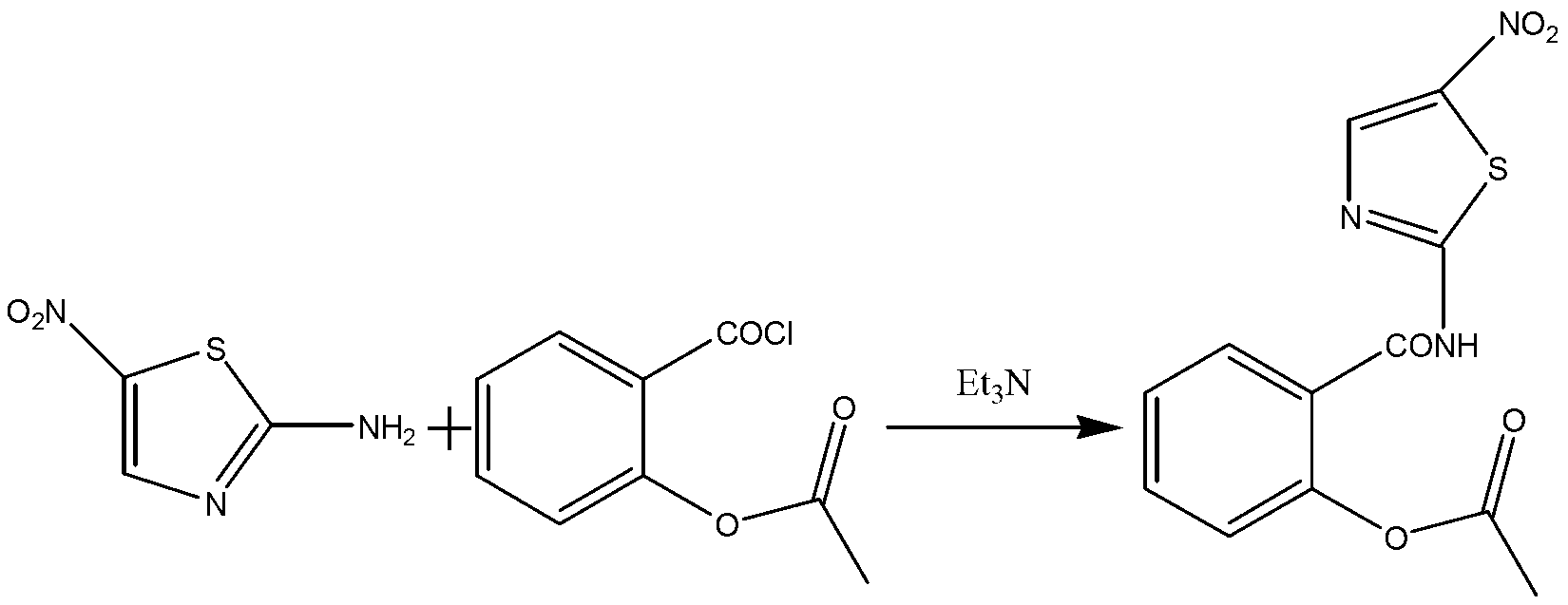
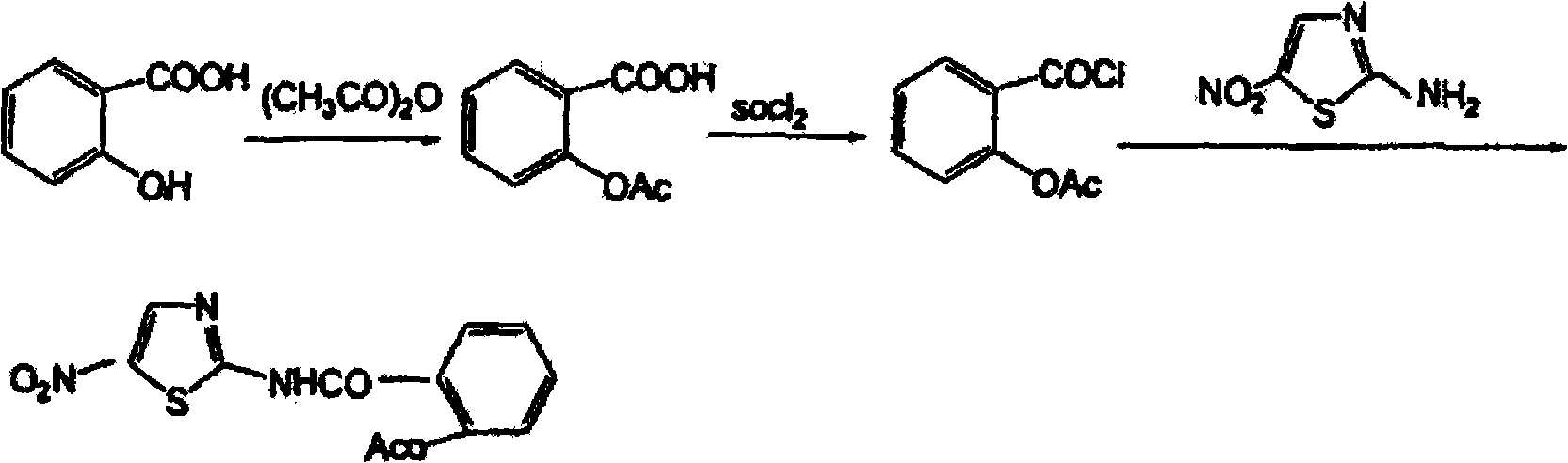
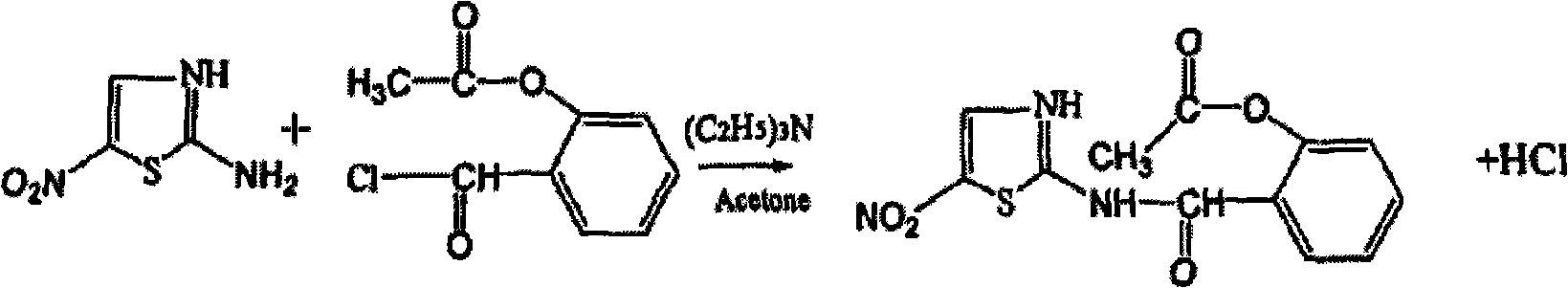
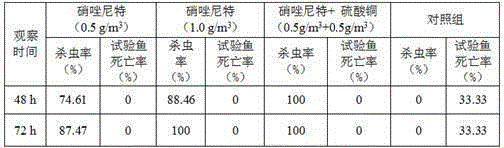
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
46 results about "Nitazoxanide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat diarrhea caused by certain parasite infections of the intestines (Cryptosporidium parvum and Giardia lamblia)..
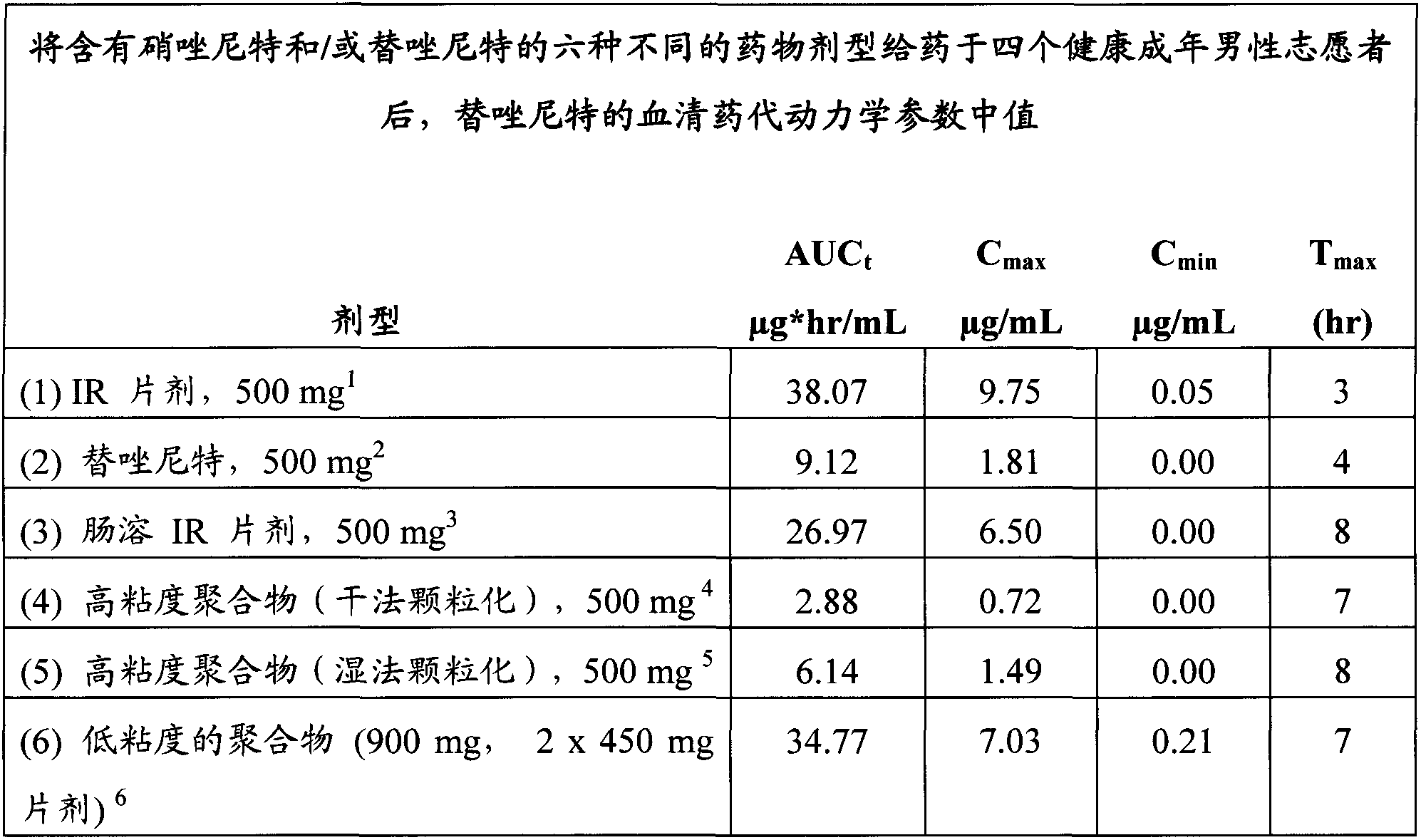
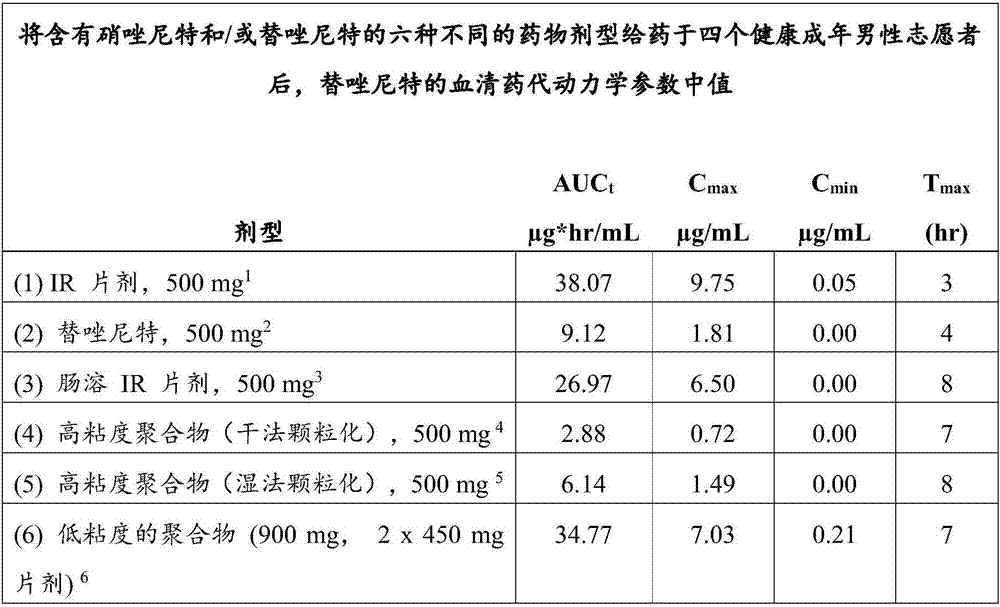
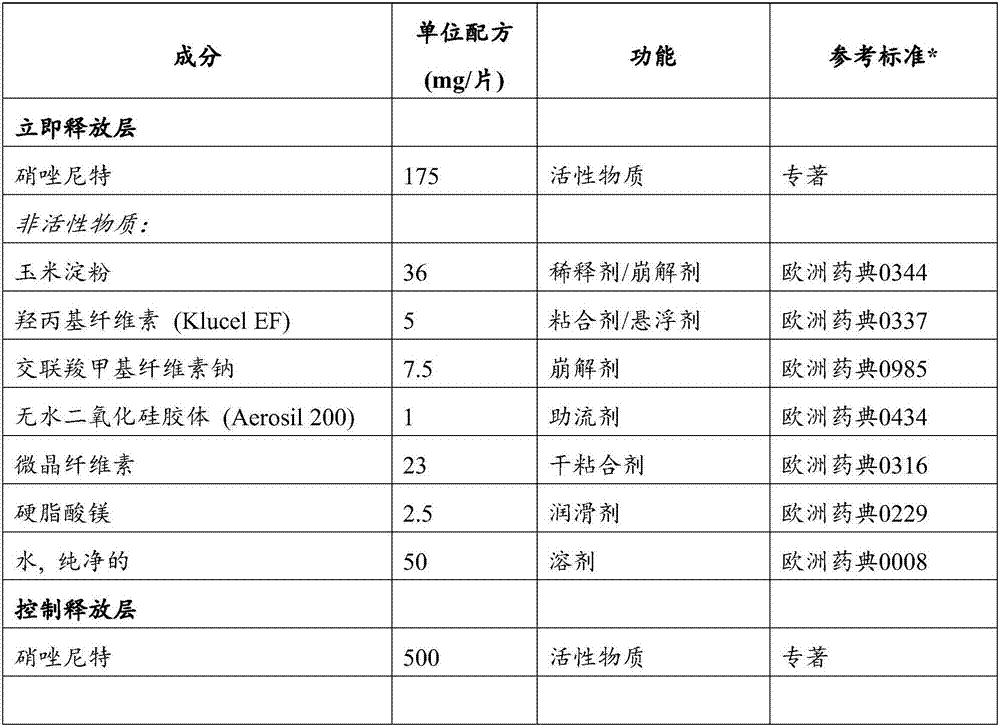
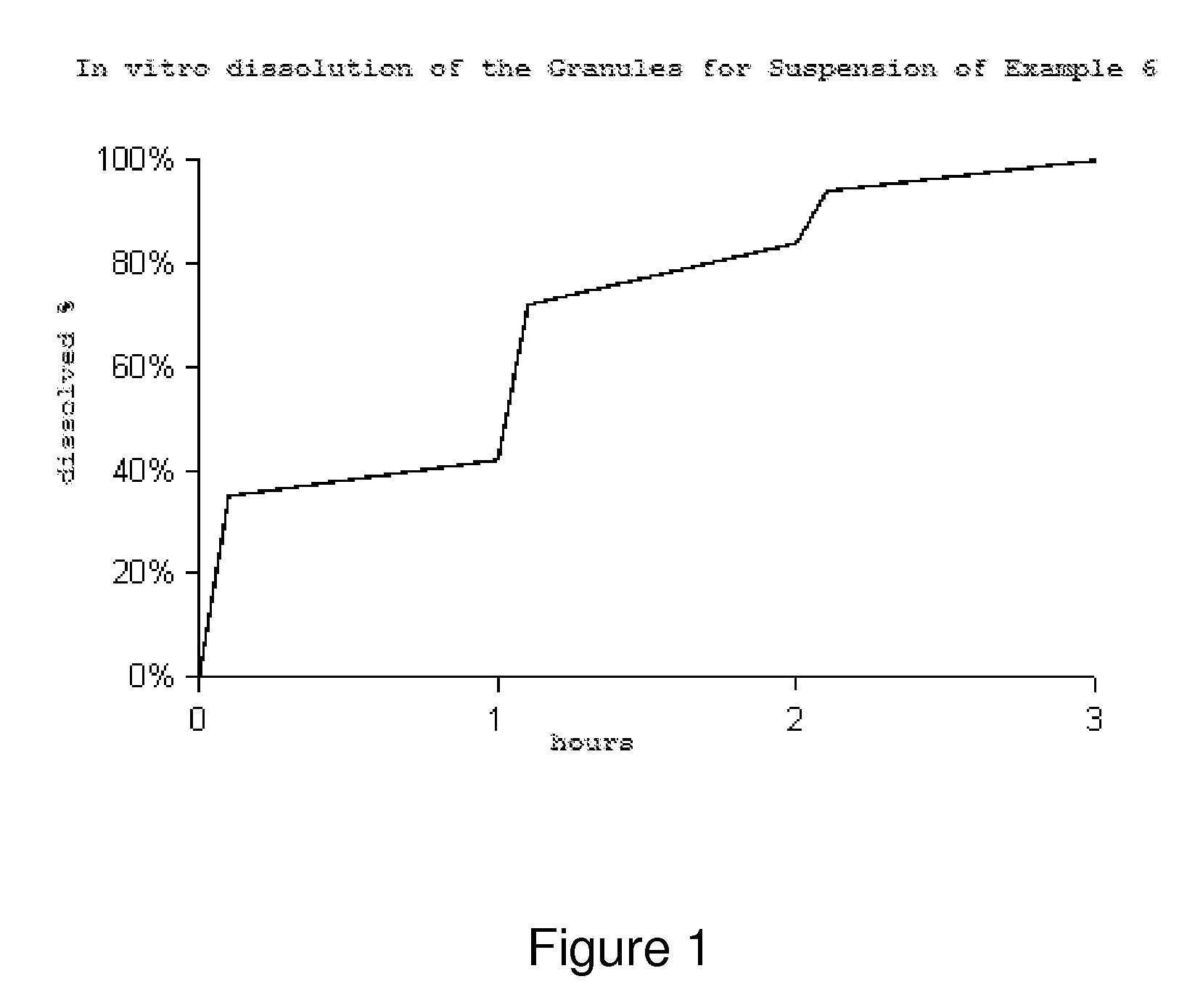
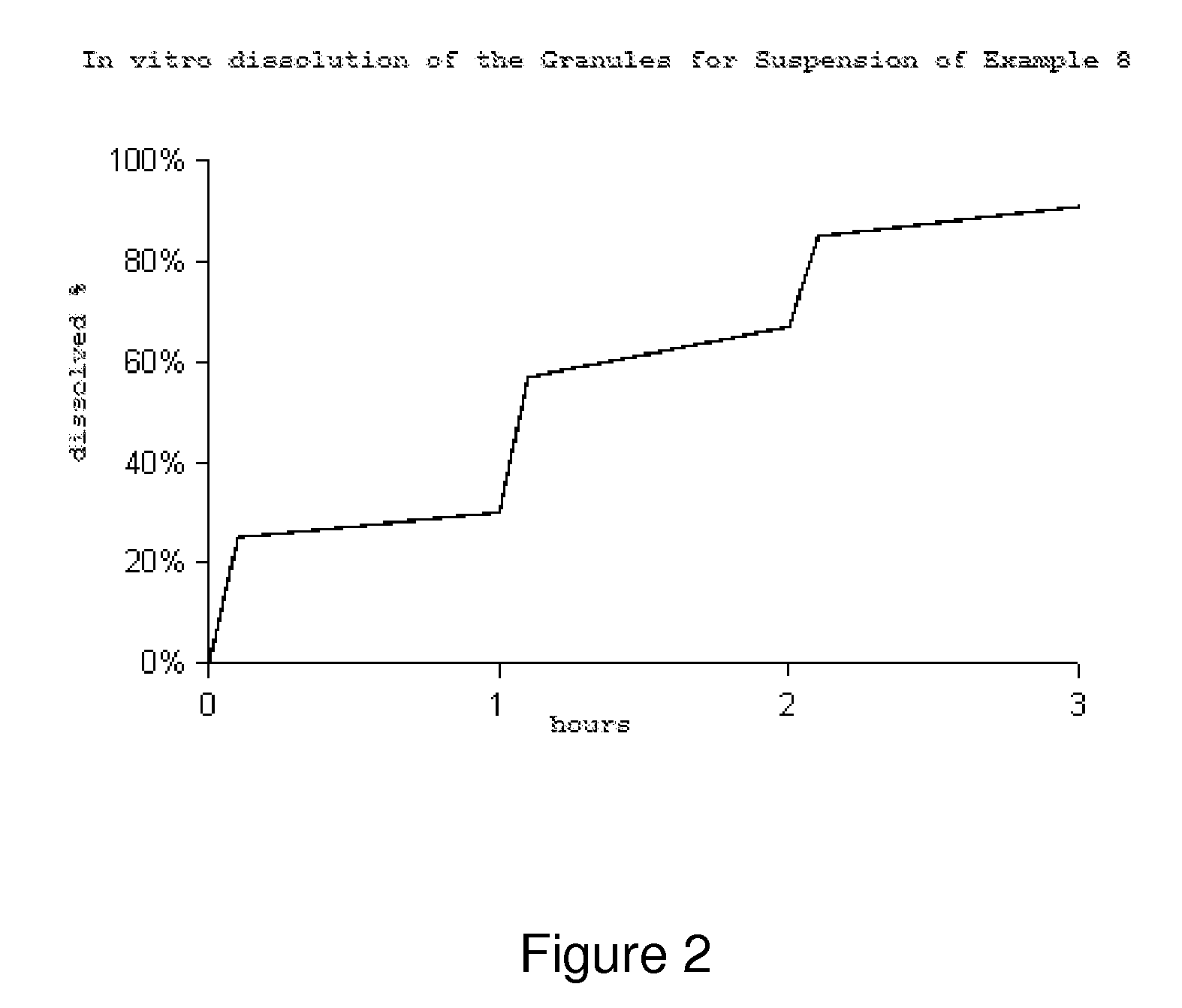
Controlled release pharmaceutical formulations of nitazoxanide
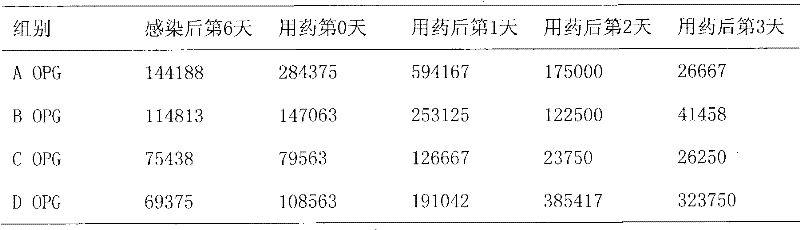
