Asarin lipid nanospheres and method for preparing same
A technology of octylenol lipid and nanospheres, which is applied in the field of asarum lipid nanospheres and their preparation, can solve the problems that are not specified, and achieve the effects of reducing drug concentration, reducing degradation, and improving targeting.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of Asarum Lipid Nanospheres:
[0027] Raw materials used: Asarone 4g, egg yolk lecithin 12g, soybean oil 50g, medium chain oil 50g, glycerin 24g, polyethylene glycol-12 hydroxystearate 35g, take asarone and medium chain oil respectively according to the above weight ratio Put oil, soybean oil, phospholipid and polyethylene glycol-12 hydroxystearate (HS 15) into a high-speed homogenizer and mix at 60°C to form an oil phase; then take glycerin according to the above weight ratio, and take 60°C Water for injection, add 825g of water for injection that has dispersed glycerin to the oil phase, homogenize at 15000-20000rpm for 3-5 minutes to form colostrum; then, use a high-pressure homogenizer to pass N 2 After 4 to 6 cycles, a uniform emulsion is formed and the pH is adjusted to 7. The prepared emulsion is firstly filtered with a 0.45 μm microporous membrane under pressure, and then filtered with a 0.22 μm microporous membrane; or packed and sealed , autoclaved...
Embodiment 2
[0031] Preparation of Asarum Lipid Nanospheres:
[0032]Raw materials used: asarone 2g, soybean oil 30g, medium chain oil 70g, egg yolk lecithin 2g, soybean lecithin 3g, polyethylene glycol-12 hydroxystearate 20g, glycerin 24g, add sterile water for injection to 1000mL , prepared according to the process method of Example 1.
