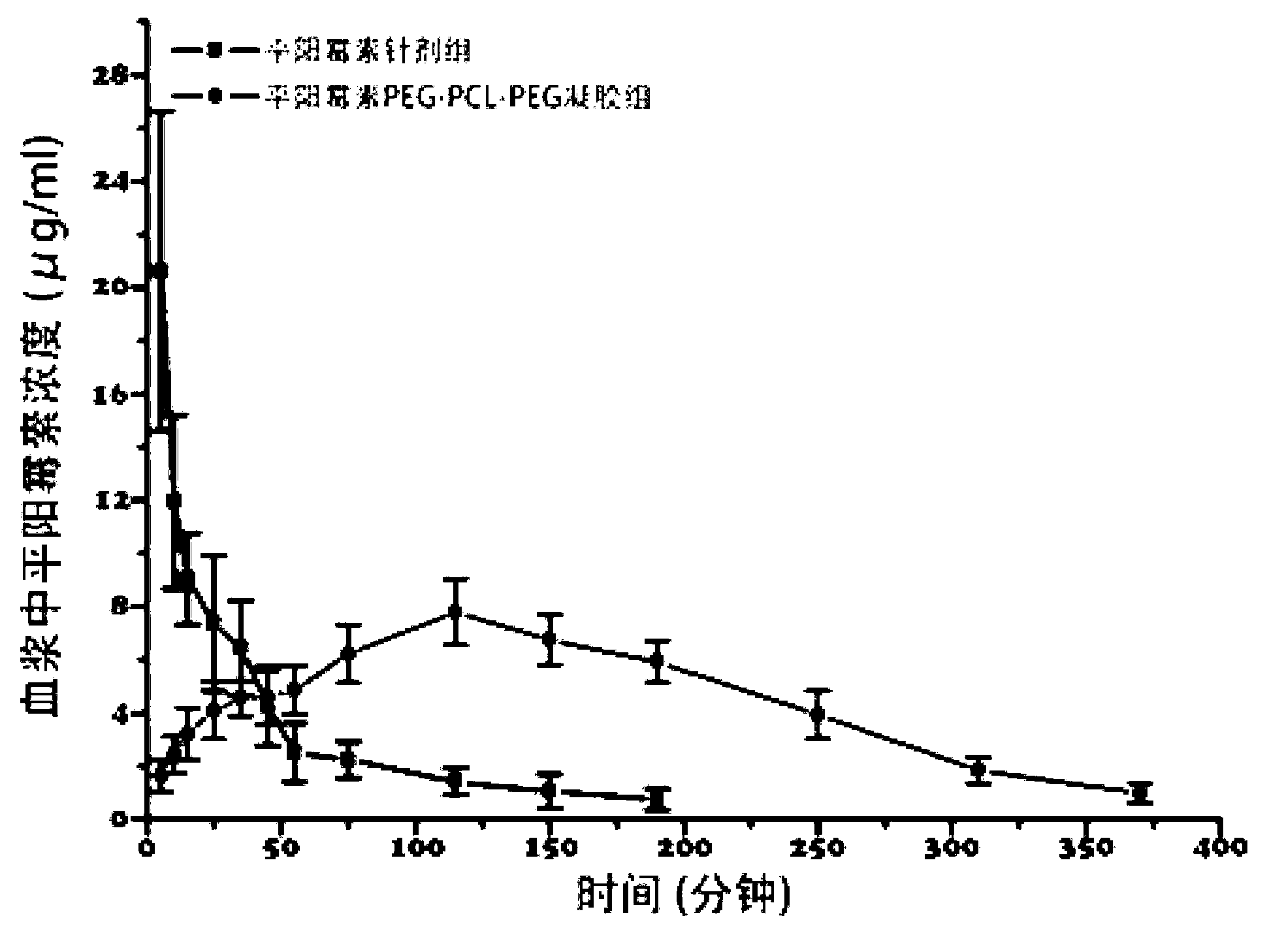
Pingyangmycin polyethylene glycol (PEG)-polycaprolactone (PCL)-polyethylene glycol (PEG) temperature-sensitive slow-release gel, as well as preparation method and application of same
ActiveCN102836418AExtended half-lifeExtension of timeAerosol deliveryOintment deliverySide effectHalf-life
The invention discloses a pingyangmycin polyethylene glycol (PEG)-polycaprolactone (PCL)-polyethylene glycol (PEG) temperature-sensitive slow-release gel, as well as a preparation method and the application of the gel. The Pingyangmycin PEG-PCL-PEG temperature-sensitive slow-release gel mainly consists of two parts comprising PEG (polyethylene glycol)-PCL (polycaprolactone)-PEG (polyethylene glycol) co-polymer and Pingyangmycin, is liquid at room temperature, and is solid gel under the in vivo 37 DEG C condition; the gel system has significant slow-release effect, thereby having functions in prolonging the half-life period and the acting time of the Pingyangmycin, reducing drug concentration in plasma, and reducing systemic toxic and side effects. The gel can be formed in situ after the medicine is injected in a high-flow-speed vessel, and then location embolism is realized, and so as to realize the, and muscularization and closing of muscle is caused, so that the sclerotherapy and the interventional