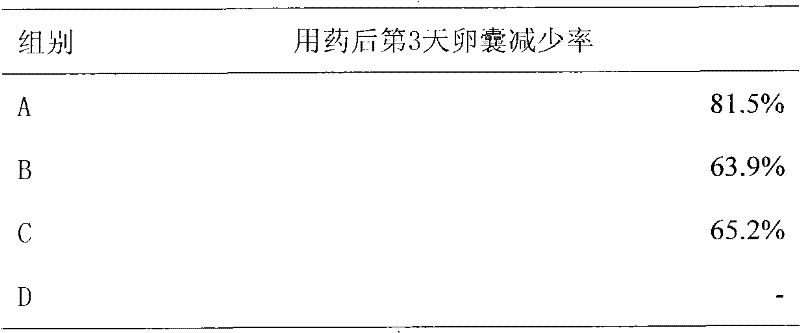
ActiveUS9351937B2Reducing one or more side-effectsBiocideOrganic active ingredientsImmediate releaseSolid Dose Form
Solid dosage formulations of nitazoxanide or a nitazoxanide analog are provided that comprise a controlled release portion and an immediate release portion. The pharmaceutical composition is typically in the form of a bilayer solid oral dosage form comprising (a) a first layer comprising a first quantity of nitazoxanide or analog thereof in a controlled release formulation; and (b) a second layer comprising a second quantity of nitazoxanide or analog thereof in an immediate release formulation. Method of using the formulations in the treatment of hepatitis C are also provided.
