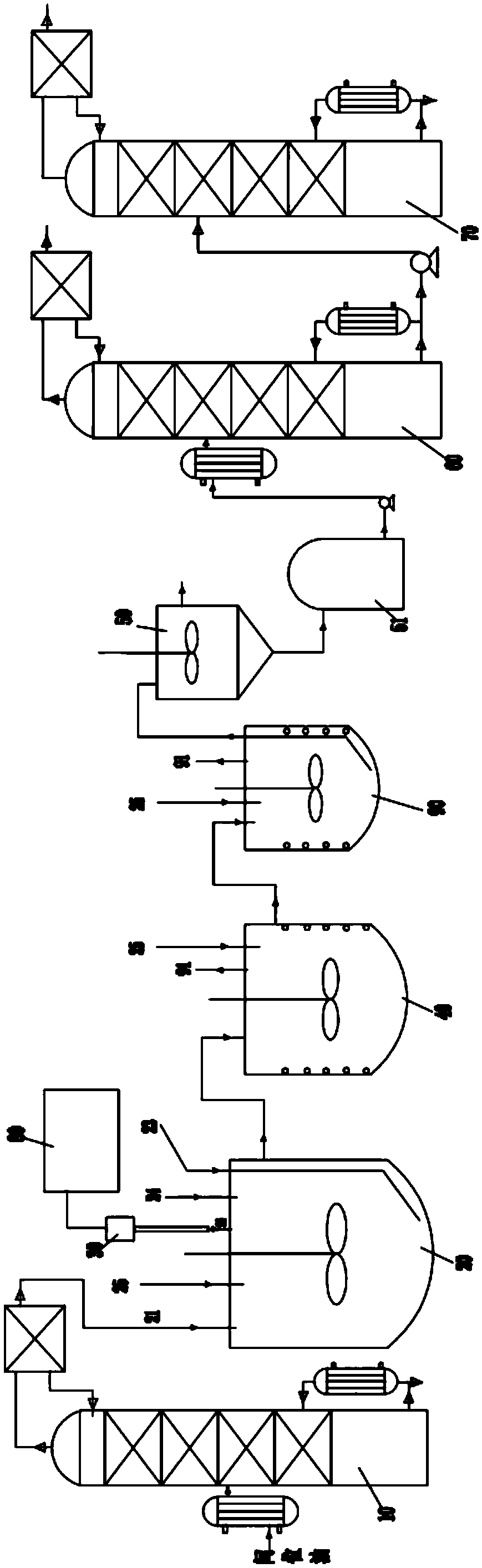
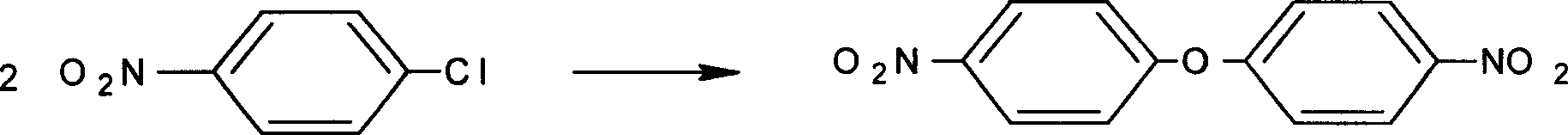Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
103 results about "P-nitrochlorobenzene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
4-Chloro-1-nitrobenzene 4-Chloronitrobenzene p-Nitrochlorobenzene PNCBO. ... 4-Nitrochlorobenzene was originally prepared by the nitration of 4-bromochlorobenzene by Holleman and coworkers. Applications. 4-Nitrochlorobenzene is an intermediate in the preparation of a variety of derivatives.
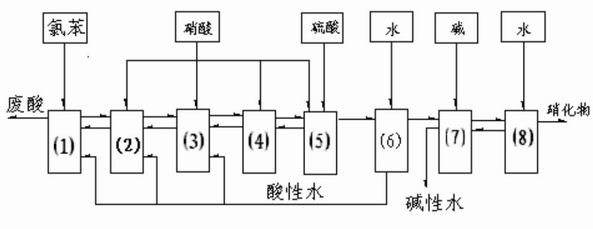
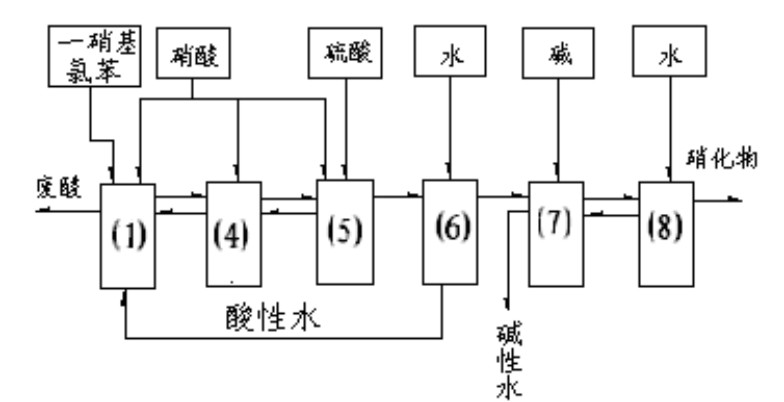
Method for continuously preparing dinitrochlorobenzene
ActiveCN102070457AIncrease profitIncrease production capacityNitro compound preparationNitrateChlorobenzene
The invention discloses a method for continuously preparing dinitrochlorobenzene. Multiple dinitration reactors are connected in series for reaction, and mononitrochlorobenzene is continuously added to the first dinitration reactor, and continuously flows through all the dinitration reactors and flows out; a mixed acid nitrating agent is prepared from 75-85% of sulfuric acid, 2-7% of nitric acid and 5-15% of water; the reaction temperature is 40-95 DEG C; and each dinitration reactor is provided with a separation device for separating an organic phase from an inorganic phase, and the inorganic phase is returned into the reactor, thereby keeping the inorganic phase and the organic phase in the required proportion. In the invention, the dinitrochlorobenzene is prepared by continuous reactions, and the reactions are continuously carried out and sequentially completed; chlorobenzene is used for extracting nitrates and nitric acid in an acid phase, thereby lowering the content of the nitric acid in the acid phase and recycling the nitrates in the acid phase; by the invention, the dinitrochlorobenzene can be continuously prepared from two raw materials (chlorobenzene and p-nitrochlorobenzene) by using one set of devices; and the invention has the advantages of low raw material consumption and high production capacity.
