Compound anti-parasitic preparation and preparation method thereof
An anti-parasitic and preparation technology, which is applied in pill delivery, anti-infective drugs, pharmaceutical formulations, etc., can solve the problems of poor treatment effect, difficulty in effectively and comprehensively treating nematode parasitic diseases, narrow anti-insect spectrum, etc., and achieves good uniformity , improve the use effect and safety performance, and promote the effect of dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] A preparation method of a compound antiparasitic preparation, comprising the following steps:
[0054] 1) Weighing and sieving: Weigh ivermectin, mebendazole, toltrazuril, disintegrants, fillers, lubricants, adhesives and other auxiliary materials according to the above parts by weight, and the weighed ivermectin Vermectin, mebendazole, toltrazuril and auxiliary materials are respectively pulverized and sieved through a 60-120 mesh sieve. Optionally, the adjuvant also includes a flavoring agent and a preservative, and the flavoring agent and the preservative are respectively weighed by the above parts by weight.
[0055] 2) Preparation of ivermectin solid dispersion: Add the sieved ivermectin to absolute ethanol to dissolve completely, then add part of the sieved filler and mix evenly, and then dry, pulverize and sieve , to prepare the ivermectin solid dispersion, wherein the content of the ivermectin in the ivermectin solid dispersion is 1%-5%.
[0056] In one embodi...
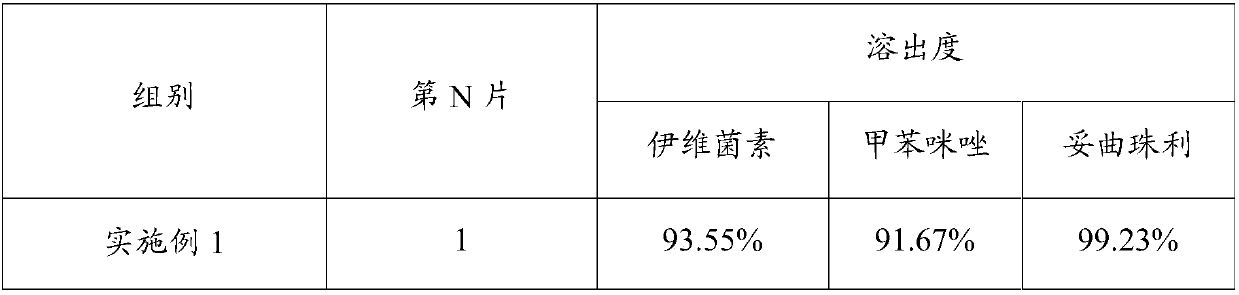
Embodiment 1~13
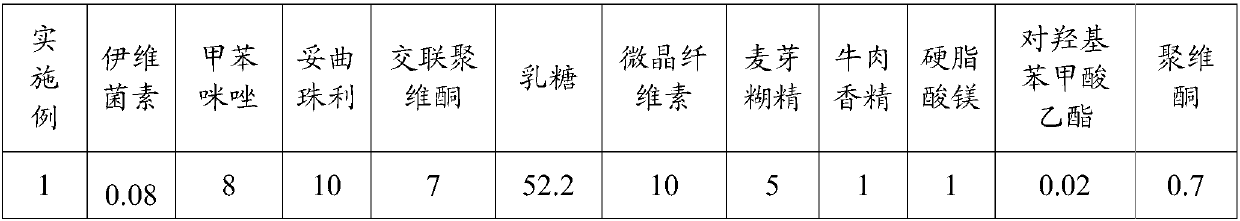
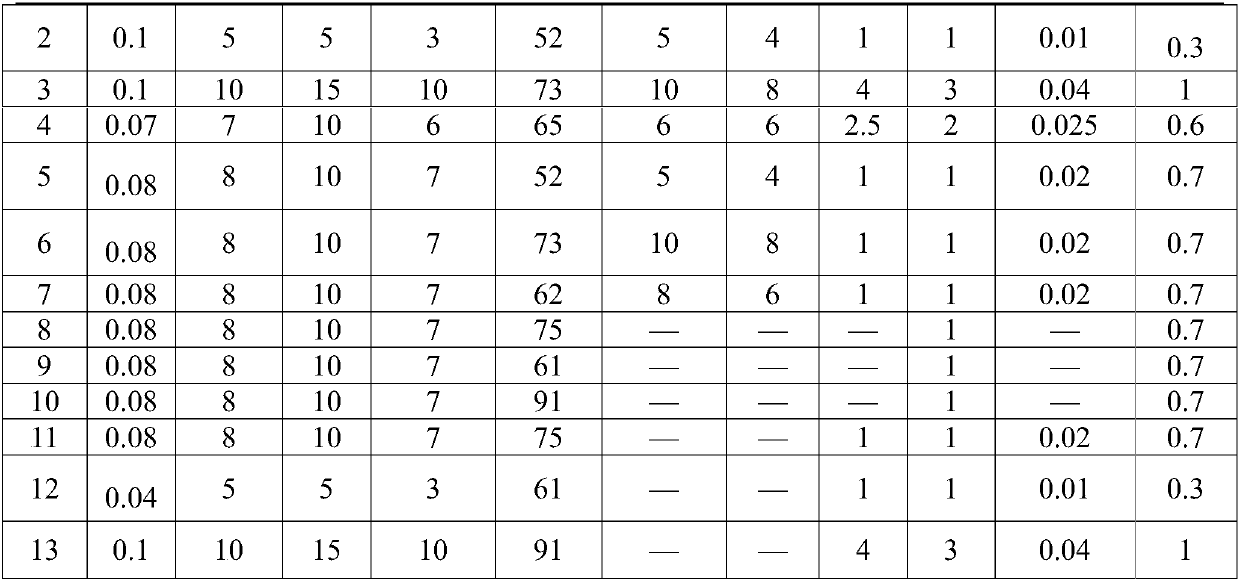
[0077] The composition of the compound antiparasitic preparations in Examples 1-13 is shown in Table 1. The corresponding numerical value of various raw materials in table 1 is the parts by weight of this raw material.