Owner:ROMARK LAB L C
Compositions and methods for treating tuberculosis
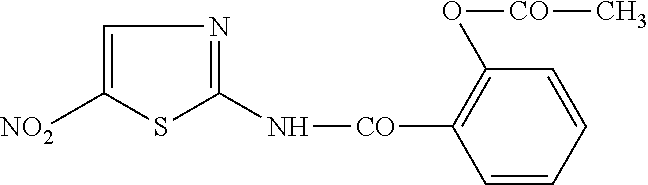
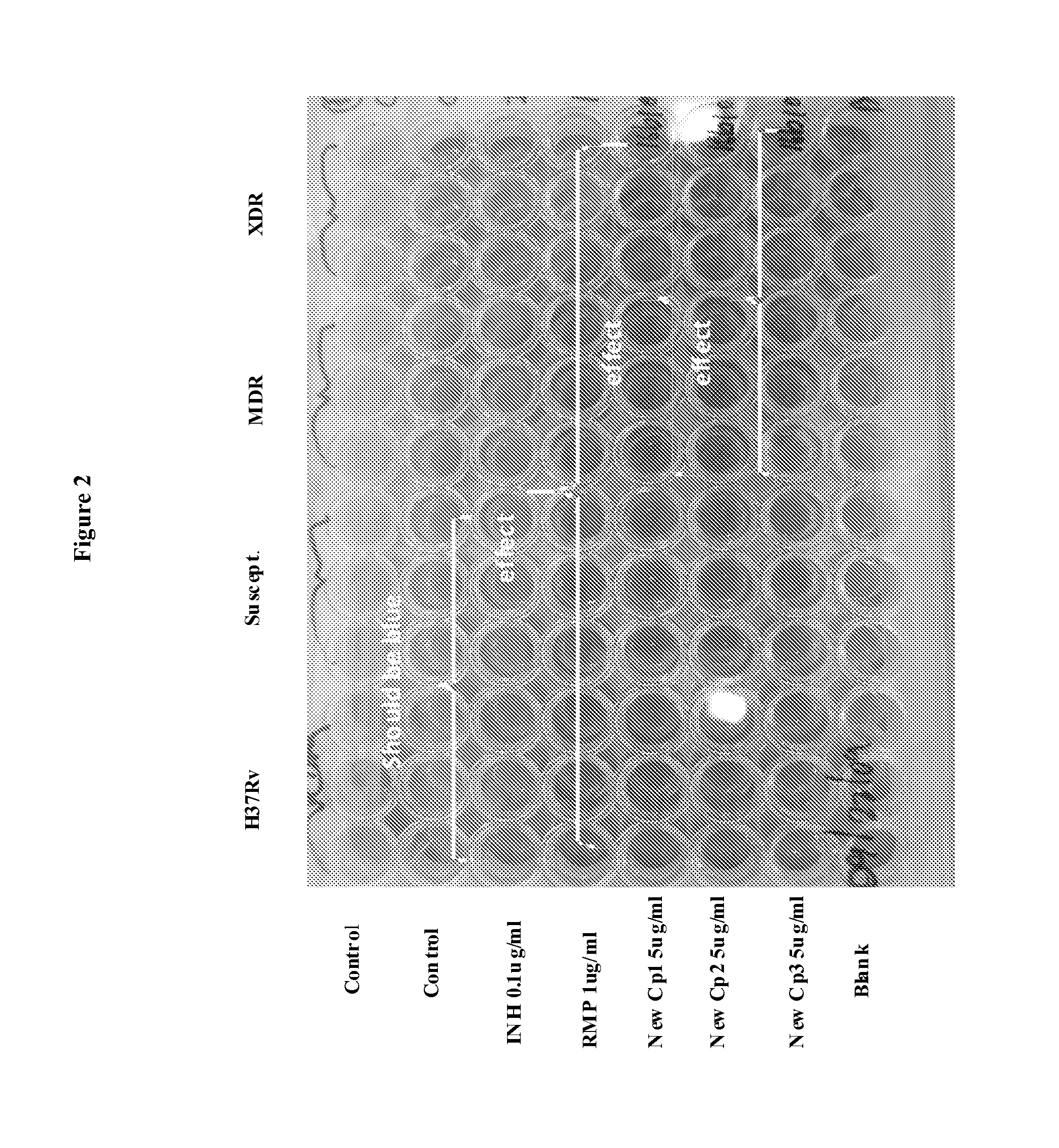
The invention provides for the use of antimicrobial chemical entities based on a nitrothiazolide backbone that exhibit anti-mycobacteria activity, including the mycobacterium causing tuberculosis. Multiple compounds were synthesized and screened for anti-tuberculosis activity. Disclosed herein are a series of compounds with anti-tuberculosis activity, including six leads that completely inhibited bacterial growth at 5 micrograms per ml or less. Three of these compounds were tested to determine MIC and these ranged between 1 and 4 micrograms per ml against both drug susceptible Mycobacterium tuberculosis strains and strains that are multi-drug resistant (MDR) including XDR strains. The compounds developed are derived from parent compound nitazoxanide, which had no inhibitory activity in the stringent testing format used herein. The derivatives were synthesized using a di-nitro-thiophene or 4-Chloro-5-Nitro-thiazole scaffold and R groups connected via a peptide bond (NHCO) to cyclic compounds such as benzene, thiophene or furans. Many of these compounds have broad spectrum activity against Gram positive bacteria including Staphylococcus aureus (MRSA) and Staphylococcus epidermidis. Several of these lead compounds were not toxic for mice at 200 mg / Kg doses administered over a period of three days.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
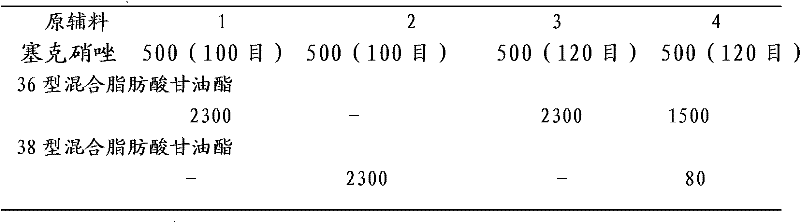
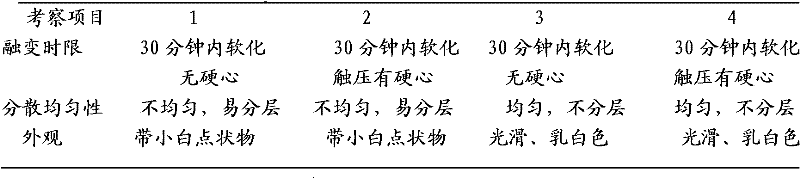
Secnidazole vaginal suppository and its preparation process
InactiveCN102266284ASimple recipeSimple production processOrganic active ingredientsAntimycoticsSide effectMonoglyceride
The secnidazole vaginal suppository of the present invention is composed of the following components: main ingredient secnidazole 500g, auxiliary material 36 type mixed fatty acid glyceride 2300g, made into 1000 suppositories, each weighing 2.8 grams, the 36 type mixed Fatty acid glycerides are a mixture of triglycerides, diglycerides and monoglycerides, and the melting point of type 36 mixed fatty acid glycerides is 35-37°C. The drug of the secnidazole suppository of the present invention is directly absorbed through the vagina, and will not cause damage to the heart, kidney and liver after testing. The curative effect is good, there is basically no side effect, the proportion is simple, and the use is convenient. The invention also discloses the preparation process of the secnidazole vaginal suppository at the same time.
Owner:HUBEI TUNGSHUN PHARMA
Combination chemotherapy for helminth infections
InactiveUS20050171169A1Good curative effectImprove the level ofOrganic active ingredientsBiocideAlbendazoleHelminth infections
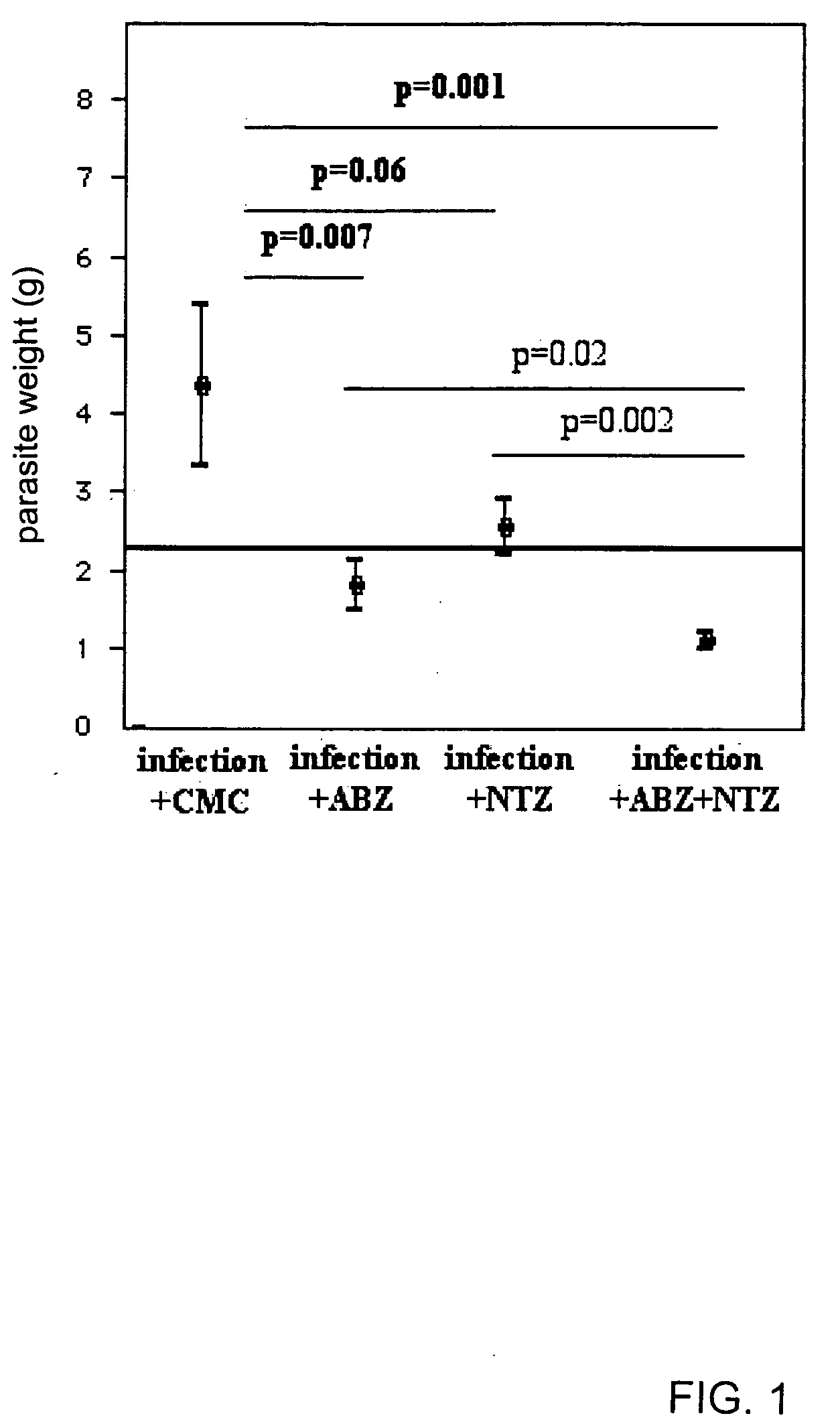
Methods for the treatment of a helminthic infection, by the administration, in either single or multiple dosages, of a synergistic combination comprising albendazole according to formula (I): and nitazoxanide according to formula (II): in which the efficacy and range of parasites treated is enhanced by the instant combination compared to the sum of the effects of the subject drugs administered separately.