Owner:LIANYUNGANG DIPU CHEM
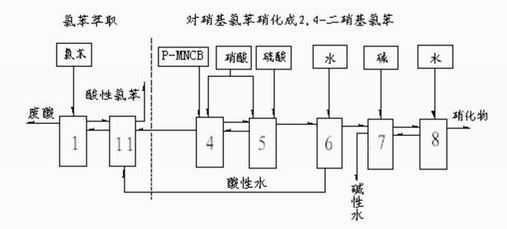
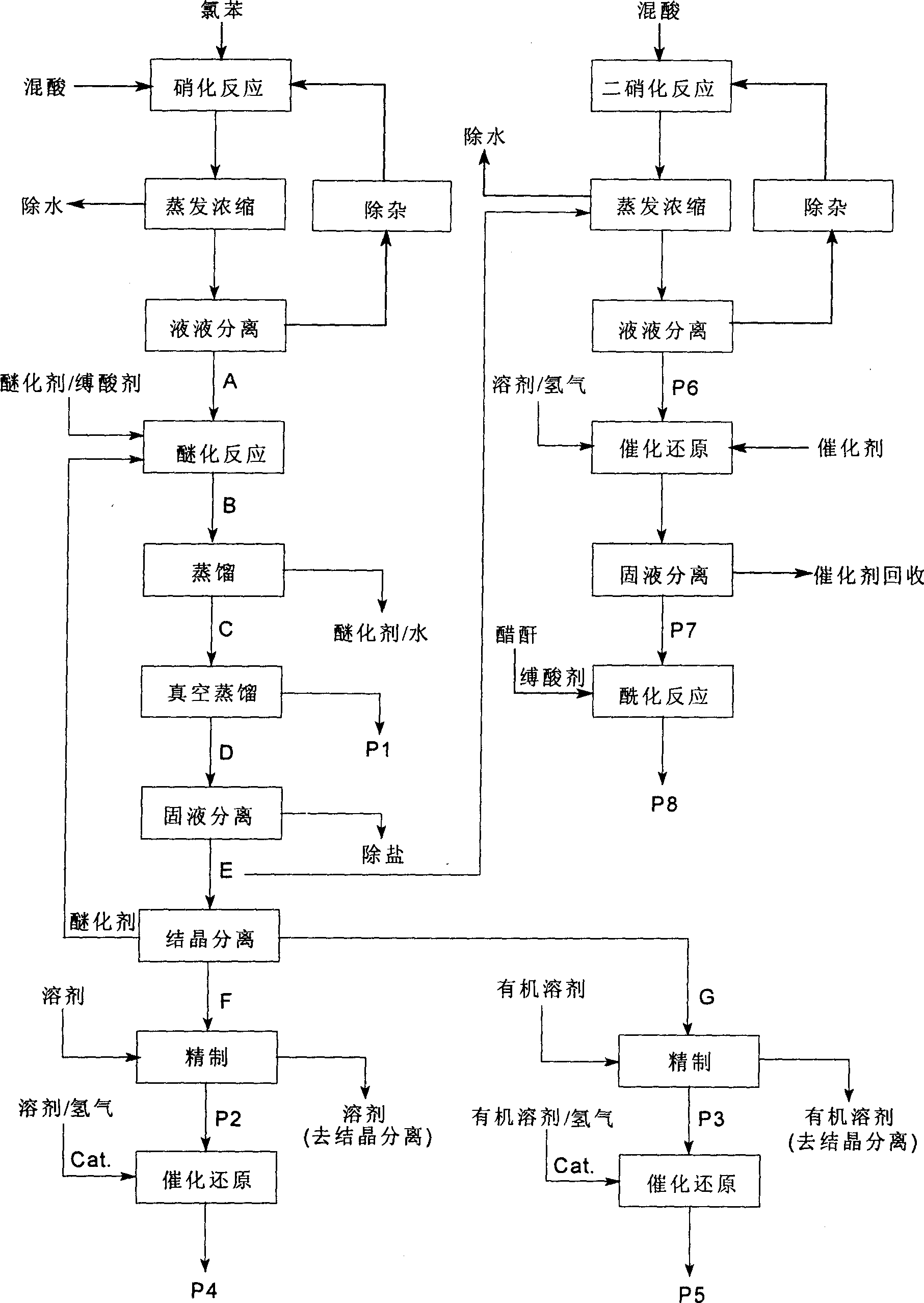
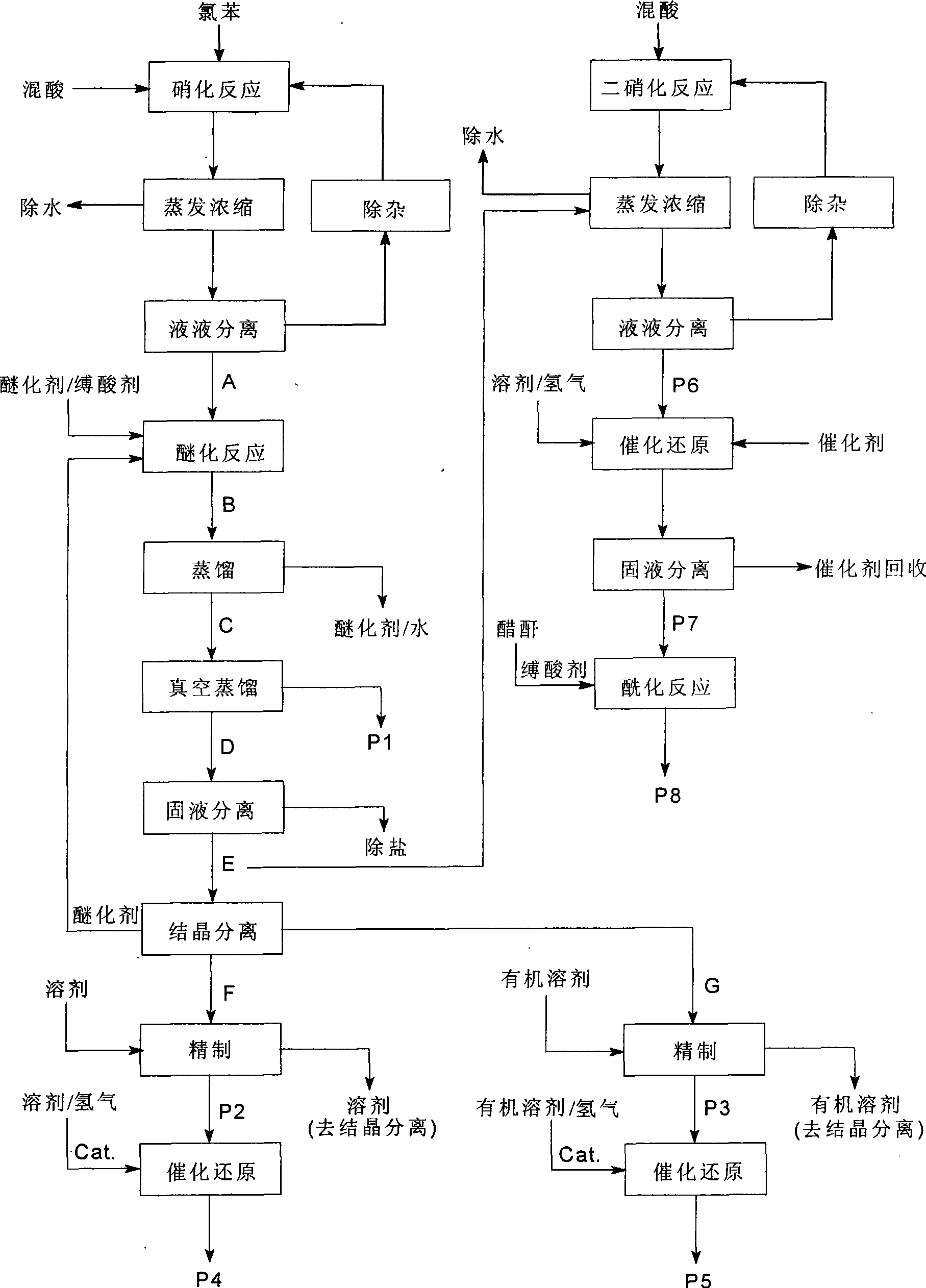
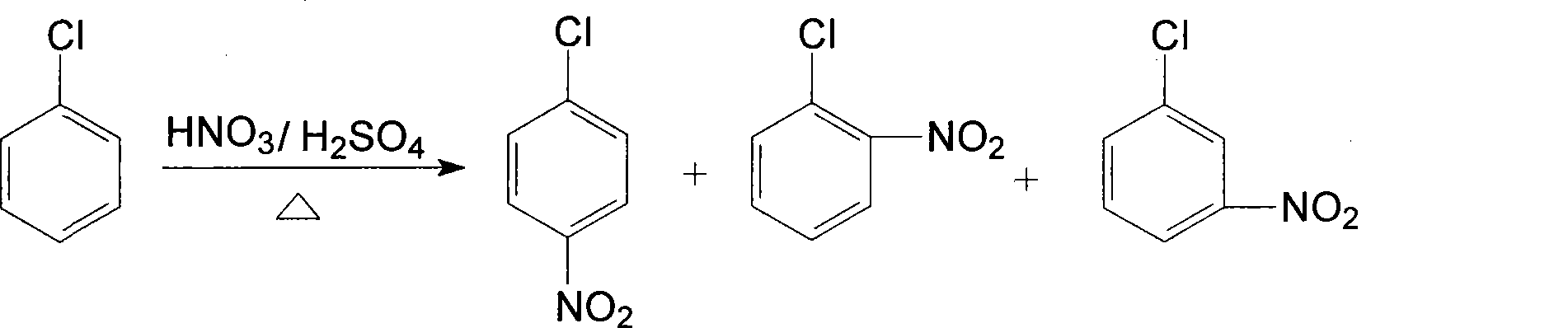
Process of producing nitrobenzether aminobenzether amidobenzether from chlorobenzene
InactiveCN1861562AAvoid conditionsAvoid investment in equipmentOrganic compound preparationCarboxylic acid amides preparationO-nitrochlorobenzeneChloride salt
A process for preparing nitrophenylether, aminophenylether and amidophenylether between chlorobenzene and acid mixture removing water, liquid-liquid separation to obtain organic phase consisting of o-, p- and meta-nitro chlorobenzene compounds, etherifying said mixture, recovering etherifying agent, vacuum distilling, separating and refining meta-nitro chlorobenzene, removing chloride salt generated in etherification, crystallizing separation, recrystallizing p- and o-nitro phenylether compounds, catalytic hydrogenating reaction, nitrifying reaction to obtain 2,4-drinitro phenylether, catalytic hydroreducing reaction to obtain 2,4-diamino phenylether, and aceylating reaction to obtain 2-amino-4-acetylamino phenylether.
Owner:CHANGZHOU JIASEN CHEM +1
Preparation method of 2,4-dinitochloro benzene
InactiveCN1513830AImprove product qualityImprove efficiencyOrganic chemistryOrganic compound preparationBenzeneNitration
A process for preparing 2,4-benzene dinitrochloride from nitrochloro-benzene includes nitrifying reacting between said nitrochlorobenzene and the mixture of nitric acid and inorganic at 60-90 deg.C for 5-10 hr to generate 2,4-benzene dinitrochloride, separating out the waste acid at lower layer, and neutralizing to obtain high-purity product.