[0078] The composition of table 1 compound antiparasitic preparation
[0079]
[0080]
[0081] 1. the preparation method of embodiment 1-7 is as follows:
[0082] 1) Weigh respectively ivermectin, mebendazole, toltrazuril, crospovidone, lactose, microcrystalline cellulose, maltodextrin, beef essence, magnesium stearate, polydextrin according to the above-mentioned parts by weight. Vitone and ethyl paraben. The weighed raw materials were respectively pulverized and sieved through a 100-mesh sieve.
[0083] 2) Add the ivermectin and ethyl p-hydroxybenzoate into absolute ethanol to dissolve completely, then add part of lactose and mix evenly, dry in an oven at a temperature of 55°C-60°C for 4-5 hours, pulverized, and sieved through a 100-mesh sieve to ...
Embodiment 8-10
[0088] 2. The preparation method of embodiment 8-10 is as follows:
[0089] 1) Take respectively ivermectin, mebendazole, toltrazuril, cross-linked povidone, lactose, magnesium stearate and povidone by the above-mentioned parts by weight. The raw materials weighed were pulverized respectively and sieved through a 100 mesh sieve.
[0090] 2) Add the ivermectin to absolute ethanol to dissolve completely, then add some lactose and mix evenly, dry it in an oven at 55°C-60°C for 4-5 hours, crush it, and pass through a 100-mesh sieve After sieving, an ivermectin solid dispersion containing 1% ivermectin was prepared.
[0091] 3) Add the povidone sieved in step 1) into purified water to prepare an aqueous solution of povidone with a concentration of 3% (w / v).
[0092] 4) Mix the sieved mebendazole, toltrazuril, crospovidone, remaining lactose, and step 2) the ivermectin solid dispersion prepared in step 1) according to an equal incremental method, and add Step 3) The prepared aque...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com