Owner:ROMARK LAB L C
Application of nitazoxanide in preparing drug for resisting eimeria coccidium
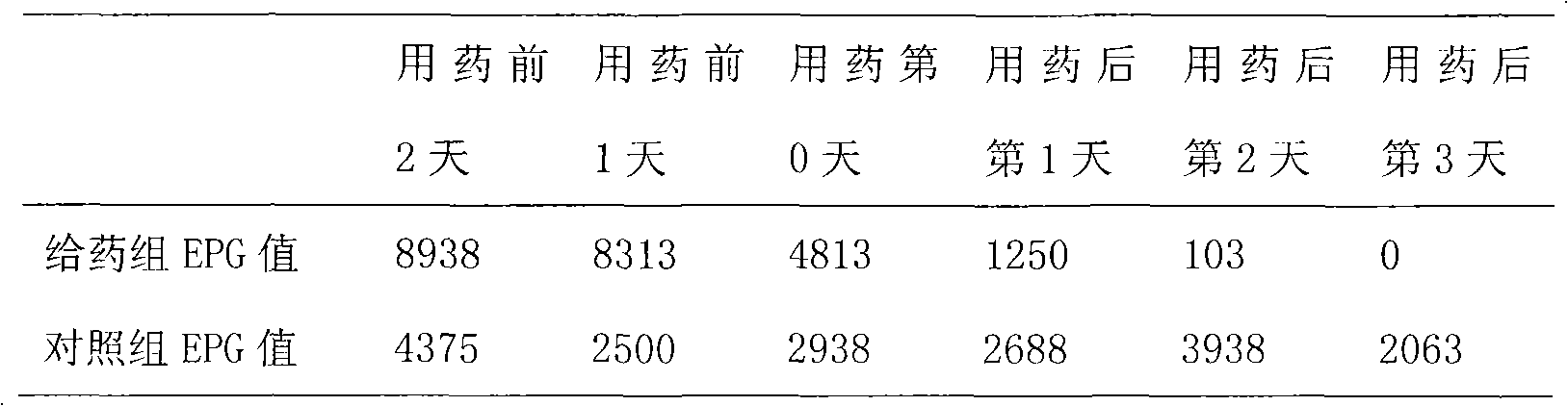
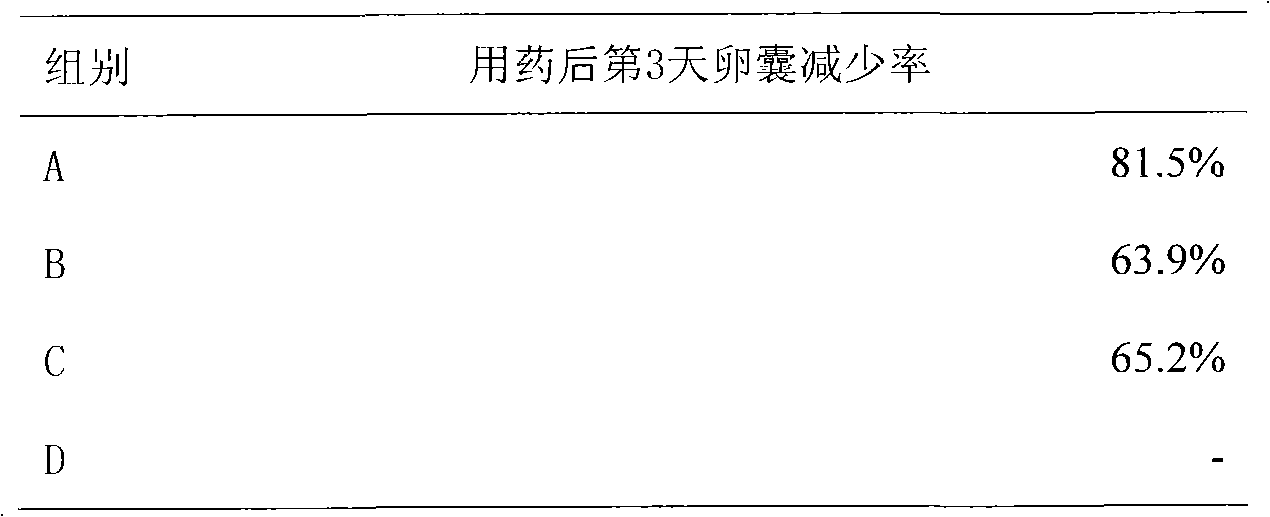
The invention provides a new medicinal application of nitazoxanide and particularly discloses an application of nitazoxanide in preparing a drug for resisting eimeria coccidium. The application can serve for the prevention and treatment of coccidiosis of animals such as chickens and rabbits, and the like.
Owner:JILIN UNIV
Senolytic compounds
PendingCN110678187AHalogenated hydrocarbon active ingredientsCyclic peptide ingredientsDiseaseNitrofurazone
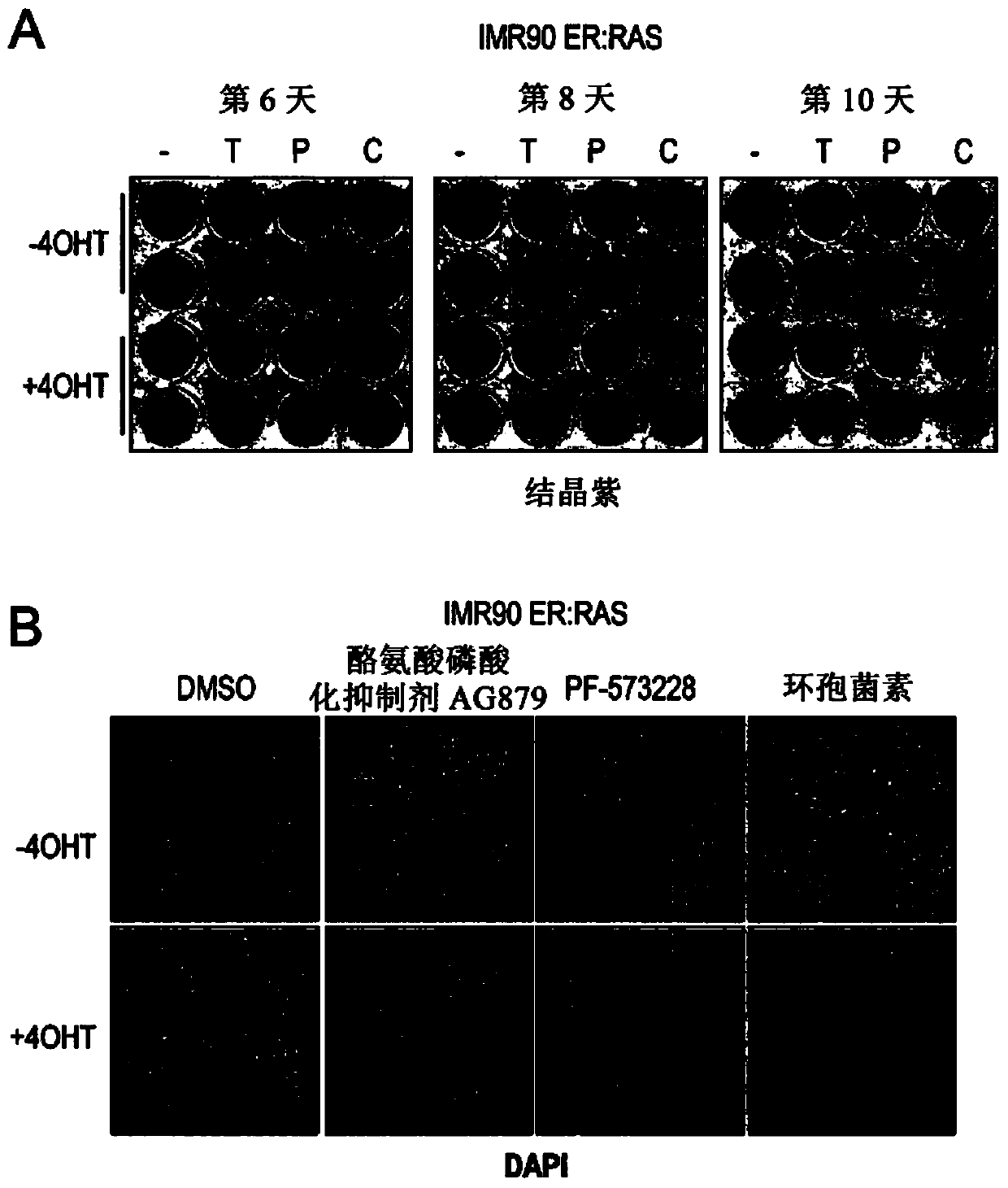
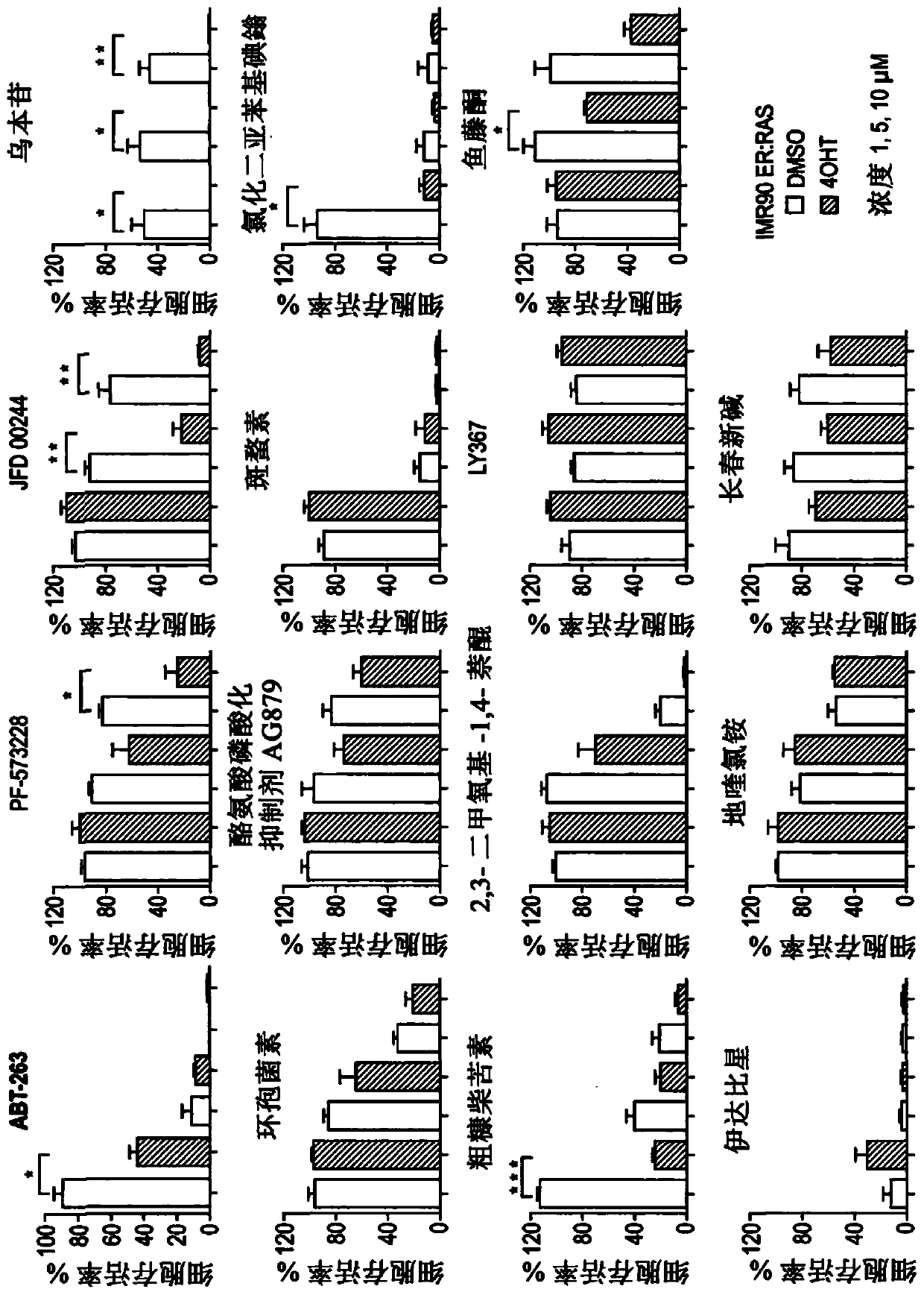
The present invention relates to an agent for use in selectively killing one or more senescent cells, wherein the agent is selected from the following: a cardiac glycoside or alglycone, a focal adhesion kinase (FAK) inhibitor, an HMG-CoA reductase inhibitor, JFD00244, Cyclosporine, Tyrphostin AG879, Cantharidin, Diphenyleneiodonium chloride, Rottlerin, 2,3-Dimethoxy-1,4-naphthoquinone, LY-367,265,Rotenone, Idarubicin, Dequalintum chloride, Vincristine, Nitazoxanide, Nitrofurazone, Temsirolimus, Eltrombopag, Adapalene, Azacyclonol, Enoxacin and Raltegravir, and pharmaceutically acceptable salts thereof. Another aspect relates to compounds for use in treating or preventing a senescence- associated disease or disorder, and methods relating thereto.