Owner:徐德军
Method of producing toltrazuril
A preparation method of the Toltrazuril is provided. The Toltrazuril is the 1-[3-methyl-4-(4- Trifluoromethylthiobenzoxy) benzyl]-3-methyl-1, 3, 5, -triazine-2, 4, 6(1H, 3H, 5H)-trione. The methyl sulfide chloro compounds, fluoro-compounds, amides, phenol, methyl nitrate chlorobenzene, benzene aether, ammonia benzene aether, isonitrile acid ester and methyl urea from the reactions of 4-nitrochlorobenzene, sulfur, sodium sulfide and dimethyl sulfate. The detailed preparation is that reaction 1: the methanol of 410g and 4-nitrochlorobenzene of 158g are mixed and heated until soluble and are added with sulfur, sodium sulfide and methanol mixed liquor by drops, and kept for 2h under temperature of 60 DEG C. to 65DEG C.; reduce the temperature, add water of 880g and add dimethyl sulfate of 192g; during the period, the pH value of the sodium hydroxide is adjusted over 9, and the methyl sulfide of 153.6 is gained by filtering after reaction with yield of 91.1 per cent.
Owner:PU LIKE BIO ENG +1
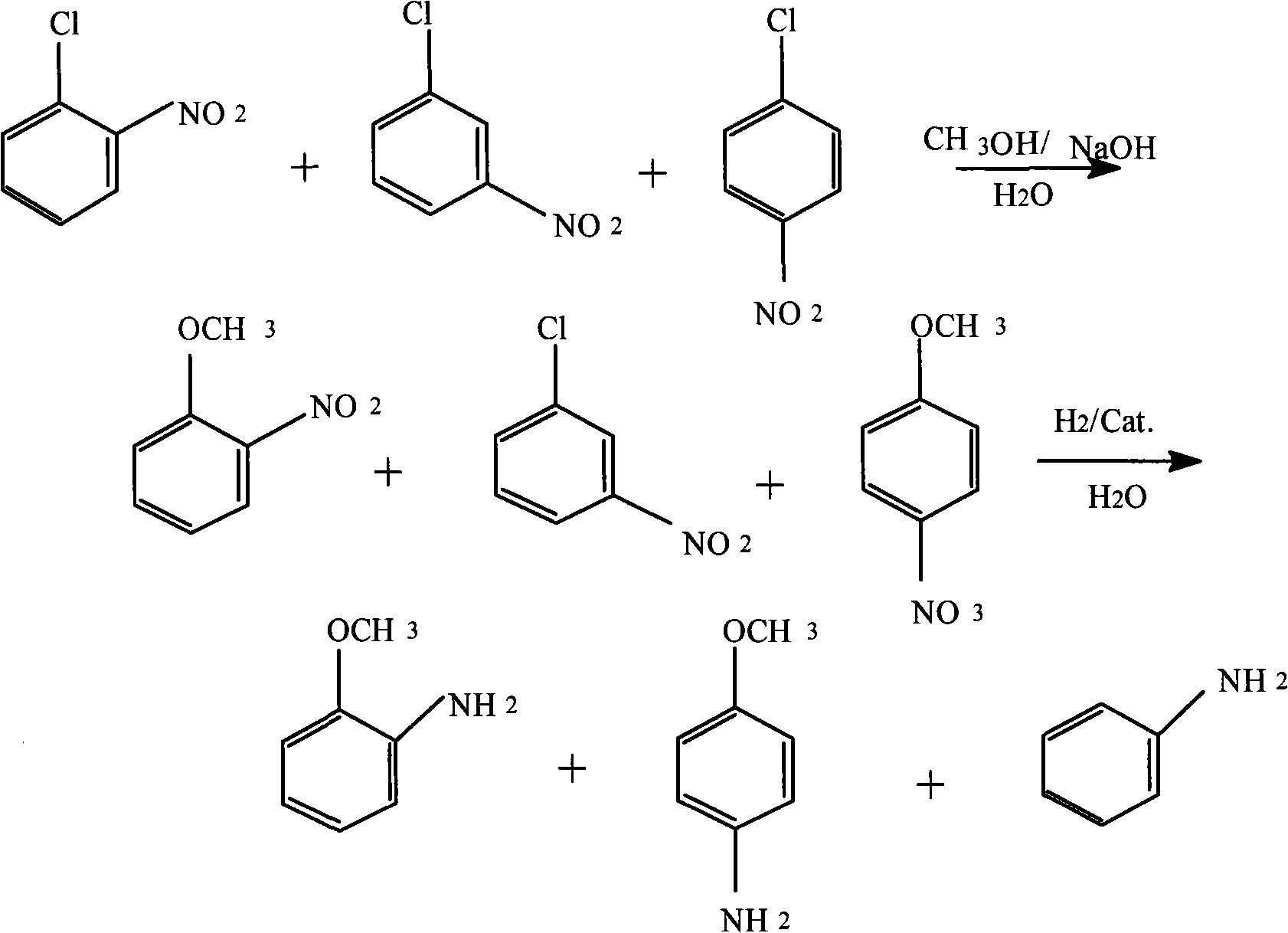
Method for producing anisidine by mixed nitrochlorobenzene reacting in aqueous solvent
InactiveCN101607919ALow ingredient requirementsReduce pollutionOrganic compound preparationAmino-hyroxy compound preparationO-nitrochlorobenzeneDistillation
The invention relates to a method for producing anisidine by mixed nitrochlorobenzene (comprising o-nitrochlorobenzene, p-nitrochlorobenzene and m-nitrochlorobenzene) in an aqueous solvent through steps of etherification, hydrogenation, distillation separation, and the like. The method comprises the technical processes: (1) enabling the mixed nitrochlorobenzene and methanol to react, using water as a solvent and sodium hydroxide as a catalyst; (2) separating an aqueous phase; (3) catalyzing and hydrogenating etherified oil, and directly hydrogenating and reducing the etherified oil by using water as the solvent without washing to remove alkaline by water; (4) filtering the catalyst; (5) separating crude products, cooling and precipitating an organic phase, and separating and removing the water phrase; and (6) rectifying and separating an organic phase, and rectifying the organic phase obtained by separating water to obtain pure p-anisidine and pure o-anisidine with the purity over 99 percent. The method for producing anisidine by mixed nitrochlorobenzene reacting in an aqueous solvent has simple technology, low cost and energy consumption, high product purity, environmental protection and low toxicity.
Owner:扬州铭睿达化工科技有限公司 +1
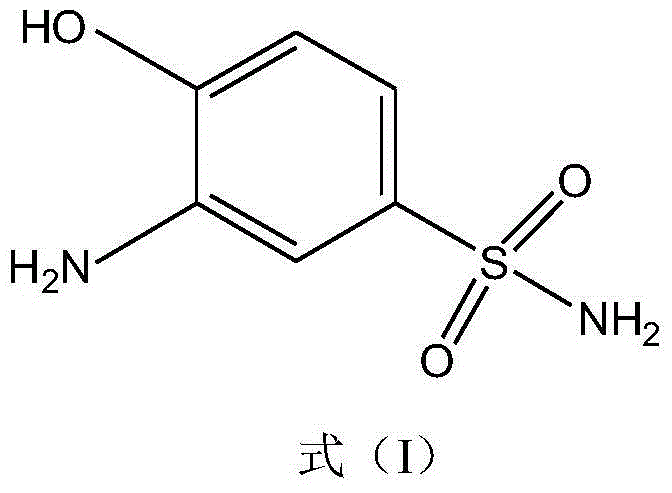
Synthetic method of 2-aminophenol-4-sulfonamide
InactiveCN104592064AReduce dosageReduce wasteOrganic compound preparationSulfonic acid amide preparationO-nitrochlorobenzeneChlorosulfuric acid
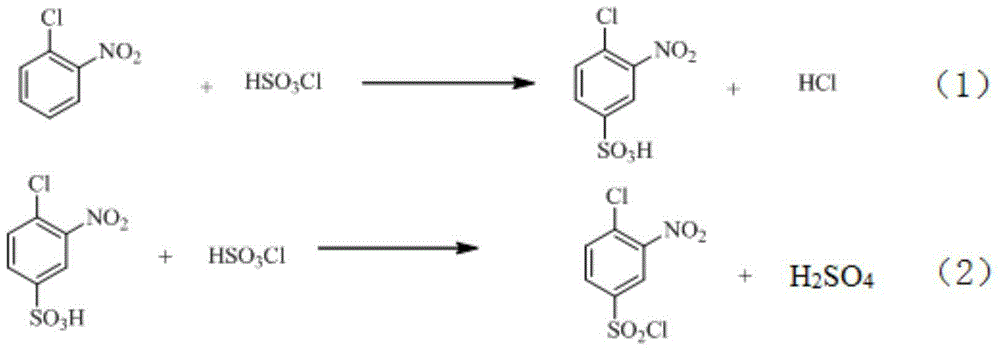
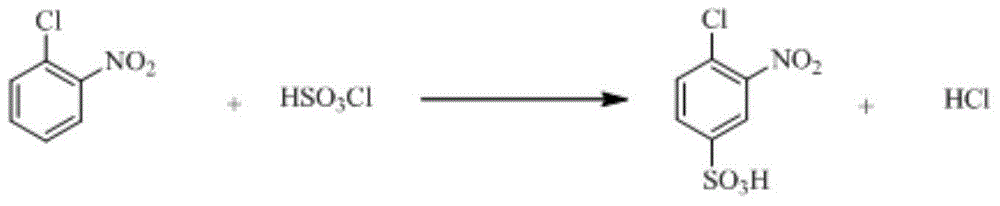
The invention relates to a synthetic method of 2-aminophenol-4-sulfonamide. The synthetic method comprises the steps of chlorosulfonation, ammoniation, hydrolysis, acidification and reduction, wherein the chlorosulfonation comprises the following two steps: (a) adding chlorosulfonic acid into p-nitrochlorobenzene which serves as a raw material, and carrying out sulfonation reaction to obtain 4-chloro-3-nitrobenzene sulfonic acid; and (b) adding sulfoxide chloride into 4-chloro-3-nitrobenzene sulfonic acid obtained in the step (a), and carrying out chlorination to obtain 4-chloro-3-nitrobenzenesulfonyl chloride. According to the synthetic method, a chlorosulfonation process is improved, and the chlorosulfonation is carried out in two steps, namely p-nitrochlorobenzene is taken as the raw material, is firstly sulfonated by chlorosulfonic acid and is then chloridized by sulfoxide chloride, so that the consumption of chlorosulfonic acid is reduced, the unnecessary wasting is reduced, the production cost is saved, and the yield of products is increased to reach up to 96.88%.
