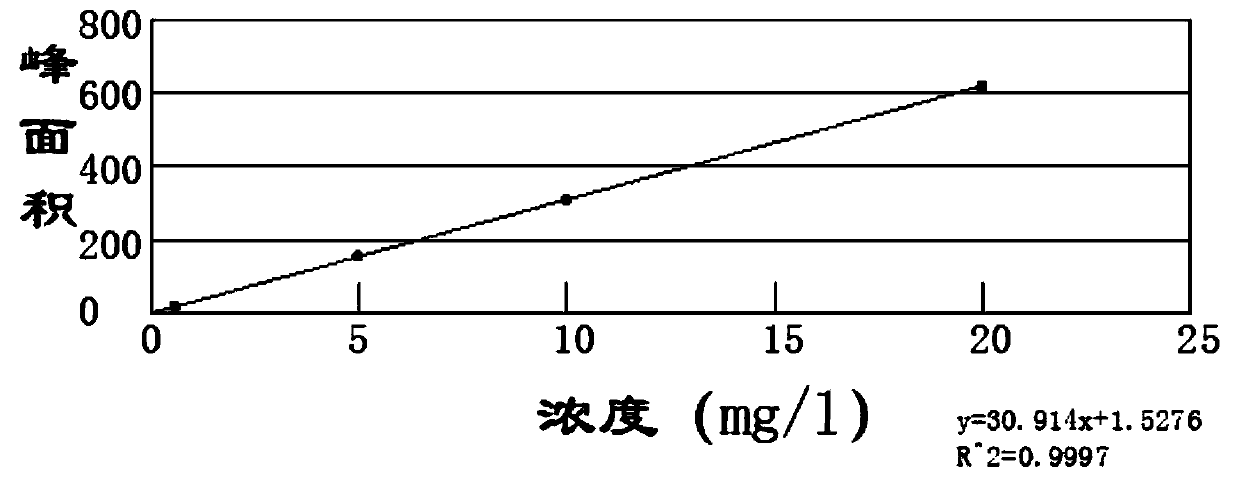
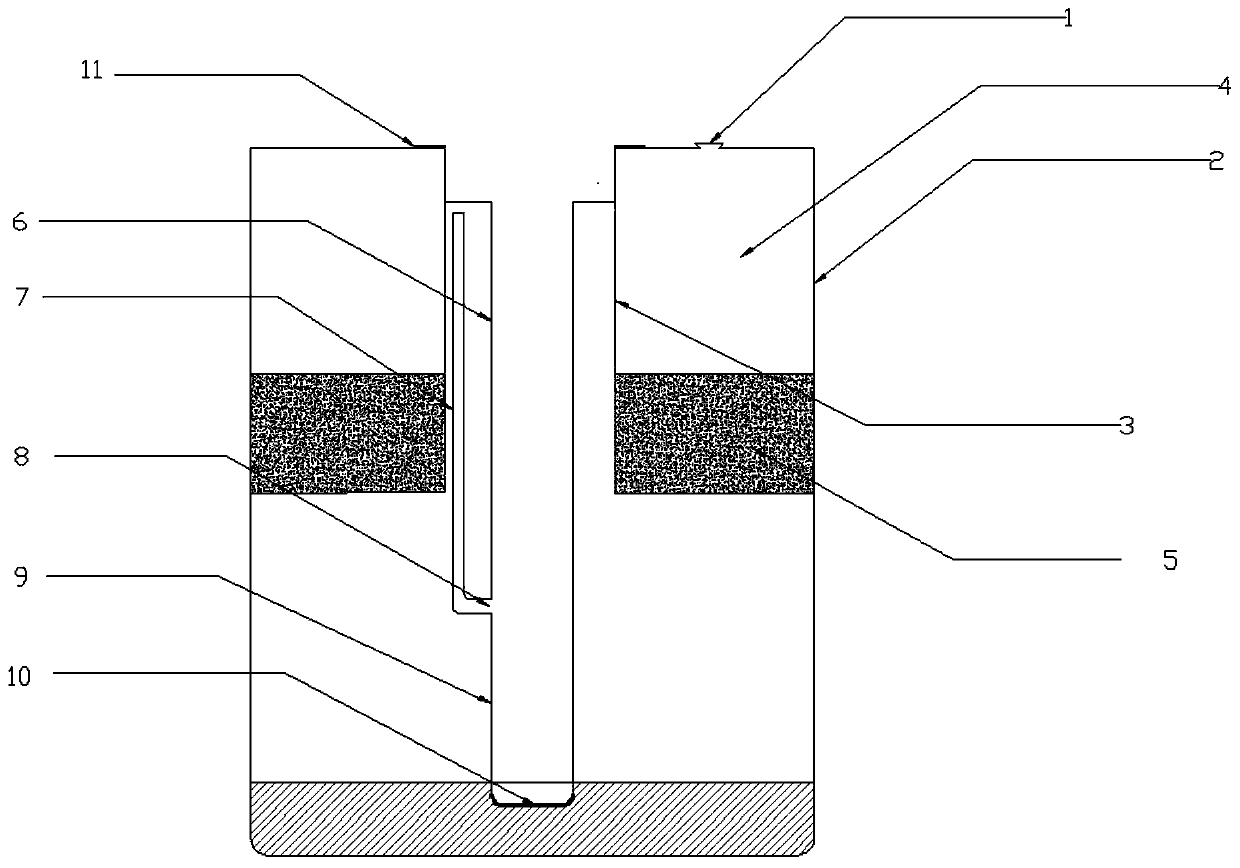
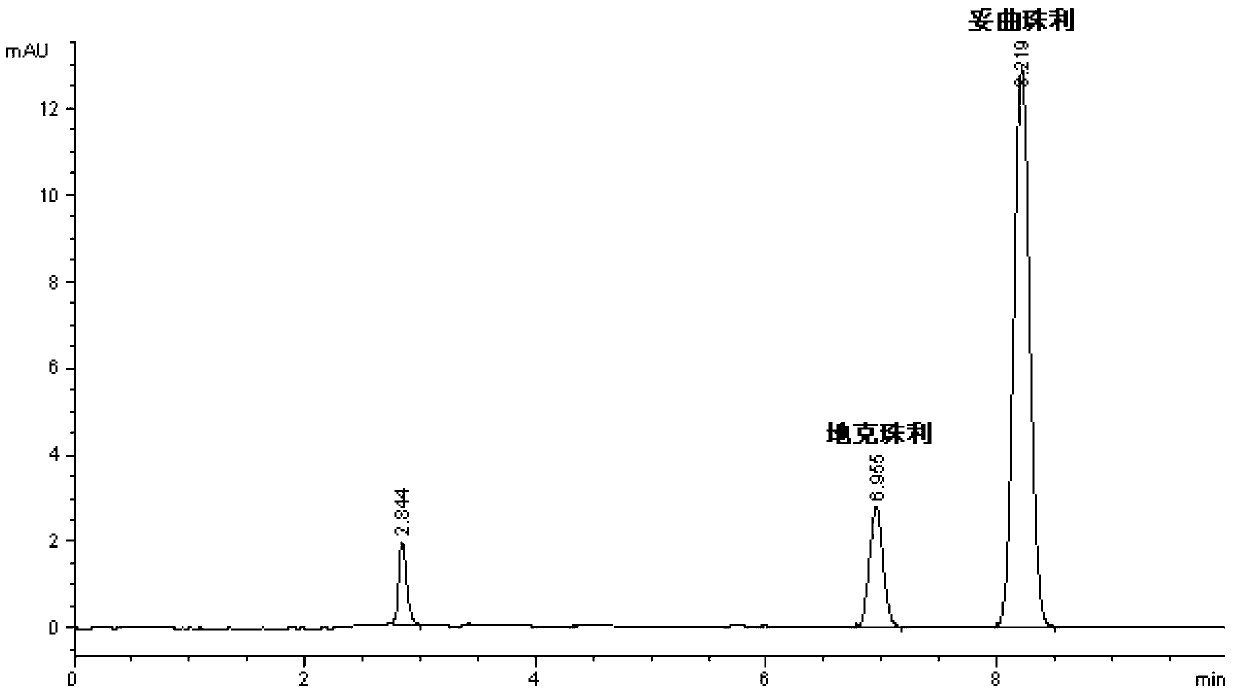
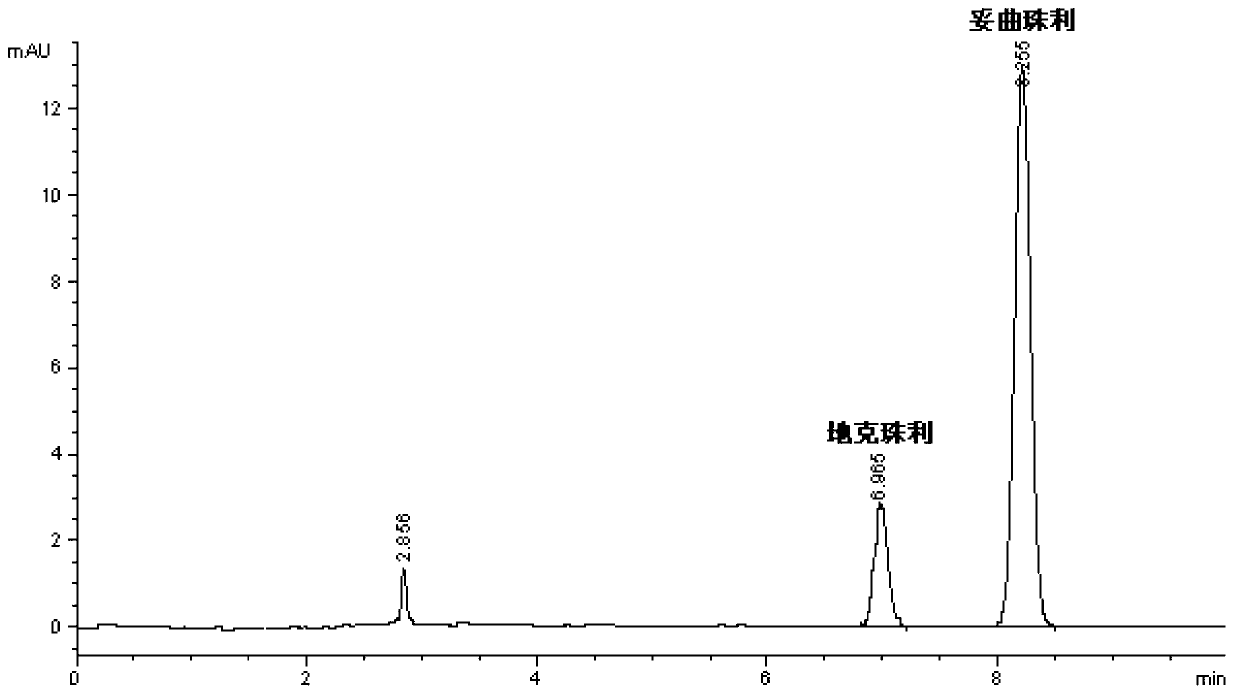
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
62 results about "Toltrazuril" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Toltrazuril is a coccidiostat.