therapy are combined effectively, the gel has excellent biocompatibility and degradability, is beneficial for treating hemangiomas, vascular malformations and cancers, particularly, a new selection is provided to the treatment of the vein malformation and partial malformation of cancers.
Owner:WUHAN UNIV
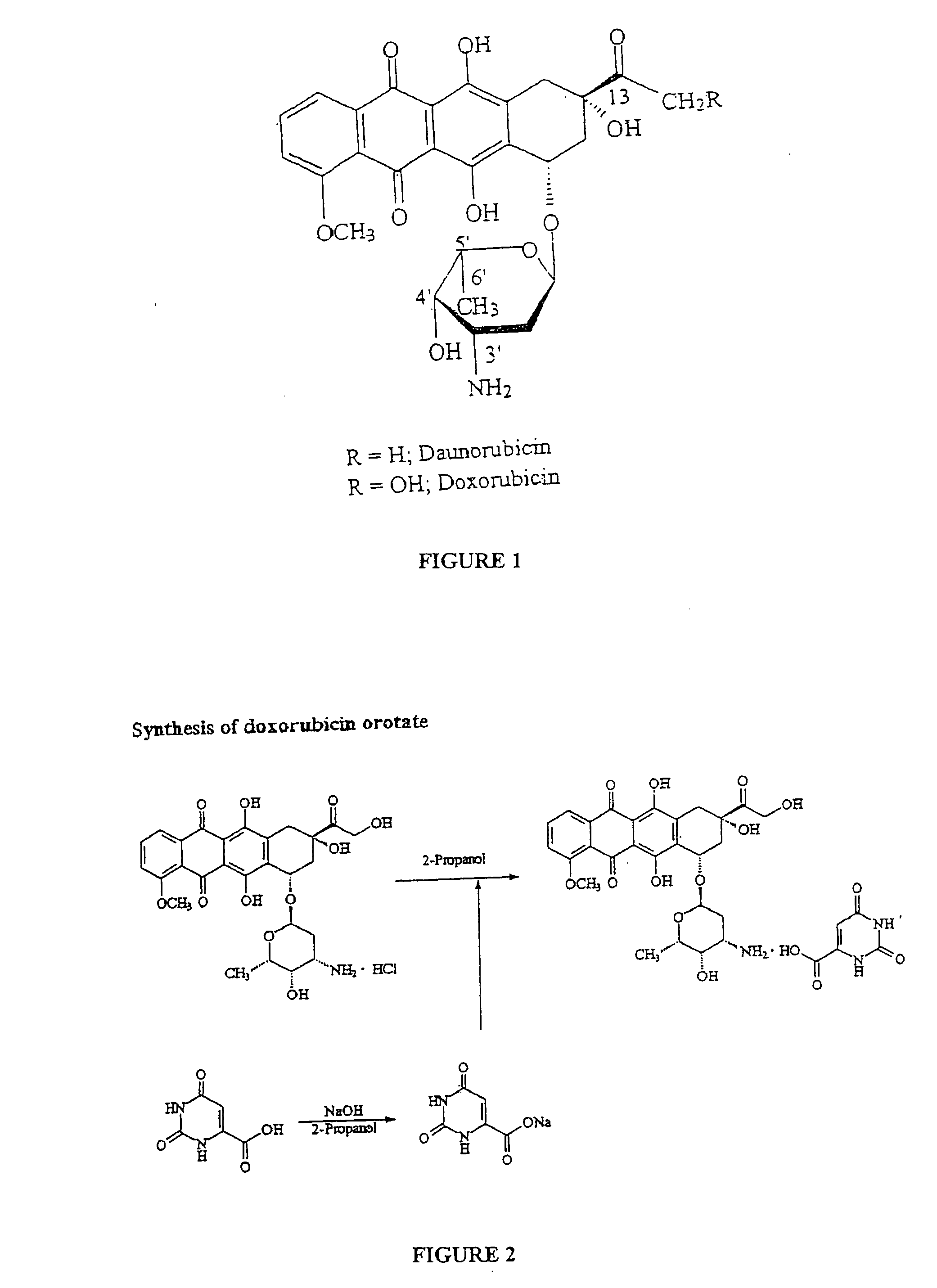
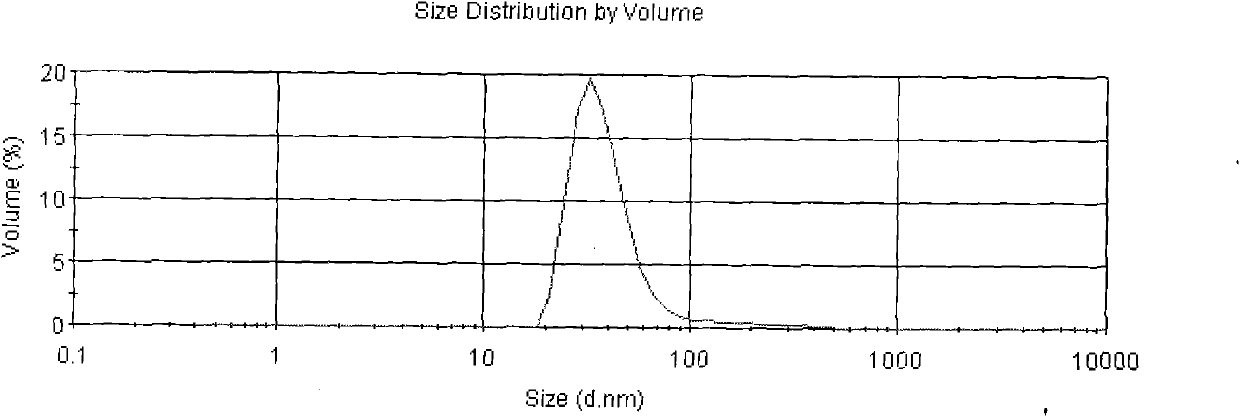
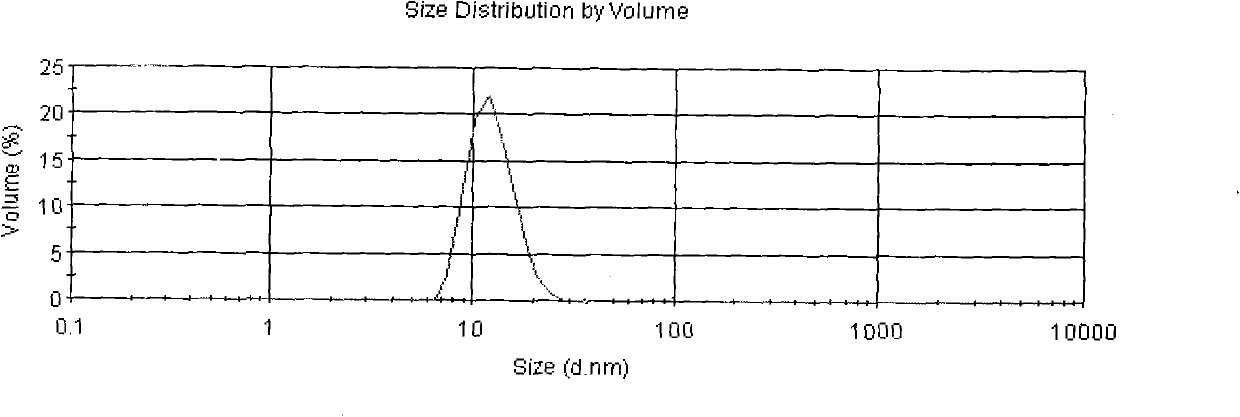
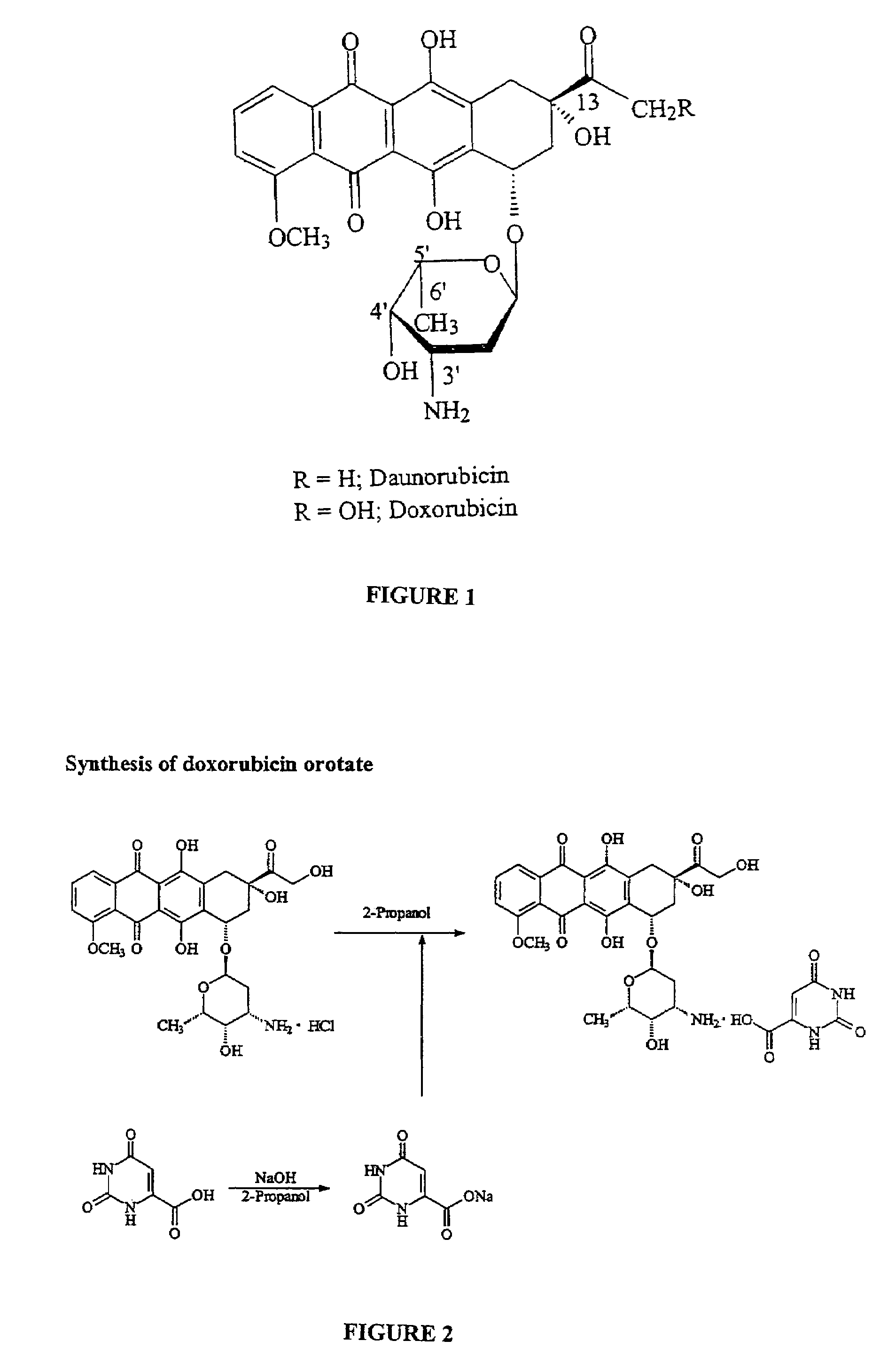
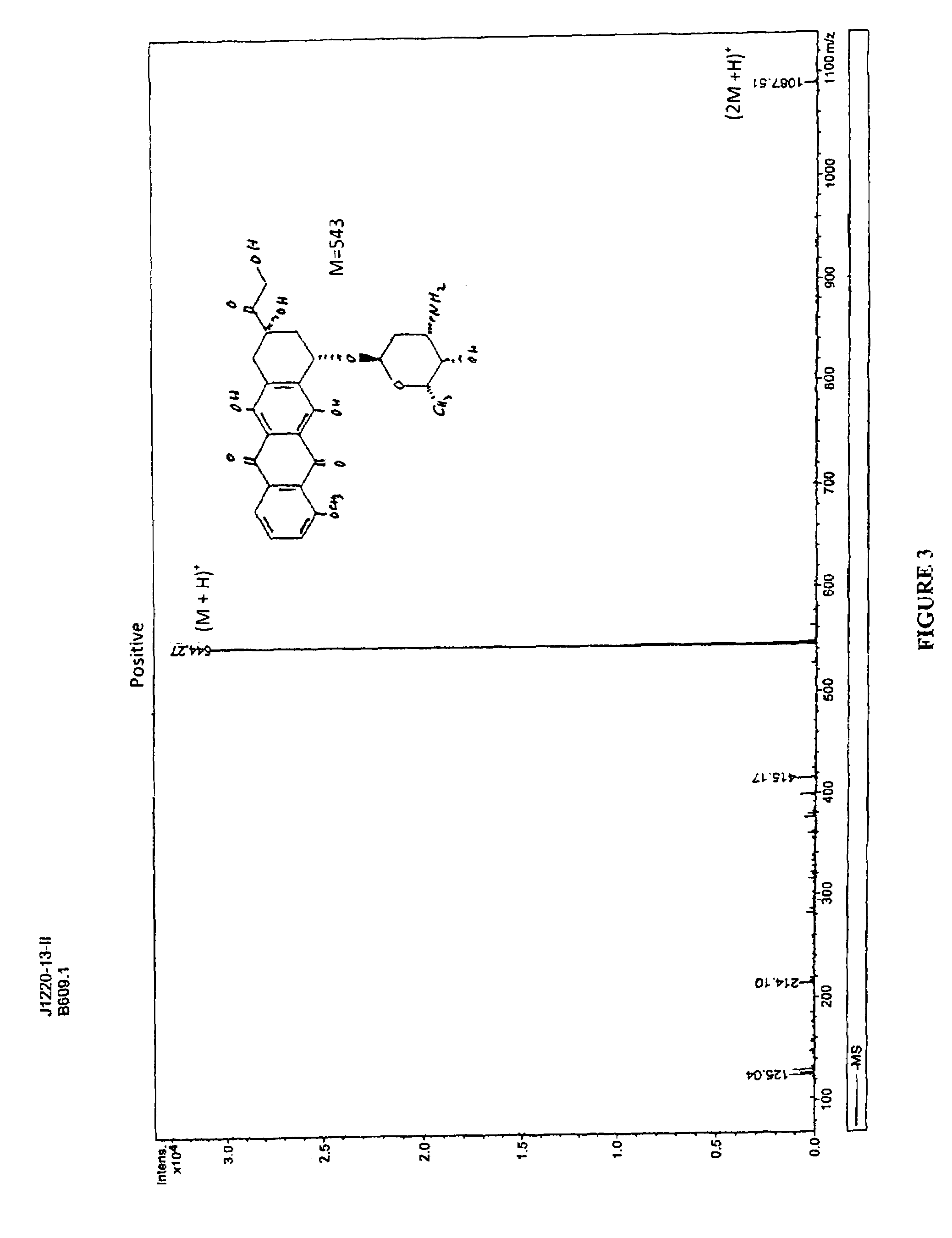
Compositions and methods of reducing tissue levels of drugs when given as orotate derivatives
ActiveUS20090131344A1Low toxicityToxic effectsSalicyclic acid active ingredientsBiocideSide effectTissue toxicity
This invention is in the field of chemical restructuring of pharmaceutical agents known to cause tissue toxicity as a side effect, by producing their orotate derivatives. More particularly, it concerns orotate derivatives of the anthracyclines, doxorubicin and daunorubicin, that are found to reduce levels of the pharmaceutical agent in noncancerous tissues. These orotate derivatives are equally efficacious in inhibiting the SCCAKI-1 kidney tumor in animals and the reduction in the heart tissue of doxorubicin compared with doxorubicin HCl suggests a reduction in toxicity induced by free radical generation by the anthracyclines.