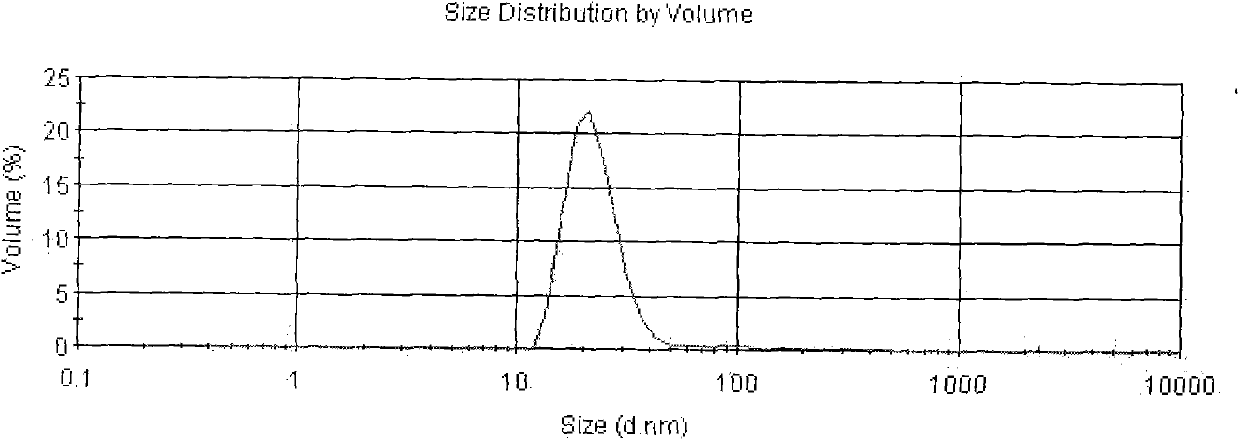
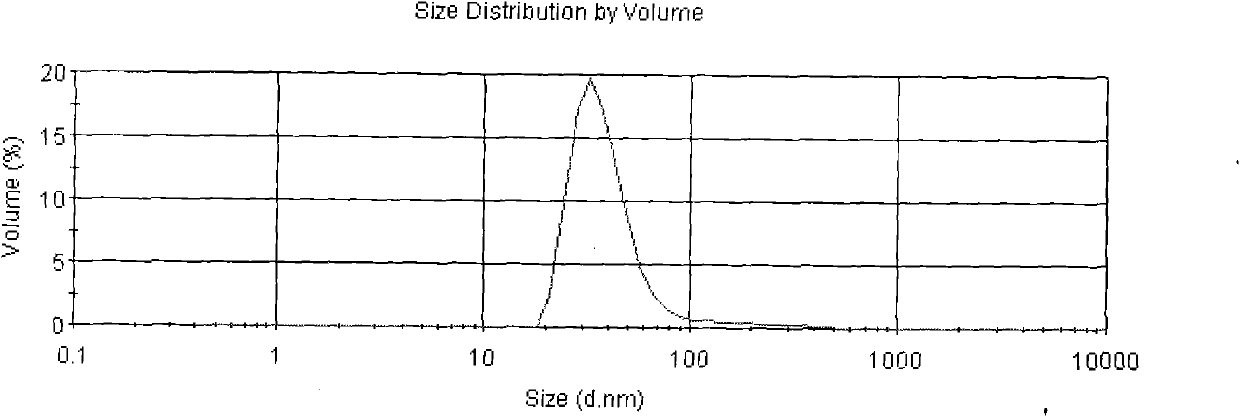
[0033] The Asarone lipid nanospheres particle size assay method prepared by the present embodiment is to get Asarone lipid nanospheres 0.1ml, add purified water (with a pore diameter of 0.22 μm membrane filter in advance) and dilute to 5000 times, Mix well, as the test solution, and use Malvern Nano-ZS 90 particle size analyzer to measure. The average particle size is 94nm, see the results figure 2 .
[0034] The medium-chain oil in this example is caprylic / capric glyceride (abbreviated as medium-chain triglyceride MCT)
Embodiment 3
[0036] Preparation of Asarum Lipid Nanospheres:
[0037] Raw materials used: Asarone 5g, medium chain oil 150g, egg yolk lecithin 50g, polyethylene glycol-12 hydroxystearate 300g, glycerin 22g, add sterilized water for injection to 1000mL, and prepare according to the process of Example 1.
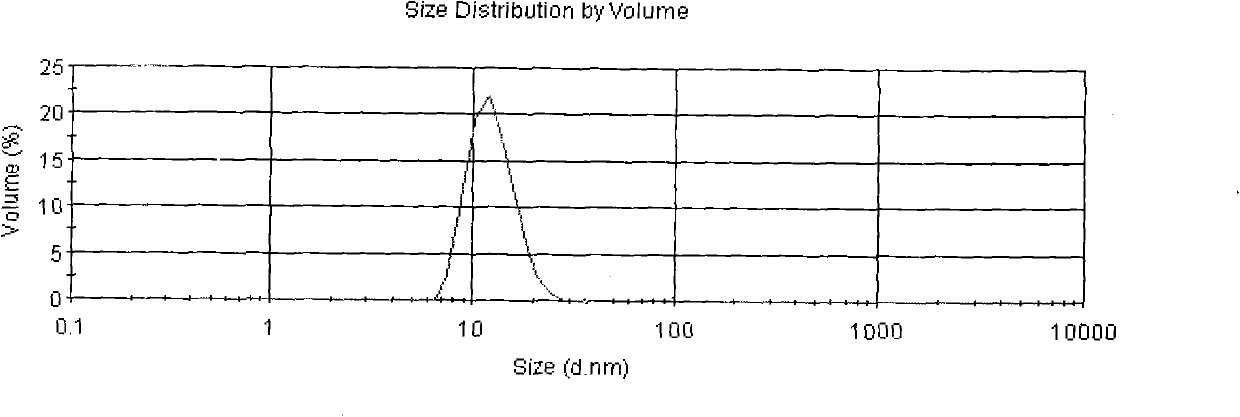
[0038] The Asarone lipid nanospheres particle size assay method prepared by the present embodiment is to get Asarone lipid nanospheres 0.1ml, add purified water (with a pore diameter of 0.22 μm membrane filter in advance) and dilute to 5000 times, Mix well, as the test solution, and use Malvern Nano-ZS 90 particle size analyzer to measure. After measurement: the average particle size is 35nm, see the results image 3 .
[0039] The medium chain oil in this embodiment is caprylic / capric glyceride (abbreviated as medium chain triglyceride MCT).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com