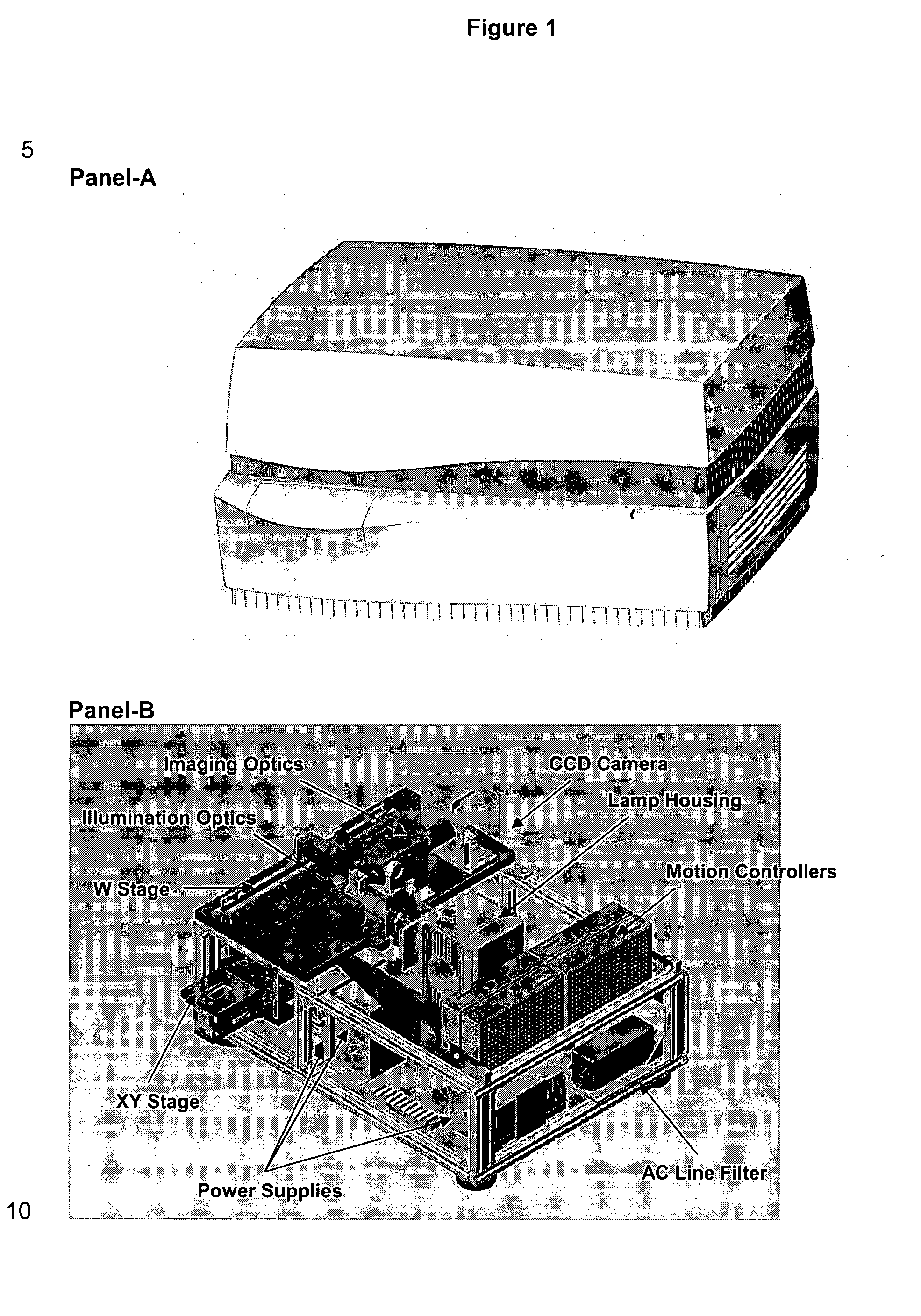
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
42 results about "Alternative therapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
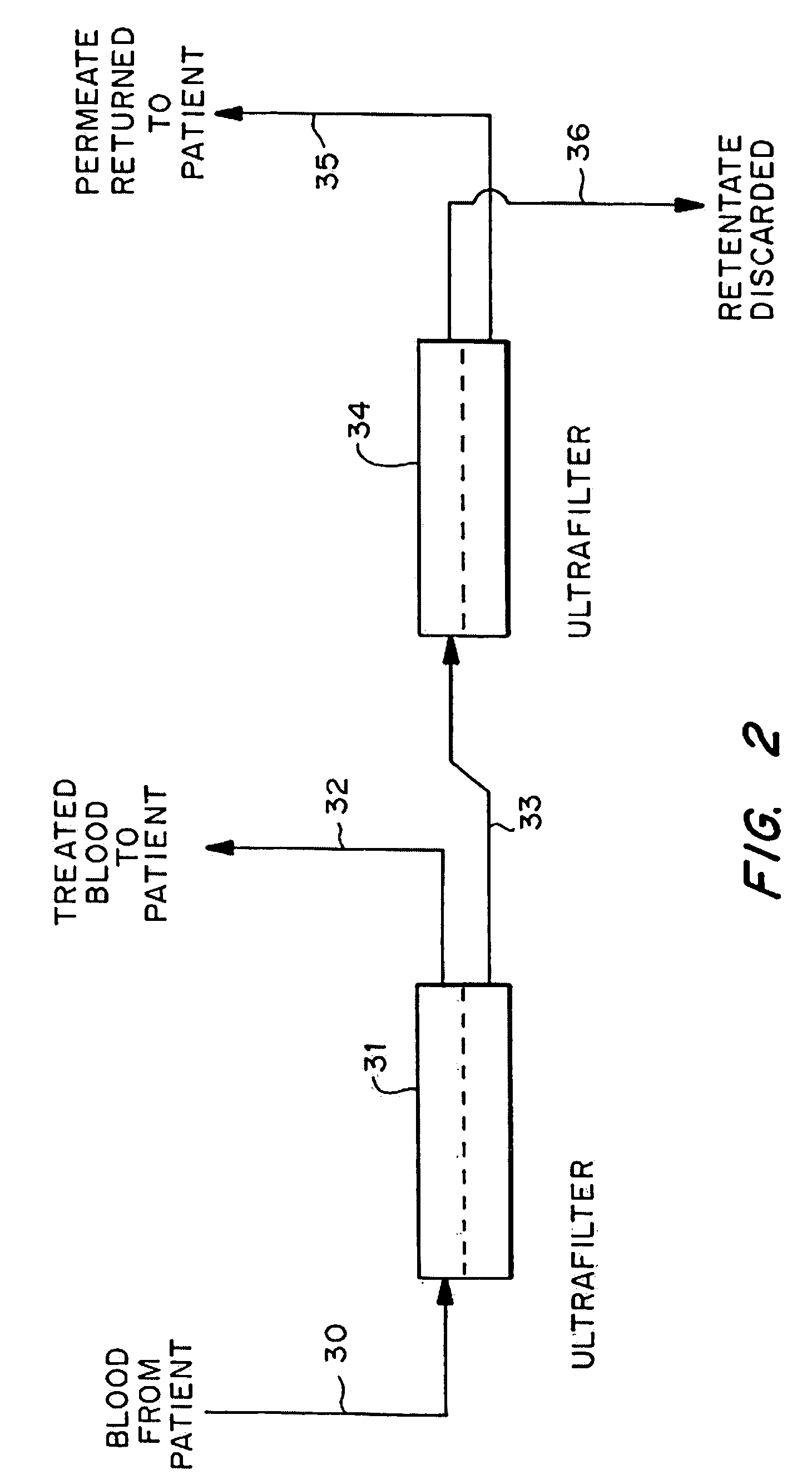
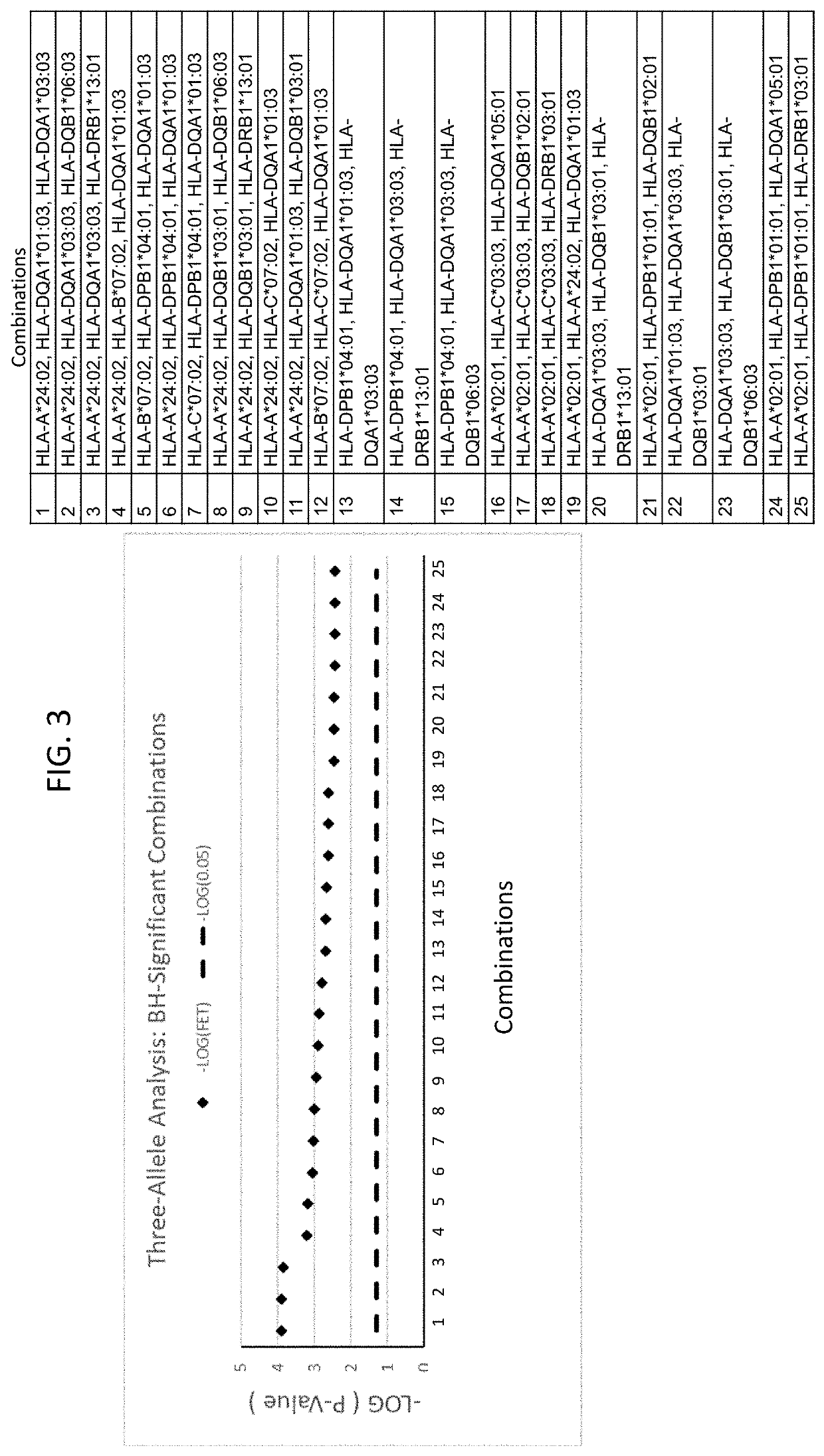
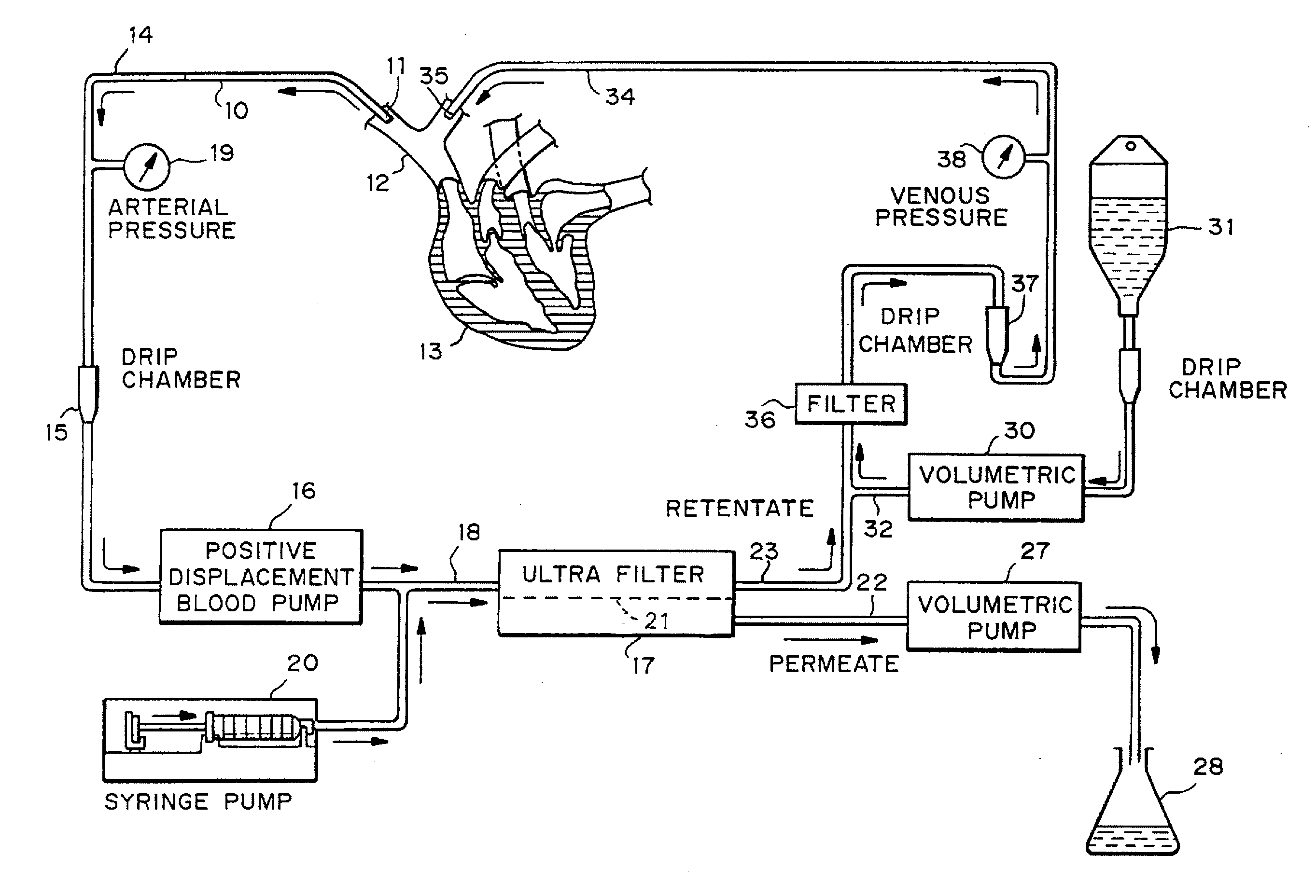
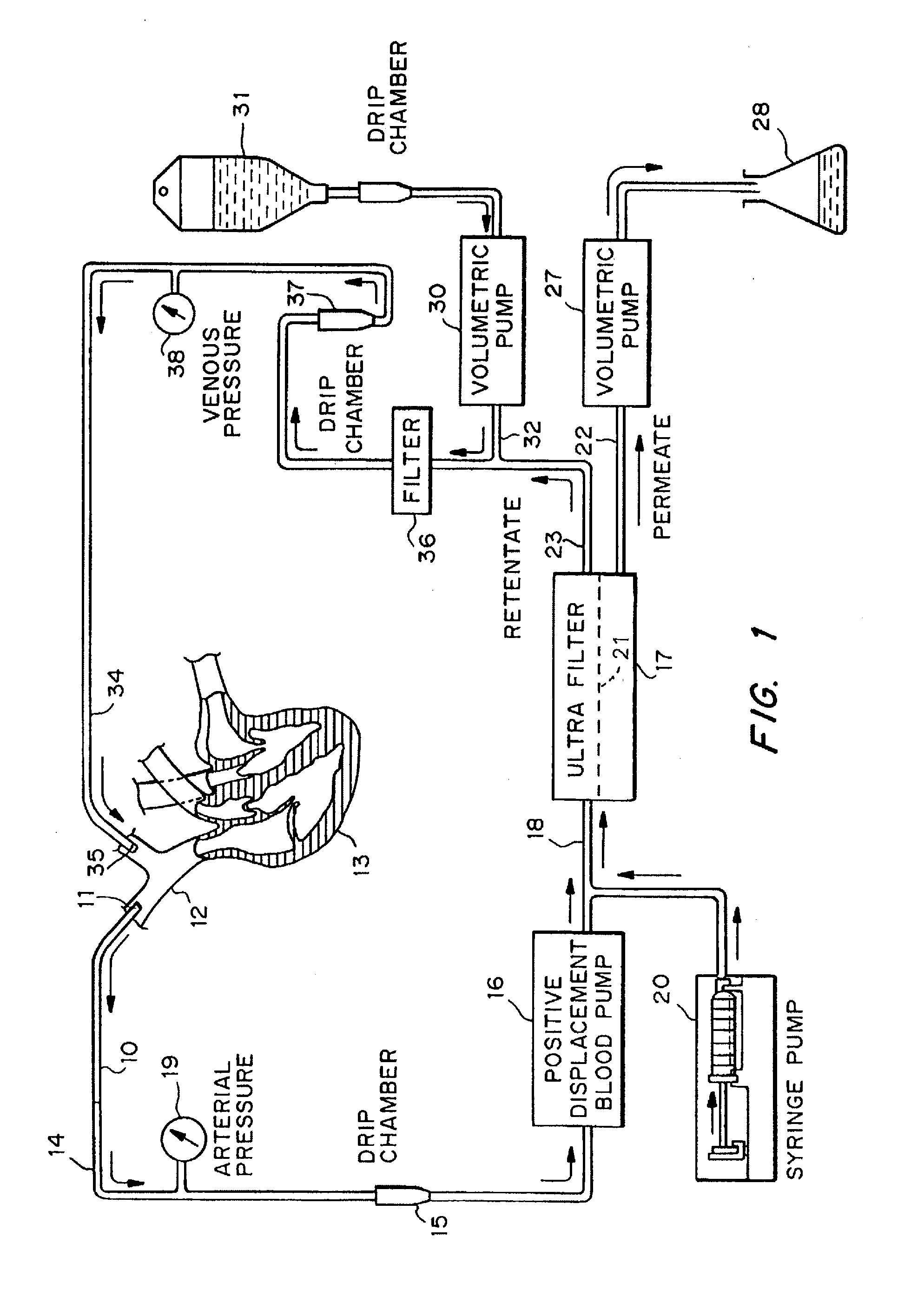
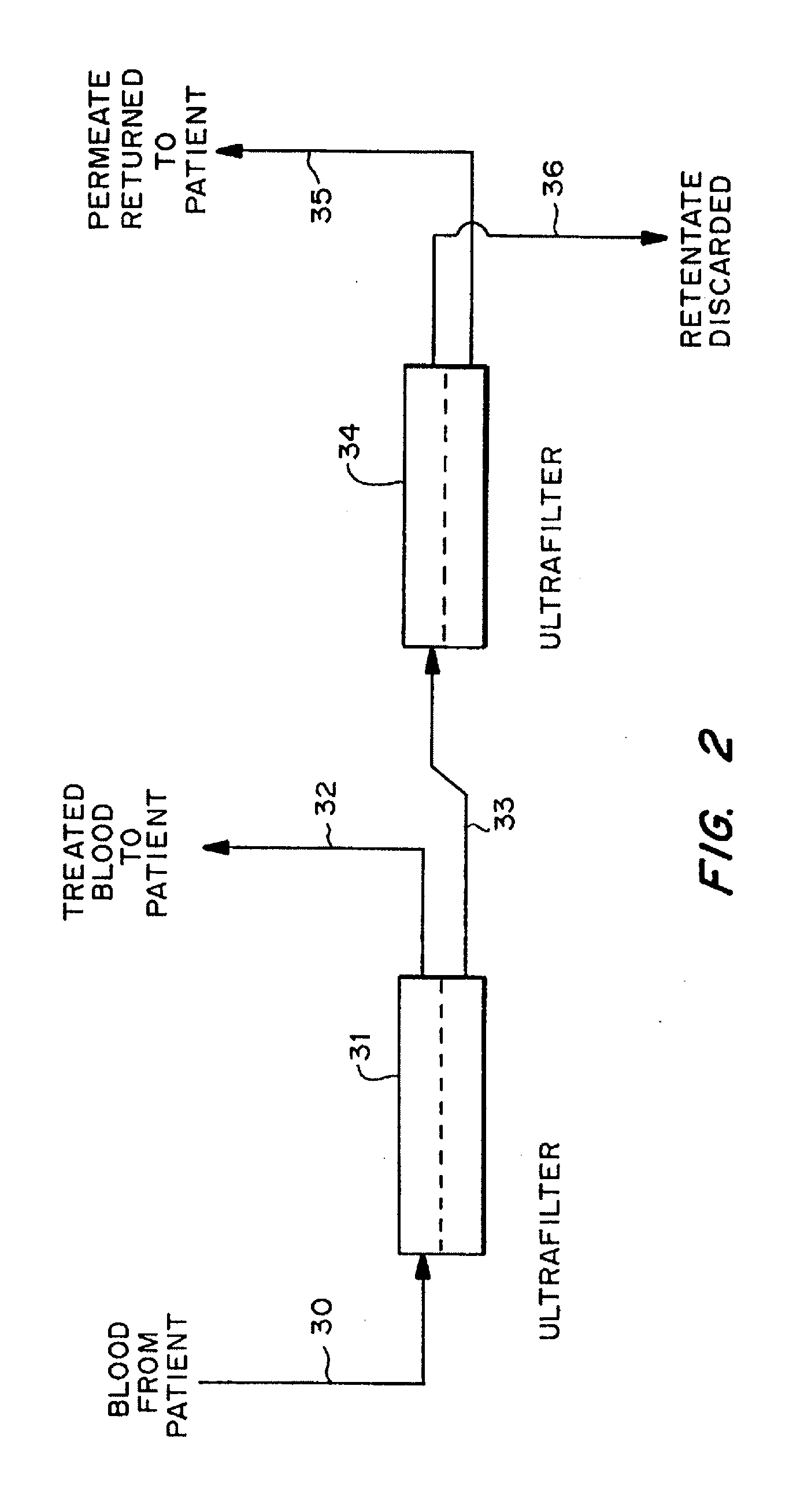
Cardiac rhythm management device and sensor-suite for the optimal control of ultrafiltration and renal replacement therapies