Owner:SAVVIPHARM INC
Tnp-470 polymer conjugates and use thereof
InactiveUS20050169881A1Avoid problemsPromote accumulationBiocideOrganic active ingredientsAbnormal tissue growthWater soluble
The present invention relates to conjugates of water-soluble polymers and o(chloracetyl-carbamoyl) fumagillol (TNP-470) and use of those conjugates as specific intracellular carriers of the TNP-470 into tumor vessels. The present invention further relates to use of those conjugates to lower the neurotoxicity of TNP-470. Preferably, the polymer has a molecular weight in the range of 100 Da to 800 kDa. More preferably, the polymer has a molecular weight no greater than 60 kDa. Most preferably, the polymer has a molecular weight in the range of 15 kDa to 40 kDa.
Owner:CHILDRENS MEDICAL CENT CORP
Bracket with drug temperature-sensitive controlled-release function and application thereof
InactiveCN101862477AGood temperature sensitivityGood drug release functionStentsCoatingsDrugSpray coating
The invention relates to a bracket with a drug temperature-sensitive controlled-release function, which consists of a supporting bracket and a drug-carrying coating which covers the surface of the bracket, wherein the drug-carrying coating is formed by applying the coating with the drug temperature-sensitive controlled-release function on the surface of the bracket in the modes of spin coating, dip coating or spray coating. The invention also provides a coating with the drug temperature-sensitive controlled-release function and the application thereof. The coating with the drug temperature-sensitive controlled-release function has good temperature sensibility and drug controlled release function, can be evenly applied on the surface of bracket material. The drug-carrying bracket can effectively kill cancer cells and restrain the proliferation of nude mouse cancer transplantation tumor.
Owner:XIN HUA HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Composition and process for production thereof
InactiveCN102470335AImprove permeabilityLower drug concentrationAntibacterial agentsBiocideActive agentEfficacy
Disclosed are: a composition which enables the more effective development of the efficacy of a water-soluble medicinal agent in a solution containing the medicinal agent; and a dispersion in which a hydrophobic medicinal agent can be dispersed stably without requiring the use of any surfactant. Specifically disclosed are: a composition comprising ultra-fine air bubbles having a most frequent particle diameter of 500 nm or less, a medicinal agent and water; and a process for producing a composition comprising ultra-fine air bubbles having a most frequent particle diameter of 500 nm or less, a medicinal agent and water, which utilizes an ultra-fine air bubble generation apparatus.
Owner:莱格瑞克株式会社 +1
Slow-release pain-easing antibacterial absorbable dressing and preparation method thereof
InactiveCN109745576ASlow down the spreadExtended release timeAbsorbent padsConjugated synthetic polymer artificial filamentsFiberElectrospinning
The invention provides a preparation method of a slow-release pain-easing antibacterial absorbable dressing. The preparation method includes the following steps that a, a spinning solution A is prepared; b, a spinning solution B is prepared; c, electrostatic spinning is conducted, wherein the spinning solution A in the step a and the spinning solution B in the second b are added into an electrostatic spinning device comprising two single-channel injection pumps and subjected to electrostatic spinning, so that electrostatic spinning fiber membranes are obtained, wherein the included angle formed by single-axis spray nozzles (2) of the two single-channel injection pumps (1) is 60-180 degrees; d, the electrostatic spinning fiber membranes are crosslinked fixedly. Through the preparation method of the slow-release pain-easing antibacterial absorbable dressing, the speed of diffusing medicine in a carrier is decreased, release time is prolonged, the medicine is released gradually on the wounded part, and the effective medicine concentration of the wounded part can be kept for a long term. The invention further provides the slow-release pain-easing antibacterial absorbable dressing.
Owner:HUAINAN UNITED UNIVERSITY
Enzyme formulation for use as food supplement
ActiveUS20160114012A1Lower drug concentrationAffect occurrencePeptide/protein ingredientsDigestive systemFood supplementMedicine
The present document describes an enzyme formulation comprising an enzyme mixture comprising from about 5% to about 45% (wt / wt) of a fungal protease enzyme; and from about 1.5% to about 50% (wt / wt) of at least one polysaccharide digesting enzyme; in combination with an acceptable pharmaceutical carrier. The present document also describes the use of the formulation of the present invention for the prevention or treatment of digestive disorder.
Owner:BRYSON PATENTS
Compound docetaxel ester microsphere injection and preparation method thereof
ActiveCN102008434AChange in body distributionImprove solubilityOrganic active ingredientsSolution deliveryHemolysisMicrosphere
The invention discloses a compound docetaxel ester microsphere injection and a preparation method thereof, belonging to the technical field of medicines. The compound docetaxel ester microsphere injection is characterized by being prepared from the following raw materials in weight parts: 0.5 to 5 parts of docetaxel, 30-150 parts of Brucea javanica oil, 30-150 parts of middle-chain triglyceride, 10-30 parts of lecithin, 10-60 parts of polyethylene glycol surface active agents, 20-35 parts of glycerine and 700-950 parts of sterilized water for injection. The compound docetaxel ester microsphere injection of the invention is oil in water type microsphere preparation. The docetaxel as a main drug is coated in the Brucea javanica and middle-chain triglyceride compound oil phase of the oil in water type microsphere. The microsphere has a smaller grain diameter (which is lower than 100nm), and the preparation has better stability. The compound oil phase compositions play a cooperative anticancer role, and reduce the stimulation reaction of the injection and the side reactions such as hemolysis, allergy and the like. The invention has targeting function and increases the medicine effect.
