Medicine composite used for embolotherapy and acesodyne and preparation method thereof
A composition and drug technology, applied in the field of interventional medicine, can solve problems such as inability to maintain pain relief, and achieve the effects of reducing toxic side effects and reducing drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: microsphere preparation method
[0042] Using the reverse phase suspension polymerization method, add 50ml of liquid paraffin and an appropriate amount of Span80 in a three-necked flask, nitrogen, and then dissolve polyvinyl alcohol, acrylic acid, N, N'-methylenebisacrylamide (crosslinking agent), 12.5 ml of a solution of potassium persulfate (initiator) was added dropwise to the oil phase at 55° C., and after pre-crosslinking for 10 minutes, tetramethylethylenediamine (catalyst) was added and reacted for 4 hours under stirring at 500 rpm. Wash the microspheres, sieve out different specifications according to the particle size, and collect the microspheres in each particle size range.
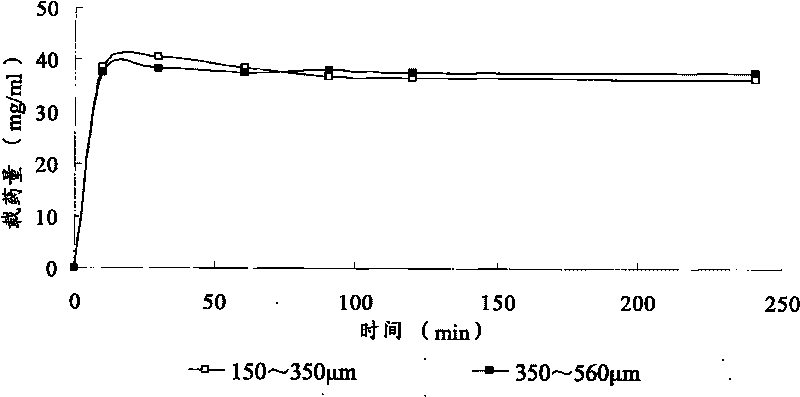
[0043] By changing the amount of each component, two batches of microspheres were prepared. After sieving, the microspheres with particle sizes ranging from 150-350 μm, 350-560 μm and 560-710 μm were collected for use in the following examples.
Embodiment 2
[0044] Embodiment 2: the mensuration of microsphere exchange capacity
[0045]Accurately weigh 100mg of dry microspheres, fully soak in hydrochloric acid solution, wash with distilled water, blot the surface moisture, place in a 50ml stoppered Erlenmeyer flask, add 25ml of 0.1mol / L sodium hydroxide solution, heat in a water bath at 60°C for 2h, After cooling to room temperature, take out 5ml of the solution, use phenolphthalein as indicator, and titrate with 0.1mol / L hydrochloric acid standard solution; at the same time, conduct blank control test. The volume of consumed hydrochloric acid standard solution is denoted as V 样品 and V 空白 . The formula for calculating the exchange capacity Q is: Q=(V 空白 -V 样品 )×5×0.1 / 100. The two batches of microspheres prepared in Example 1 were measured according to the above method, and their exchange capacities were 10.6 mol / mg and 12.6 mol / mg respectively.
Embodiment 3
[0046] Embodiment 3: microsphere loading lidocaine hydrochloride
[0047] Establishment of the standard curve: Dilute the lidocaine hydrochloride stock solution into 100, 200, 250, 300, 350, 400, 450 and 500 μg / ml sample solutions respectively, and measure the absorbance at the point of maximum absorption at 261 nm. Do linear regression with drug concentration (C) to absorbance (A), and the resulting standard curve equation is: A=0.0015C+0.0025, R 2 = 0.9999.
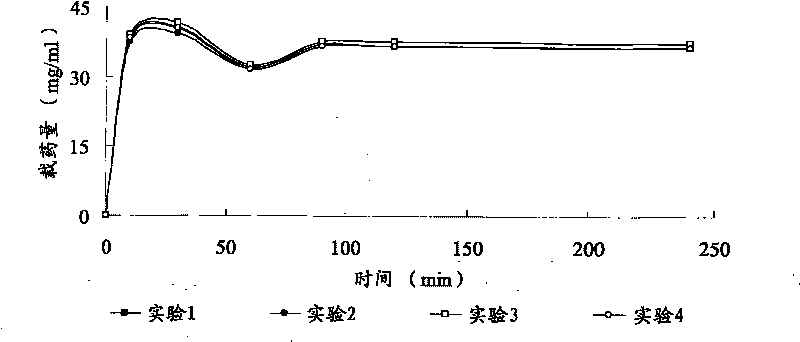
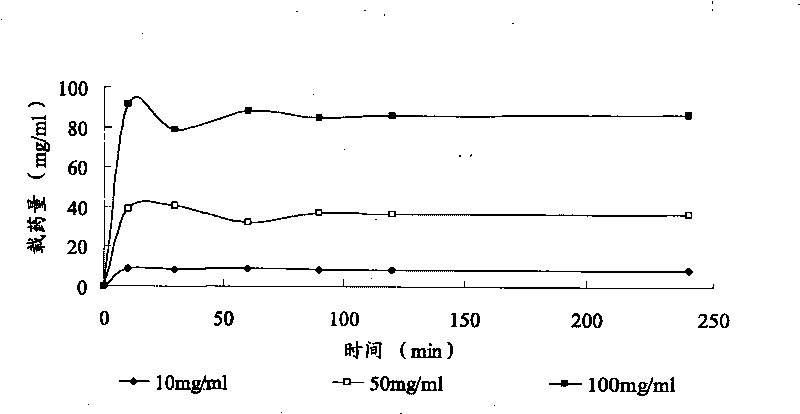
[0048] Drug loading method: Take 1ml of microspheres with a certain particle size range, put them into a 10ml vial, add 5ml of lidocaine hydrochloride solution of known concentration, and take samples regularly at 0, 10, 30, 60, 90, 120, and 240 minutes 50 μl, the absorbance was measured at 261 nm, and the absorbance value was substituted into the standard curve to calculate the drug concentration contained in the sample.
[0049] Calculation of drug loading: drug loading = (drug content in solution before drug loadin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com