Method of producing toltrazuril
A preparation method of the Toltrazuril is provided. The Toltrazuril is the 1-[3-methyl-4-(4- Trifluoromethylthiobenzoxy) benzyl]-3-methyl-1, 3, 5, -triazine-2, 4, 6(1H, 3H, 5H)-trione. The methyl sulfide chloro compounds, fluoro-compounds, amides, phenol, methyl nitrate chlorobenzene, benzene aether, ammonia benzene aether, isonitrile acid ester and methyl urea from the reactions of 4-nitrochlorobenzene, sulfur, sodium sulfide and dimethyl sulfate. The detailed preparation is that reaction 1: the methanol of 410g and 4-nitrochlorobenzene of 158g are mixed and heated until soluble and are added with sulfur, sodium sulfide and methanol mixed liquor by drops, and kept for 2h under temperature of 60 DEG C. to 65DEG C.; reduce the temperature, add water of 880g and add dimethyl sulfate of 192g; during the period, the pH value of the sodium hydroxide is adjusted over 9, and the methyl sulfide of 153.6 is gained by filtering after reaction with yield of 91.1 per cent.
Owner:PU LIKE BIO ENG +1
Preparation method of toltrazuril-cyclodextrin inclusion compound
InactiveCN101518652AImprove solubilityQuality improvementPharmaceutical non-active ingredientsAntiparasitic agentsHigh volume manufacturingOrganic solvent
The invention relates to a preparation method of a toltrazuril-cyclodextrin inclusion compound. The method comprises the following steps: the toltrazuril is dissolved in the dilute alkaline solution and performs inclusion with cyclodextrin or the derivative thereof at the temperature of 20 to 80 DEG C, the entire system is neutralized with acid, the resultant is dried and crushed and then the toltrazuril-cyclodextrin inclusion compound is obtained. The invention does not utilize the organic solvent for dissolving and has the advantages of simple process, easy control, low cost and mass production; and the prepared inclusion compound has high security, good stability and good dissolvability and is convenient to use.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Preparation method of freeze-dried powder sample of toltrazuril in chicken meat and toltrazuril sulphone residue
InactiveCN104155163AEasy to transportEasy to usePreparing sample for investigationVitamin CFreeze-drying
The invention belongs to the technical field of a standard sample of an animal-sourced substrate drug in detection of veterinary drug residue and discloses a preparation method of a freeze-dried powder sample of toltrazuril in chicken meat and a toltrazuril sulphone residue. The method includes following steps: feeding chicken with the toltrazuril; enabling the toltrazuril and a metabolite toltrazuril sulphone to remain in chicken after toltrazuril sulphone with time controlling; killing the chicken to obtain breast muscle; performing a skin removing and blood draining process; adding a vitamin C solution; performing a homogenizing process to obtain meat paste; performing a vacuum freeze-drying process and a crushing and sieving process; performing a nitrogen-filling packaging process in an anaerobic work station; performing a vacuum packaging process to a polyethylene bottle with an aluminium foil bag after a sealing process being carried out to obtain a standard sample of the freeze-dried powder. The sample can be transported at normal temperature and is long in preserving time. A situation of combination of a target object and the substrate is coincided with that of an actual detection sample. The sample can be used of quality controlling of relative detection and analysis, is homogeneous and stable, is convenient to transport, is reliable in use and has significant economic value and market competitiveness.
Owner:威海出入境检验检疫局检验检疫技术中心 +1
Toltrazuril injection and preparation method thereof
InactiveCN102743334AReduce systemic toxicityLittle systemic toxicityPharmaceutical delivery mechanismAntiparasitic agentsDiseaseTreatment effect
Owner:ZHENGZHOU HOUYI PHARMA
Anti-coccidium microemulsion containing toltrazuril and preparation method thereof
ActiveCN101336901ALow costNo precipitationAntiparasitic agentsEmulsion deliveryOrganic solventOil phase
The invention provides an anti-coccidial microemulsion, which contains toltrazuril, an oil phase, a surfactant, a cosurfactant and water. The invention also provides a method for preparing the anti-coccidial microemulsion, which comprises the steps of dissolving toltrazuril in a small quantity of oil phase, adding a certain amount of surfactant and cosurfactant, emulsifying with a certain method to make into nanometer oil-in-water type microemulsion. The inventive anti-coccidial microemulsion remarkably reduces the cost of pharmaceutical preparations, reduces the use of organic solvents, and reduces environmental pollution. The microemulsion can be added into the drinking water for livestock and poultry may with uniform dispersion and without drug separation out, resulting in uniform ingestion of the livestock and poultry and no toxic action.
Owner:长沙伟嘉饲料有限公司 +1
High-content toltrazuril soluble powder, as well as preparation method and application thereof
InactiveCN103142487AHigh drug contentImprove bioavailabilityPowder deliveryAntiparasitic agentsSolubilityFreeze-drying
The invention provides a high-content toltrazuril soluble powder, as well as a preparation method and application of the high-content toltrazuril soluble powder, relates to the field of the soluble powders, preparation methods and application of the soluble powders, and aims at solving the problems of the existing preparation method that the toltrazuril preparation is low in water solubility, low in bioavailability, poor in stability, and easy to generate precipitate and discolor so that the treatment effects and application of the toltrazuril preparation are greatly limited. The high-content toltrazuril soluble powder is prepared from toltrazuril, an alkaline pH (Potential of Hydrogen) regulator, a cosolvent and a soluble filler in parts by weight. The preparation method comprises the following steps of: 1, weighing; 2, mixing and preparing solution; and 3 preparing the powder by the spray drying process. The application is the application of the high-content toltrazuril soluble powder serving as the raw material in tablet preparations, granular preparations and freeze-dried powder preparations. The preparation method is simple in preparation process; the prepared powder contains the toltrazuril up to 60% and is quick to dissolve; and the preparations can be placed for three years at the room temperature without packing and discoloring. The high-content toltrazuril soluble powder is applicable to the field of veterinary drug preparations.
Owner:HEILONGJIANG UNIV
Novel compound anti-coccidiosis medicament and preparation method thereof
InactiveCN101933930ALower doseReduce dosageAntiparasitic agentsHeterocyclic compound active ingredientsDrug synergismCurative effect
The invention discloses a medicament for treating coccidiosis, particularly a novel compound anti-coccidiosis medicament and a preparation method thereof. The novel compound anti-coccidiosis medicament is characterized by being prepared from the following components: decoquinate, toltrazuril, polymer carrier, solubilizing agent and anhydrous dextrose. Therefore, the medicament has the advantages that: the indissoluble decoquinate and toltrazuril are prepared into water-soluble substances by using solid dispersion technology and inclusion technology, and the decoquinate and toltrazuril are mixed to fulfill the aim of reducing dosage, improving curative effect and reducing drug resistance; moreover, the synergistic effect of the medicaments can be realized by combining the decoquinate and the toltrazuril, the killing effect is enhanced and the dosage of each medicament is reduced by half.
Owner:山东步步赢生物科技有限公司
Preparation method of toltrazuril soluble powder
InactiveCN101773474AImprove solubilityEasy to usePowder deliveryPharmaceutical non-active ingredientsSolubilitySocial benefits
Owner:HUBEI LONGXIANG PHARMA TECH CO LTD
Synthesis technique for toltrazuril
ActiveCN101265236ASimple and fast operationReduce pollutionOrganic chemistryAntiparasitic agentsHydrogenMedicinal chemistry
The invention relates to the field of chemically synthesized drug, in particular to a new synthetic process of drug for preventing and curing poultry coccidiosis, i.e., toltrazuirl. The synthetic process includes allowing trifluoromethylthiophenol and 2-chloro-5-nitrotoluene as raw material to condense, performing the reduction reaction with hydrogen in presence of Pd / C catalyst, reacting with bis (trichloromethyl) carbonate for esterifying isocyanic acid, and finally adding diethyl carbonate and methylurea for synthesizing toltrazuirl. The new synthetic process of toltrazuirl has easy operation, simple required equipment, low pollution, easily the prior raw material, low cost, and high yield with a total one of up to about 50%.