ActiveUS20070175827A1ElectrotherapyMechanical/radiation/invasive therapiesUltrafiltrationOptimal control
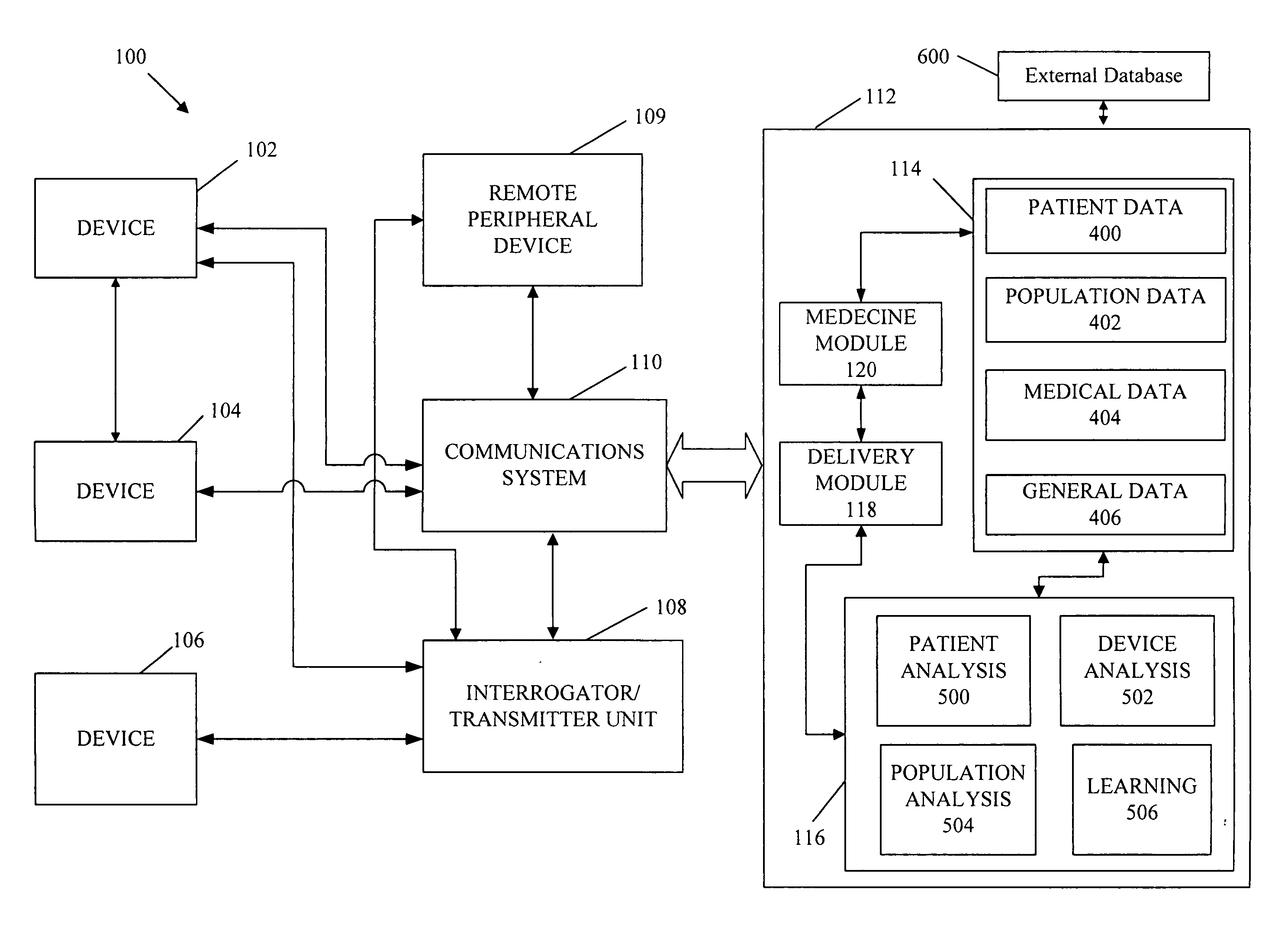
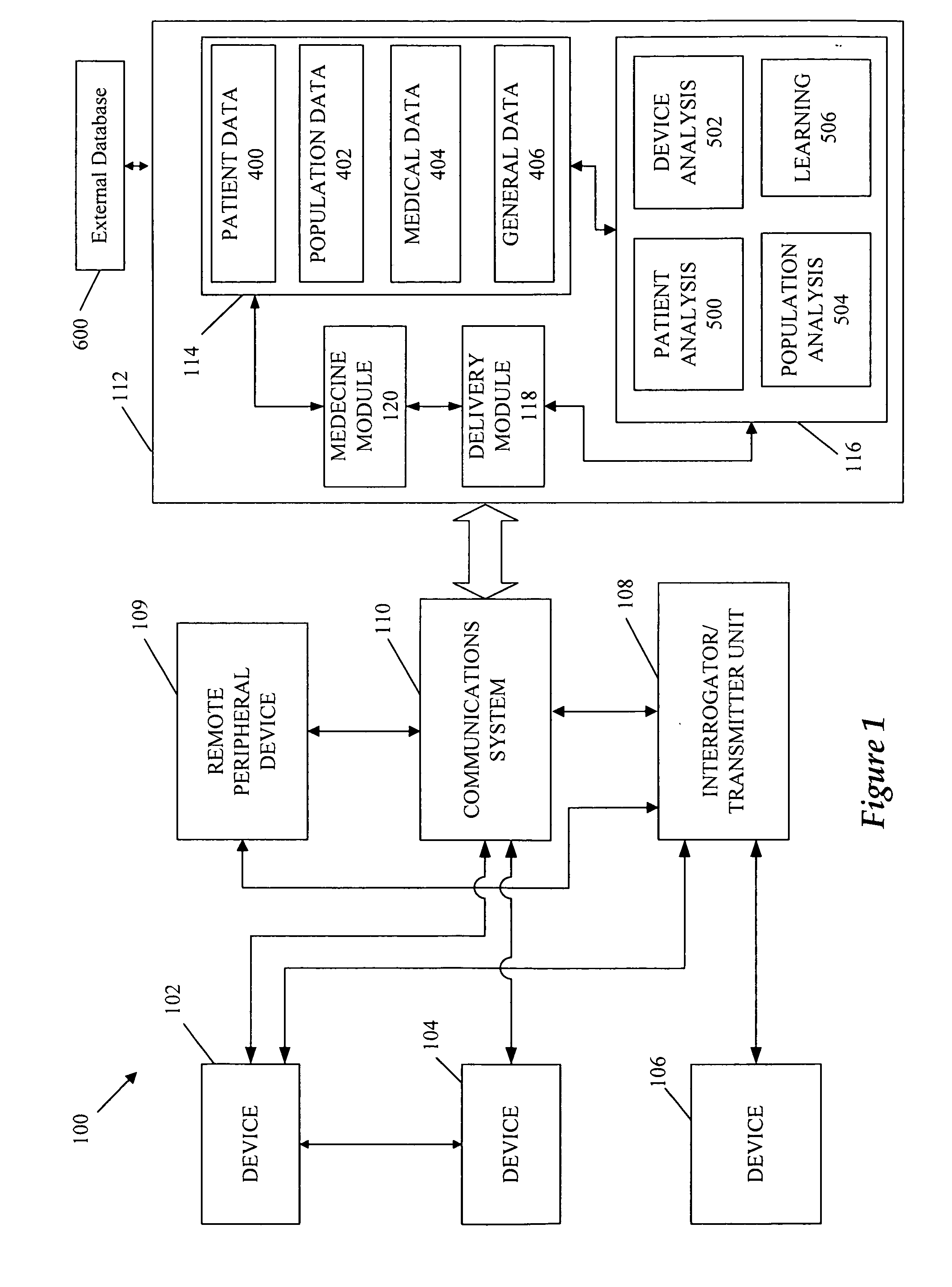
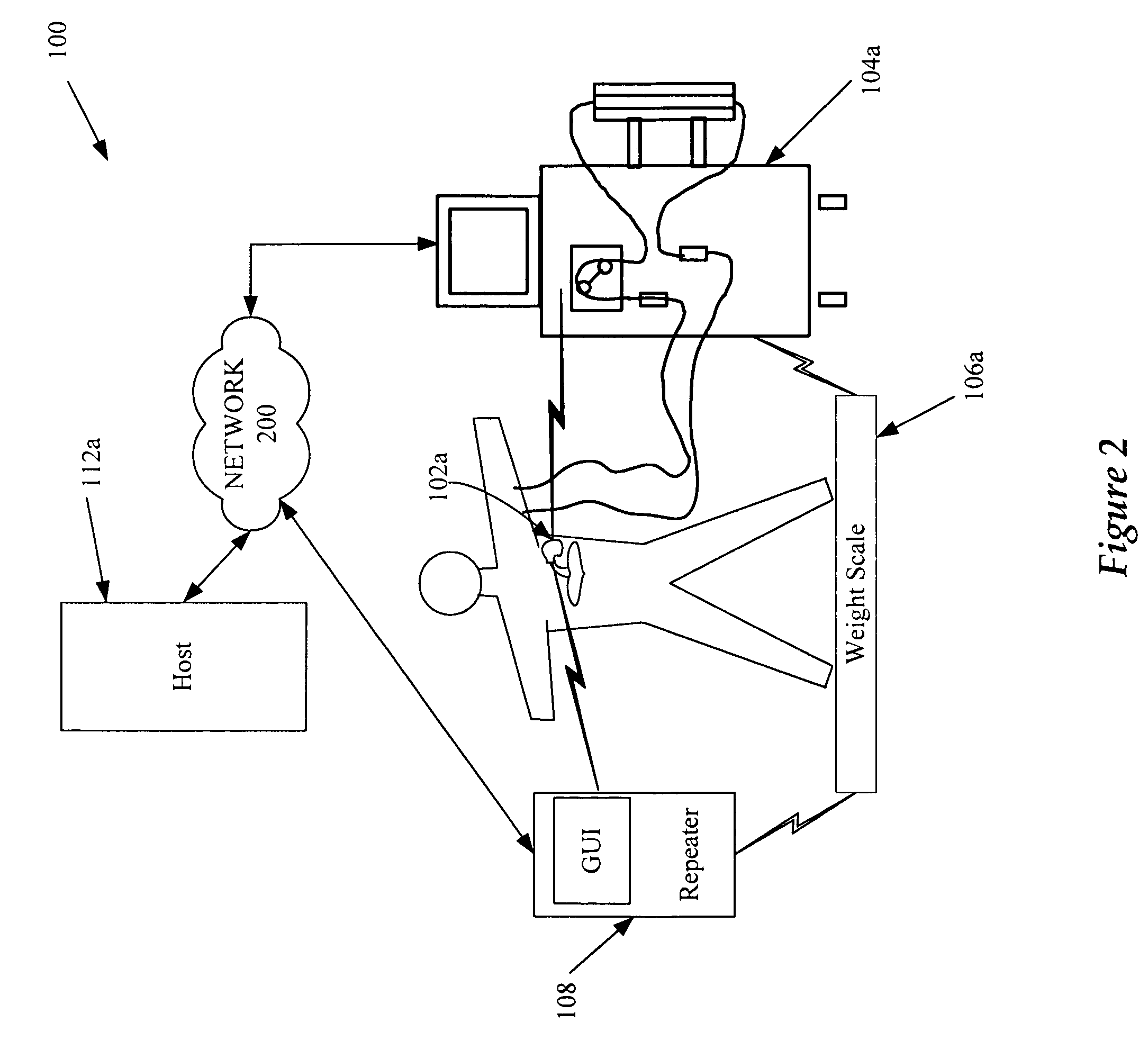
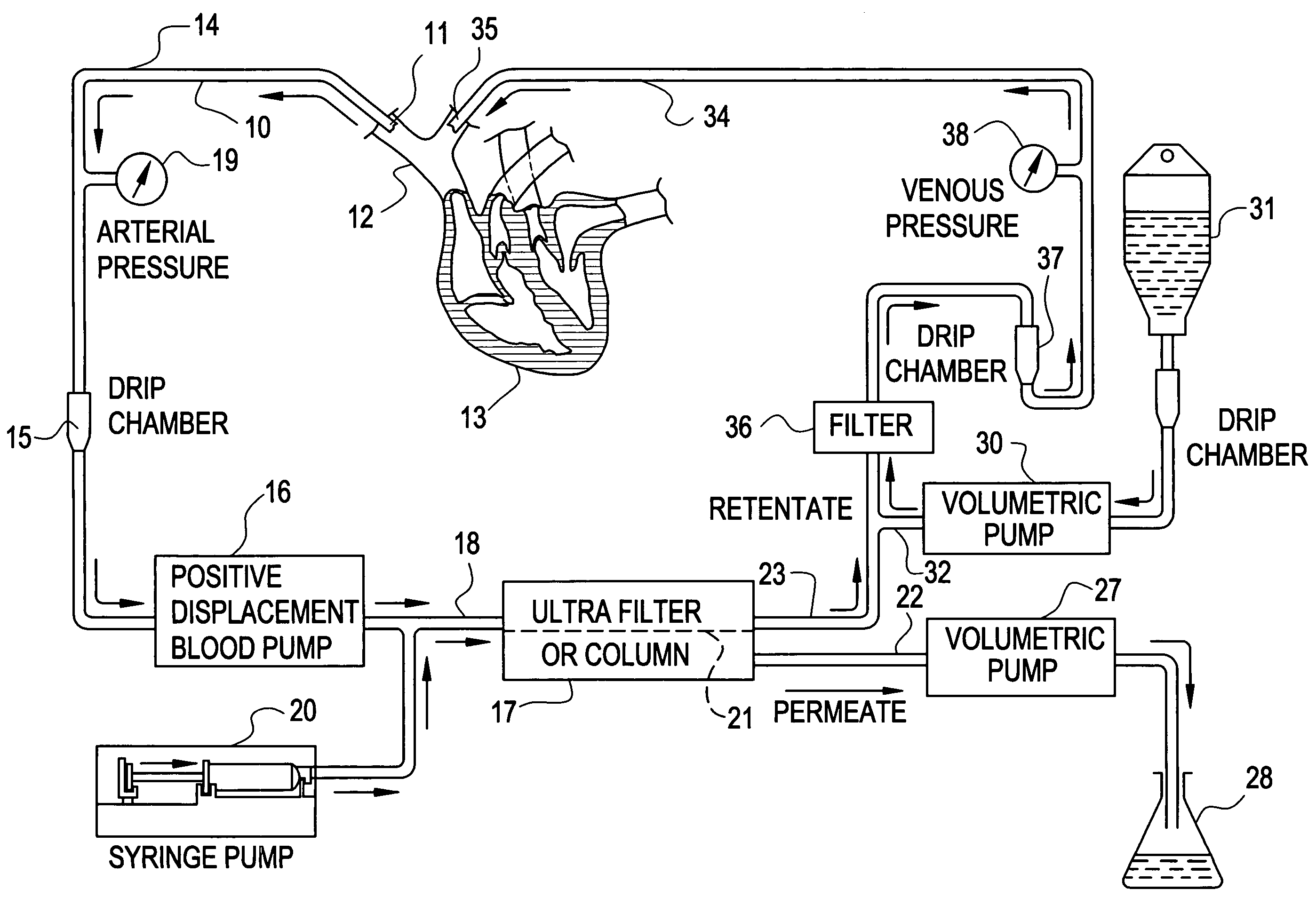
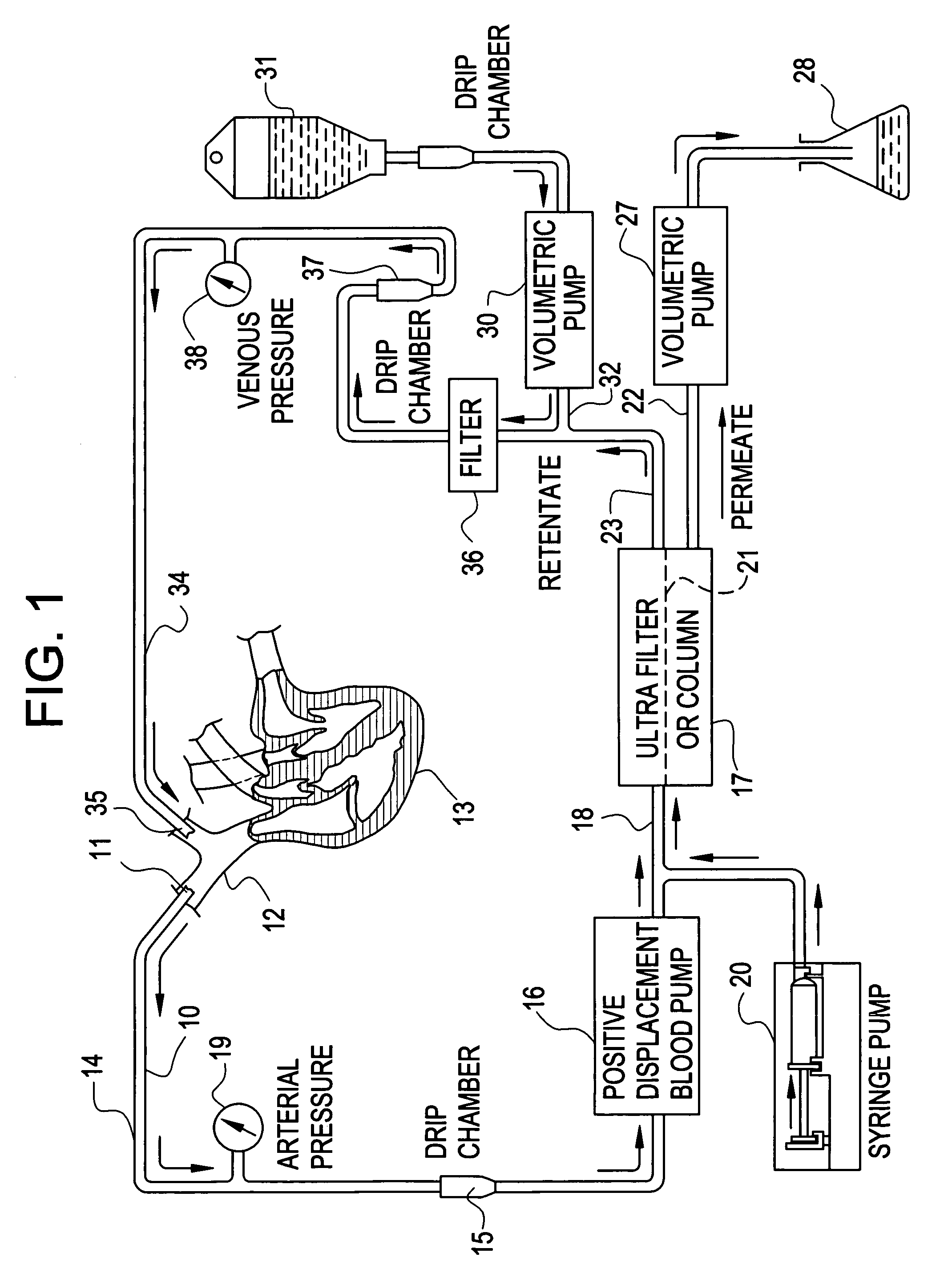
A cardiorenal patient monitoring system comprising, either implanted or non-implanted device(s), remote peripheral