Owner:英国研究与创新公司
Controlled release pharmaceutical formulations of nitazoxanide
Solid dosage formulations of nitazoxanide or a nitazoxanide analogue are provided that comprise a controlled release portion and an immediate release portion. The pharmaceutical composition is typically in the form of a bilayer solid oral dosage form comprising (a) a first layer comprising a first quantity of nitazoxanide or analogue thereof in a controlled release formulation; and (b) a second layer comprising a second quantity of nitazoxanide or analogue thereof in an immediate release formulation. Method of using the formulations in the treatment of hepatitis C are also provided.
Owner:ROMARK LAB L C
Method for preparing nitazoxanide
InactiveCN103159697AReduce manufacturing costEasy to makeOrganic chemistry2-Amino-5-nitrothiazoleRoom temperature
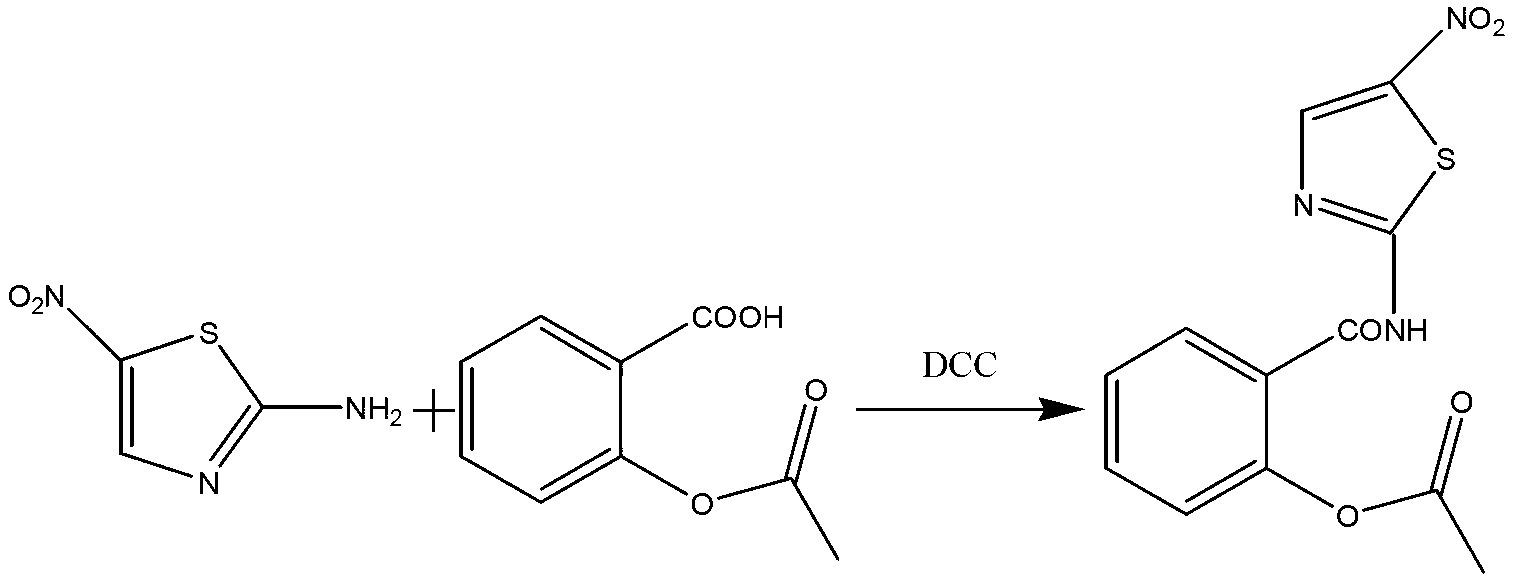
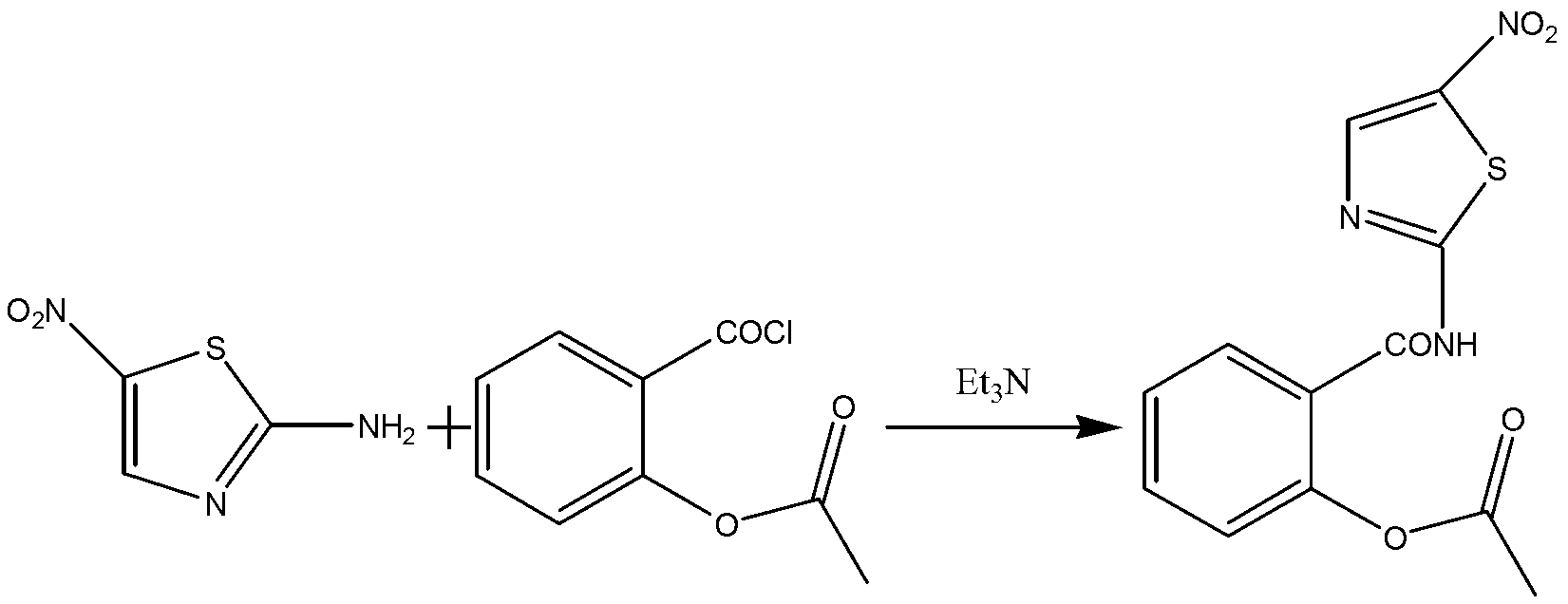
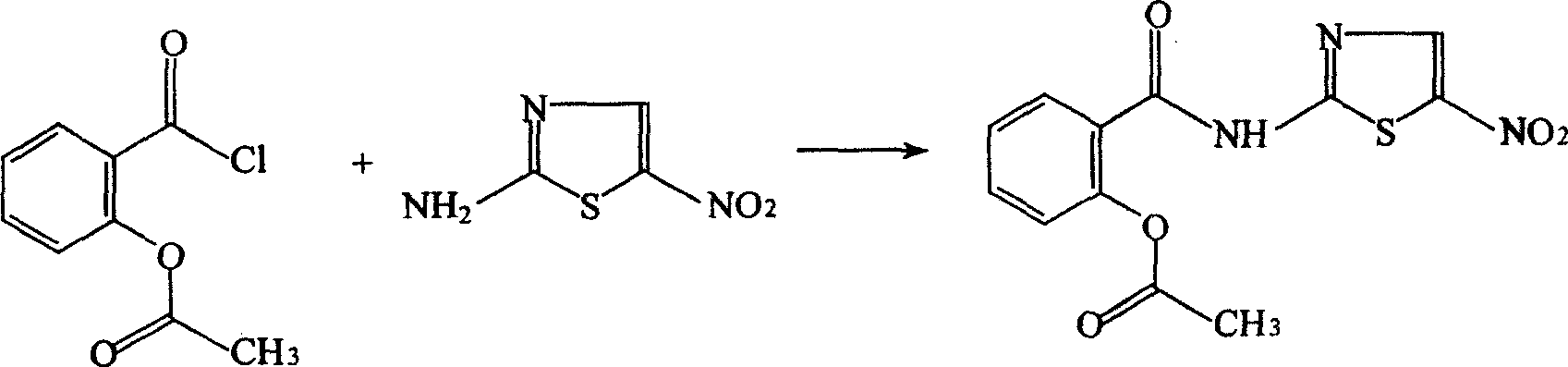
The invention discloses a method for preparing nitazoxanide. The method comprises the following steps of: reacting acetylsalicylic acid with thionyl chloride at 50 DEG C-70 DEG C so as to generate O-acetylsalicylryl chloride, then preparing an O-acetylsalicylryl chloride solution by utilizing 1, 4-dioxane or N, N-dimethyl formamide (DMF), dripping the O-acetylsalicylryl chloride solution into a mixed liquid of 2-amino-5-nitrothiazole, triethylamine, 1, 4-dioxane or N, N-dimethyl formamide, continuously stirring for 20-60 minutes at room temperature after the dripping is ended, thus obtaining the nitazoxanide. The method has the advantages of moderate reaction conditions, low cost and simple post-processing method and is convenient for mass industrial production.
Owner:ANHUI ZHONGSHENG PHARMA
Preparation method of nitazoxanide
The invention relates to a preparation method of nitazoxanide. The preparation method is characterized in that 2-amido-5-nitro thiazole and anhydrous acetone are evenly mixed, a solution composed of the anhydrous acetone and o-acetyl salicylic acid chloride is added into the obtained mixed liquid, then anhydrous triethylamine is slowly dropped into the mixture when being stirred, a reaction is carried out at constant temperature, the reaction temperature is kept after the anhydrous triethylamine is dropped, the stirring is continuously carried out, the obtained reaction liquid is poured into ice water when being stirred, and the mixture stands, is filtered, is dried and is recrystallized by using ethanol to obtain the nitazoxanide. The method is simple and feasible, the yield of a final product is about 75%, the reaction step is simplified, and the production efficiency is improved.