Owner:HUBEI LONGXIANG PHARMA TECH CO LTD
High-efficiency water-solubility toltrazuril solution and preparation method thereof
InactiveCN101766564AImprove solubilityImprove stabilityPharmaceutical delivery mechanismAntiparasitic agentsSolubilityAntistatic agent
The invention relates to a high-efficiency water-solubility toltrazuril solution which comprises the following components in percentage by weight: 2-6 percent of toltrazuril, 2-10 percent of inclusion agent, 1 percent of antistatic agent, 7-17 percent of composite emulsifying agent, 1-3 percent of pH regulator, 1 percent of antioxidant, 35-70 percent of composite organic solvent and the balance of purified water. In the invention, an advanced microemulsion preparation process is adopted, and the water insoluble toltrazuril is prepared into a water-insoluble microemulsion solution which can be mixed with water in any proportion, therefore, the dilution and precipitation problems are effectively solved, the bioavailability of the product is improved, and the medicine consumption is reduced; and compared with the prior art, the invention reduces the consumption of the organic solvent and the harmful pharmacological action, saves the cost and improves the security.
Owner:QINGDAO ZHONGREN PHARMA
Toltrazuril suspension emulsion and preparation method thereof
InactiveCN102512366ASimple manufacturing methodReduce manufacturing costAntiparasitic agentsEmulsion deliveryVegetable oilOil phase
The invention discloses a toltrazuril suspension emulsion, which comprises the following components by weight part: 1-5 parts of toltrazuril, 20-40 parts of vegetable oil, 5-20 parts of emulsions and 74-45 parts of water. The preparation method of the toltrazuril suspension emulsion comprises the following steps of: (1) taking the toltrazuril and the vegetable oil according to the proportion, grinding the toltrazuril and the vegetable oil through a colloid mill to prepare an oil phase; (2) weighing the emulsions, adding a little bit of water into the emulsions for stirring and dissolving to prepare a water phase; (3) adding the water phase into the oil phase, grinding through the colloid mill, and uniformly mixing; and (4) adding the residual water and stirring to obtain the toltrazuril suspension emulsion. The toltrazuril suspension emulsion has the beneficial effects of good stability and good biocompatibility, the domestic technical blank is filled, and a novel technical field is opened for application of toltrazuril. The preparation method of the toltrazuril suspension emulsion is simple and easy for operation, and the manufacturing cost is low.
Owner:GUANGZHOU HESHENGTANG ANIMAL PHARMA
Toltrazuril nano-suspension and preparation method thereof
InactiveCN102973502AIncrease profitExtended staySolution deliveryAntiparasitic agentsOrganic solventMedicine
The present invention belongs to the field of veterinary medicine preparations, and particularly relates to a toltrazuril nano-suspension and a preparation method thereof. The nano-suspension is prepared by micronizing the main drug toltrazuril and accessories. The massic volume percentage content of toltrazuril in the nano-suspension is 0.01-5.00%. The accessories include by volume: 10-70% of an organic solvent, 0.01-20.0% of a surfactant, 0.1-5.0% of a suspending agent, 0.1-2.5% of an antioxidant, and the balance being water. The toltrazuril nano-suspension of the present invention is convenient for administration, high in medicine utilization, and accurate in dosage of administration, helps to improve the bioavailability of the medicine, and is simple in production process and suitable for industrial production.
Owner:HENAN SOAR VETERINARY PHARMA
Compound anti-parasitic preparation and preparation method thereof
InactiveCN107693532ASuitable adhesionMobility is suitablePill deliveryPharmaceutical non-active ingredientsAdhesiveAnti parasitic
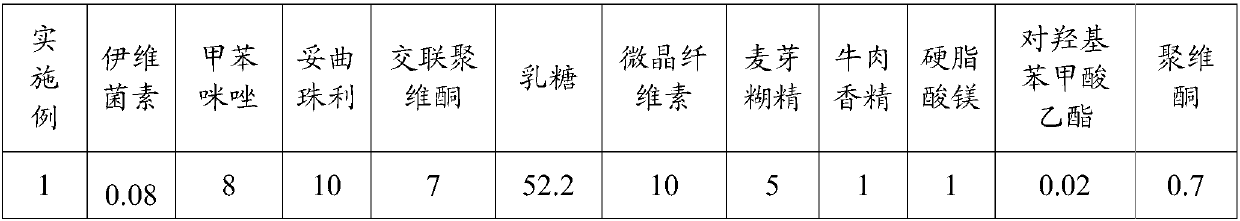
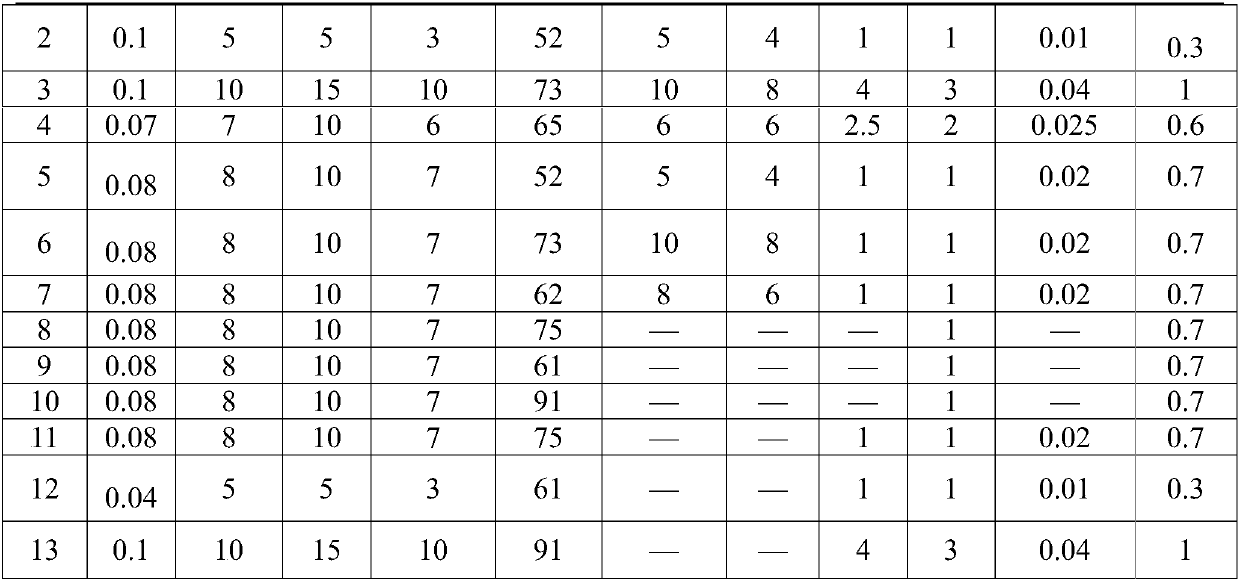
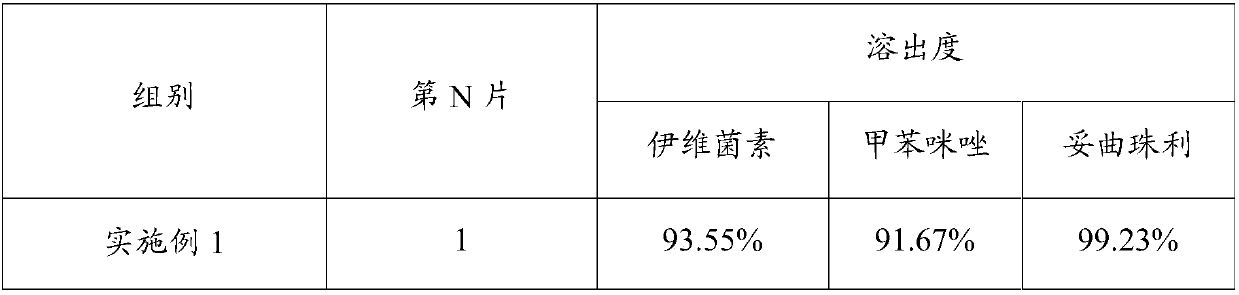
The invention relates to a compound anti-parasitic preparation, and a preparation method and application thereof. The compound anti-parasitic preparation is mainly prepared from the following raw materials in parts by weight: 0.04 to 0.1 part of ivermectin, 5 to 10 parts of mebendazole, 5 to 15 parts of toltrazuril, 3 to 10 parts of a disintegrating agent, 61 to 91 parts of a filling agent, 1 to 3parts of a lubricating agent and 0.3 to 1 part of an adhesive. The compound anti-parasitic preparation has high uniformity and has a good anti-parasitic effect.