device(s), computer network(s), host, and communication means between the device(s), computer network(s), and host. The preferred embodiment shows an advanced patient monitoring system for using an implanted cardiac device and a dialysis machine in renal therapy. In addition, the method of advanced patient monitoring is in conjunction with the advanced patient monitoring system is disclosed.
Owner:CARDIAC PACEMAKERS INC
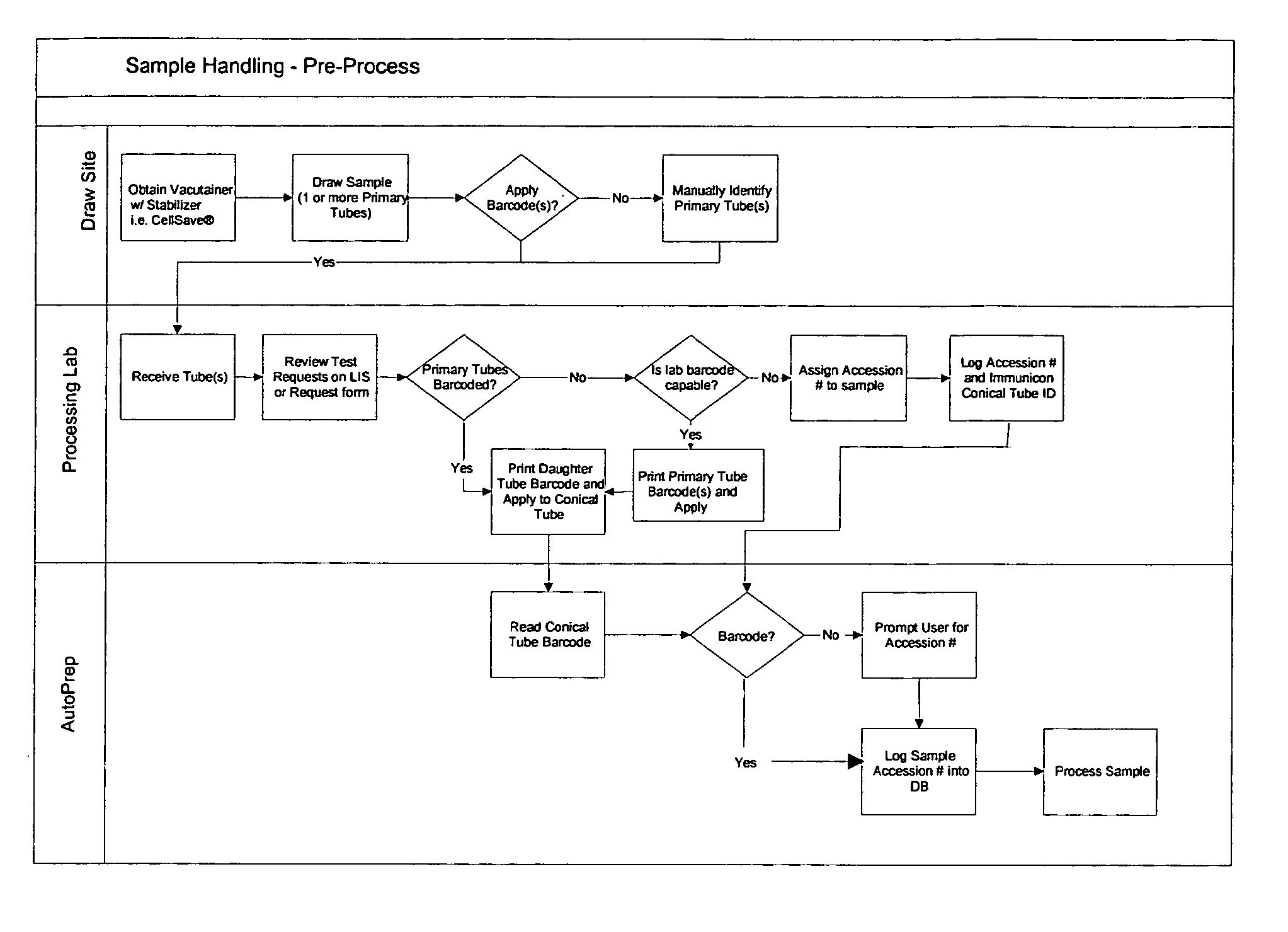