Owner:SHENYANG WANJIA INST OF BIOLOGICAL TECH RES
Antitumor slow-release implantation agent and preparation method thereof
ActiveCN106236699AIncrease the area under the curveRapid drug actionOrganic active ingredientsInorganic non-active ingredientsWhole bodyTumor cells
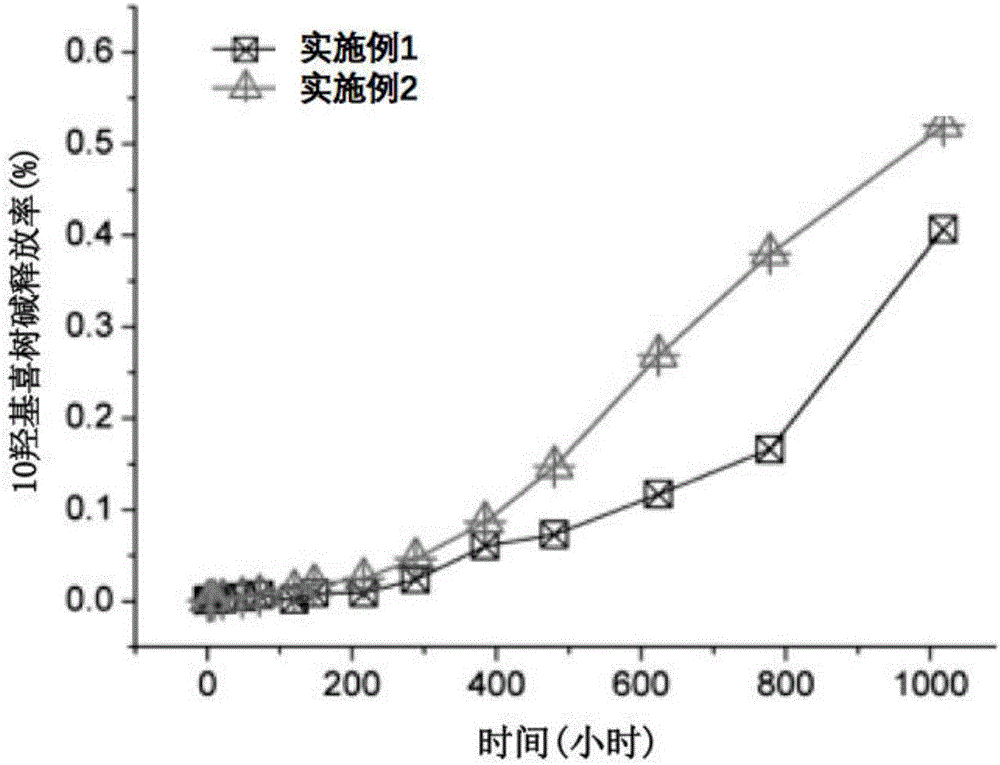
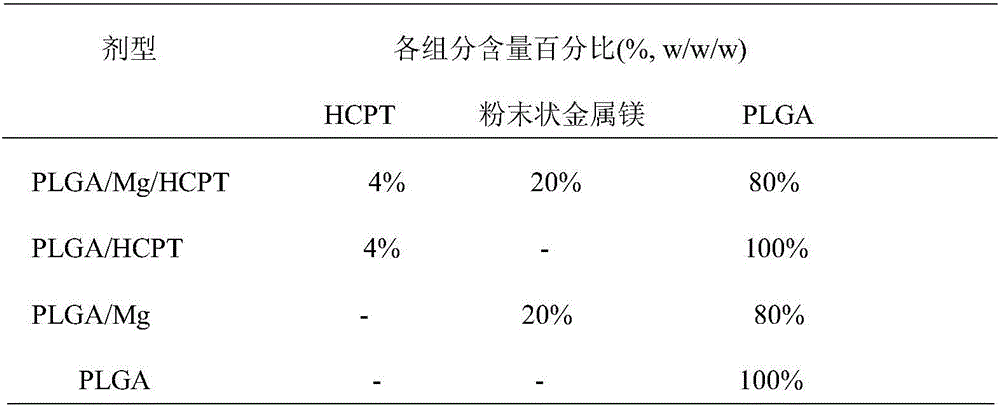
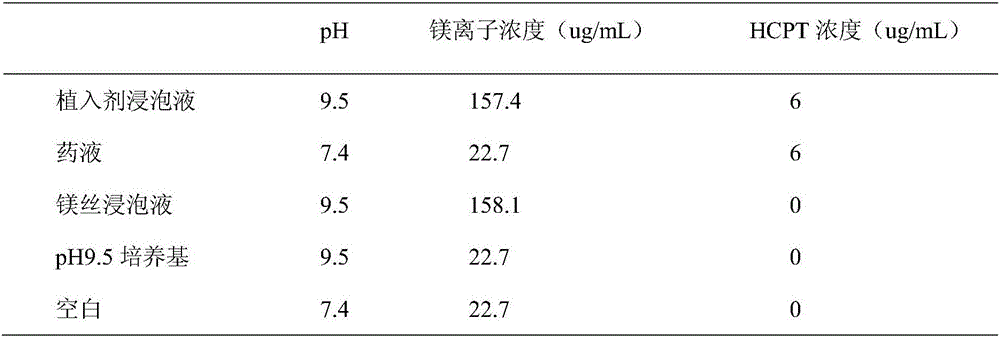
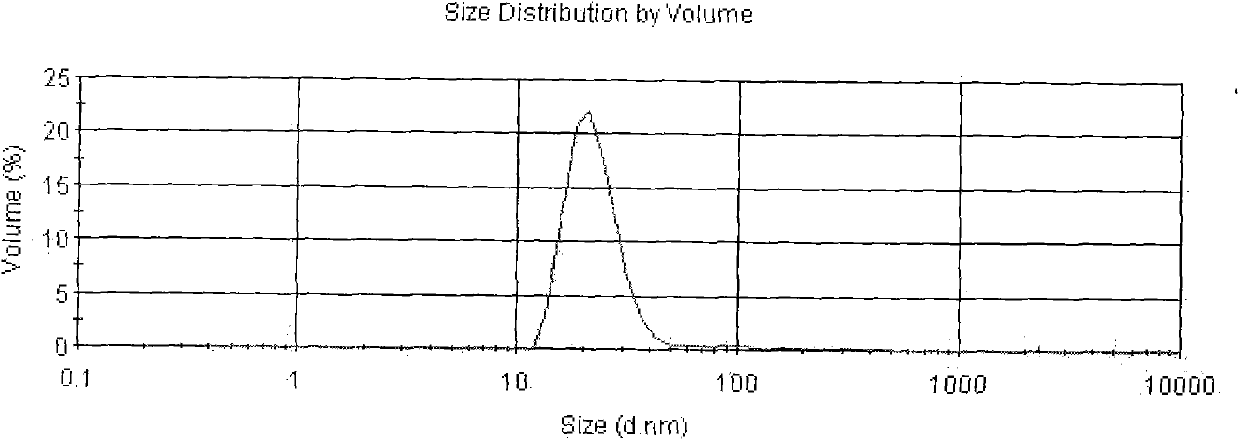
The present invention belongs to the field of biomedicine, and particularly relates to an antitumor slow-release implantation agent, which comprises 10-hydroxycamptothecin, a degradable polymer and metal magnesium, and is obtained by a solution spraying or hot-melting extrusion process. According to the present invention, after the implantation agent is subjected to the surgical implantation, the slow release of the10-hydroxycamptothecin can be achieved, and compared with the traditional administration mode, the administration mode of the present invention has the following characteristics that the drug systemic toxicity can be substantially reduced in the premise of the effective maintenance of the local drug concentration of the tumor; the metal magnesium in the implantation agent can react with water in the body environment to generate magnesium hydroxide, such that the environment around the tumor can be adjusted to achieve the alkaline state so as to inhibit the tumor cell growth; and the implantation agent can be used alone or can be combined with other treatment methods to provide the treatment or tumor recurrence prevention effect.
Owner:THE FIRST HOSPITAL OF CHINA MEDICIAL UNIV +2
Asarin lipid nanospheres and method for preparing same
ActiveCN102085183AGood physical and chemical stabilityReduce degradationNervous disorderEther/acetal active ingredientsLipid formationGlycerol
The invention discloses asarin lipid nanospheres and a method for preparing the same, and belongs to the technical field of medicaments. The asarin lipid nanospheres are prepared by the homogenization of the following raw materials in part by weight: 0.2 to 1.0 part of asarin, 5 to 15 parts of medium chain oil, soybean oil or the mixture of the medium chain oil and the soybean oil, 0.5 to 5 parts of lecithin, 1 to 30 parts of polyethylene glycol-12-methyl hydroxystearate, 1 to 4 parts of glycerol and 40 to 100 parts of water for injection. The preparation method comprises the following steps of: adding the oil phase into the aqueous phase, stirring the mixed solution to obtain the coarse product, adjusting the pH value, and repeatedly homogenizing the coarse product by a homogenizer to obtain the finished product. The average grain size of the lipid nanospheres is less than 100nm; compared with asarin injections and big-grain size emulsions which are sold on markets, the asarin lipid nanospheres obviously improve the targeting property in the lung, obviously reduce the distribution in the hepatic tissue, lower the potential hepatic toxicity and improve the drug efficacy.
Owner:SHENYANG WANJIA INST OF BIOLOGICAL TECH RES
Compositions and methods of reducing tissue levels of drugs when given as orotate derivatives
ActiveUS7470672B2Low toxicityToxic effectsSalicyclic acid active ingredientsBiocideSide effectTissue toxicity
This invention is in the field of chemical restructuring of pharmaceutical agents known to cause tissue toxicity as a side effect, by producing their orotate derivatives. More particularly, it concerns orotate derivatives of the anthracyclines, doxorubicin and daunorubicin, that are found to reduce levels of the pharmaceutical agent in noncancerous tissues. There orotate derivatives are equally efficacious in inhibiting the SCCAKI-1 kidney tumor in animals and the reduction in the heart tissue of doxorubicin compared with doxorubicin HCl suggests a reduction in toxicity induced by free radical generation by the anthrracyclines.
Owner:SAVVIPHARM INC
Elemene cabazitaxel compound flexible emulsion and preparation method thereof
ActiveCN111888331AReduce dosageLess irritatingOrganic active ingredientsPharmaceutical non-active ingredientsPhospholipinCabazitaxel
The invention discloses an elemene cabazitaxel compound flexible emulsion and a preparation method thereof. The emulsion is prepared from the following raw materials: 0.1-2g / 100mL elemene, 0.05-0.3 g / 100mL cabazitaxel, 2-20g / 100mL oil, 1-3g / 100mL phospholipid, 0.3-4g / 100mL polyethylene glycol derivative, 2-10g / mL osmotic pressure regulator and the balance of water. The preparation method comprisesthe following steps: (1) melting the oil, the phospholipid, the polyethylene glycol derivative, the elemene and the cabazitaxel to obtain an oil phase; (2) mixing the osmotic pressure regulator withthe water to obtain a water phase; and (3) adding the oil phase into the water phase, performing shearing and homogenizing under high pressure, adjusting the pH value, and performing sub-packaging. The elemene and the cabazitaxel are encapsulated in the flexible emulsion at the same time, so that the pharmacokinetic behavior of medicines is remarkably improved, the anti-drug-resistance effect of the medicines and the permeability of the medicines entering cells are improved, the curative effect is enhanced, and the safety of the medicines is remarkably improved.