Owner:FOSHAN NANHAI EASTERN ALONG PHARMA CO LTD
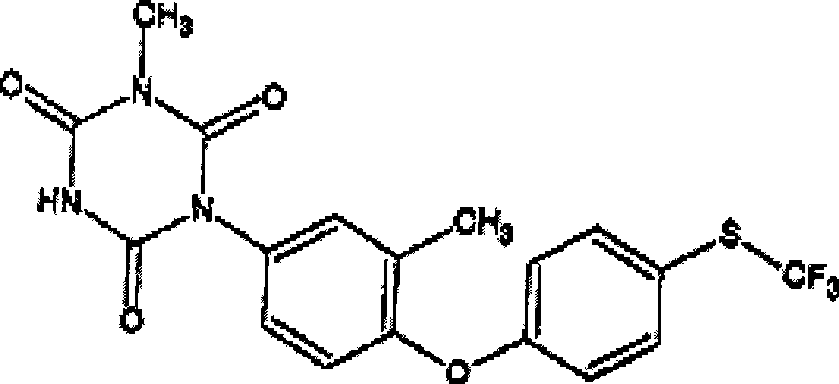
Preparation method for 1-methyl-5-[3-methyl-4-(4-trifluoromethylthio-phenoxy)-phenyl]-biuret, and application thereof
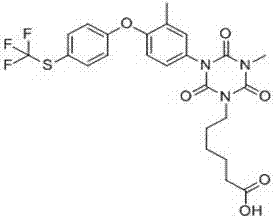
The present invention mainly discloses a preparation method for 1-methyl-5-[3-methyl-4-(4-trifluoromethylthio-phenoxy)-phenyl]-biuret, and an application thereof. According to the present invention, 3-methyl-4-(4-trifluoromethylthio-phenoxy)-aniline reacts with a cyanate in the presence of an acid to obtain N-[3-methyl-4-(4-trifluoromethylthio-phenoxy)-phenyl]-urea; the N-[3-methyl-4-(4-trifluoromethylthio-phenoxy)-phenyl]-urea reacts with methylaminoformyl chloride in a solvent to obtain 1-methyl-5-[3-methyl-4-(4-trifluoromethylthio-phenoxy)-phenyl]-biuret; the 1-methyl-5-[3-methyl-4-(4-trifluoromethylthio-phenoxy)-phenyl]-biuret and diethyl carbonate are subjected to condensation under an alkaline condition to obtain an anti-coccidian drug toltrazuril. The method of the present invention has characteristics of simple process, mild conditions, high total conversion rate, good product quality, cheap and available raw materials, and green environmental protection, and is a competitive industrialization synthesis route.
Owner:ZHEJIANG GUOBANG PHARMA
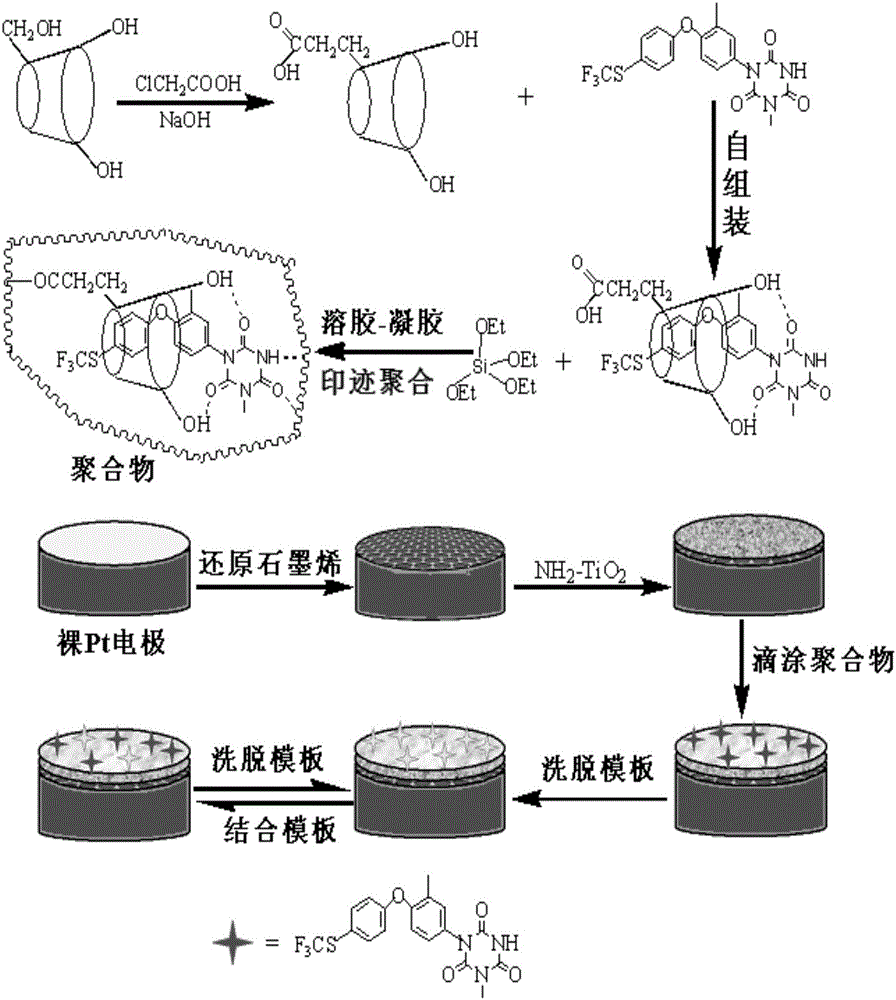
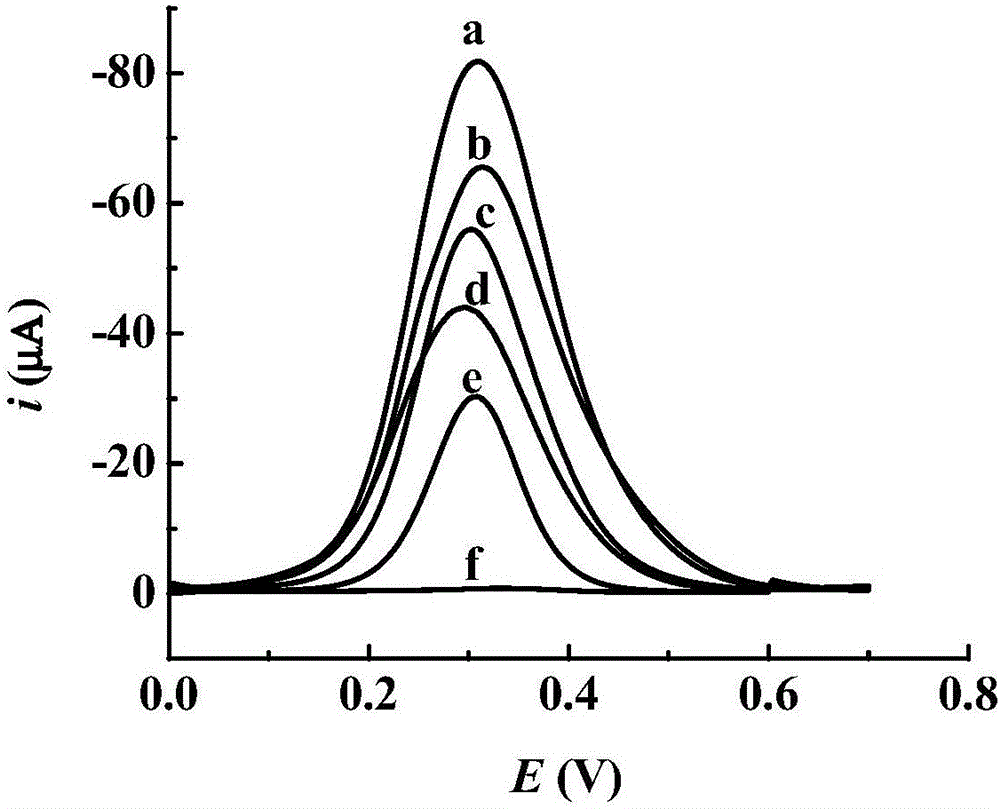
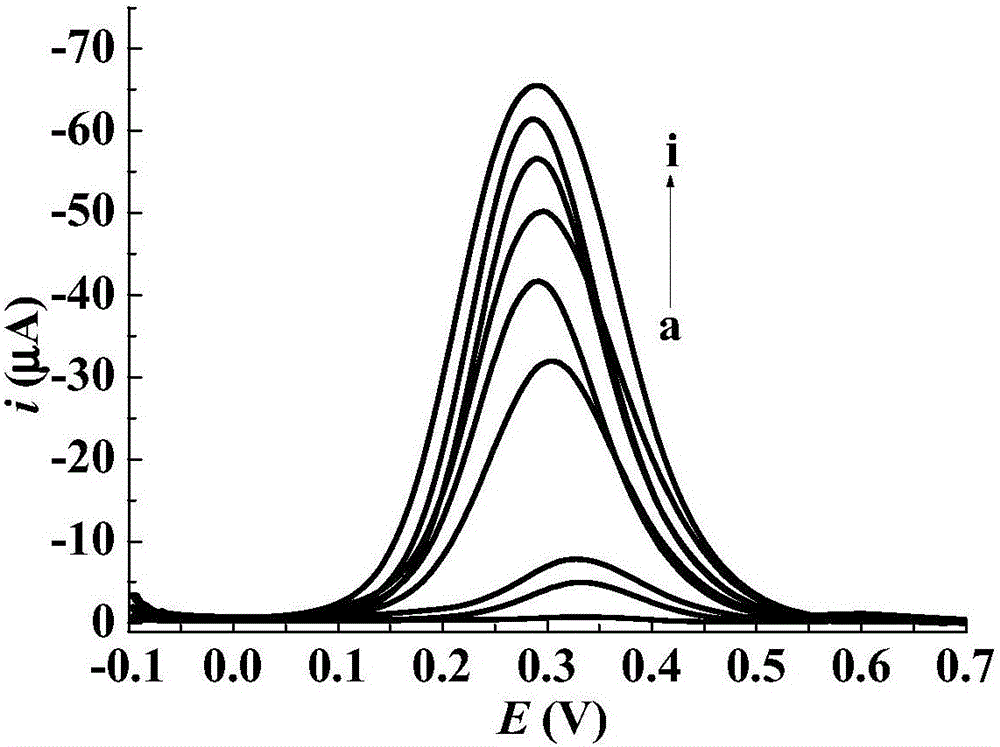
Preparation method of toltrazuril molecular imprinting electrochemical sensor
InactiveCN106226369AImprove stabilityGood compatibilityMaterial electrochemical variablesWater bathsFunctional monomer
The invention relates to a preparation method of a toltrazuril molecular imprinting electrochemical sensor. The preparation method comprises the following steps: taking toltrazuril as the template molecule, carboxylated beta-cyclodextrin as the functional monomer, and tetraethyl ortho-silicate as the crosslinking agent, carrying out reactions under an acidic condition to obtain a toltrazuril sol imprinted polymer; dropwise painting a reduced graphene dispersion liquid on a platinum electrode (phi 2mm), drying the platinum electrode under an infrared lamp; then dropwise painting an aminated titanium dioxide dispersion liquid on the platinum electrode, drying the platinum electrode under an infrared lamp; then dropwise painting the toltrazuril sol imprinted polymer on the platinum electrode, drying the platinum electrode at a room temperature; placing the platinum electrode in a HCl solution, and washing off the template molecules in a thermostatic water bath to obtain a toltrazuril molecular imprinting electrochemical sensor. The operation is simple, the coating is firm, the template molecules can be easily washed out, and the prepared molecular printing electrochemical sensor has the advantages of stability, good repeatability, high sensitivity, and good selectivity, and can be used to detect toltrazuril in food and feed.