Owner:QINGDAO VLAND BIOTECH INC +2
Composite preparation used for treating larimichthys crocea cryptocaryon irritans disease
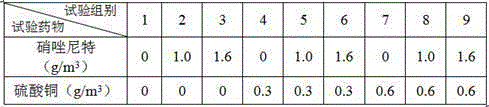
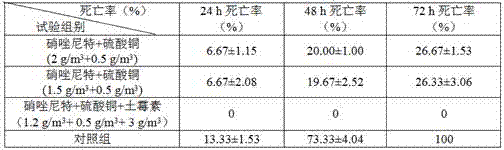
ActiveCN105267237AEfficient killingAvoid secondary infectionAntibacterial agentsInorganic active ingredientsDiseaseCryptocaryon
The invention relates to a composite preparation used for treating larimichthys crocea cryptocaryon irritans disease and application thereof. The composite preparation comprises, 10 wt%-15 wt% of nitazoxanide, 5 wt%-7 wt% of copper sulfate, 25 wt%-30 wt% of terramycin, and 48 wt%-60 wt% of a solvent, and the sum of the above raw materials is 100%. The usage method comprises after larimichthys crocea is definitely diagnosed to be attacked by cryptocaryon irritans disease, splashing the above preparation according to the amount of 8-10 g / m<3>, continuously performing dipping bath for 3 days, so as to effectively cure larimichthys crocea cryptocaryon irritans disease and prevent bacterial secondary infection. The preparation is simple in preparation method and convenient to use, and is capable of substantially reducing mortality of larimichthys crocea attacked by cryptocaryon irritans disease and raising culture economic benefit.
Owner:福建省淡水水产研究所
Novel thiazole derivatives, and preparation method and application thereof
InactiveCN106632133AImprove the bactericidal effectAntibacterial agentsOrganic active ingredientsPositive controlDrug biological activity
The invention relates to a structure of novel thiazole derivatives, a preparation method of the thiazole derivatives and application of the thiazole derivatives in the aspect of bioactivities. The invention also relates to pharmaceutically acceptable salts, a solvate or prodrug, and a pharmaceutical composition of the derivatives. Thiazolyl, uramido, thioureido and other active groups are introduced to obtain the series thiazole derivatives (I) and (II). The test on the activities of the compounds for some representative pathogenic bacteria and parasites shows that the series compounds have parasite resisting activities; and the CC50 / IC50 of partial compounds for Toxoplasma gondii is greater than 1 or even higher, so the partial compounds have favorable inhibiting activities. Besides, the compounds XQH-2-97, XQH-3-13 and XQH-3-14 have obvious inhibitory effects on Streptococcus mutans, have obviously higher bactericidal capacities than nitazoxanide in the positive control group, and thus, are hopeful to be developed into new antibacterial compounds.
Owner:SHANDONG UNIV
Compound sulfa-nitazoxanide soluble powder for pigs and poultry
ActiveCN105998036ASusceptible to drug resistanceImprove antibacterial propertiesAntibacterial agentsPowder deliveryBacteroidesSolubility
The invention provides compound sulfa-nitazoxanide soluble powder for pigs and poultry. The soluble powder is prepared from the following components: 20-30g of sulfa-drugs, 4-10.0g of TMP sodium, 1.5-5g of nitazoxanide, 5-25g of a co-solvent and 1-7.5g of a stabilizing agent, and the total amount after the components and auxiliaries are mixed is 100g. The soluble powder can be used for overcoming the defect that sulfa-drugs are invalid to anaerobic bacteria during infection of coccidium mixed with anaerobic bacteria, has excellent effects in preventing and treating protozoon infection and mixed infection of protozoon and bacteria of pigs and poultry, and is valid to drug-resistance coccidium infection which is poor or invalid to sulfa-drugs; nitazoxanide has the defects of water insolubility and alkali solubility, while the co-solvent can be used for improving the water solubility of nitazoxanide, and the stability of nitazoxanide can be improved by adding the stabilizing agent. The compound sulfa-nitazoxanide soluble powder is administrated by drinking, is convenient to use, and has excellent effects on preventing and treating protozoon infection and mixed infection of protozoa and bacteria of pigs and poultry.
Owner:洛阳市兽药厂
A compound preparation for treating large yellow croaker irritating Cryptocaryoniasis
ActiveCN105267237BEfficient killingAvoid secondary infectionAntibacterial agentsInorganic active ingredientsCryptocaryonCoccidiosis
The present invention relates to a compound preparation for treating large yellow croaker irritating cryptocystosis and its application. The compound preparation is composed of 10 wt% to 15 wt% of nitazoxanide and 5 wt% to 7 wt% of copper sulfate , oxytetracycline 25 wt%~30 wt%, dissolving agent 48%~60%, the sum of the above raw materials is 100%. The method of use is: after the diagnosis of cryptocystosis irritant, sprinkle the above compound preparation in an amount of 8~10 g / m3, and soak in the bath continuously for 3 days, which can effectively cure cryptocystosis irritant of large yellow croaker and prevent secondary infection of bacteria . The invention has the advantages of simple preparation method and convenient use, can significantly reduce the death rate of large yellow croaker stimulated by cryptocystosis, and improve the economic benefits of breeding.
Owner:福建省淡水水产研究所
Compound toltrazuril solution and method for preparing same
InactiveCN101548976AGood anti-coccidial effectEasy to usePharmaceutical product form changePharmaceutical delivery mechanismAntigenCoccidiostats
The present invention provides a compound toltrazuril solution. Each 100 ml solution contains medicinal ingredient as follows: toltrazuril 2.5-5 g, nitazoxanide 3-8 g. The compound toltrazuril solution has advantages of low cost, better coccidiostat effect, wide antigen insert, low dose and no-easy drug-tolerance, the compound toltrazuril solution also can be added into drinking water for animal which is more convenient without adding and stirring to feed stuff.
Owner:HENAN SOAR VETERINARY PHARMA
Oil-in-water-type compound nitazoxanid nanoemulsion and preparation method thereof
InactiveCN102526039AImprove stabilityGood stability over timeAntibacterial agentsOrganic non-active ingredientsBiotechnologyProtozoa
The invention discloses an oil-in-water-type compound nitazoxanid nanoemulsion combination, wherein, the particle size of nanoemulsion ranges from 1 to 100 nm, and the oil-in-water-type compound nitazoxanid nanoemulsion combination comprises the following raw materials by mass percent: surface-active agents accounting for 19.0 to 35.0 percent, cosurfactants accounting for 0 to 7.0 percent, nitazoxanid accounting for 0.01 to 0.21 percent, cinnamic aldehyde accounting for 0.1 to 8.0 percent and distilled water that is the rest ingredient; and the sum of the mass percent of the raw materials is 100 percent. The nanoemulsion improves the solvability of nitazoxanid and the medical bioavailability, improves the curative effects of medicines, reduces the medicine dose, has higher activity of resisting protozoa, worms, bacteria, virus and the like, and is used for treating diseases of people or livestocks caused by protozoa, the worms, the bacteria, virus and the like. The oil-in-water-type compound nitazoxanid nanoemulsion combination is safe to use, has the advantages of obvious curative effects, simple preparation method and wide market prospect in medicine field, and is convenient to popularize and apply.
Owner:NORTHWEST A & F UNIV
Nitazoxanide suspension for expelling parasite in livestock body and preparation method thereof
InactiveCN101606905AMeet the needs of parasitic disease prevention and control drugsSimple production processOrganic active ingredientsSolution deliveryHigh volume manufacturingHerd
The invention relates to a nitazoxanide suspension for expelling parasite in livestock body, comprising the following components by weight percent: 3-5 percent of nitazoxanide, 0.2-0.5 percent of xanthan gum, 0.2-0.6 percent of Carbomer, 0.3-0.6 percent of citric acid, 0.15-0.3 percent of trisodium citrate, 0.15-0.3 percent of stabilizing agent, 4-9 percent of flavoring agent and the balance of water. During preparation, water is added in the xanthan gum and Carbomer to prepare a glue solution; the glue solution is placed in an emulsifier, and then, the citric acid, trisodium citrate, stabilizing agent, nitazoxanide and flavoring agent are added for shearing. The invention has simple producing process and stable product quality, can be stored for a long time, needs no preparation in use, can be produced in large quantities, and can provide the herdsmen in pasturing areas and stockmen to prevent and treat parasite for herd and sheep flock.
Owner:QINGDAO VLAND BIOTECH INC +2
Application of nitazoxanide in preparation of medicine for preventing and treating interstitial lung diseases
PendingCN111544431AAdd new usesIncrease varietyOrganic active ingredientsAntiviralsInterstitial lung diseasePharmaceutical drug
The invention belongs to the field of medicines, and particularly relates to application of nitazoxanide in preparation of a medicine for preventing and treating interstitial lung diseases. Accordingto the application of nitazoxanide in preparing the medicine for preventing and treating interstitial lung diseases, further, nitazoxanide is nitazoxanide and an in-vivo active metabolite of nitazoxanide. The interstitial lung diseases comprise disease complications caused by coronavirus infection. According to the invention, detailed research is carried out on the improvement of the interstitiallung disease by the nitazoxanide, the variety of medicines for treating the interstitial lung diseases is expanded, and the new application of nitazoxanide is also expanded.
Owner:REYOUNG PHARMA
Use of nitazoxanide and pharmaceutically acceptable salt thereof in preparation of drug for treating bladder cancers
InactiveCN111973593APrevent proliferationInhibition of clonogenicityOrganic active ingredientsAntineoplastic agentsCancer cellPharmaceutical medicine
The invention discloses use of nitazoxanide or pharmaceutically acceptable salt thereof in preparation of a drug for treating bladder cancers. The nitazoxanide or the pharmaceutically acceptable saltthereof is capable of inhibiting proliferation and clonality of different bladder tumor cell lines, so that the potential of a mitochondrial membrane is decreased, ROS is generated and increased, andapoptosis of bladder cancer cells is caused; meanwhile, the nitazoxanide or the pharmaceutically acceptable salt is capable of effectively inhibiting stemness maintenance of the bladder cancer cells;and the nitazoxanide and the pharmaceutically acceptable salt still have an effect of inhibiting growth of bladder tumors in vivo, appearances of reduction of activities and feeding and the like of nude mice do not appear under a therapeutic dose, and phenomenon of obvious sliming or death of the nude mice do not appear as well, so that the nitazoxanide and the pharmaceutically acceptable salt have better treatment and application prospects to the bladder cancers.