Owner:HANGZHOU NORMAL UNIVERSITY
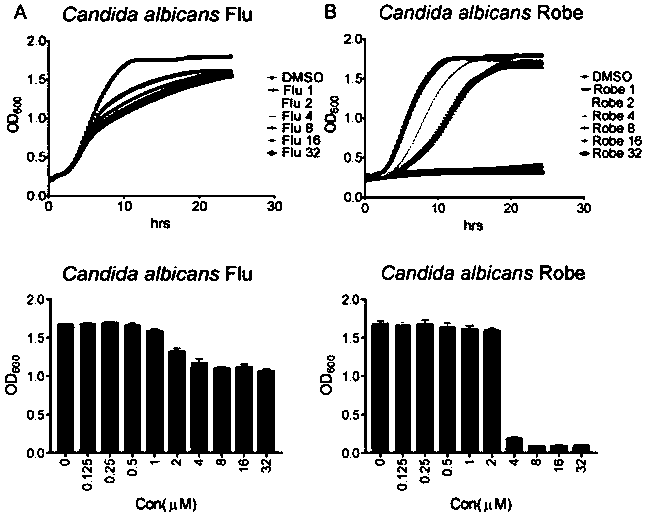
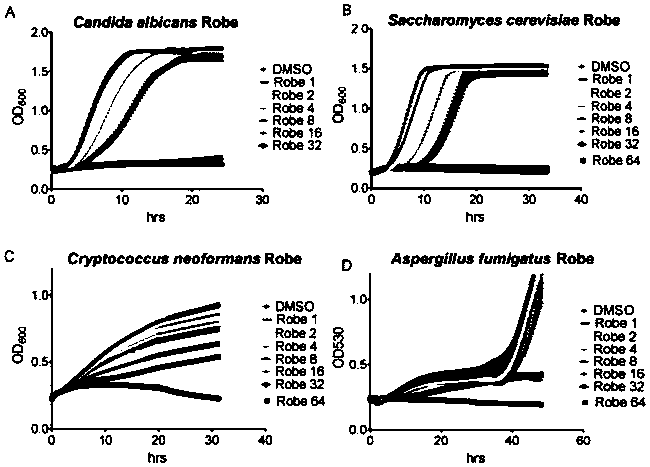
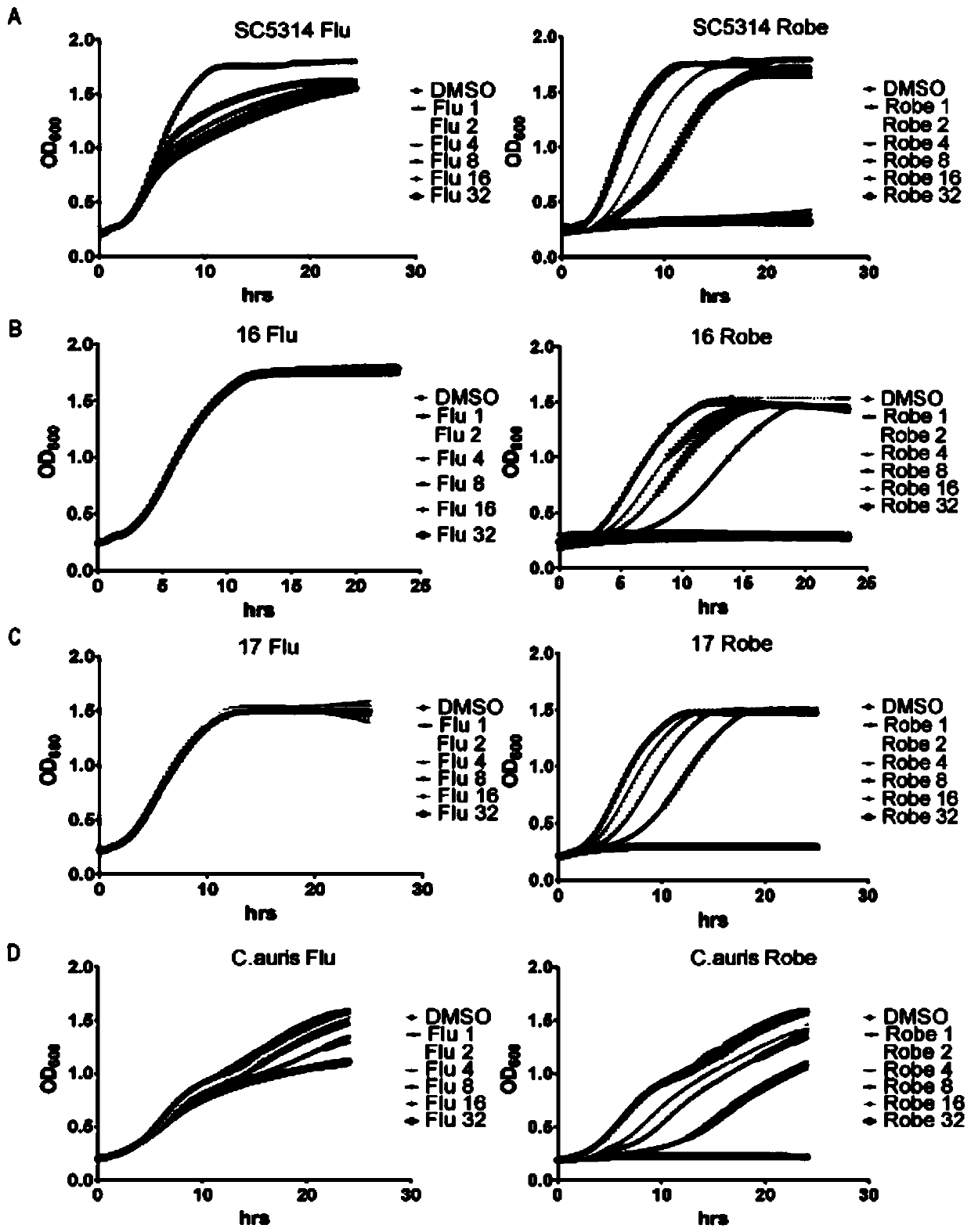
Application of robenidine hydrochloride in preparation of medicine for treating fungal infection
ActiveCN110251497AGood antibacterial effectIncrease lethalityOrganic active ingredientsAntimycoticsAntifungal drugsFluconazole
The invention belongs to the field of medicine preparation, and particularly relates to application of robenidine hydrochloride in preparation of medicine for treating fungal infection. Experiments prove that the minimum bacteriostatic concentration of the robenidine hydrochloride is 4 mu M, and is lower than that of fluconazole which is a common antifungal drug, the bacteriostatic effect of the robenidine hydrochloride is more obvious at the same concentration dose, the robenidine hydrochloride still has a good killing effect on drug-resistant fungal strains and super fungi, the toxicity is low, and the robenidine hydrochloride is safe and reliable. The invention also finds that the cell wall and the transcription factor RLM1 are drug targets of robenidine hydrochloride for treating fungal infection, and provides broad prospects for drug treatment of fungal infection.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
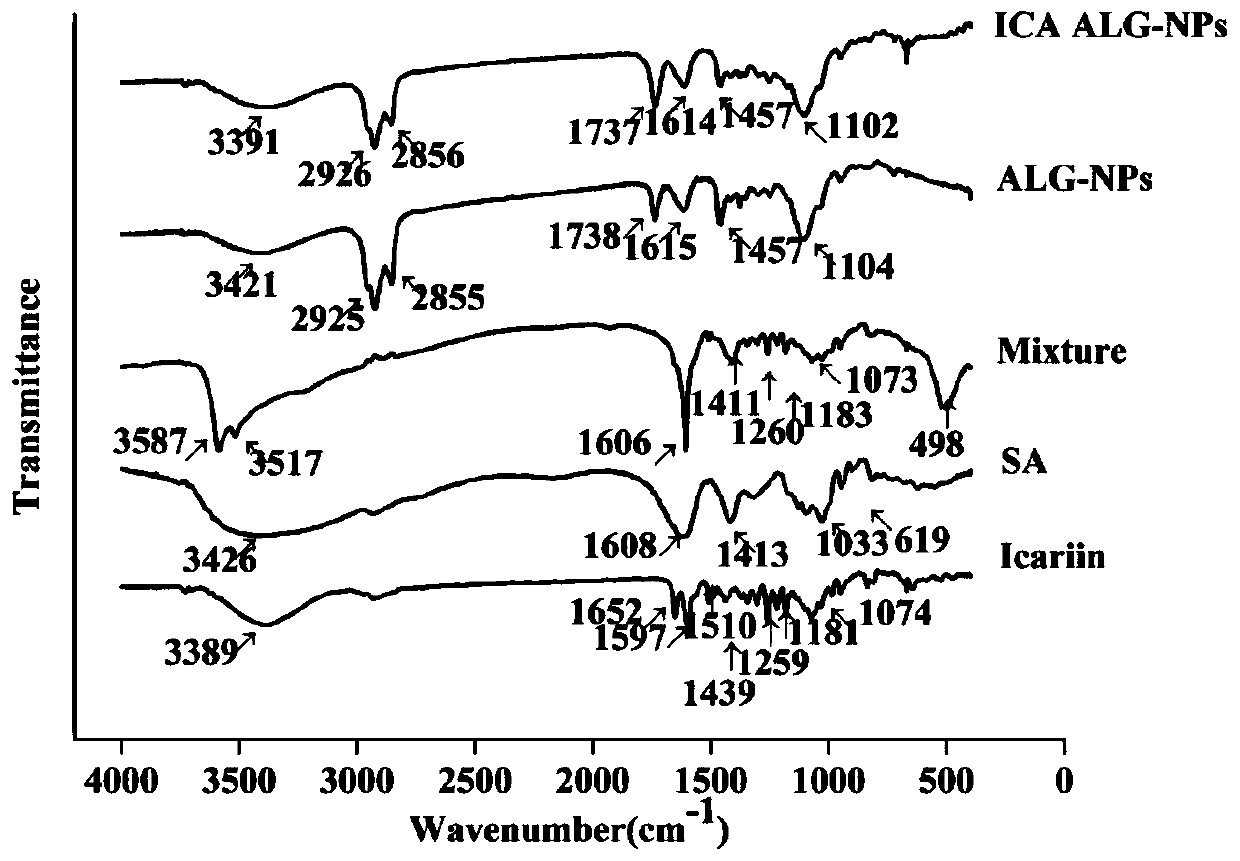
Preparation method and application of icariin sustained-release nano-gel by aerosol targeted drug delivery
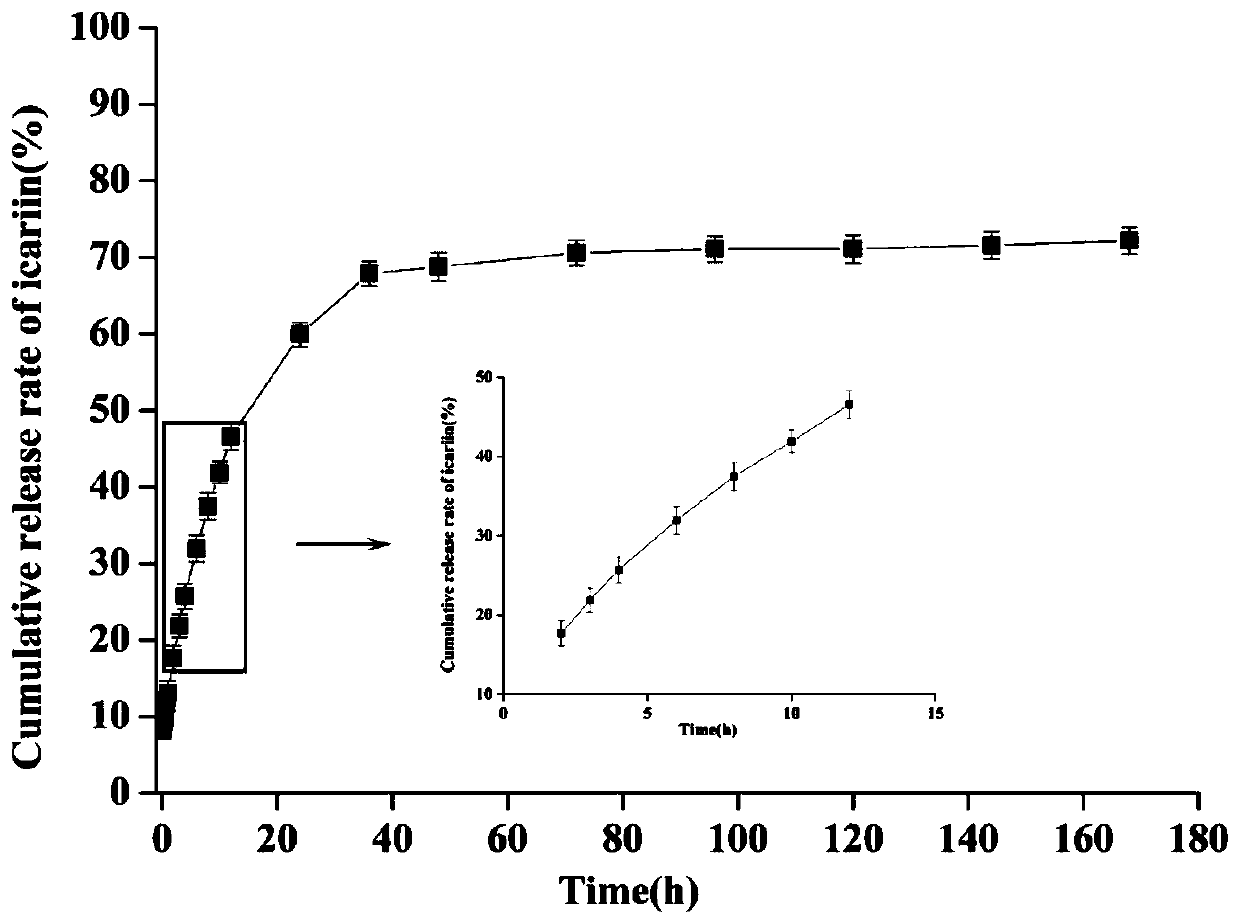
PendingCN111437397AGood sustained releaseGood bioadhesionPowder deliveryOrganic active ingredientsIcariinDisease
The invention discloses a sustained-release nano-gel by aerosol targeted drug delivery for treating respiratory system diseases. Sodium alginate high-molecular polymer is used as a vector material toconstruct a zinc alginate nano-gel drug delivery system to serve as a drug release storage cavern. Icariin-zinc alginate nano-gel is delivered to the lung in a targeted manner by adopting a drug delivery mode of ultrasonic aerosol inhalation, so that the liver first-pass effect of drugs can be avoided, response is rapid, and clinical drug delivery characteristics are accorded. The icariin-zinc alginate nano-gel can be directly and quickly distributed to the whole lung after ultrasonic aerosol inhalation, and can be