Owner:GUANGDONG FOOD & DRUG VOCATIONAL COLLEGE
Specific medicine used for treating coccidiosis and preparation method of specific medicine
InactiveCN104997784AUse low concentrationDoes not affect immunityPharmaceutical non-active ingredientsAntiparasitic agentsSolubilityDrug withdrawal
The invention discloses a specific medicine used for treating coccidiosis and a preparation method of the specific medicine. The specific medicine is mainly prepared from diclazuril, decoquinate and toltrazuril. Every 100 ml of the medicament comprises, by weight, 0.45-0.55 g of diclazuril, 3.6-4.4 g of decoquinate and 1.8-2.2 g of toltrazuril. The preparation method comprises the fours steps of aqueous preparation, oil phase preparation, emulsion and dissolution promotion. The pecific medicine is mainly prepared from diclazuril, decoquinate and toltrazuril. The effect of treating the coccidiosis is improved through the synergistic effect of diclazuril, decoquinate and toltrazuril, and the drug resistance is lowered. Formed double solvents can effectively improve the solubility of the main medicine components in water, and main medicine can be stable in the preparation and is easily dissolved in water. Meanwhile, the special medicine has the advantages of being capable of taking effects fast, short in drug withdrawal period, high in death rate control and reduction, high in drug bioavailability and the like.
Owner:FUJIAN BRADY PHARMA CO LTD
Toltrazuril suspension and preparation method thereof
ActiveCN102198099ADosage is accurateUniform dosagePowder deliverySolution deliveryOrganic solventMedicine
The invention discloses toltrazuril suspension, which belongs to the field of veterinary medicines and comprises the following components in percentage by mass: 2.5 to 15 percent of toltrazuril, 15.0 to 30.0 percent of suspending aid, and 65.0 to 82.5 percent of auxiliary materials. The invention also discloses the preparation method of the suspension. The suspension is administrated by mixing with drinking water, the use is convenient, the suspension is suitable for mass administration, and the labor force and materials are saved; the dosage is accurate, the toltrazuril disperses uniformly in water, and the concentration of the medicine is uniform; the absorption of the toltrazuril is desirable, the effectiveness is quick, and the bioavailability is high; in addition, compared with liquid preparation, the toltrazuril suspension is very stable and maintains a dry state constantly in a process from production to storage, and the package is sealed to prevent the suspension from contact with moisture and air. The suspension does not contain organic solvent, so the possibility of adverse pharmacological effect that may be produced by the organic solvent is lowered and the safety is high.
Owner:江苏基宇生物医药科技有限公司
Preparation method and application of triazine anticoccidial medicinal composite molecularly imprinted polymer