Owner:QINGDAO DOUBLE PEACH SPECIALTY CHEM GRP
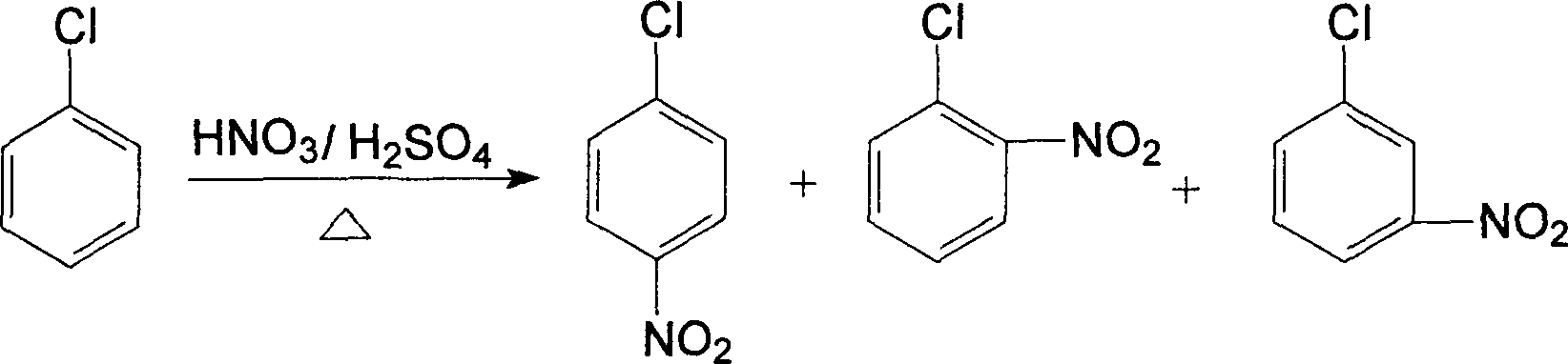
Method for preparing p-nitrochlorobenzene by nitrifying chlorobenzene by using nitrogen dioxide
InactiveCN103086892AHigh reaction yieldHigh selectivityNitro compound preparationChlorobenzeneNitrogen dioxide
The invention relates to a method for preparing p-nitrochlorobenzene by nitrifying chlorobenzene by using nitrogen dioxide. The p-nitrochlorobenzene is prepared by taking chlorobenzene as raw material and nitrogen dioxide as a nitration reagent. The nitrogen dioxide nitration reagent is used for replacing the traditional nitration reagent of nitric acid-sulphuric acid mixed acid, not only is utilization rate of reaction atoms improved, but also environmental economical efficiency of industrial preparation is improved, as well as the environment pollution is reduced. Simultaneously, the invention provides a nitration method of an acid sensitive compound, and the nitration method can be applied to the nitration of a plurality of aromatic compounds, and even applied to the nitration of aromatic compounds containing a plurality of deactivating groups.
Owner:ANHUI HUAIHUA
Production method of highly pure 2,4-dinitrochlorobenzene
InactiveCN104045563AReduce productionEasy to useNitro compound preparationChemical industryNitration
A production method of highly pure 2,4-dinitrochlorobenzene relates to the technical field of the chemical industry. The method comprises the following steps: adding a nitric acid and sulfuric acid mixed acid into a nitration kettle, adding p-chloronitrobenzene in a dropwise manner, heating the nitration kettle to 105DEG C, stopping stirring, allowing the obtained material to stand for layering, adding a waste acid into an extraction pot, adding p-nitrochlorobenzene into the extraction after the extraction in order to react with residual nitric acid in the waste acid, extracting organic matters in the extracted acid, allowing the obtained solution to stand for layering, inputting the waste acid into a concentration workshop section, concentrating, mechanically using, recovering the extracted p-nitrochlorobenzene as a next batch nitration material, adding a qualified nitrated liquid from the nitration kettle into a neutralization water washing pot, adding Na2CO3.10H2O, washing to neutrality, stopping stirring, and allowing the obtained solution to stand for layering in order to obtain a finished product. The method has the advantages of simple and convenient preparation, small waste acid output, less equipment investment, high out degree and convenient operation, and the prepared 2,4-dinitrochlorobenzene has the advantages of good use effect, safety and reliability.
Owner:ANHUI HUARUN PAINTS
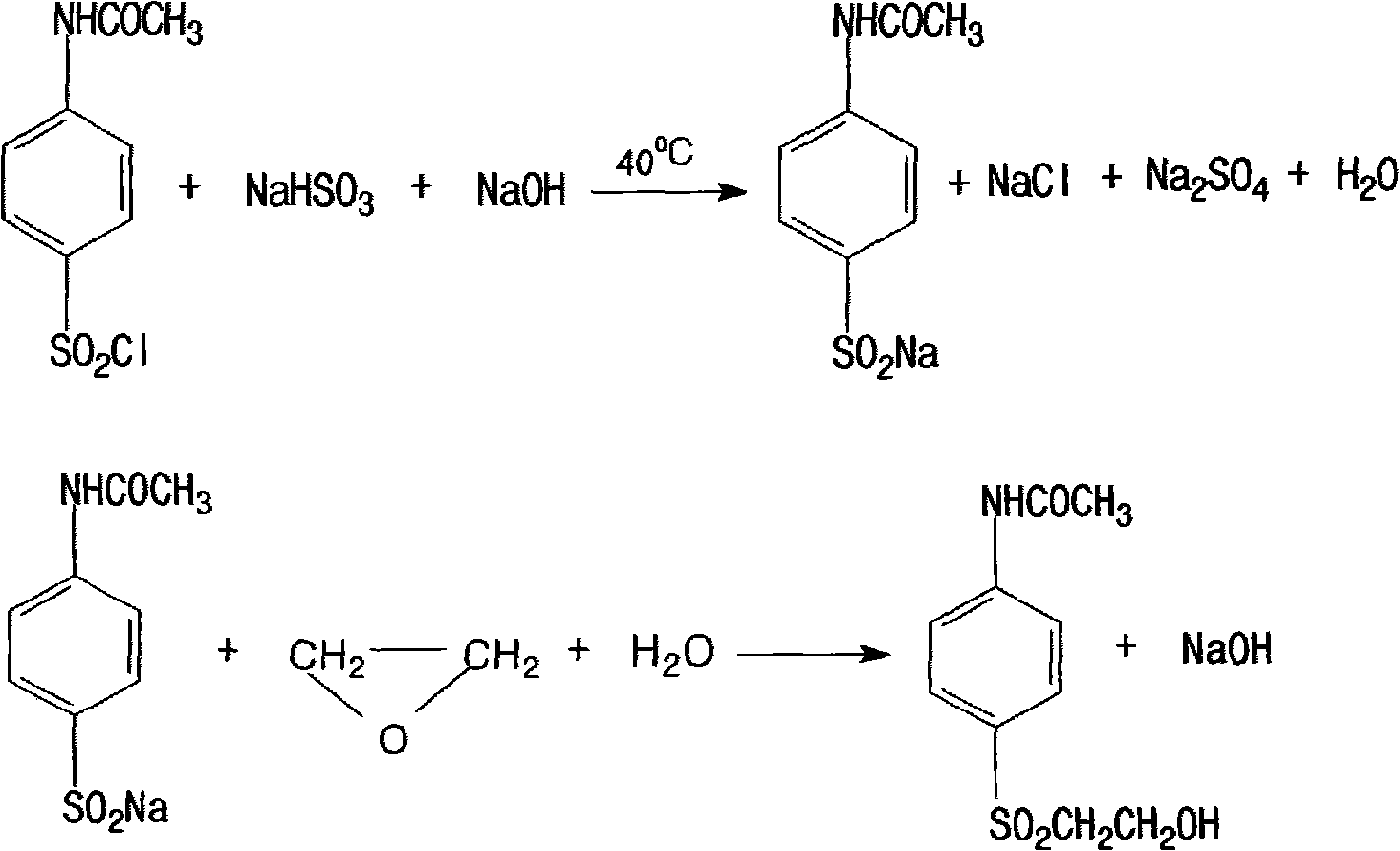
Method for producing amino-phenyl-beta-hydroxyethyl sulfone sulfate
ActiveCN101525309AIncrease profitReduce consumptionOrganic chemistryOrganic compound preparationDistillationTungstate
The invention relates to a method for producing amino-phenyl-beta-hydroxyethyl sulfone sulfate. The method comprises: nitrochlorobenzene and mercaptoethanol are added into DMF and then slowly added with sodium hydroxide, and the mol ratio among the nitrochlorobenzene, the mercaptoethanol, the DMF and the sodium hydroxide is 1:1-1.2:1.5-2:1; after the sodium hydroxide is added, the reaction lasts for 3-5h at the temperature of 50-80 DEG C; the preliminary product is obtained by reduced pressure distillation; the preliminary product, tungstate and hydrogen peroxide are added into an oxidation pot to react for 1-3h at the temperature of 95-100 DEG C, and oxidized crystallizing material is separated out by cooling, crystallizing and vacuum filtration; after that, the obtained oxidized crystallizing material is treated by hydrogenation reaction and esterification reaction, so that the amino-phenyl-beta-hydroxyethyl sulfone sulfate is obtained. The yield coefficient of the product reaches about 85%, and byproducts such as sodium chloride as well as three wastes which are waste acid, waste water and the like are less; as the quantity of the waste water is reduced to 1-2 t from 6-8 t produced by using the original product, the method reduces the production cost and is beneficial to promotion and application.