adhered to the lung surface to constantly and stably release the icariin, so that the bioavailability of drugs can be improved, and the effect of treating respiratory system diseases can be achieved. Therefore, a proper and efficient novel preparation is provided to icariin treated respiratory system diseases, and targeted drug delivery can be performed through ultrasonic aerosol inhalation, and the application range of the sustained-release nano-gel can be enlarged.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
Compound anticancer sustained releasing agent containing mesenchyme hydrolytic reagent
InactiveCN1961862AInhibitionAvoid damagePeptide/protein ingredientsPharmaceutical delivery mechanismTherapeutic effectHyaluronidase
Owner:JINAN KANGQUAN PHARMA TECH
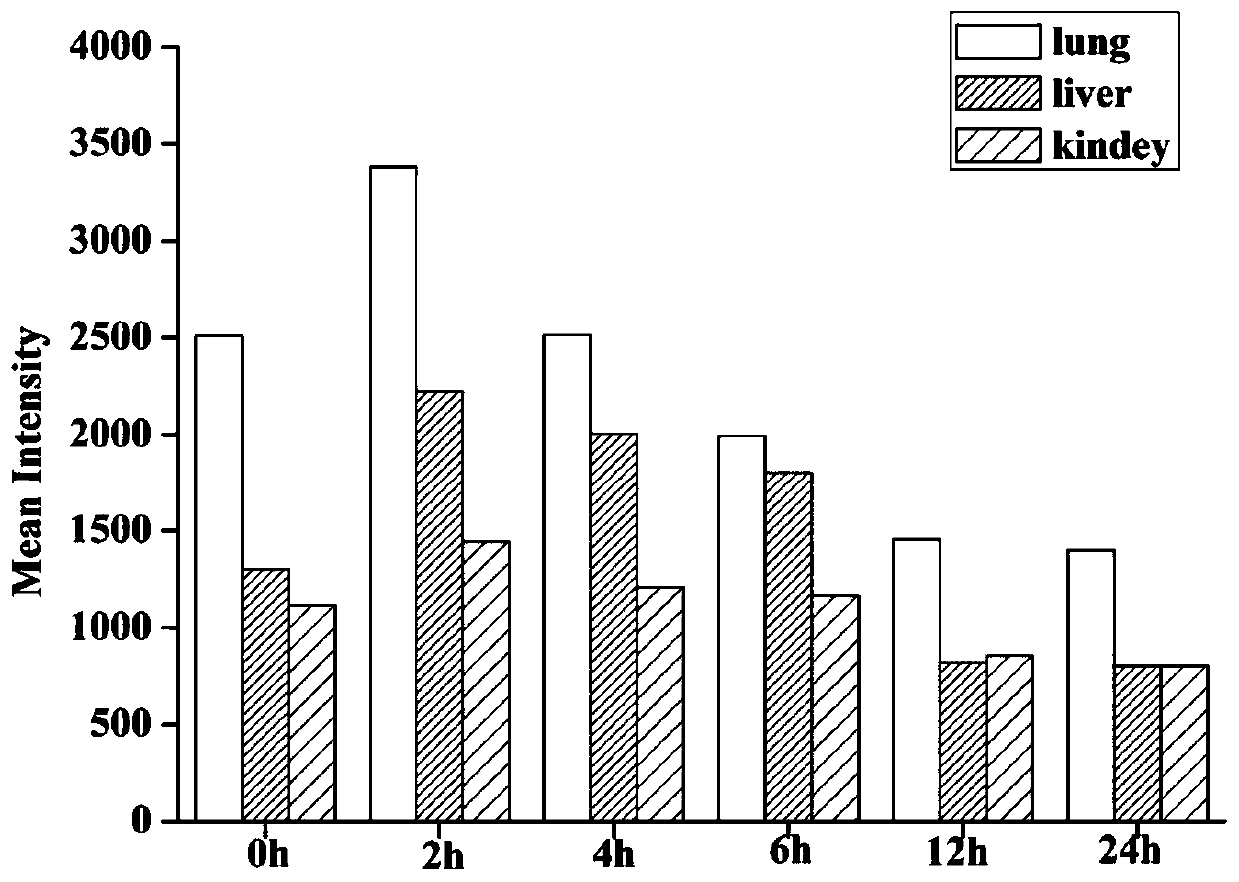
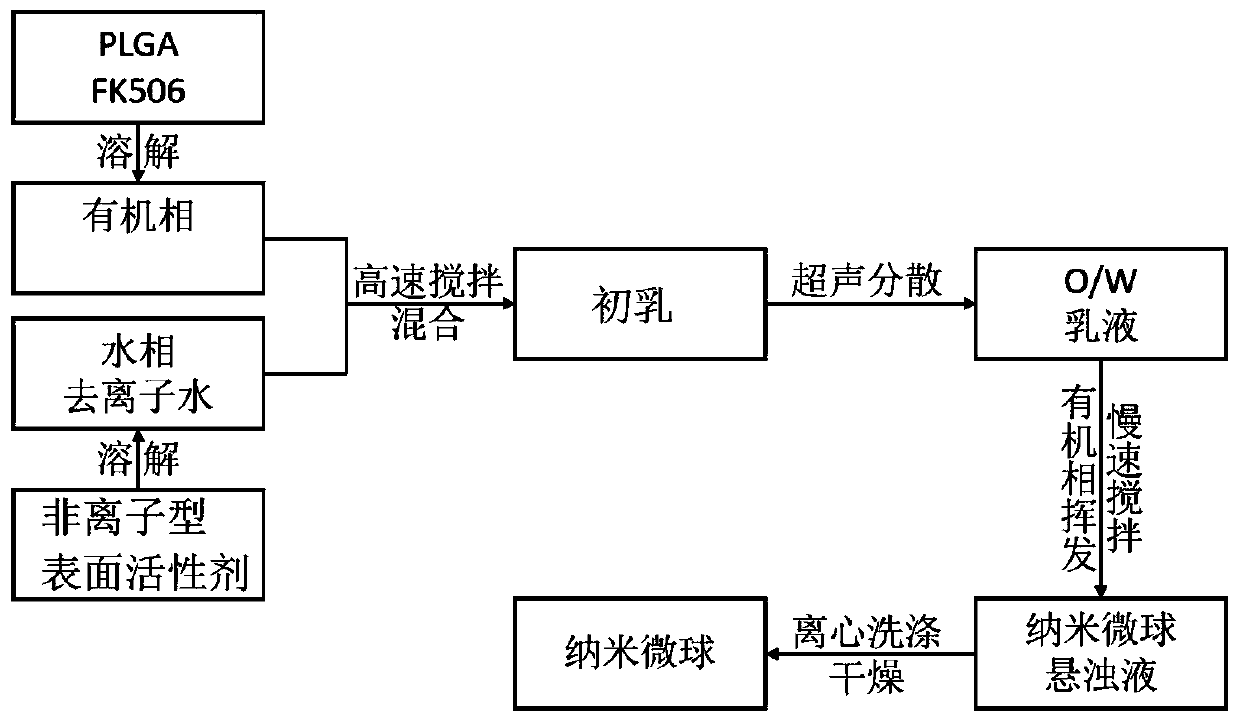
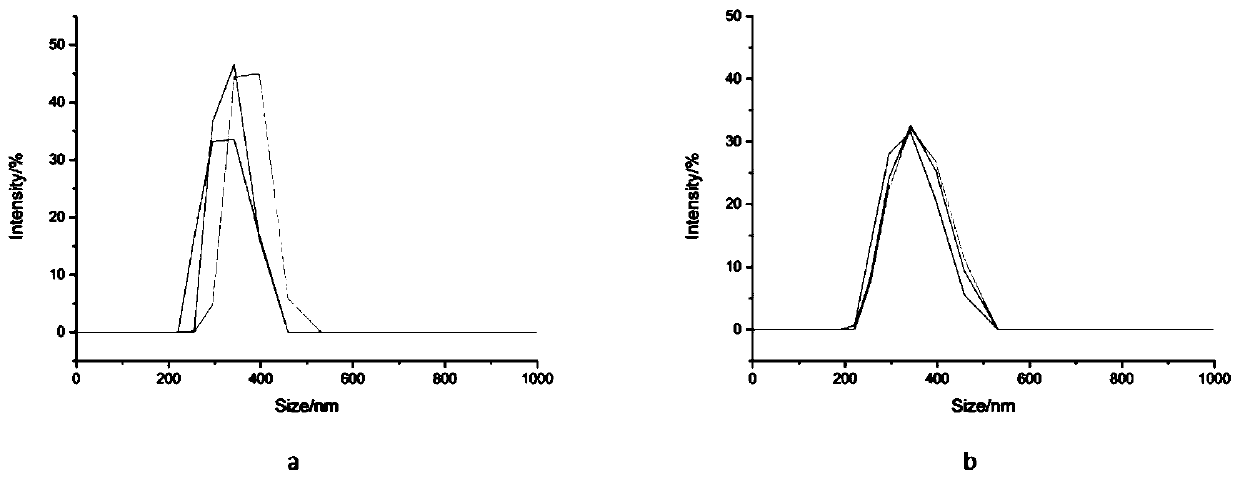
PLGA/FK506 drug-loading nano microsphere and preparation method and application thereof
ActiveCN110051652AReduce releaseGood slow releaseOrganic active ingredientsPharmaceutical non-active ingredientsOrganic solventLow speed
The invention relates to a PLGA / FK506 drug-loading nano microsphere and a preparation method and an application thereof. The preparation method for the drug-loading nano microsphere comprises the following steps: firstly adding a nonionic surfactant to deionized water and forming a water phase, enabling PLGA and FK506 to be successively dissolved in an organic solvent and forming an oil phase, after that, dropwise slowly adding the oil phase to a vortex center of the water phase, after high-speed stirring and ultrasonic treatment, acquiring O / W-type emulsion; and stirring the acquired O / W-typeemulsion in a room temperature condition under a low speed so that the organic solvent is completely volatilized, acquiring nano microsphere turbid liquid, finally centrifuging, washing, and drying to obtain a target product, wherein a particle size of the product is 100-200 nm preferably. Drug-loading capacity of the prepared PLGA / FK506 drug-loading nano microsphere reaches 17.64%, and encapsulation efficiency reaches 77.08%. Active targeting performance of the drug-loading microsphere is advantageously improved, and drug slow release and stronger effect of inhibiting growth of scar cells are realized.
