Composition and process for production thereof
A composition and drug technology, applied in the direction of botanical equipment and methods, mixing methods, detergent compositions, etc., can solve the problems of unsatisfactory, air bubbles cannot exist stably for a long time, etc., achieve low manufacturing cost, safe and applicable , cost reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
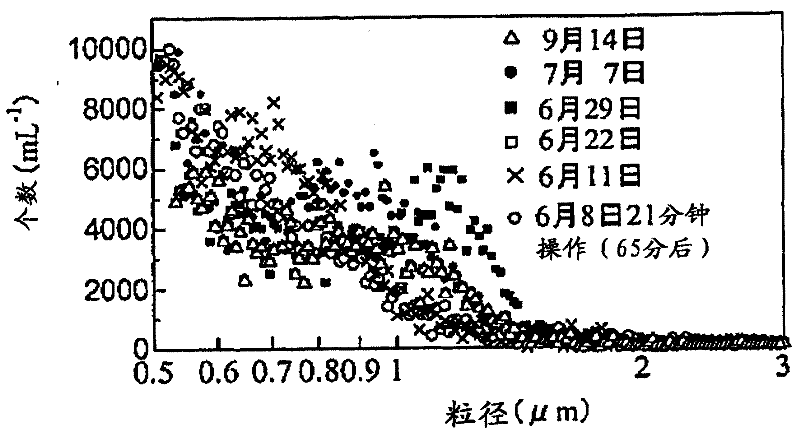
[0064] "BAVITAS" manufactured by Kyowa Co., Ltd. was used as an ultrafine bubble generating device utilizing a gas-liquid mixed shear method, and ultrafine bubbles were generated using pure water of 18.2 MΩ / cm. The particle size distribution at the time of generation and the change in the particle size distribution of bubbles after 3 months are shown in figure 1 middle. The measurement of the particle size distribution was performed using Multisizer 3 (manufactured by Beckman Coulter). No change in the number of bubbles in the portion having a particle diameter of 1 μm or less was observed.
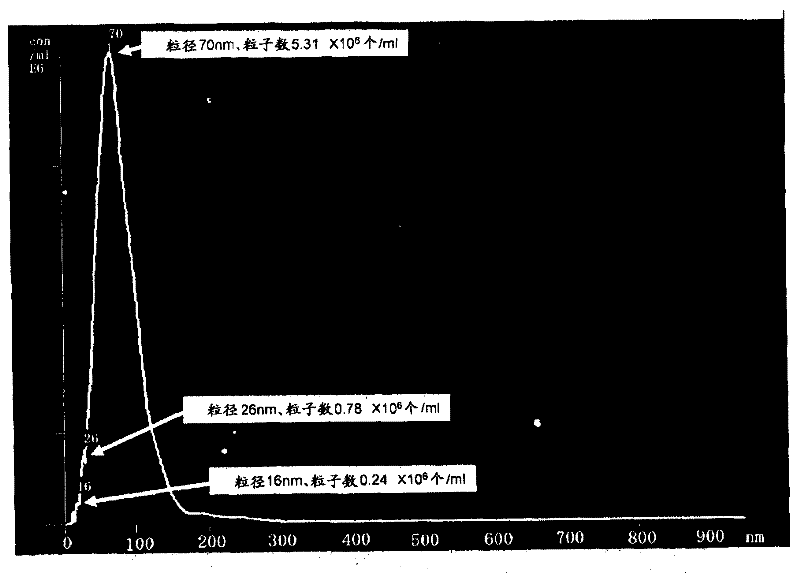
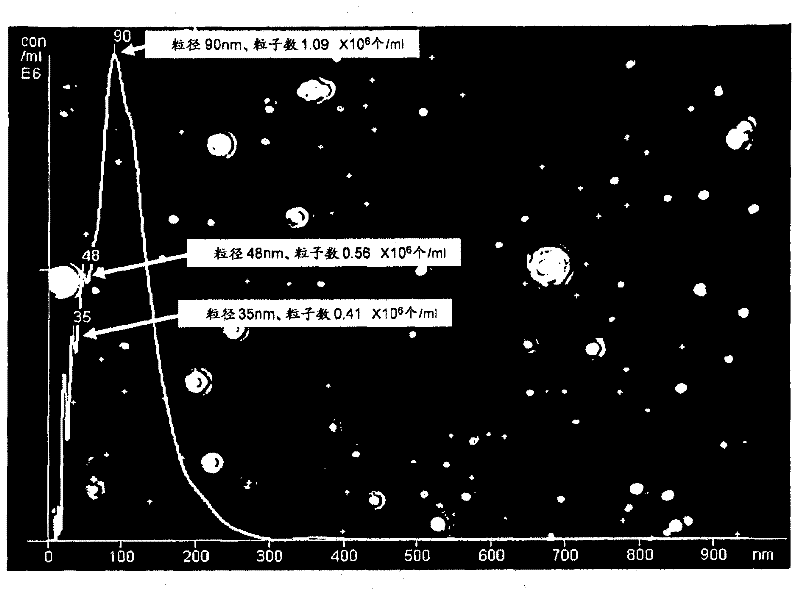
[0065] At the same time, the particle size of the generated ultrafine bubbles was measured with a nanoparticle analysis system NanoSight Series (manufactured by NanoSight Corporation). The measurement results are shown in figure 2 and image 3 middle. The horizontal axis of the figure represents the particle size in nm, and the vertical axis represents the number of particles per 1m...
Embodiment 2-5
[0068] The mixture having the composition shown in Table 1 below was treated under the same conditions as in Example 1 using "BAVITAS" manufactured by Kyowa Machinery Co., Ltd. Here, the water used is not pure water but distilled water. The results are shown in Table 1.
[0069] [Table 1]
[0070]
[0071] ※1 Micro-bubble generating device: "BAVITAS" manufactured by Kyowa Machinery Co., Ltd.
[0072] In Examples 2-4, drugs exhibiting hydrophobicity were stably dispersed. Both the drug stored at room temperature (RT) and the drug stored at 40°C maintained a good emulsified state.
[0073] Figure 5-13 shows the results of measuring the particle size distribution of the dispersions obtained in Examples 2-4 immediately after preparation and after storage at room temperature and 40° C. using a particle size distribution measuring device LS 13 320 (manufactured by Beckman Coulter Co., Ltd.). The abscissa represents the particle diameter, and the volume % (upper graph) and n...
Embodiment 6-8
[0077] As the samples used in Examples 6-8, the components shown in the following Table 2 in parts by weight were sequentially blended and treated under the same conditions as in Example 1 using "BAVITAS" manufactured by Kyowa Corporation. However, the water used is not pure water but distilled water.
[0078] Surfactants were used in Comparative Examples 2 and 3, and volatile components were emulsified in the same manner as in Examples 6 and 7. In addition, in Comparative Example 4, the same drug as in Example 8 was dissolved in a homomixer.
[0079] [Table 2]
[0080]
[0081] 1: "BAVITAS" established by Concorde Co., Ltd.
[0082] 2: Thiabendazole
[0083] 3: Water-soluble plant extract
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com