Operator independent programmable sample preparation and analysis system
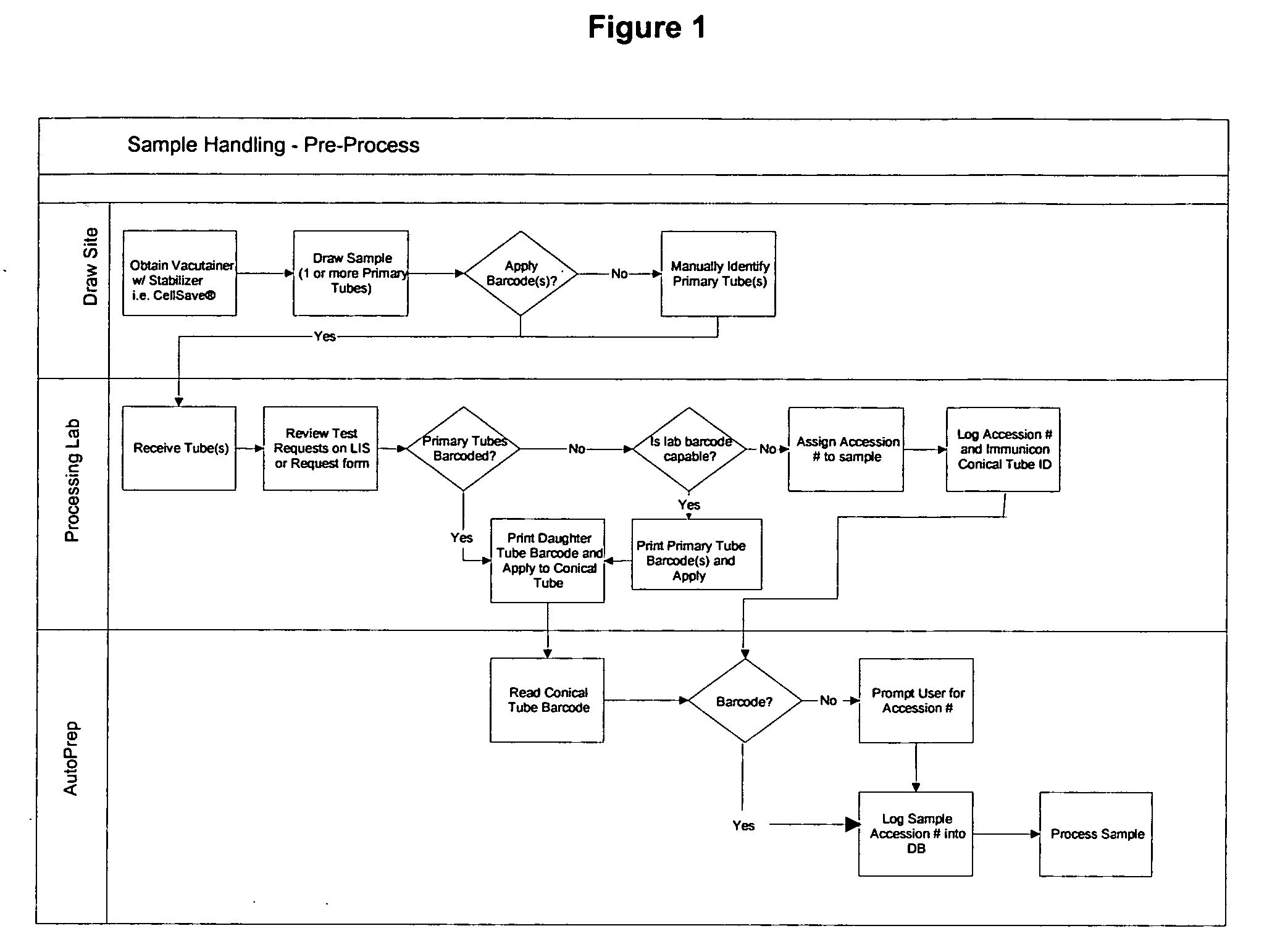
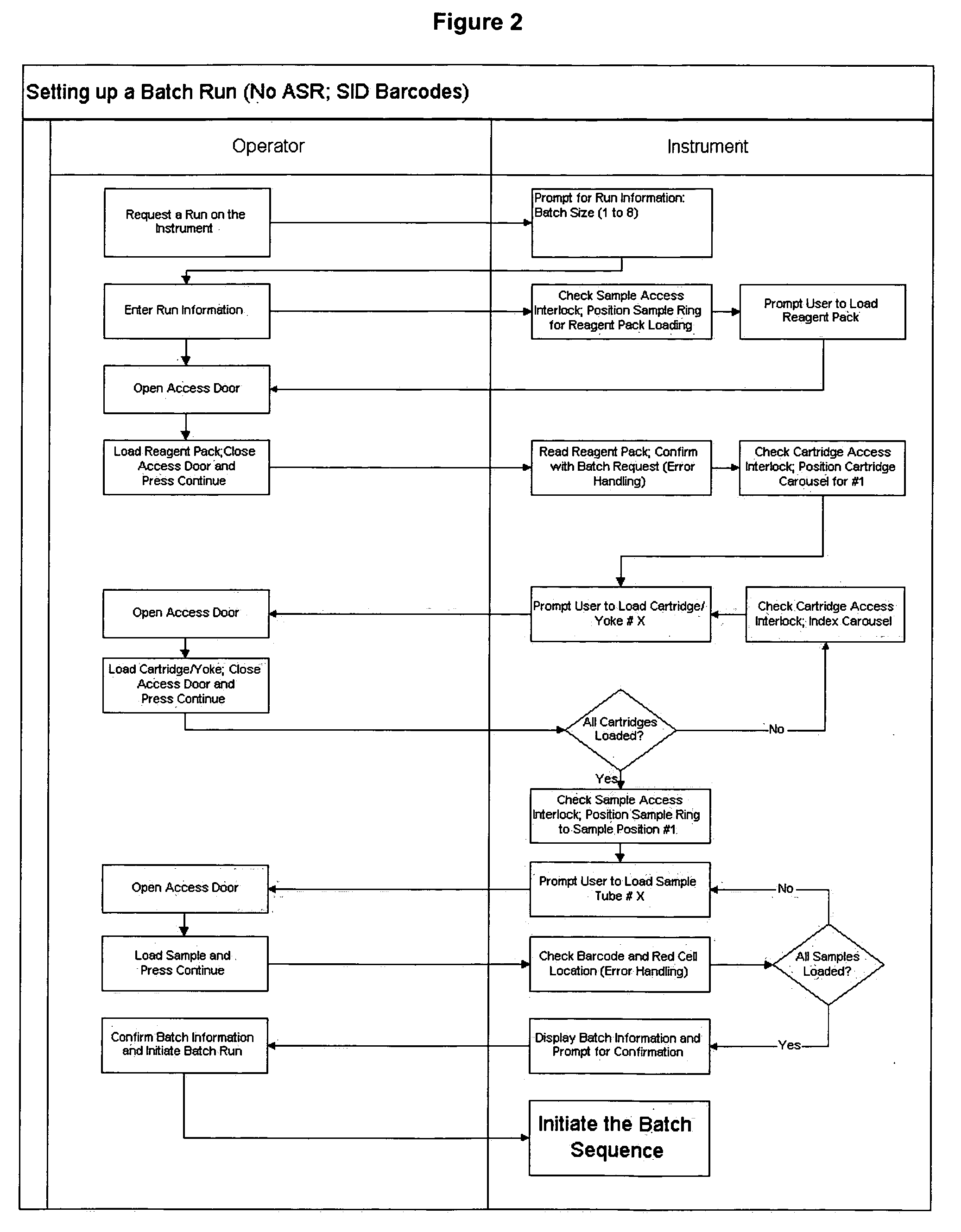
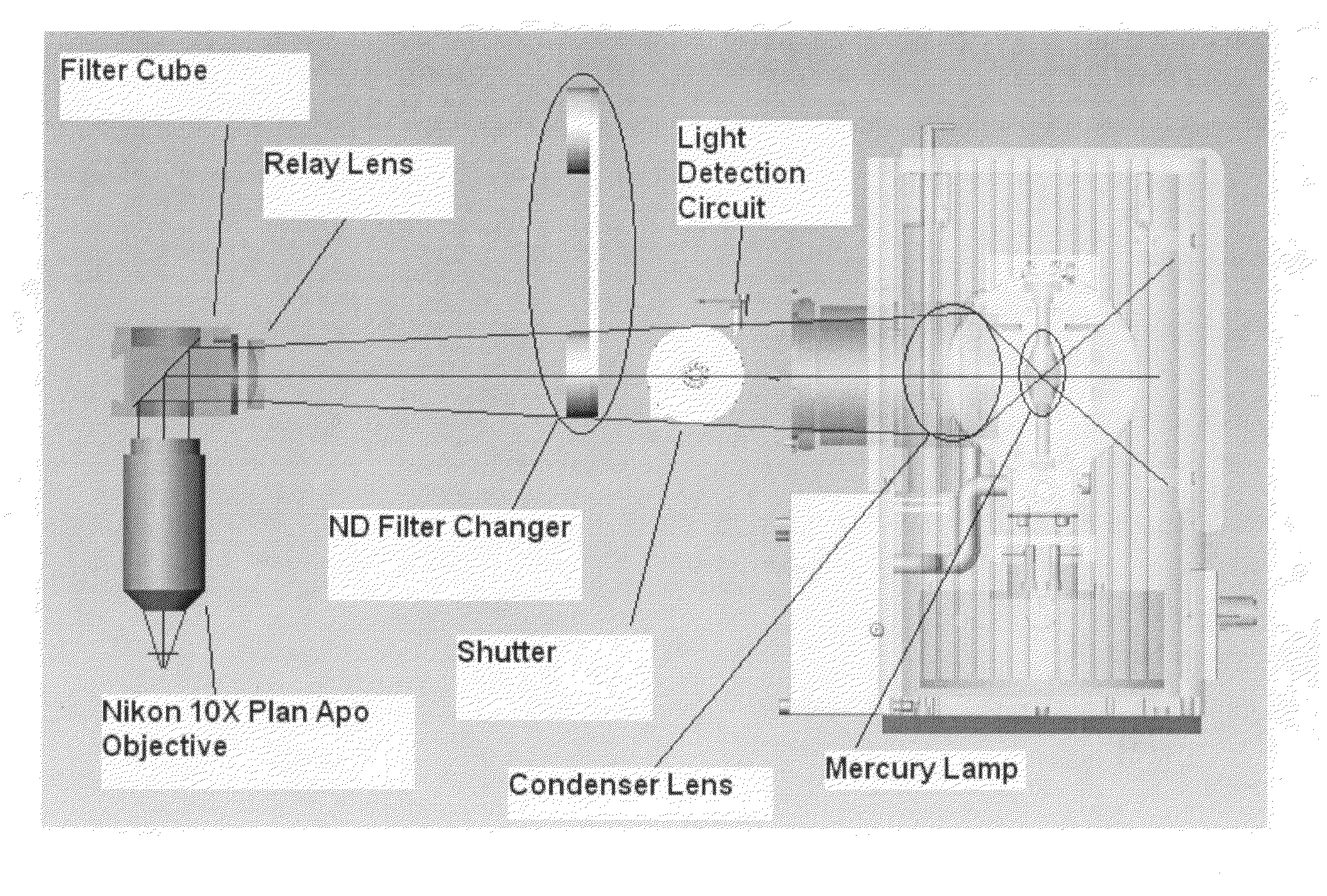
ActiveUS20070212698A1Minimal operator interactionImprove processing efficiencyBioreactor/fermenter combinationsBiological substance pretreatmentsRegimenHandling system
The present invention provides a protocol and apparatus for enriching circulating tumor cells and other rare cells from blood, including debris and other components, from samples with high precision and at high throughput rates. This invention discloses an improved processing system from previously described semi-automated sample processing. The system further reduces operator intervention and hands-on time from prior systems. While this system has general utility in processing diverse materials, the system is configured for sample processing of biological specimens to provide an enriched fraction suitable for detection, enumeration and identification of target cells by appropriate analytical methodologies. The presence and quantities of such target cells in a sample specimen can utilized for screening and detection in disease such as cancer, assessing early stage pre-metastatic cancer, monitoring for disease remission in response to therapy and selection of more effective dose regimens or alternative therapies in case of relapse.