Owner:KAIFENG LONGXING CHEM
Continuous synthetic method of high-purity p-nitroaniline
InactiveCN107619373AReduce energy consumptionImprove product qualityOrganic compound preparationChemical recyclingFixed bedP-Nitroaniline
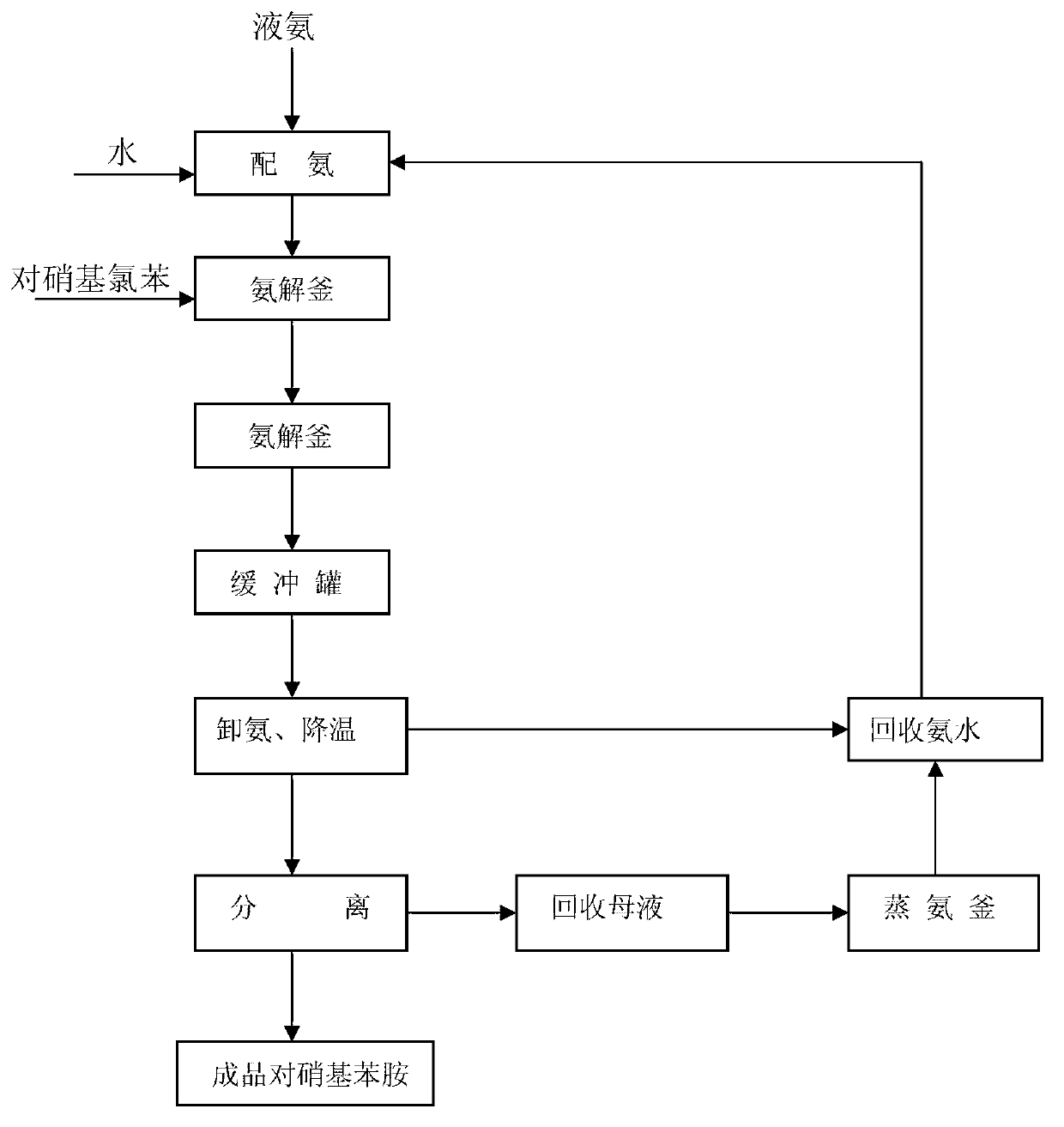
The invention provides a new process for synthesizing p-nitroaniline. The process comprises: taking p-nitrochlorobenzene and ammonia water as raw materials, in a fixed bed reactor or shell-and-tube reactor and in the presence of a catalyst, carrying out an ammonification reaction to prepare a p-nitroaniline crude product, performing separation recycling to the crude product to prepare p-nitroaniline and obtain ammonium chloride as a by-product, and recycling excess ammonia for cyclic application. According to the process, the p-nitroaniline conversion rate can reach 100%, and the purity of p-nitroaniline can reach 99.9%. The process is green, eco-friendly and new.
Owner:CHINA PETROLEUM & CHEM CORP +1
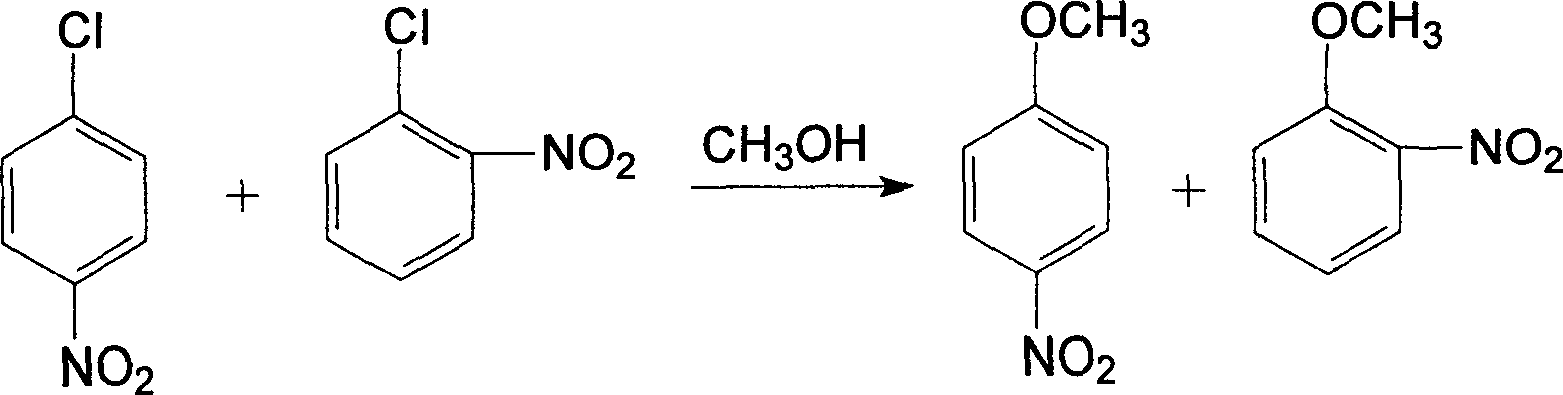
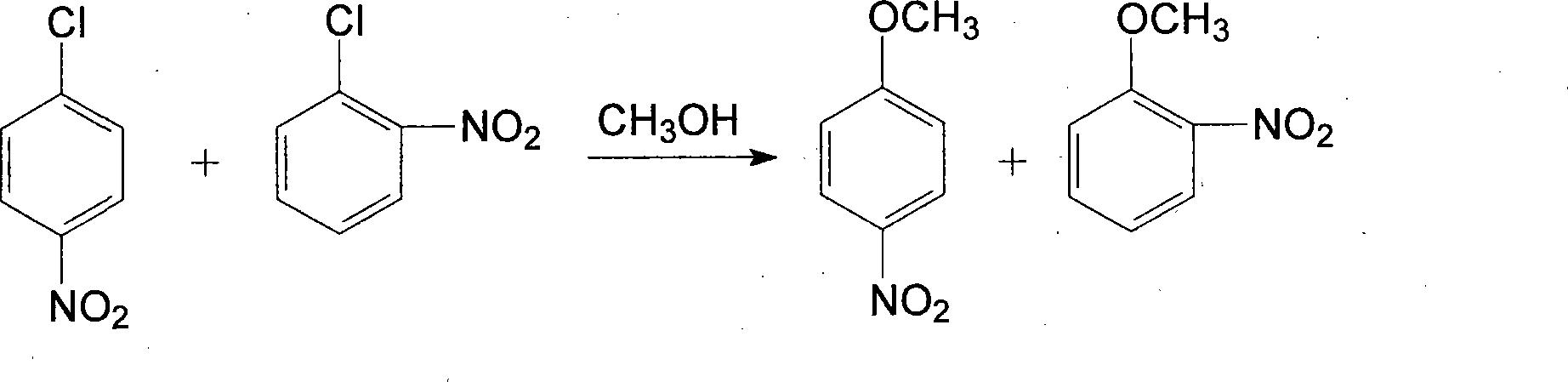
Method for preparing o-nitroanisole and p-nitroanisole from mixed nitrochlorobenzene
InactiveCN103073431AAvoid costly separationsSave energyOrganic chemistryOrganic compound preparationO-nitrochlorobenzeneChlorobenzene
The invention relates to a method for preparing o-nitroanisole and p-nitroanisole from mixed nitrochlorobenzene. According to the invention, mixed nitrochlorobenzene consisting of a product obtained after nitration of chlorobenzene, o-nitrochlorobenzene, m-nitrochlorobenzene and p-nitrochlorobenzene is used as a raw material, and in a methanol system, 30% of alkali lye and a phase-transfer catalyst are added for a reaction to prepare products of o-nitroanisole and p-nitroanisole, wherein m-nitrochlorobenzene does not participate in the reaction. The method provided by the invention overcomes the problems of great investment for rectifying tower equipment, great energy consumption in rectification, high cost, long process flow, harsh operation conditions, poor operational safety performances and generation of waste residues hardly to treat in rectification-crystallization separation of mixed nitrochlorobenzene in conventional production processes for o-nitroanisole and p-nitroanisole and overcomes the technical problems of generation of considerable alkaline waste water containing sodium hyposulfite and production of a poor-quality product in a sodium sulfide reduction process.