Owner:WUHAN UNIV OF TECH
New detection index for drug sensitivity under state of inflammatory bowel disease and application of new detection index in design of drug therapy scheme
InactiveCN104535774ALower drug concentrationInhibition of therapeutic effectDigestive systemBiological testingTherapeutic effectDrug efficiency
The invention provides a new detection index for drug sensitivity under the state of an inflammatory bowel disease and application of the new detection index in the design of a drug therapy scheme, relates to multiple fields of pharmaceutical pharmacokinetics, pharmacology, cytobiology and molecular biology, creatively reveals natural drug-resistant phenomena of the inflammatory bowel disease and expounds related mechanisms. According to the new detection index, the phosphorylation of STAT3 is promoted by virtue of cell factors TNF-a, IL17 and LPS, which are excessively secreted in mice with the inflammatory bowel disease, in a way of STAT3 / Nf-kb, then the phosphorylation and the nuclear translocation of P65 are induced, the expression of peripheral blood monouclear cells P-gp is promoted, and the drug concentration of an immune inhibitor in the cells is reduced, the treatment effect of the immune inhibitor is inhibited, and the natural drug resistance is generated; by administering a P-gp specific inhibitor, the drug resistance phenomenon can be reverted. The new detection index prompts that P-gp expression quantity of peripheral blood lymphocyte can serve as an evaluation index of the natural drug resistance of patients with IBD (inflammatory bowel disease), and by virtue of the combination of the P-gp inhibitor, the decrease of the pharmaceutical effect caused by the natural drug resistance can be reverted, so that a new thought is provided for the treatment scheme of IBD.
Owner:CHINA PHARM UNIV
Composition and preparation of brain-targeted tanshinone oral oil-in-oil nanoemulsion
InactiveCN102293744ALow toxicityAvoid inconvenienceOrganic active ingredientsNervous disorderDiseaseVascular disease
The invention relates to a brain-targeted tanshinone oral oil-in-oil type microemulsion preparation, which is suitable for preventing and treating cerebrovascular diseases and central nervous system diseases. The preparation of the present invention utilizes the combination of the characteristics of nanoparticles and the increase of the fat solubility of the preparation to promote the penetration of the drug through the blood-brain barrier, improve the selective enrichment of the drug in the brain, provide a higher concentration of the drug in the brain, and significantly increase the concentration of the drug in the brain. residence time, thereby improving the prevention and treatment of corresponding brain diseases.
Owner:ANHUI UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
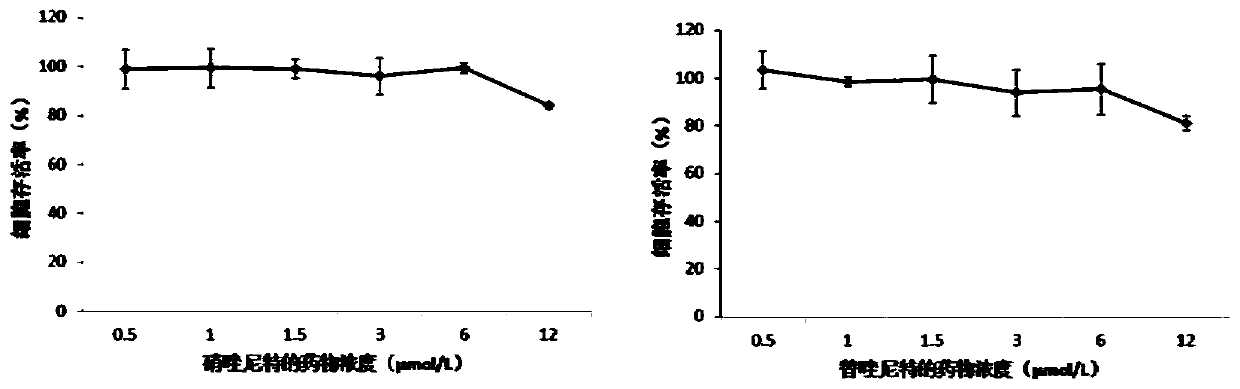
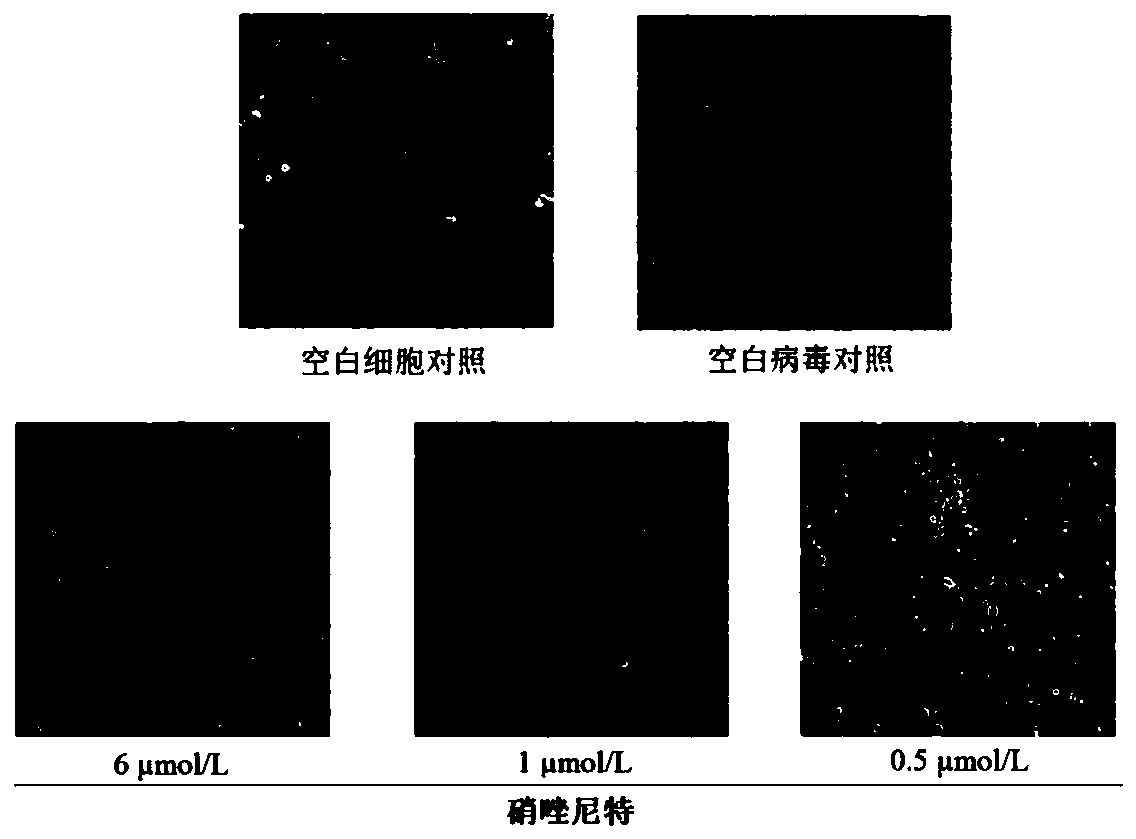
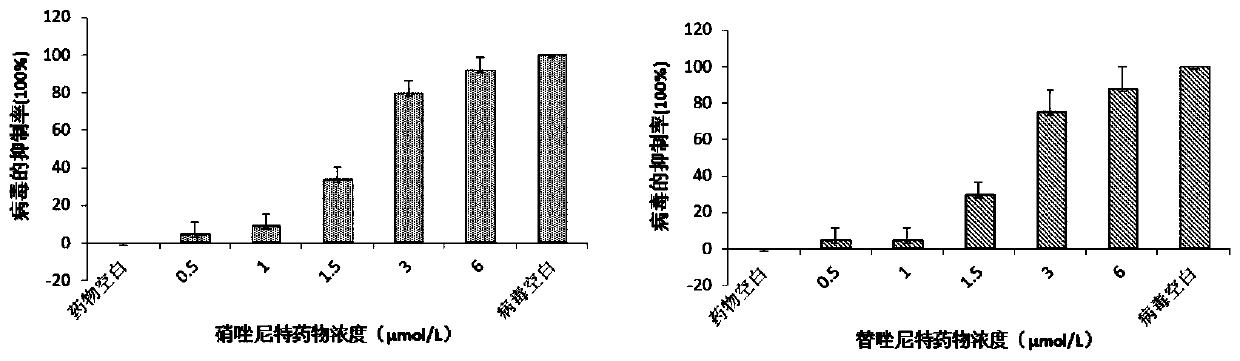
Application of nitazoxanide and tizoxanide in preparation of drug for resisting porcine reproductive and respiratory syndrome viruses
ActiveCN111012788ALower drug concentrationReduce the amount of nucleic acidOrganic active ingredientsAntiviralsPharmaceutical drugPharmaceutical medicine
The invention discloses an application of nitazoxanide and tizoxanide in the preparation of a drug for resisting porcine reproductive and respiratory syndrome viruses, and an application of a pharmaceutical preparation in the preparation of a drug for resisting porcine reproductive and respiratory syndrome virus infection. It is found in the invention that nitazoxanide and / or tizoxanide and pharmaceutically acceptable salts thereof can effectively protect animal cells at a low drug concentration, and the drug concentration can reach a micromole concentration level. It is also found in the invention that nitazoxanide and / or tizoxanide and pharmaceutically acceptable salts thereof can protect cells from virus infection and reduce the amount of nucleic acid of viruses in cells.