Owner:ANHUI AGRICULTURAL UNIVERSITY
Suspension containing toltrazuril and diclazuril and preparation method of suspension
The invention belongs to the technical fields of veterinary antiparasitic agents and preparation methods of the veterinary antiparasitic agents, and particularly relates to a water suspension oral liquid containing toltrazuril and diclazuril and a preparation method of the water suspension oral liquid. The preparation comprises the following components: (a) toltrazuril and diclazuril crude drugs, (b) a suspending agent, (c) a wetting agent, (d) a preservative, and (e) a preservative. The water suspension oral liquid containing toltrazuril and diclazuril disclosed by the invention is prepared by adopting a dispersion method, and is a water suspension with an off-white color and slight viscosity. The suspension oral liquid containing toltrazuril and diclazuril developed according to the invention is good in stability, good in palatability, long in elimination half life and high in bioavailability, and can be used as a veterinary oral preparation.
Owner:QINGDAO VLAND BIOTECH INC
Toltrazuril inclusion compound lyophilized powder and preparation method thereof
InactiveCN108653217AMentioned stabilityBioavailabilityPowder deliveryPharmaceutical non-active ingredientsSolubilityEvaporation
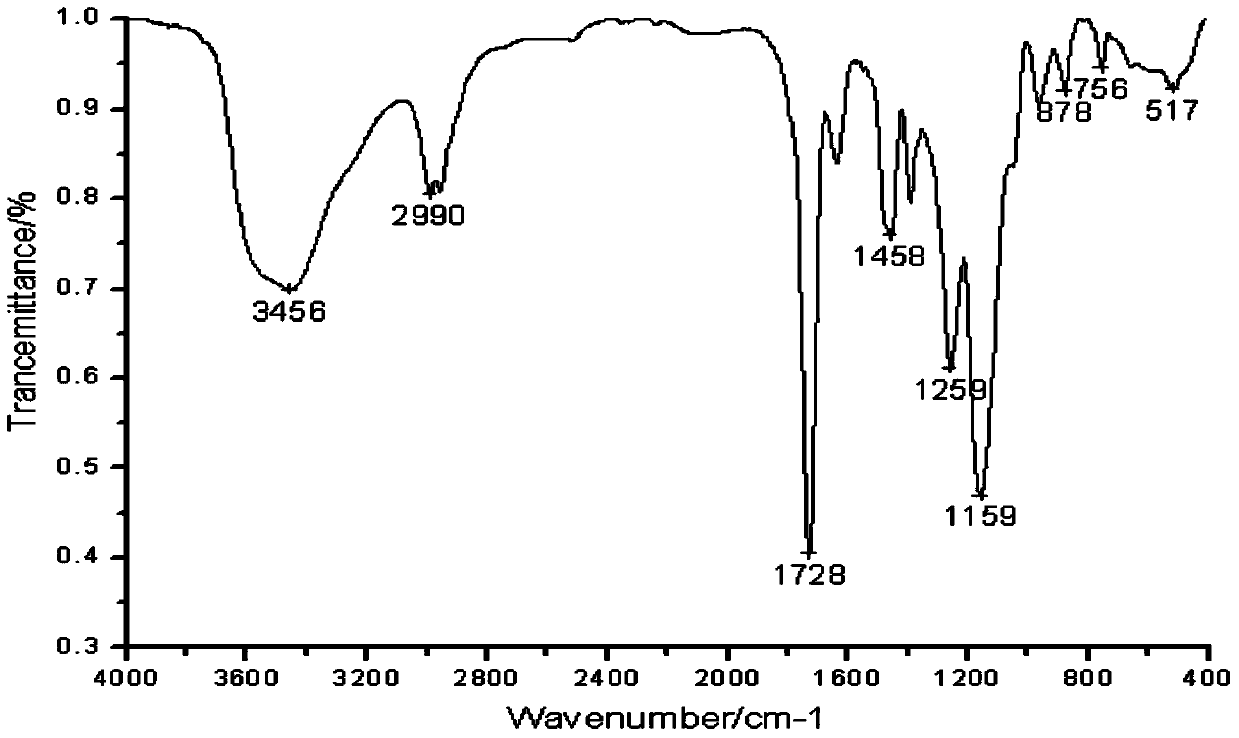
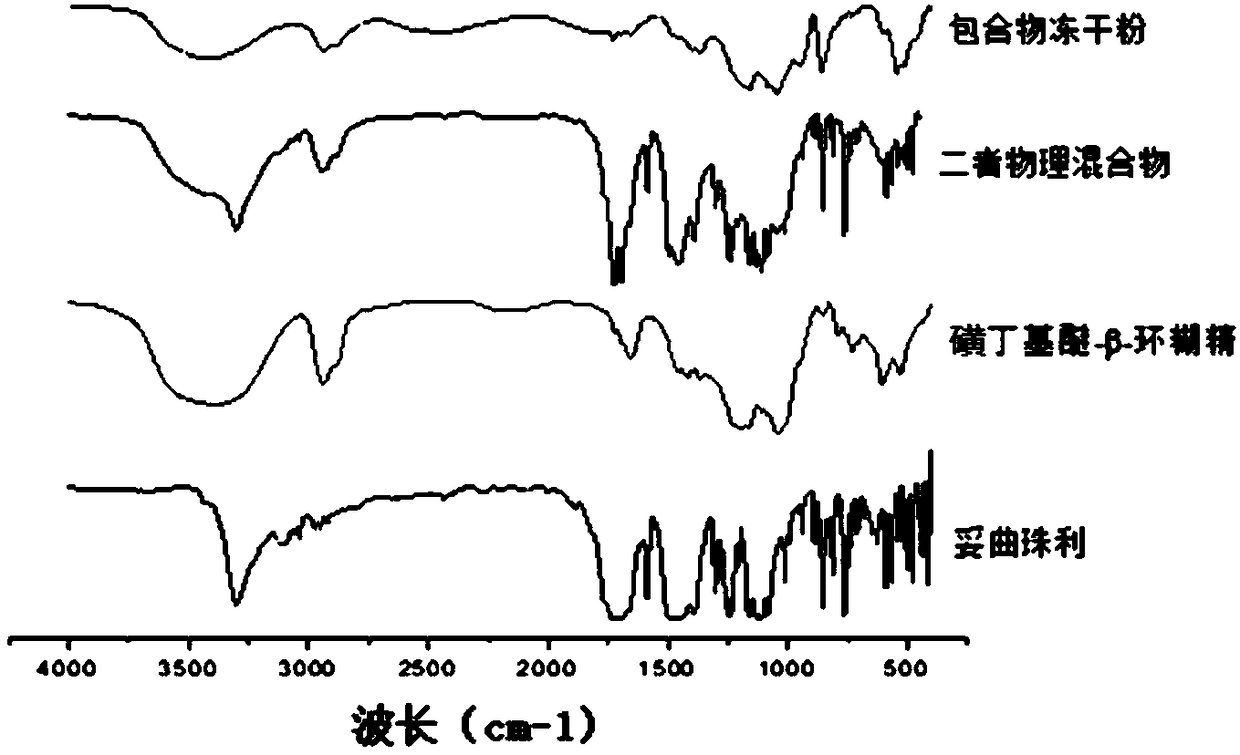
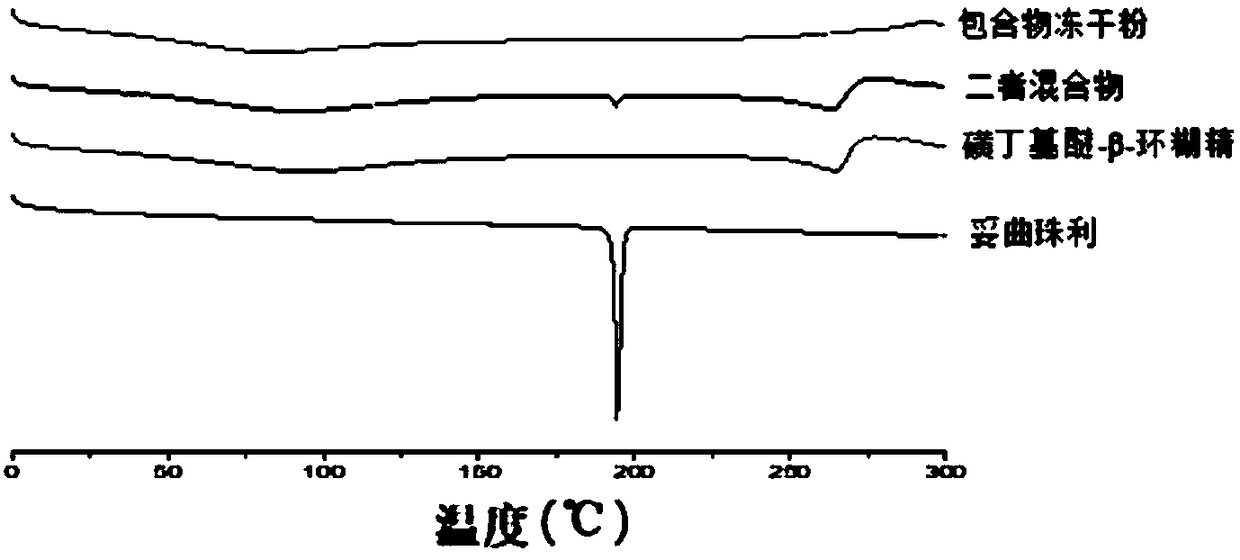
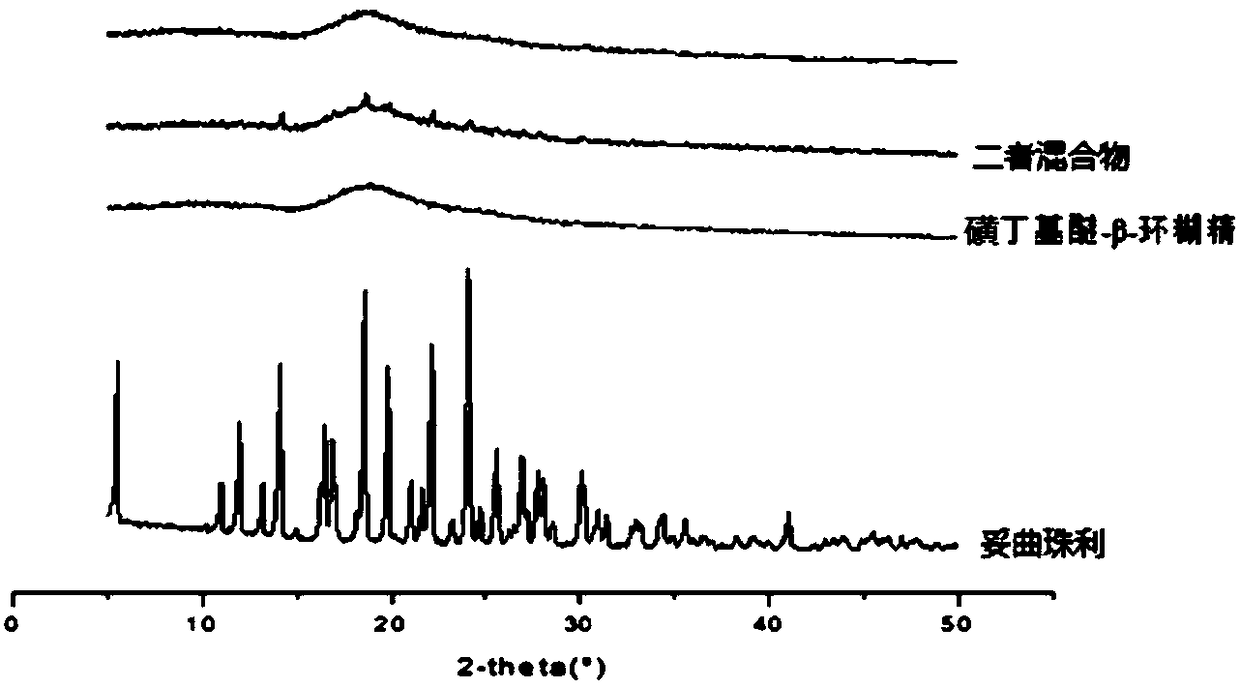
The invention discloses toltrazuril inclusion compound lyophilized powder. The toltrazuril inclusion compound lyophilized powder is obtained by adopting sulfobutyl ether-beta-cyclodextrin to perform inclusion on toltrazuril and then carrying out a lyophilization process. A preparation method of the toltrazuril inclusion compound lyophilized powder concretely comprises the following steps: dissolving the toltrazuril by an organic solvent to obtain an organic solution of the toltrazuril; adding the organic solution of the toltrazuril into an aqueous solution of the sulfobutyl ether-beta-cyclodextrin, and magnetically stirring for carrying out a reaction; carrying out rotary evaporation on the materials obtained after the reaction so as to remove the organic solvent, then filtering by using amicrofiltration membrane and drying to obtain a toltrazuril inclusion compound; carrying out the lyophilization process to obtain the toltrazuril inclusion compound lyophilized powder. According to the preparation method, the sulfobutyl ether-beta-cyclodextrin is adopted to perform inclusion on the toltrazuril, so that the solubility of the indissolvable drug-the toltrazuril is improved; by adopting the lyophilization process, the stability and bioavailability of the toltrazuril are improved; the toltrazuril inclusion compound lyophilized powder enriches the preparations of antiparasitic drugs.