Owner:CHANGZHOU JIASEN CHEM
Process for treating waste water of nitrobenzene, 2,4-dinitrophenol, p-nitro-chlorebenzene
ActiveCN1559933AShorten the migration pathElectrocatalytic Oxidative Degradation Speed ImprovementWater/sewage treatmentMultistage water/sewage treatmentIndustrial waste waterNitrobenzene
The invention is a method of processing the waste water containing nitrobenzene, 2, 4- dinitrophenol and para-nitrochlorobenzene, pumping the basic waste water in a water distributing tank or the neutral water produced by using 98% sulphuric acid to regulate the pH value up to 7-8, into an electro-catalyzing device with activated carbon particle group, the voltage and current of the device, staying for a short time and then making the outgoing water pass through a medium tank for buffering, adding in 98% sulphuric acid to regulate the pH value up to primary effluent standard in the list 4 of 'Sewage Synthetic Effluent Standard' of the national GB8978-1996 and discharging. It has characters of effective degrading the industrial waste water containing nondegradable basic or neutral organic pollutants like nitrobenzene, 2, 4- dinitrophenol, para-nitrochlorobenzene, etc.
Owner:江苏环保产业股份有限公司
Production process for preparing parachloroaniline by catalytic hydrogenation of para-nitrochlorobenzene
InactiveCN102757352AInhibition of hydrodechlorinationHigh yieldOrganic compound preparationAmino compound preparationMeta-chloroanilineSolvent
The invention discloses a production process for preparing chloroaniline by catalytic hydrogenation of para-nitrochlorobenzene. The meta-chloroaniline is prepared by catalytic hydrogenation of para-nitrochlorobenzene as raw material in methanol as solvent in the presence of Raney-Ni catalyst and dicyandiamide as a dechlorination inhibotor at 55 DEG C under 1 MPa. According to the process, the selectivity of parachloroaniline is more than 98.5%, and the dechlorination rate is less than 0.5%.
Owner:JIANGSU KANGHENG CHEM
Method for separating nitrochlorobenzene and nitrophenol waste water by HZSM-5 zeolites and resource recovery
InactiveCN1490255AEasy to separateHigh efficiency and low consumption separationWater/sewage treatment by sorptionResource recoveryWastewater
A process for recovering the p-nitrochlorobenzene from the sewage containing nitrochlorobenzene and nitrophernol by the adsorption of HZSM-5 zeolite includes such steps as treating the granular H2SM-5 zeolite by diluted HNO3, baking, loading it in an enclosed adsorptive container, delivering said sewage into the container, and adsorbing at 275-340 deg.K and 50-200 rpm for 10s-12hr. Its advantages are high effect and low consumption.
Owner:NANJING UNIV +1
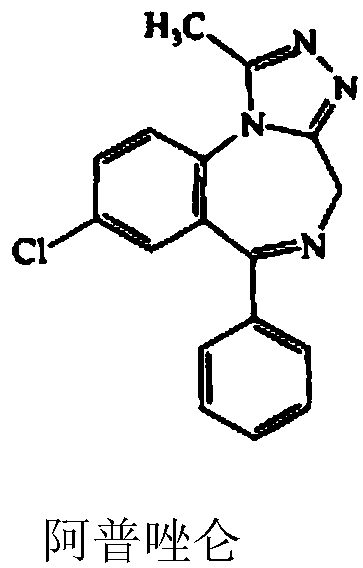
Preparation method of alprazolam intermediate
InactiveCN108250091AInhibition of dissolutionReduce pollutionOrganic chemistryOrganic compound preparationSodium hydroxideMethanol
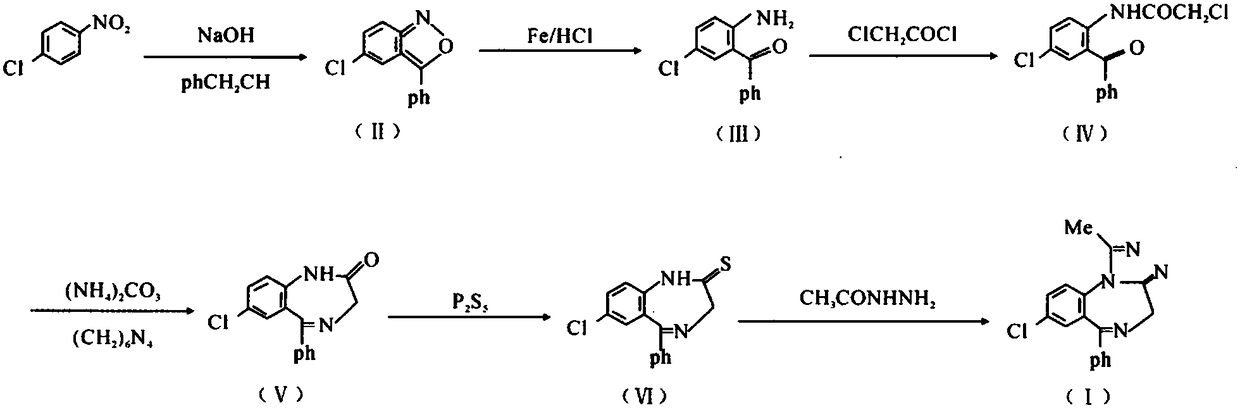
The invention discloses a preparation method of an alprazolam intermediate. The method comprises the following steps: sequentially adding methanol and p-nitrochlorobenzene in a sodium hydroxide watersolution, dropwise adding phenylacetonitrile under stirring, reacting at 40 + / -5 DEG C after the dropwise addition is finished, and carrying out post-treatment to obtain an intermediate (II) ; and dissolving the intermediate (II) in ethanol, adding iron powder, refluxing, dropwise adding sulfuric acid, refluxing for 1 hour, and carrying out post-treatment to obtain an intermediate(III). Accordingto the method disclosed by the invention, the sodium hydroxide water solution is used in the synthesis of the intermediate (II) to replace a dangerous mode of refluxing and dissolving solid sodium hydroxide by using ethanol, a small amount of methanol is added into the reaction system, and the problem of two-phase reaction is successfully solved. The sulfuric acid is used for replacing volatile hydrochloric acid during the synthesis of the intermediate (III), the reaction time is effectively shortened, and the reaction efficiency is improved. The method disclosed by the invention is safer to operate, short in reaction time and more suitable for industrial production.
Owner:山东安信制药有限公司
Method for continuous production of paranitroaniline by series-connected kettles
ActiveCN103130655AIncreased chance of collisionGuaranteed pressureOrganic compound preparationAmino compound preparationP-NitroanilineHigh pressure
The invention provides a method for continuous production of paranitroaniline by series-connected kettles. The method comprises the following steps of: adding ammonia water with mass percentage concentration of 20%-50% to two or more than two high-pressure ammonolysis kettles which are connected in series, stopping charging when ammonia water in each ammonolysis kettle accounts for 50% of the volume of the kettle, slowly starting the stirring of the ammonolysis kettle till the speed of the ammonolysis kettle rises to 200-600 r / m, then adding p-nitrchlorobenzene and ammonia water according to a molar ratio of 1: (2-10) to the first high-pressure ammonolysis kettle connected in series for carrying out continuous ammonolysis reaction when the temperature of each ammonolysis kettle is heated to 120-200 DEG C and the pressure of each ammonolysis kettle is increased to 3-9.5MPa, enabling materials reacted in the last ammonolysis kettle to enter a buffering tank for buffering, then entering a pressure relief cooling kettle for pressure relief and temperature reduction, separating the cooled materials to obtain a solid material, and drying to obtain paranitroaniline. The method has the advantages of being capable of improving the working efficiency and reducing the labor intensity and simultaneously solving the problems of unstable quality, potential safety hazards and the like caused by manual operation.