Owner:武汉世纪金辉农业科技有限公司
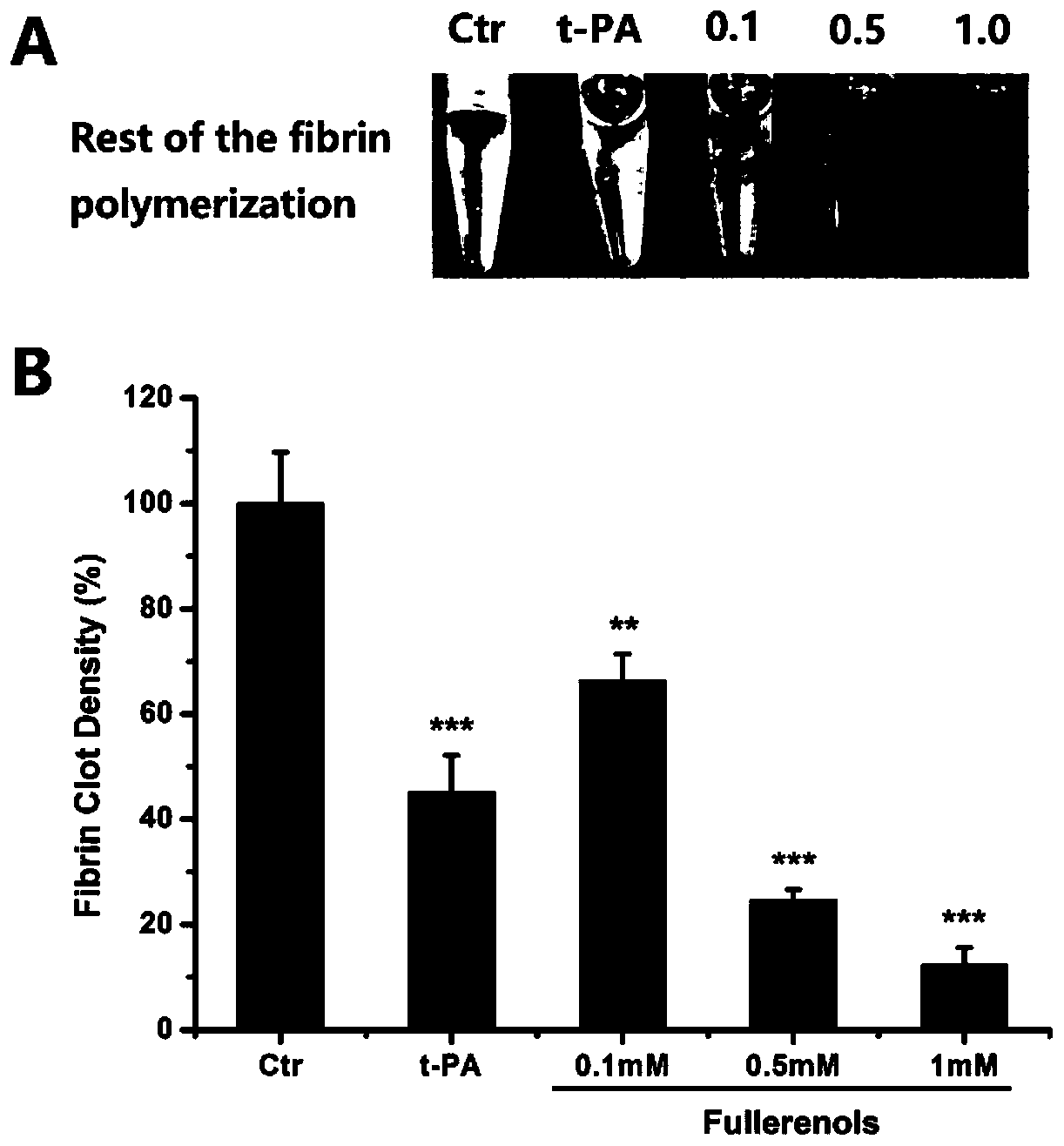
Fullerol and composition thereof in preparing antithrombotic drugs
ActiveCN110292583AGood biosecurityGood blood safetyPowder deliveryCarbon active ingredientsSolventDrug
The invention discloses application of fullerol and a composition thereof in preparing antithrombotic drugs, and relates to the technical field of medicine. Specifically, the application of the fullerol C60(OH)X in preparing drugs for dissolving thrombus and / or inhibiting thrombosis is studied, wherein X is larger than or equal to 10 and less than 40, and the drugs comprise the fullerol and / or meso-porous silicon and / or cytomembrane; further more, it is found that the components of a composition used for inhibiting and / or dissolving the thrombus mainly comprise the fullerol, the meso-porous silicon, erythrocyte membrane and platelet membrane, and can also comprise a solvent and / or a pharmaceutically acceptable carrier. In the application of the composition in preparing the antithrombotic drugs, the drugs can be prepared into various dosage forms, and the dosage amounts corresponding to the dosage forms are 0.4 mg / kg / day in terms of the fullerol. It is found through studies that the fullerol has obvious thrombolytic and antithrombotic effects, the targeting and enrichment of the thrombus can be enhanced by loading biological membranes with fullerol nanomedicine, and a good thrombolytic effect is achieved.
Owner:INST OF HIGH ENERGY PHYSICS CHINESE ACADEMY OF SCI
A kind of tilmicosin drug inclusion compound and its preparation and application
ActiveCN105311047BSimple processEasy to makeAntibacterial agentsOrganic active ingredientsAlcoholAdditive ingredient
Owner:LUOYANG HUIZHONG ANIMAL MEDICINE
Triptolide compound composition as well as preparation method and application thereof
InactiveCN111494319AImprove stabilityPromote absorptionOrganic active ingredientsEmulsion deliveryAmmonium glycyrrhizateLiver and kidney
The invention relates to a triptolide compound composition as well as a preparation method and application thereof, and belongs to the technical field of medicines. The triptolide compound compositionhas an obvious effect-enhancing and toxicity-reducing effect. The triptolide compound composition is prepared from triptolide, an ammonium glycyrrhizinate phospholipid compound, an emulsifier, oil, awater-soluble stabilizer, a fat-soluble stabilizer, glycerol, a co-emulsifier and water according to the following ratio, by weight percentage, 0.01%-0.02% of triptolide, 0.1%-0.9% of the ammonium glycyrrhizinate phospholipid complex, 5%-15% of oil, 0.9%-2.7% of the emulsifier, 0.3%-0.9% of the fat-soluble stabilizer, 2.5% of glycerol, 0.05%-0.1% of the co-emulsifier, 0%-0.2% of the water-solublestabilizer and the balance of water. The triptolide and the ammonium glycyrrhizinate are matched for use, the ammonium glycyrrhizinate is used for protecting cells such as livers and kidneys of organisms from being damaged by the triptolide, and the phospholipid complex prepared from the special structure of the ammonium glycyrrhizinate is high in lipophilicity, high in bioavailability and high in preparation stability, so that the treatment effect is improved.
Owner:SHENYANG PHARMA UNIVERSITY
Abamectin transdermal solution and preparation method thereof
InactiveCN113209012ALower drug concentrationImprove stabilityAntibacterial agentsOrganic active ingredientsPyrrolidinonesAbamectin
The invention provides an abamectin transdermal solution and a preparation method thereof. The abamectin transdermal solution comprises abamectin, azone, sec-butyl acetate, ethyl acetate, N, N-dimethylacetamide, glycerol methylal and methyl pyrrolidone. The azone and the methyl pyrrolidone are used as composite penetrating agents, and sucrose acetate isobutyrate and benzyl alcohol are used as sustained-release transdermal enhancers, so that the treatment effect is prolonged under the action of the sustained-release transdermal enhancers while the rapid penetration treatment of the abamectin transdermal solution is ensured. The sec-butyl acetate, the ethyl acetate, the N, N-dimethylacetamide and the glycerol methylal are used as a mixed solvent, so that the stability of veterinary drugs can be improved, the viscosity can be reduced, drug residues and toxic and side effects can be reduced, and a synergistic effect can be achieved.
Owner:WEIDA HUNAN TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com