Owner:SHENZHEN LUOHU PEOPLELS HOSPITAL
Screening method of medicament for preventing prostatic cancer and application of nitazoxanide in pharmacy
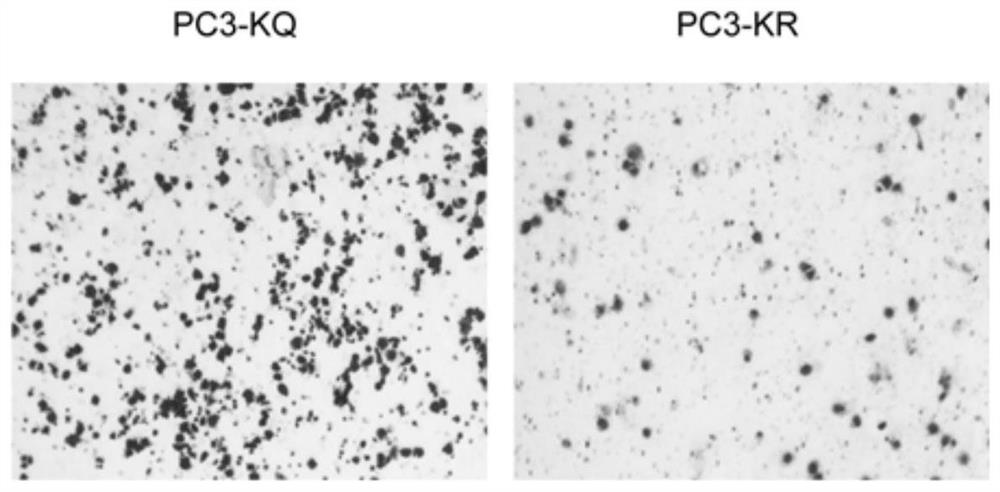
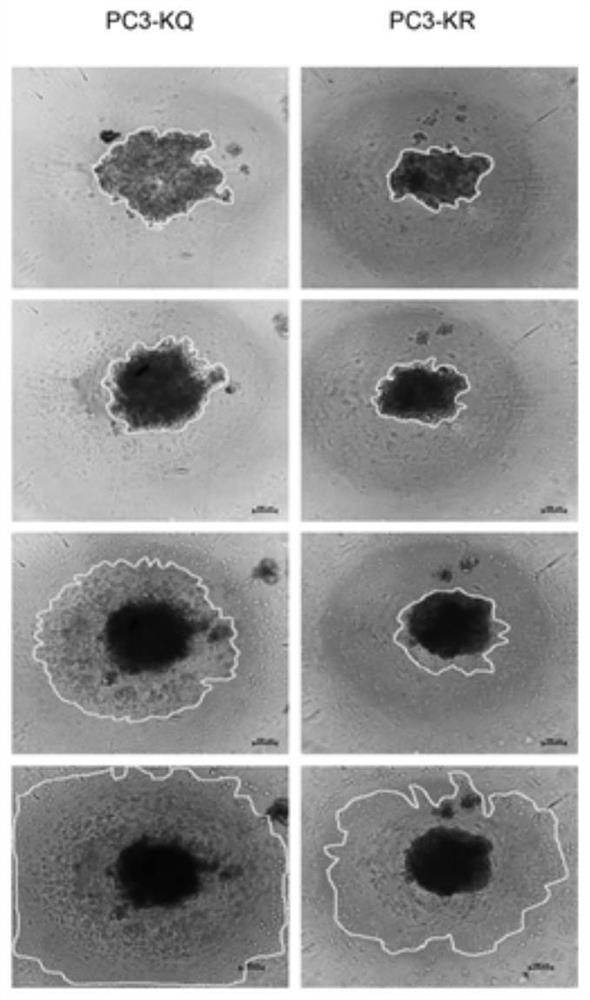
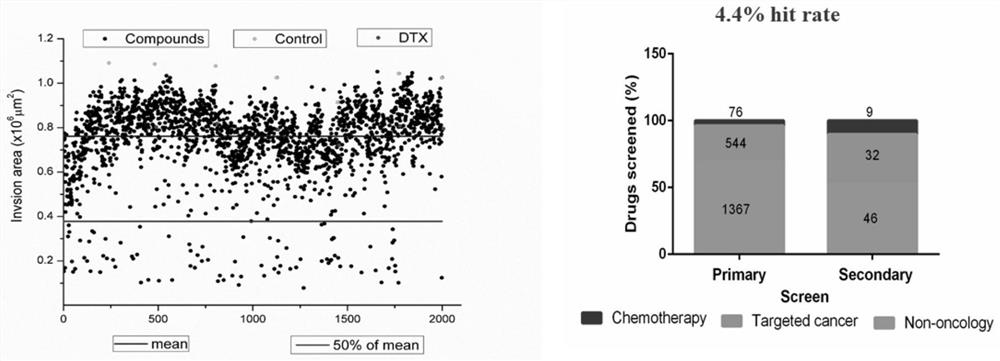
PendingCN114134195ALower acquisition costsSimulate the realOrganic active ingredientsCompound screeningProstate cancer cellCell invasion
The invention discloses a screening method of a medicament for preventing prostate cancer and application of nitazoxanide in pharmacy, and the screening method comprises the following steps: S10, performing 3D cell culture on prostate cancer cells to obtain tumor spheroids; s20, a to-be-screened medicament is prepared into a solution with the concentration of 10 mu M, the solution is used for carrying out a cell invasion experiment on the tumor spheroids, the invasion area of the tumor spheroids is calculated, the medicament corresponding to the tumor spheroids with the invasion area smaller than 50% is screened out, and the to-be-screened medicament is selected from FDA marketing and pharmacopoeia recorded compound libraries; s30, preparing the screened medicaments into a plurality of solutions with different concentration gradients of 0-10 [mu] M, respectively adding the solutions into the tumor spheroids for culture, calculating the invasion areas of the tumor spheroids, and screening seven medicaments with the invasion areas less than 70%; and S40, measuring the IC50 of the seven medicaments, and screening out the medicament with the IC50 value less than 28 mu M, namely the medicament for preventing prostatic cancer. The nitazoxanide is good in safety and obvious in prevention effect.
Owner:SOUTH UNIVERSITY OF SCIENCE AND TECHNOLOGY OF CHINA
Cotton coating type rooting agent as well as preparation method and application thereof
InactiveCN110476995APromote hair root growthTo achieve the effect of strong roots and strong seedlingsBiocidePlant growth regulatorsComposite filmMANNITOL/SORBITOL
The invention discloses a cotton coating type rooting agent as well as a preparation method and an application thereof. The composition is prepared from the following components in percentage by weight: 0.001 to 1 percent of brassinolide compound, 0.003 to 3 percent of nitazoxanide, 0.005 to 5 percent of Brassinogen essence, 0.01 to 1.5 percent of mannitol, 0.1 to 5 percent of antioxidant 1010, 0.5 to 10 percent of oat B-glucan, 5 percent of composite film-forming agent and the balance of water. The cotton seed coating agent is specially used for cotton seed emergence and seedling rooting andgrowth, roots of cotton seeds are continuously stimulated to root under the protection of the coating agent, and therefore the effects of strengthening roots and seedlings are achieved. The seed coating agent is good in film-forming property, uniform in coating, low in shedding rate and very simple and convenient to operate, can continuously act on roots after seedling emergence, can promote rootstrengthening, stable growth and early emergence of seedlings during field direct seeding, and can promote rooting and rooting of the seedlings and shorten seedling recovery during seedling transplantation; therefore, the effects of high efficiency and labor saving are achieved.
Owner:江西鑫邦生化有限公司
Method for preparing nitazoxanide
InactiveCN1935796AReduce dosageReduce washing water consumptionOrganic chemistry2-Amino-5-nitrothiazoleState of art
The invention relates to a nidazolenit preparing method, mainly solving the technical problems of large utilization of tetrahydrofuran and higher cost; and also resolving the technical problem of bad quality of product by selecting organic alkali triethylamine as acid binder. And it comprises the steps of: dissolving 2-amino-5-nitrothiazole in enough nitrogen-containing compound solvent, adding in enough acid binder and blending, and lowering the temperature; adding in ortho-acetyl salicyl chloride and making full condensation reaction; dissolving idazolenit crude in nitrogen-containing compound solvent, and adding in activated carbon to decolor; filtering and adding alcohol in mother solution for alcohol precipitation, filtering and drying.
Owner:HANGZHOU SHINYANG SAMWOO FINE CHEM CO LTD
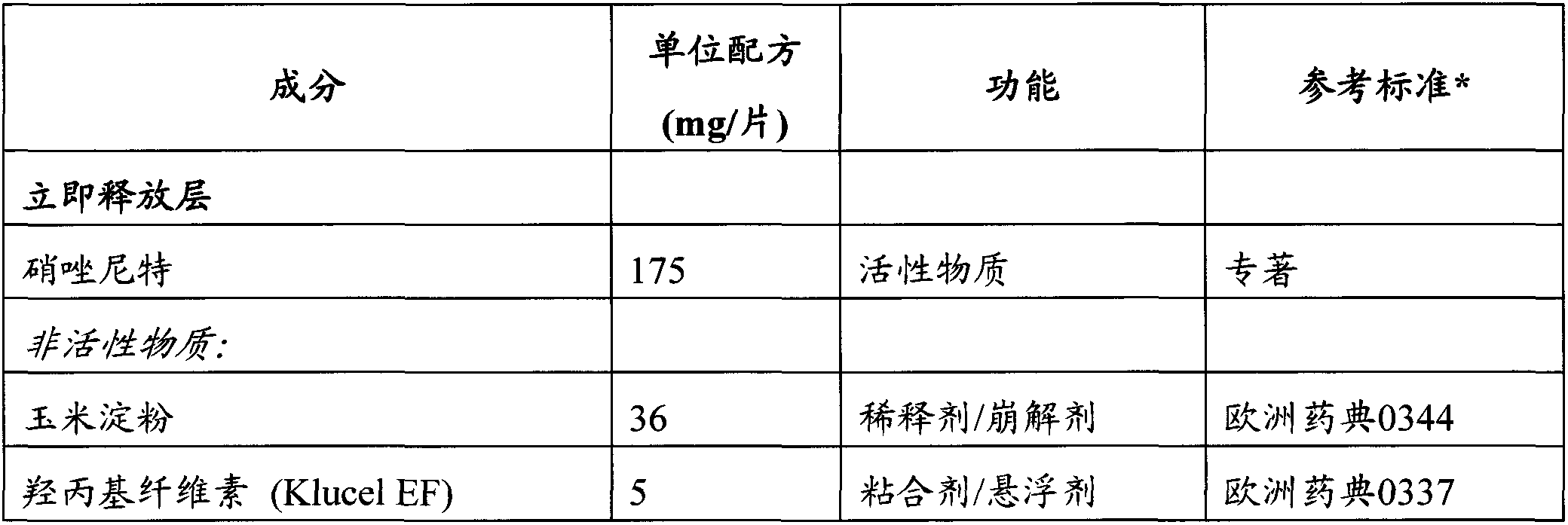
Controlled release pharmaceutical formulations of nitazoxanide
InactiveCN107260695AOrganic active ingredientsPeptide/protein ingredientsPharmaceutical formulationControlled-Release Formulations
Solid dosage formulations of nitazoxanide or a nitazoxanide analogue are provided that comprise a controlled release portion and an immediate release portion. The pharmaceutical composition is typically in the form of a bilayer solid oral dosage form comprising (a) a first layer comprising a first quantity of nitazoxanide or analogue thereof in a controlled release formulation; and (b) a second layer comprising a second quantity of nitazoxanide or analogue thereof in an immediate release formulation. Method of using the formulations in the treatment of hepatitis C are also provided.
Owner:ROMARK LAB L C
Application of nitazoxanide in pharmacy
The invention discloses application of nitazoxanide in pharmacy, and relates to the technical field of biological medicine. Nitazoxanide is a broad-spectrum anti-parasitic drug and a broad-spectrum antiviral drug, and is used for treating various worm, protozoa and virus infections in medicine. The invention provides the new application of the nitazoxanide, the nitazoxanide can be used for treating the prostate diseases, such as prostate cancer bone metastasis, a new drug for treating the prostate diseases is developed, the nitazoxanide is a drug approved by FDA, and the drug safety of the nitazoxanide is verified; the nitazoxanide can be massively obtained from plants and animals, and the obtaining cost is low.
Owner:SOUTH UNIVERSITY OF SCIENCE AND TECHNOLOGY OF CHINA
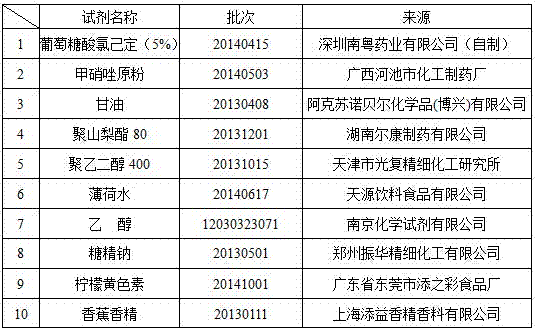
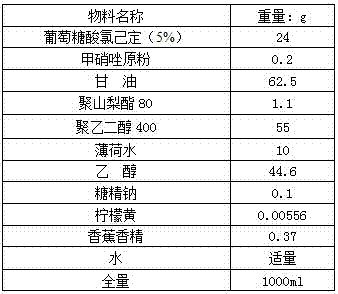
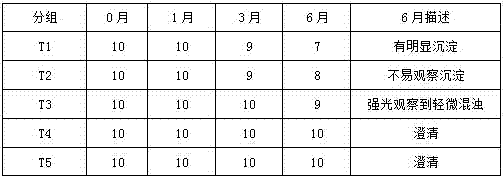
A kind of compound gargle of chlorhexidine gluconate and preparation method thereof
ActiveCN104622870BGood killing effectProlongs local therapeutic actionAntibacterial agentsOrganic active ingredientsOral diseaseDisease
Owner:SHENZHEN SOUTH CHINA PHARMA
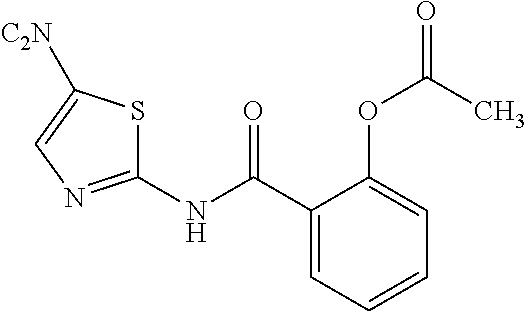
Nitazoxadine composition and process to prepare same
Disclosed is a pharmaceutical nitazoxanide composition comprising: (a) an immediate release fraction comprising nitazoxanide non-coated granules or non-granulated powder, and (b) a pH-dependent release fraction comprising granules of nitazoxanide coated with one or more polymers having a pH-dependent solubility.