Owner:绍兴齐越化工科技有限公司
Reclaiming technique by using resin to adsorb nitro chlorobenzene in wastewater from producing nitro chlorobenzene
InactiveCN1562789AReduce contentSolid sorbent liquid separationWater/sewage treatment by sorptionLiquid wasteO-nitrochlorobenzene
The method is to to get rid of mechanical impurity in prodn, waste water of nitrochlorobenzene by pretreatment, then to make it passing through adsorption column filled by styrene-divinylbenzene absorbing resin in flow quantity less than 10 BV / h, the nitrochlorobenzene is selected absorbed on the resin, nitroxylene is not absorbed, quantity of absorbed nitrochlorobenzene is less than 2 mg / L, the resin absorbing nitrochlorobenzene is deadsorbed and regenerated by using water vapour as deadsorpting agent, the generated gas-liquid mixture is cooled and separate to recover nitrochlorobenzene. More than 99 percent of o-nitrochlorobenzene and para-nitrochlorobenzene can be recovered from waste water by this invention. The recovered matter can be send back to recti-ficating production process to be rectificated separated to obtain o-nitrochlorobenzene and para-nitrochlorobenzene products.
Owner:NANJING UNIV
4,4-dinitrodiphenyl ethers synthesis method
InactiveCN1762976AImprove reaction efficiencyEasy maintenanceOrganic chemistryOrganic compound preparationDiphenyl etherPolyol
The present invention discloses the synthesis of 4, 4'-dinitro diphenyl ether. The basic synthesis route is that under the action of proper catalyst and co-catalyst monobasic alcohol or polyhydric alcohol, the material p-nitrochlorobenzene is synthesized into 4, 4'-dinitro diphenyl ether. The synthesis process has yield up to 95 %, high reaction efficiency caused by the co-catalyst, no explosive side product and no corrosive material, and is suitable for industrial production.
Owner:EAST CHINA UNIV OF SCI & TECH
Green synthesis process of 4,4'-dinitrodiphenyl ether
InactiveCN106905163AEliminate distillation recoveryAvoid explosion riskOrganic chemistryOrganic compound preparationEtherSolvent
The invention discloses a green synthesis process of a 4,4'-dinitrodiphenyl ether. The sodium para-nitro phenolate and the p-nitrchlorobenzene are taken as raw materials to conduct a condensation reaction, a condensation material liquid obtained after the reaction is cooled, crystalized and centrifugally separated to obtain a crude product and a mother solution, the crude product is washed to obtain the high-purity 4,4'-dinitrodiphenyl ether, the mother solution is directly used in the following condensation reaction without being treated. The condensation product is obtained through a temperature and speed controlled cooling and crystalizing method, a solvent in the mother solution does not need to be distilled and recovered and is directly used, a 4,4'-dinitrodiphenyl ether crystal prepared through cooling and crystalizing is low in impurity content and good in color and luster; the process flow is simplified, the product purity and the yield of the product are increased, the energy is saved, and the emission is reduced.
Owner:JIANGSU GOLD BRIDGE SALT & CHEM GRP
Method for synthesizing p-dichlorobenzene
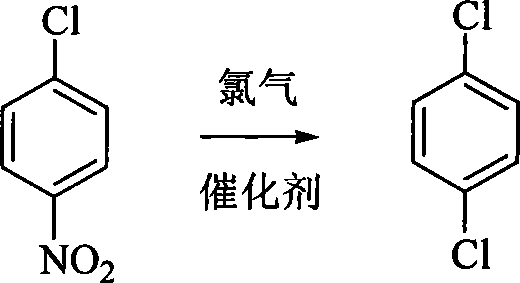
InactiveCN101085713AMild reaction conditionsEasy to operateHalogenated hydrocarbon preparationPeroxideP-dichlorobenzene
The invention provides a method for preparing benzene dichloride. It comprises following steps: ventilizing chlorine gas into dichloronitrobenzene with catalyst existance at 150- 260 Deg. C for reaction for 1- 10 hours and getting coarsebenzene dichloride, treating coarse product and getting benzene dichloride. Said catalyst is oxide or azocompound, the amount of said catalyst is 0.1- 15 of that of p-nitrochlorobenzene. The inveiton is characterized by temperate reaction condition, simple operation, simple device, high product purity of 99.5%, and avoid of difficult separation of dichlorobenzene and trichlorobenzene and even tetrachlorobenzene.
Owner:淮安嘉诚高新化工股份有限公司
Preparing process of 4,4-dinitro diphenyl ether
InactiveCN1986518AReduce pollutionLow priceOrganic chemistryOrganic compound preparationChlorobenzeneReaction temperature
The present invention is preparation process of 4, 4'-dinitro diphenyl ether, and belongs to the field of fine chemical technology. P-nitro chlorobenzene produces condensation reaction inside polar non-protonic solvent at 190-210 deg.c in the presence of catalyst alkali metal carboxylate and alkali metal carbonate, and the reaction product is crystallized to obtain 4, 4'-dinitro diphenyl ether. The present invention features the green production process with less environmental pollution and no nitroxide release, low production cost, high product quality and high product yield.
Owner:忻雪琴
Process of producing nitrobenzether aminobenzether amidobenzether from chlorobenzene
InactiveCN100494159CReduce manufacturing costImprove production stabilityOrganic compound preparationCarboxylic acid amides preparationO-nitrochlorobenzeneChloride salt
Owner:CHANGZHOU JIASEN CHEM +1
Paddy rice herbicide
InactiveCN106561704AReduce drug damageDoes not affect growth and developmentBiocideAnimal repellantsPhytotoxicitySulfite salt
Aiming to liberate hand labor, the invention aims to provide a paddy rice herbicide, which is composed of potassium hydroxide, p-nitrchlorobenzene, phenol, chlorine gas and sodium sulfite. The paddy rice herbicide can kill weeds at the germination period of weeds, has small phytotoxicity to paddy rice, and does not affect the growth and development of paddy rice.
Owner:范玉华
Rectification and separation device and method
The invention discloses a rectification and separation device and method. The rectification and separation device and method are used for separating three isomers, namely m-nitrochlorobenzene, p-nitrochlorobenzene and o-dichlorobenzene. According to the device and the method, a rectifying tower is separated by a partition board arranged in the vertical direction, and majorized technology parameters are adopted, so that using one rectifying tower to complete whole separation of the m-nitrochlorobenzene, p-nitrochlorobenzene and o-dichlorobenzene is realized. Therefore, the rectification and separation device and method provided by the invention can reduce energy consumption, equipment investment and occupied area.
Owner:扬州通扬化工设备有限公司
Preparation method of p-nitrophenol
ActiveCN101759570AEmission reductionReduce water consumptionOrganic chemistryOrganic compound preparationWater dischargeNitrophenol
The invention relates to a preparation method of p-nitrophenol. The method comprises the following technological steps: (1) hydrolysis reaction: putting p-nitrochlorobenzene and caustic soda into a hydrolysis kettle with the molecule mol ratio of p-nitrochlorobenzene to caustic soda being 1:(2.16-2.18) and concentration of caustic soda being 180-300g / L, raising the temperature in the kettle to 150-155 DEG C, stopping raising, then letting natural temperature rise to 162-165 DEG C and pressure to 0.7-0.9MPa, preserving the temperature of the hydrolysis kettle, controlling the temperature in the kettle and pressure respectively at 163-173 DEG C and 0.7-0.9MPa for 2-4h, agitating continuously during reaction to obtain reactants; (2) crystallization; (3) primary separation; (4) slurry conditioning; (5) acidification; and (6) secondary separation. Compared with background technology, the preparation method in the invention has the advantages of high production efficiency, less waste water discharge and low energy consumption.
Owner:ANHUI BAYI CHEM IND
System for separating and reutilizing meta-position oil in nitrochlorobenzene production and meta-position oil separating method
PendingCN109369409ALower conversion rateHigh yieldOrganic chemistryOrganic compound preparationSodium methoxideEngineering
The invention provides a system for separating and reutilizing meta-position oil in nitrochlorobenzene production. The system comprises a first rectifying tower, an etherification device, a metering input device, a first dealcoholization kettle, a washing tank, a second rectifying tower and a third rectifying tower, wherein the metering input device is used for storing a sodium methylate solutionand is connected with a sodium methylate inlet so as to control the adding mode of the sodium methylate solution entering the etherification device. Meta-position oil is firstly subjected to rectification separation, and then p-nitrchlorobenzene and nitrochlorobenzene are fed into the etherification device for reaction; in an etherification step, due to the fact that the reaction activity of the sodium methoxide and p-nitrchlorobenzene are highest and the reaction activity of the nitrochlorobenzene is lower, sodium alkoxide is added by adopting a metering and continuously dropping way, excessive sodium methoxide for nitrochlorobenzene reaction is not provided, the sodium methoxide does not react with the nitrochlorobenzene, the way of converting the nitrochlorobenzene into other heavy component impurities is cut off, and the yield of the nitrochlorobenzene is ensured.