Owner:SICHUAN AGRI UNIV
Toltrazuril solution and preparation method thereof
InactiveCN102973497ADoes not affect utilizationLower resistancePharmaceutical delivery mechanismAntiparasitic agentsMortality rateToltrazuril
The invention aims to disclose a toltrazuril solution and a preparation method thereof. Each 100mL of toltrazuril solution comprises the following components by weight: 2 to 5g of toltrazuril, 20 to 50mL of polyethylene glycol 400, 20 to 50g of propylene glycol, 0 to 40mL of anhydrous ethanol and a proper amount of ethanolamine. Compared with the prior art, the toltrazuril solution has the advantages that medicament resistance is avoided, the intracellular development stage of various coccidia and various medicament-resistant coccidium strains can be controlled by only using the toltrazuril solution for two days, and the safety is high, so that the toltrazuril solution can exert an optimal effect without affecting the growth and the utilization rate of a feed; and the toltrazuril solution can be mixed with multiple medicaments for use, remarkably reduces the death rate, realizes quick recovery and quick growth, saves resources, is easy to control and suitable for large-batch production, and fulfills the aims of the invention.
Owner:JIANGSU HFQ BIO TECH CO LTD
Toltrazuril-resistant monoclonal antibody hybridoma cell strain K-5 and application thereof
ActiveCN106906185AHigh detection sensitivityImprove featuresTissue cultureImmunoglobulinsIc50 valuesMicroorganism
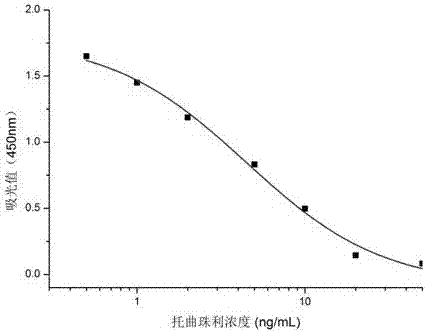
The invention discloses a toltrazuril-resistant monoclonal antibody hybridoma cell strain K-5 and application thereof, and belongs to the field of food safety immunodetection. The toltrazuril-resistant monoclonal antibody hybridoma cell strain K-5 is preserved in the China General Microbiological Culture collection center which is called CGMCC for short, and the preservation number is CGMCC No.13095. A monoclonal antibody excreted by the cell strain has good specificity and detecting sensitivity (the IC50 value is 5.0 ng / mL) on toltrazuril, and can be used for detecting residues of toltrazuril in food safety.
Owner:JIANGNAN UNIV +1
Compound toltrazuril solution and method for preparing same
InactiveCN101548976AGood anti-coccidial effectEasy to usePharmaceutical product form changePharmaceutical delivery mechanismAntigenCoccidiostats
The present invention provides a compound toltrazuril solution. Each 100 ml solution contains medicinal ingredient as follows: toltrazuril 2.5-5 g, nitazoxanide 3-8 g. The compound toltrazuril solution has advantages of low cost, better coccidiostat effect, wide antigen insert, low dose and no-easy drug-tolerance, the compound toltrazuril solution also can be added into drinking water for animal which is more convenient without adding and stirring to feed stuff.
Owner:HENAN SOAR VETERINARY PHARMA
Veterinary drug composition, and preparation method and application thereof
ActiveCN108175793AWide range of action sitesInhibition of nuclear fissionAntiparasitic agentsHeterocyclic compound active ingredientsSide effectTherapeutic effect
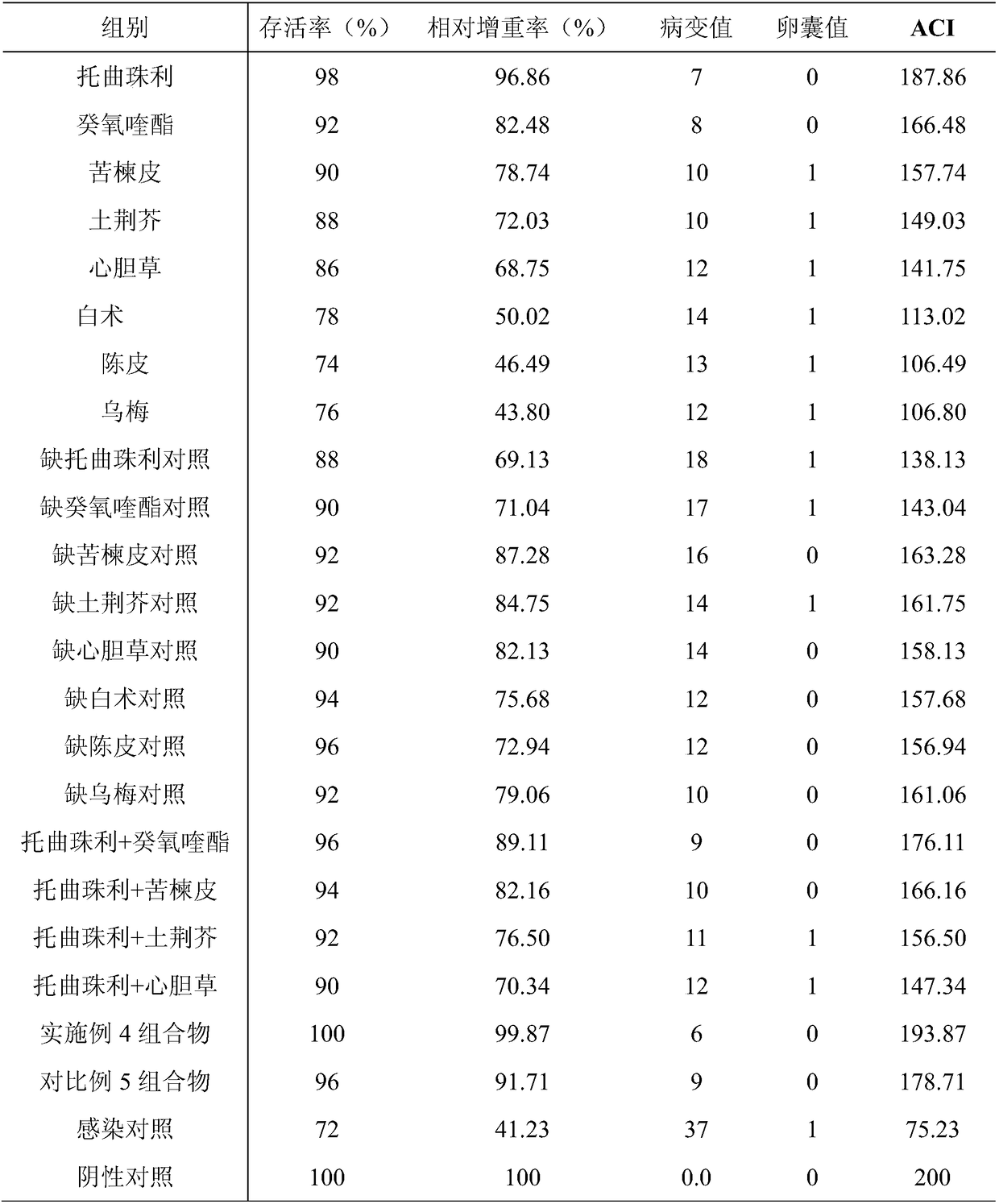
The invention provides a veterinary drug composition. The veterinary drug composition comprises, by mass, 5-25 parts of toltrazuril, 2.5-15 parts of decoquinate, 1-12 parts of pyrantel, 8-15 parts ofCortex Meliae, 2-8 parts of Chenopodium ambrosioides, 2-6 parts of Herb of Longseed Willowweed, 5-10 parts of Rhizoma Atractylodis Macrocephalae, 3-8 parts of dried orange peel and 5-10 parts of darkplum. The invention also provides a preparation method and an application of the veterinary drug composition. The veterinary drug composition has the advantages of reasonable compatibility, quick effect, minimal toxic and side effects, effective reduction of the drug resistance and drug residues, very clear therapeutic effect on intestinal injuries, reduced appetite and lean bodies of animals, caused by coccidiosis, low cost, and easiness in use. The invention further provides the application of the composition in the treatment of histomoniasis.