Owner:SIEGFRIED RHEIN DE C V
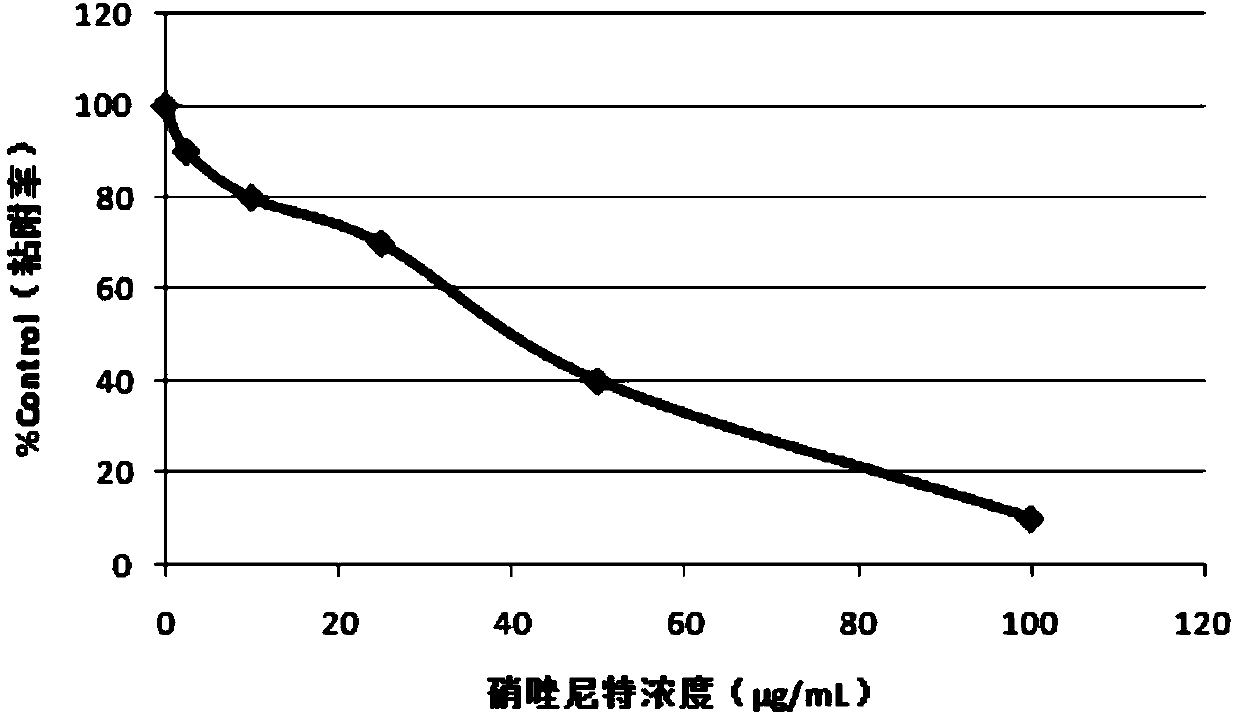
Application of nitazoxanide in preparation of medicines, foods or daily necessities for preventing and treating decayed teeth and disinfecting oral cavity
PendingCN111494373AEffective assistanceEffective controlAntibacterial agentsCosmetic preparationsBiotechnologyFood science
The invention relates to an application of nitazoxanide in preparation of medicines, foods or daily necessities for preventing and treating decayed teeth and disinfecting oral cavities, and belongs tothe technical field of new application of medicines. The invention discloses the application of nitazoxanide in preparing medicines, foods or daily necessities for preventing and treating decayed teeth. The nitazoxanide is streptococcus mutans. The invention also discloses an application of nitazoxanide in preparation of medicines, foods or daily necessities for oral disinfection. The types of the viruses inhibited by the nitazoxanide are coronaviruses including novel coronaviruses, influenza viruses, hepatitis viruses and HIV viruses. According to the invention, detailed research on inhibition of streptococcus mutans by nitazoxanide is carried out, the types of decayed tooth prevention and treatment and oral cavity disinfection are expanded, and the new application of known medicines isalso expanded.
Owner:REYOUNG PHARMA
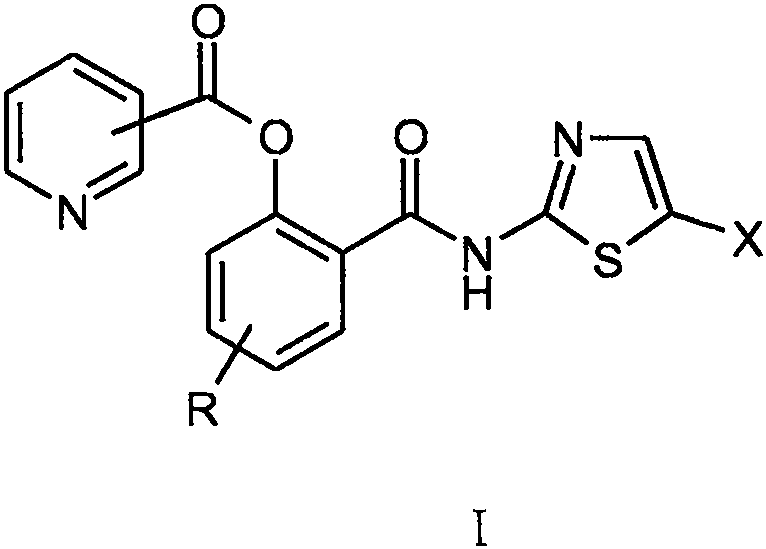
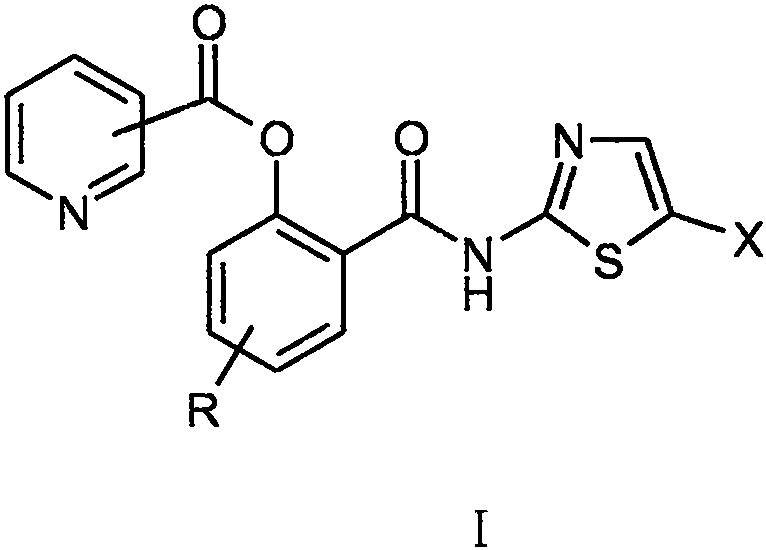
Nitazoxanide derivative and medical application thereof
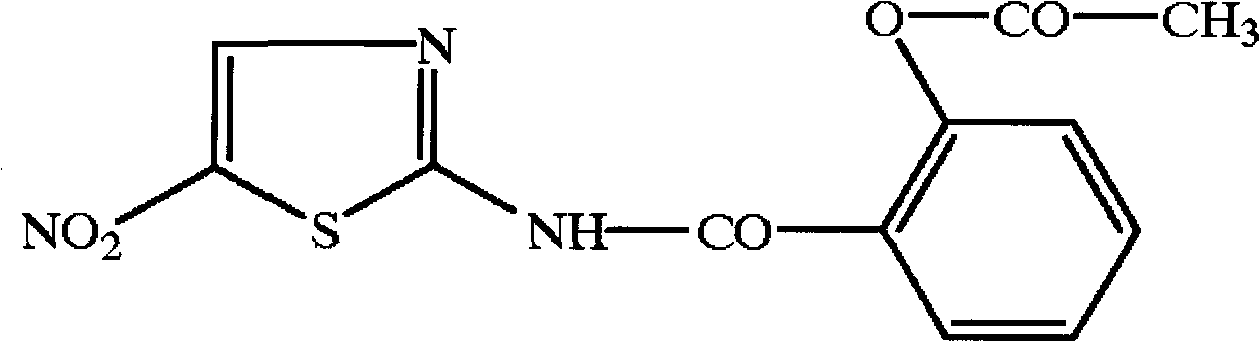
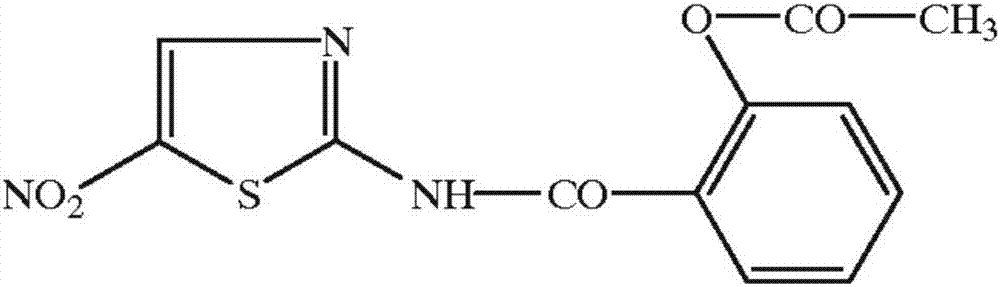
The invention relates to a novel nitazoxanide derivative represented by a formula I, wherein in the formula I, R is H, CH3, Cl or F and is connected to any position of a benzene ring, and X is NO2, Clor Br.
Owner:BEIJING MEIBEITA DRUG RES
A kind of compound sulfanitazoxanide soluble powder for pigs and poultry
ActiveCN105998036BSusceptible to drug resistanceImprove antibacterial propertiesAntibacterial agentsPowder deliveryProtozoaSolubility
Owner:洛阳市兽药厂
Application of nitazoxanide in preparing drug for resisting eimeria coccidium
The invention provides a new medicinal application of nitazoxanide and particularly discloses an application of nitazoxanide in preparing a drug for resisting eimeria coccidium. The application can serve for the prevention and treatment of coccidiosis of animals such as chickens and rabbits, and the like.
Owner:JILIN UNIV
Method for detecting genotoxic impurities of 2-amino-5-nitrothiazole in nitazoxanide
PendingCN112986449AImprove separation efficiencyHigh sensitivityComponent separationThiamazolumO-Phosphoric Acid
The invention relates to the technical field of analysis and detection, in particular to a method for detecting genotoxic impurities of 2-amino-5-nitrothiazole in nitazoxanide. According to the method for detecting the genotoxic impurities of the 2-amino-5-nitrothiazole in the nitazoxanide, detection is carried out through a high performance liquid chromatography, 2-amino-5-nitrothiazole is used as a reference substance, acetonitrile is used as a diluent, and the content of the 2-amino-5-nitrothiazole in a sample is calculated through an external standard method, wherein a chromatographic column adopts octadecyl bonded silica gel as a filling agent, and a mobile phase adopts a mixed solution of a phosphoric acid aqueous solution and acetonitrile. According to the method for detecting the genotoxic impurities of the 2-amino-5-nitrothiazole in the nitazoxanide, the blank that the genotoxic impurities are analyzed through a high performance liquid chromatography method is filled, and compared with a traditional liquid chromatography method, the method is easy to operate, convenient, efficient, wide in application range and higher in sensitivity which can reach 0.6 ppm, and guarantees the safety of drugs.
Owner:REYOUNG PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com