Owner:MENARINI SILICON BIOSYSTEMS SPA
Methods of assessing the need for and the effectiveness of therapy with antioxidants
InactiveUS20050202521A1Efficient measurementIncrease synthesisBiocideAntimycoticsProper treatmentCvd risk
The invention relates to diagnostic methods for assessing the need of a subject for treatment with an anti-oxidant, or alternatively, for determining the utilization efficiency and ultimate effectiveness of anti-oxidant therapy in subjects having been treated with antioxidants. More specifically, the methods of the present invention are particularly useful in prophylactic assessment of individuals at risk for developing diseases or conditions in which oxidative stress plays a role, such that an appropriate therapeutic regimen can be prescribed for that individual, thus leading to alternative therapies and / or life style changes. The invention further relates to methods for assessing the need for, the utilization efficiency and the effectiveness of therapy in subjects having received therapy with specific antioxidant and immune enhancing formulations. Kits are also provided for measuring the levels of markers of oxidative stress and immune cell numbers.
Owner:CRUM ALBERT
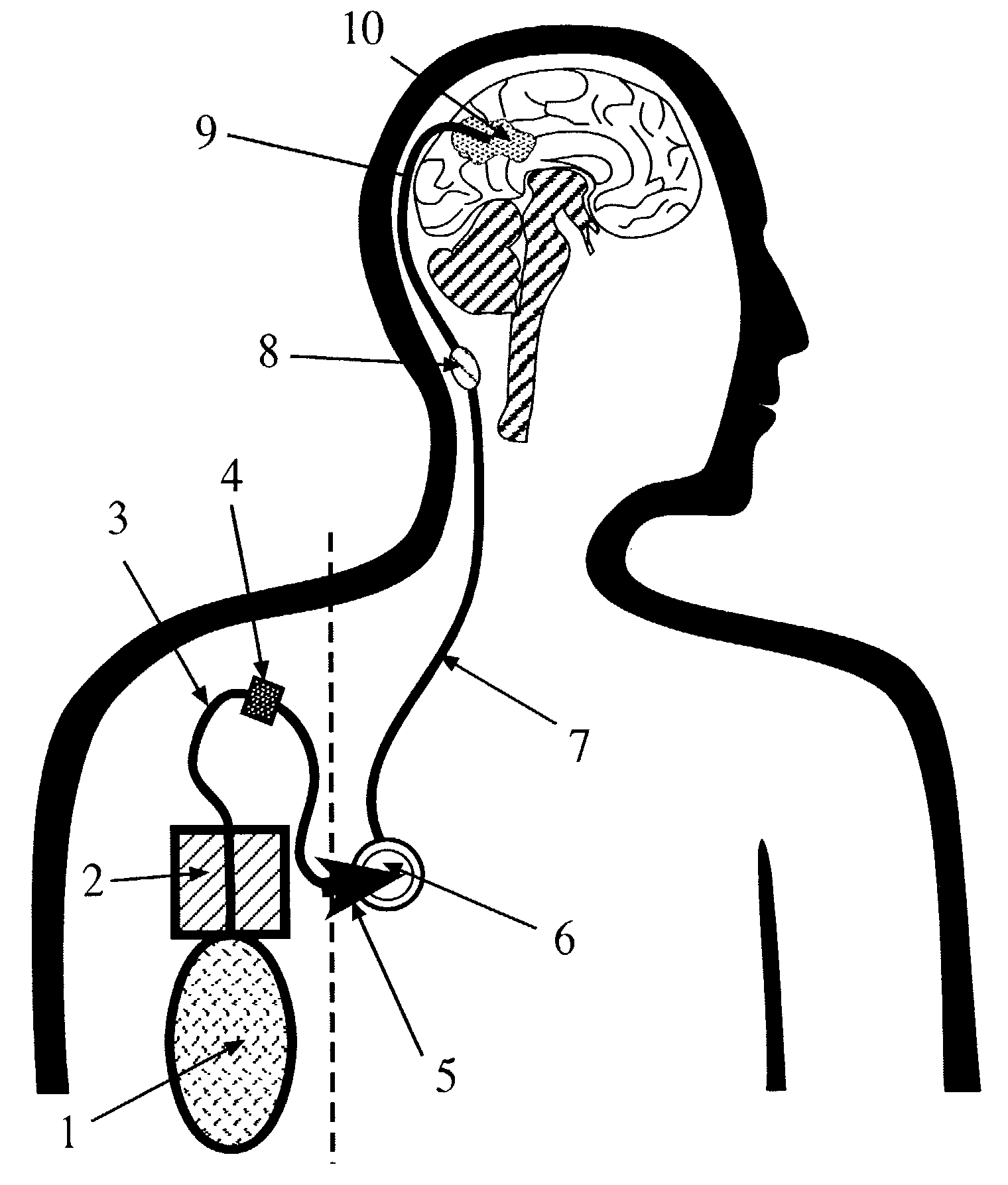
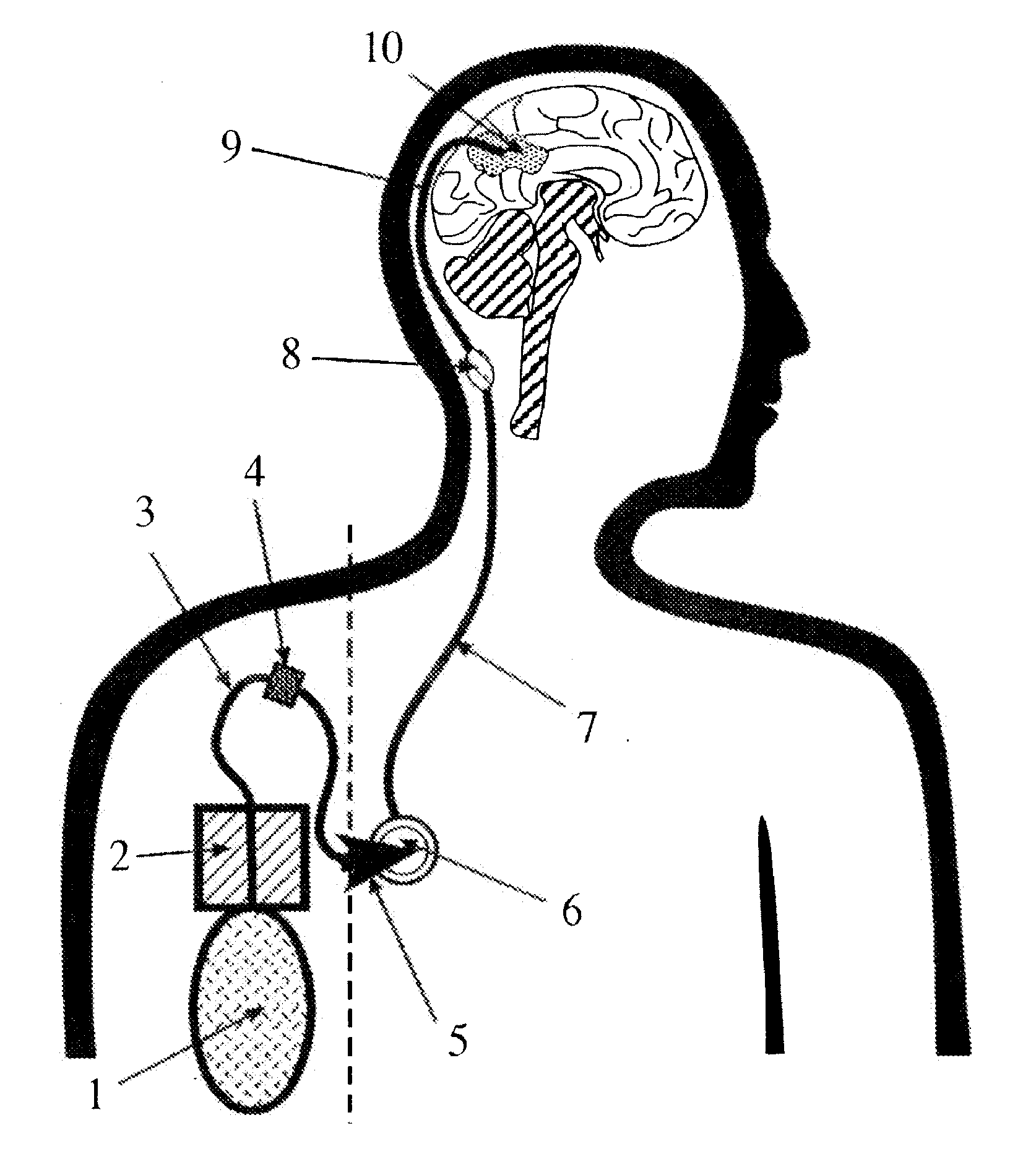