Owner:宁夏华御化工有限公司
Synthesis method of paranitroaniline
ActiveCN110372515AResponses are controllable and easily scalableFlexible quality controlOrganic compound preparationChemical recyclingSynthesis methodsReaction temperature
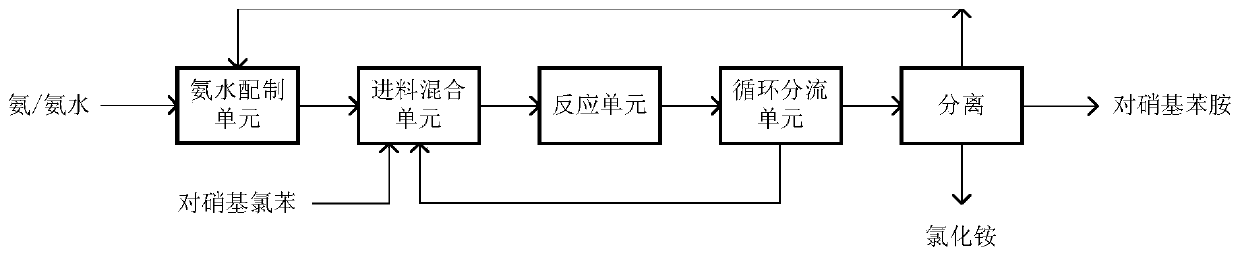
The invention discloses a continuous synthesis method of paranitroaniline. The continuous synthesis method comprises the following steps: 1) preparing ammonia water by using an ammonia water preparation unit, respectively and continuously inputting the prepared ammonia water and molten p-nitrochlorobenzene into a feeding and mixing unit, carrying out preheating to a reaction temperature, and then,mixing the materials; 2) making the mixed materials flow into a tubular reactor for a reaction, then, making the reacted materials flow into a shunt circulation unit, and dividing the materials intoa circulating material and an outflow material according to a certain recycle; and 3) returning the circulating material to the feeding and mixing unit, and sequentially carrying out continuous onlinemixing on the circulating material as well as the ammonia water and the p-nitrochlorobenzene preheated to the reaction temperature; and cooling the outflow material to crystallize to obtain paranitroaniline with the yield reaching up to 99% or above. The method disclosed by the invention simultaneously has the advantages such as high production efficiency, good safety, low equipment material requirement, flexible and easily-controlled production process and low comprehensive cost and meets the requirements for safety, greenness and high efficiency.
Owner:ZHEJIANG DIBANG CHEM +1
Rice herbicide
InactiveCN105454229AReduce drug damageDoes not affect growth and developmentBiocideAnimal repellantsSulfite saltPotassium hydroxide
The invention aims to liberate manual labor, and provides a rice herbicide, comprising potassium hydroxide, p-nitrchlorobenzene, phenol, chlorine and sodium sulfite. The invention has the advantage that weed killing at the germination period of weeds has little injury on rice, and does not affect the growth and development of rice.
Owner:丁成斌
Preparation method of high-purity p-nitrochlorobenzene
PendingCN113024383AImprove qualityIncrease economic benefitsOrganic compound preparationNitro compound preparationChlorobenzeneNitration
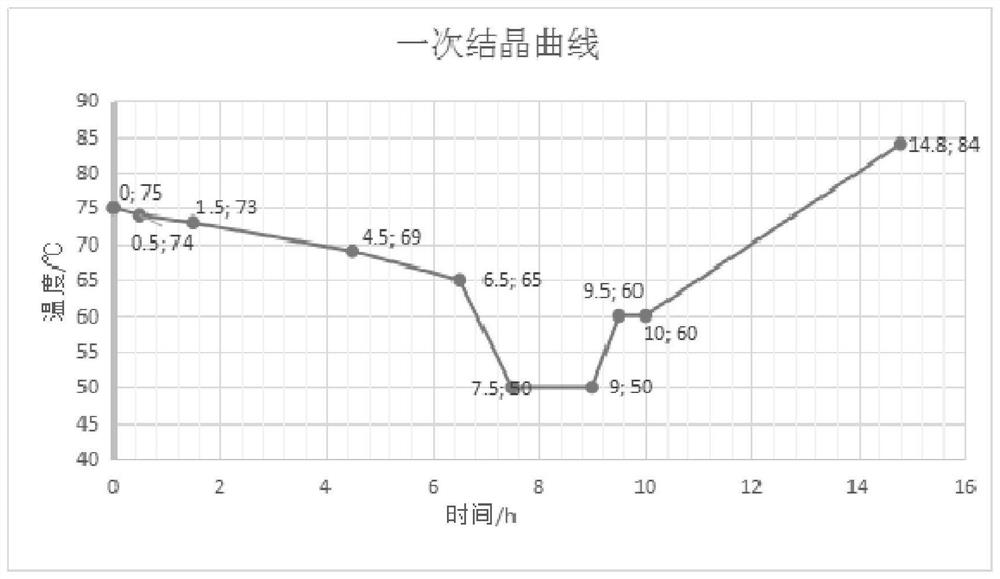
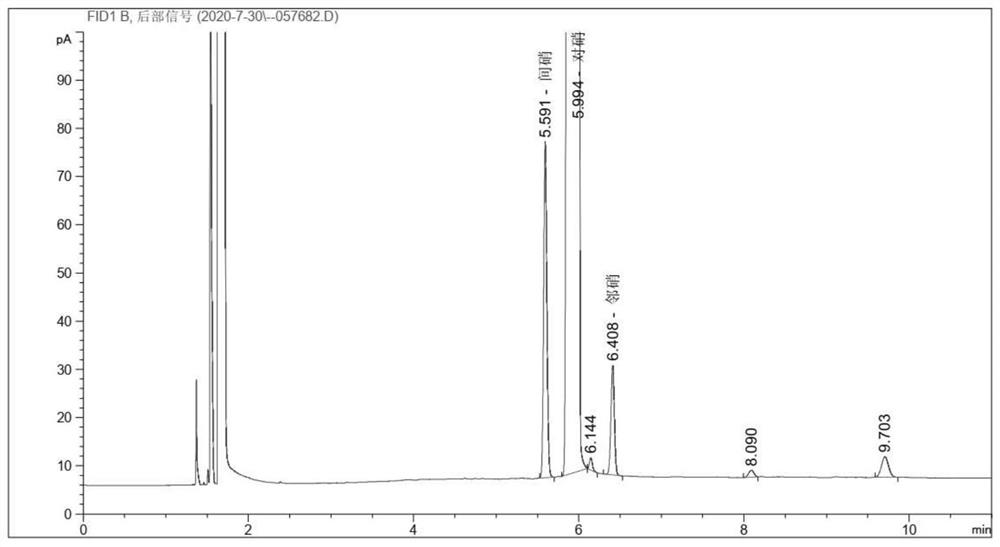
The invention provides a preparation method of high-purity p-nitrochlorobenzene, and belongs to the technical field of chemical separation. The method comprises the following steps: preparing nitric acid and sulfuric acid into mixed acid, and carrying out nitration reaction on the mixed acid and chlorobenzene to generate nitrochlorobenzene; making the nitrochlorobenzene sequentially subjected to oleic acid separation, alkali washing and water washing to obtain neutral nitrochlorobenzene; and drying the neutral nitrochlorobenzene, and separating the finished product p-nitrochlorobenzene by adopting a two-time crystallization process. By adopting twice crystallization and adjusting parameters of the crystallization process, the purity of the finished product p-nitrochlorobenzene reaches 99.9% or above on the basis of ensuring the yield of p-nitrochlorobenzene, so that the quality of p-aminoanisole produced by taking p-nitrochlorobenzene as a raw material is improved, the market demand is met, and the economic benefit is increased.
Owner:宁夏华御化工有限公司
Method for preparing aminodiphenyl ether by p-nitrchlorobenzene serving as raw material
ActiveCN107915644AHigh yieldHigh purityOrganic compound preparationChemical recyclingPolymer scienceEther
The invention relates to a preparation method of aminodiphenyl ether, in particular to a method for preparing aminodiphenyl ether by p-nitrchlorobenzene serving as a raw material. The method includesthe steps: etherification reaction; oil-water phase separation of etherification products; catalytic hydrogenation reaction; filtering; rectification and the like. The method for preparing the aminodiphenyl ether by the p-nitrchlorobenzene serving as the raw material is clean, environmentally friendly, simple to operate, low in energy consumption and pollution-free in environment, and the aminodiphenyl ether prepared by the method is high in yield and purity. According to the method for preparing the aminodiphenyl ether by the p-nitrchlorobenzene serving as the raw material, phase transfer catalysts are less in dosage, and fewer by-products are generated in the reaction process.
Owner:JIANGSU ZHONGDAN CHEM TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com