Owner:JIANGSU LINGYUN PHARMA
Separation tube for preparing sample for detecting content of toltrazuril in blood and detection method
InactiveCN111024848AReduce emulsificationEliminate distractionsComponent separationFiltration circuitsFluid phaseHematological test
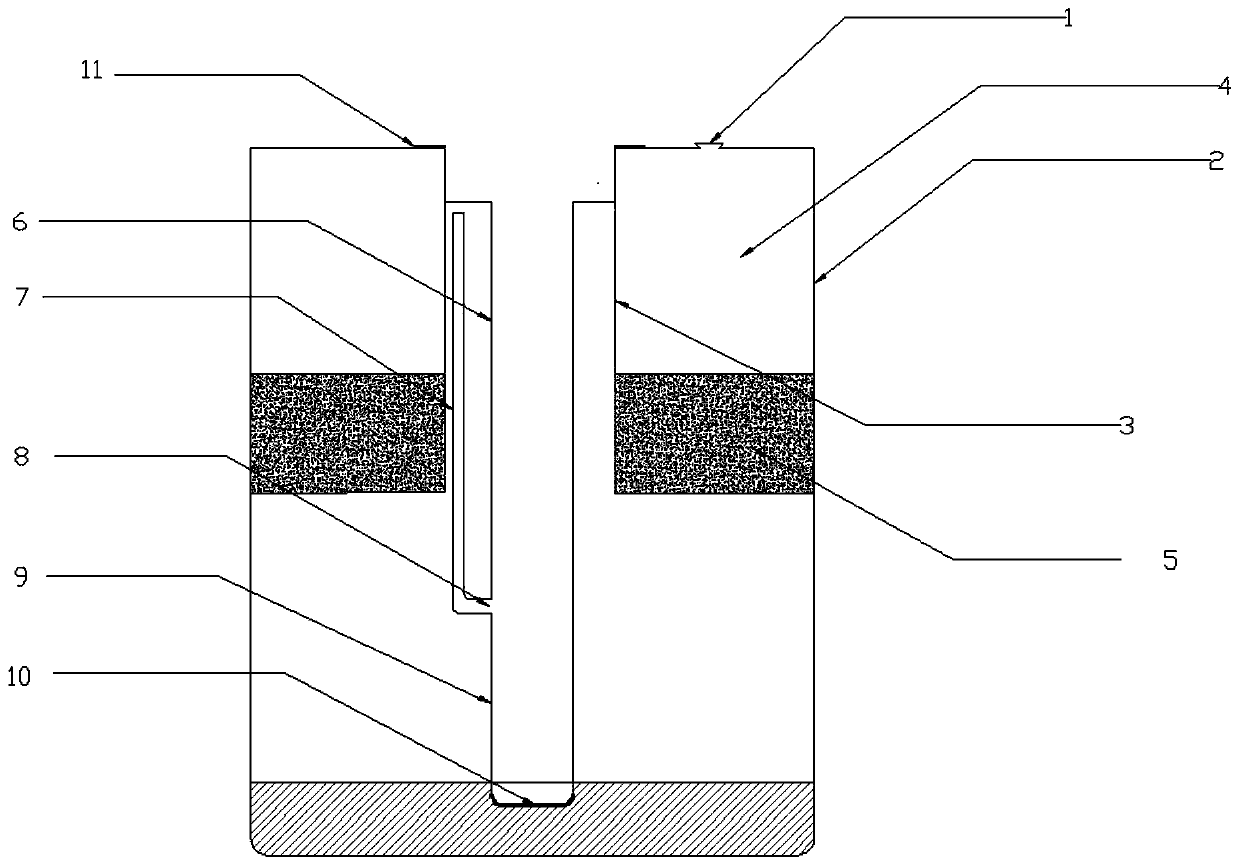
The invention discloses a separation pipe for preparing a sample for detecting the content of toltrazuril in blood. The separation pipe is composed of an inner cylinder and an outer cylinder, whereinan upper portion and a lower portion of the inner cylinder are open, the inner cylinder extends into the outer cylinder from a pipe opening of the outer cylinder, an annular cavity formed between theinner cylinder and the outer cylinder is a separation cavity, a filtering filler layer is arranged at the bottom of the separation cavity, a liquid extraction pipe is arranged in the inner cylinder body and extends into the outer cylinder body from a pipe opening of the inner cylinder, a sealing gasket is arranged between the opening of the liquid extraction pipe and the opening of the inner cylinder, the liquid extraction pipe is also provided with an air guide pipe, the bottom of the air guide pipe is connected with an air guide port which is 10-20mm away from a bottom wall, the top of the gas guide pipe is communicated with an air cavity at an upper part of the inner cylinder, and a filter film is arranged on the bottom wall of the liquid extraction pipe. The invention also discloses amethod for detecting the content of toltrazuril in the blood by using high performance liquid chromatography after the blood is treated into a sample to be analyzed by using the separation pipe, and the method for detecting the content of toltrazuril in blood has advantages of simple operation, few steps and high experiment efficiency.
Owner:镇江益生堂水产生物有限公司 +2
Preparation method of compound solution of toltrazuril and diclazuril and application thereof
ActiveCN103271921AUse low concentrationReduce use costPharmaceutical delivery mechanismAntiparasitic agentsZoologyPropanediol
The invention discloses a compound solution of toltrazuril and diclazuril and a preparation method and application thereof, belonging to the field of livestock / poultry coccidiosis prevention. The compound solution of toltrazuril and diclazuril, disclosed by the invention, consists of main medicines and an accessory, wherein the main medicines include toltrazuril and diclazuril; preferably the mass ratio of the diclazuril to the toltrazuril is 1:(3-5), and the content of toltrazuril accounts for 2-10% of the solution by mass; the accessory is a compound solvent consisting of ethanol, diethanol amine and propylene glycol; the diethanol amine is a pH regulator which regulates the pH value of the compound solution to 8-10; and the propylene glycol is a diluting solvent. According to the prepared compound solution, the actual use concentration of toltrazuril is greatly reduced, and the cost is lowered; and the detection result also shows that the prepared compound solution is an efficient anticoccidial drug and has good stability.
Owner:ZHONGSHAN TIANTIAN ANIMAL HEALTH CARE TECHNOLOGICAL
Preparation method of toltrazuril solid dispersion
InactiveCN102755293AImprove solubilitySimple preparation processPowder deliveryPharmaceutical non-active ingredientsSolubilityDissolution
A preparation method of a toltrazuril solid dispersion relates to the field of animal medicines. The toltrazuril solid dispersion is prepared by uniformly and sequentially mixing the following components in part by weight: 10-15 parts of toltrazuril (passing through an 80-mesh sieve), 20-30 parts of polyvinyl pyrrolidone and 60-100 parts of ethanol. The preparation method comprises the following steps: (1), weighing 10-15 parts of toltrazuril (passing through the 80-mesh sieve), 20-30 parts of polyvinyl pyrrolidone and 60-100 parts of ethanol in part by weight; (2), mixing the toltrazuril and the polyvinyl pyrrolidone, and stirring uniformly; and (3), quickly adding a mixture obtained in the step (2) into the ethanol, stirring uniformly, heating to evaporate the ethanol and obtain the precipitate, drying, and pulverizing to obtain the toltrazuril solid dispersion. The preparation method is simple in process; the solubility of the toltrazuril is significantly improved; and the dissolution of the toltrazuril is about 2 times that of an ordinary toltrazuril preparation (soluble powder or oral liquid).
Owner:QINGDAO LVMAN BIOLOGICAL ENG
Toltrazuril alkali metal salt soluble powder and preparation method thereof
ActiveCN103705467ASimple methodImprove stabilityPowder deliveryPharmaceutical non-active ingredientsCurative effectWater soluble
The invention discloses toltrazuril alkali metal salt soluble powder and a preparation method thereof. The soluble powder comprises the following components in percentage by mass: 2.5-50% of toltrazuril alkali metal salt, 0.1-3% of anti-caking agent and 47-97.4% of water soluble diluent. The preparation method of the soluble powder comprises the following steps: weighing the toltrazuril alkali metal salt, an anti-caking agent and a water soluble diluent according to the component proportion of the toltrazuril alkali metal salt soluble powder; and mixing the weighed components uniformly to obtain the toltrazuril alkali metal salt soluble powder. With the toltrazuril alkali metal salt as the main component, the soluble powder is soluble in water with high stability and low hygroscopicity, and is used for treating and preventing coccidiosis of chicken; the curative effect is remarkable, and the administration is convenient; the administration dosage can be accurately controlled according to the condition of the animal; and the coccidiosis of the animal can be quickly and accurately prevented and treated, and the waste of drug in the administration process can be avoided. The preparation method of the soluble powder is simple, and the cost is low.
Owner:HUBEI LONGXIANG PHARMA TECH CO LTD
Compound sodium sulfaquinoxaline composition for treating coccidiosis in young rabbits, and preparation method thereof
InactiveCN102973577AReasonable compositionEasy to makeAntiparasitic agentsHeterocyclic compound active ingredientsTrimethoprim LactateAdditive ingredient
The invention relates to a compound sodium sulfaquinoxaline composition for treating coccidiosis in young rabbits. The compound sodium sulfaquinoxaline composition is prepared by mixing the following ingredients in parts by weight: 0.5-1 part of toltrazuril, 4-6 parts of sodium sulfaquinoxaline, 0.8-1.2 parts of trimethoprim lactate, 15-25 parts of sodium citrate, 1.5-2.5 parts of sodium carboxymethyl cellulose, 0.2-0.5 part of sodium sulfite, 0.2-0.5 part of EDTA (Ethylene Diamine Tetraacetic Acid)-2Na, and a plurality of parts of distilled water. The compound sodium sulfaquinoxaline composition has the beneficial effects of being reasonable in formula, and simple to prepare, unique in medicine application mode, obvious in curative effect, and having special effects in treating the coccidiosis in young rabbits, proved clinically.
Owner:QINGDAO LVMAN BIOLOGICAL ENG
Treatments with triazines
ActiveUS9877969B2Efficient administrationEffective levelingHeavy metal active ingredientsPharmaceutical delivery mechanismDiseaseSubcutaneous injection
The invention relates to improved methods for protecting non-human animals with triazine compounds by intramuscular or subcutaneous injection(s). The invention can be used with various triazines, such as toltrazuril, in different non-human animals, such as a porcine, an ovine, a bovine, a canine, a feline, or an avian, for protecting them against infectious diseases, such as protozoan disorders.
Owner:CEVA SANTE ANIMALE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com







![Preparation method for 1-methyl-5-[3-methyl-4-(4-trifluoromethylthio-phenoxy)-phenyl]-biuret, and application thereof Preparation method for 1-methyl-5-[3-methyl-4-(4-trifluoromethylthio-phenoxy)-phenyl]-biuret, and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/45aaf550-ed23-4605-a141-46566ae7cae1/2012102149990100002DEST_PATH_IMAGE008.PNG)