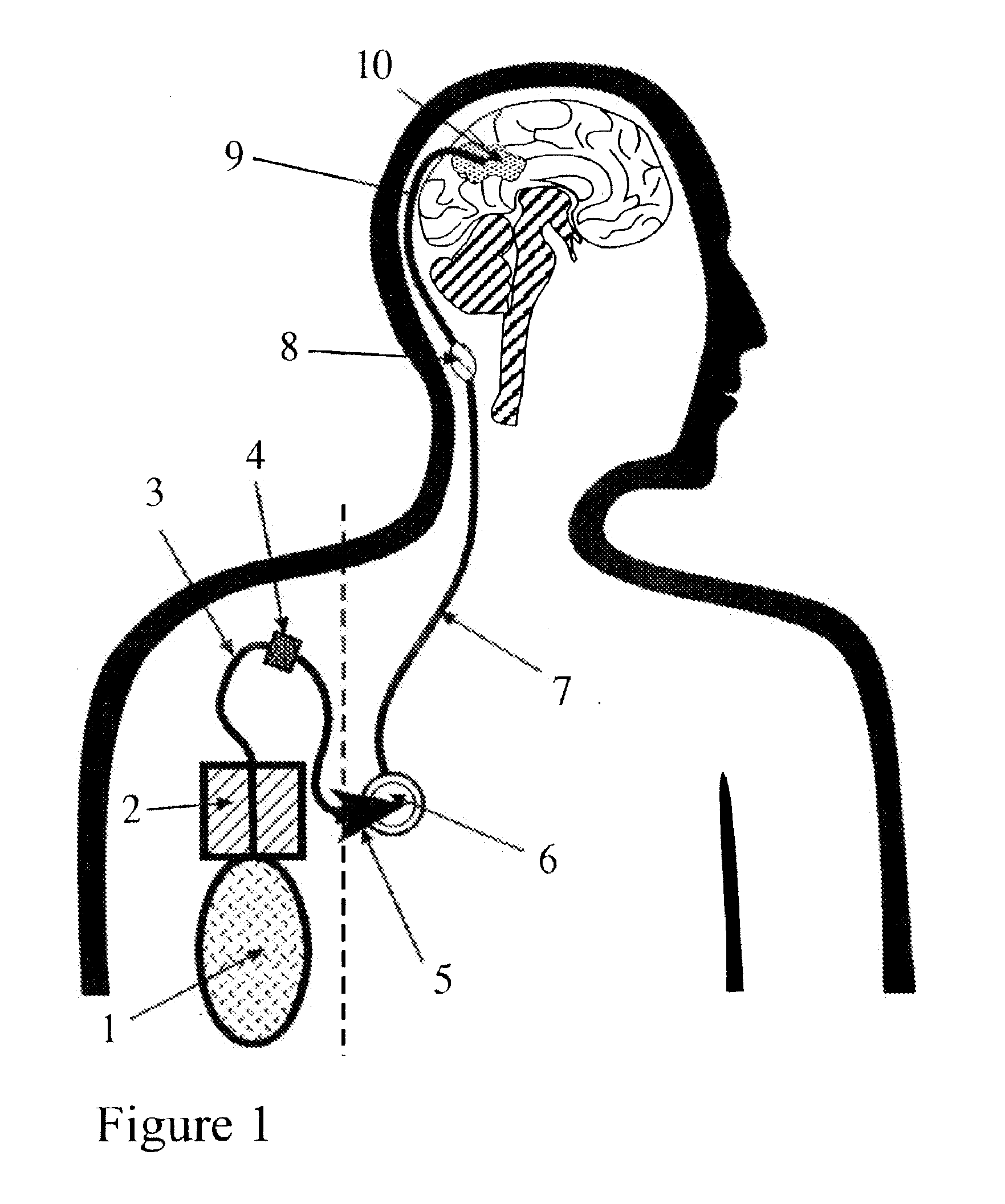
Portable equipment for administration of fluids into tissues and tumors by convection enhanced delivery technique
InactiveUS7963956B2Efficient deliveryImprove bioavailabilityMedical devicesPharmaceutical delivery mechanismEngineeringConvection-Enhanced Delivery
The present invention is directed to a device for a portable convection enhanced delivery system that allows administering liquids to specific locations within the body, especially tissues and tumors also allowing outsubject treatment. The application system comprises a portable extracorporal pump with a fluid reservoir that is connected via an infusion system to an infusion catheter placeable to any tissue or tumor the fluid should be administered to by high flow microperfusion. The system enables administration of fluids of any kind by convection enhanced delivery also in out-patient treatment. The system can be used for delivering various drugs, proteins, protein toxins, antibodies-for treatment or imaging, proteins in enzyme replacement therapy, growth factors and viruses or oligonucleotides in gene therapy etc. The application methods as well as the surgical method to implant this device are enclosed to this invention.
Owner:ANTISENSE PHARMA GMBH
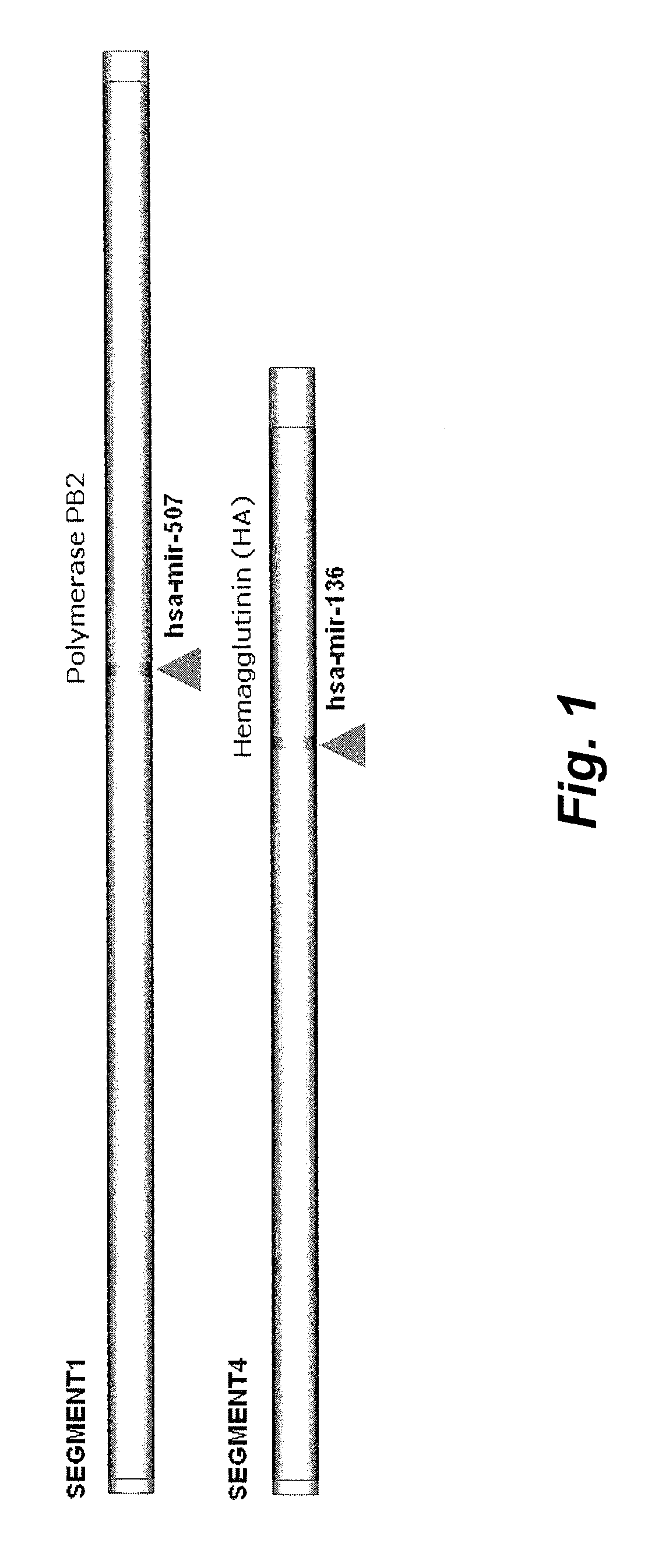
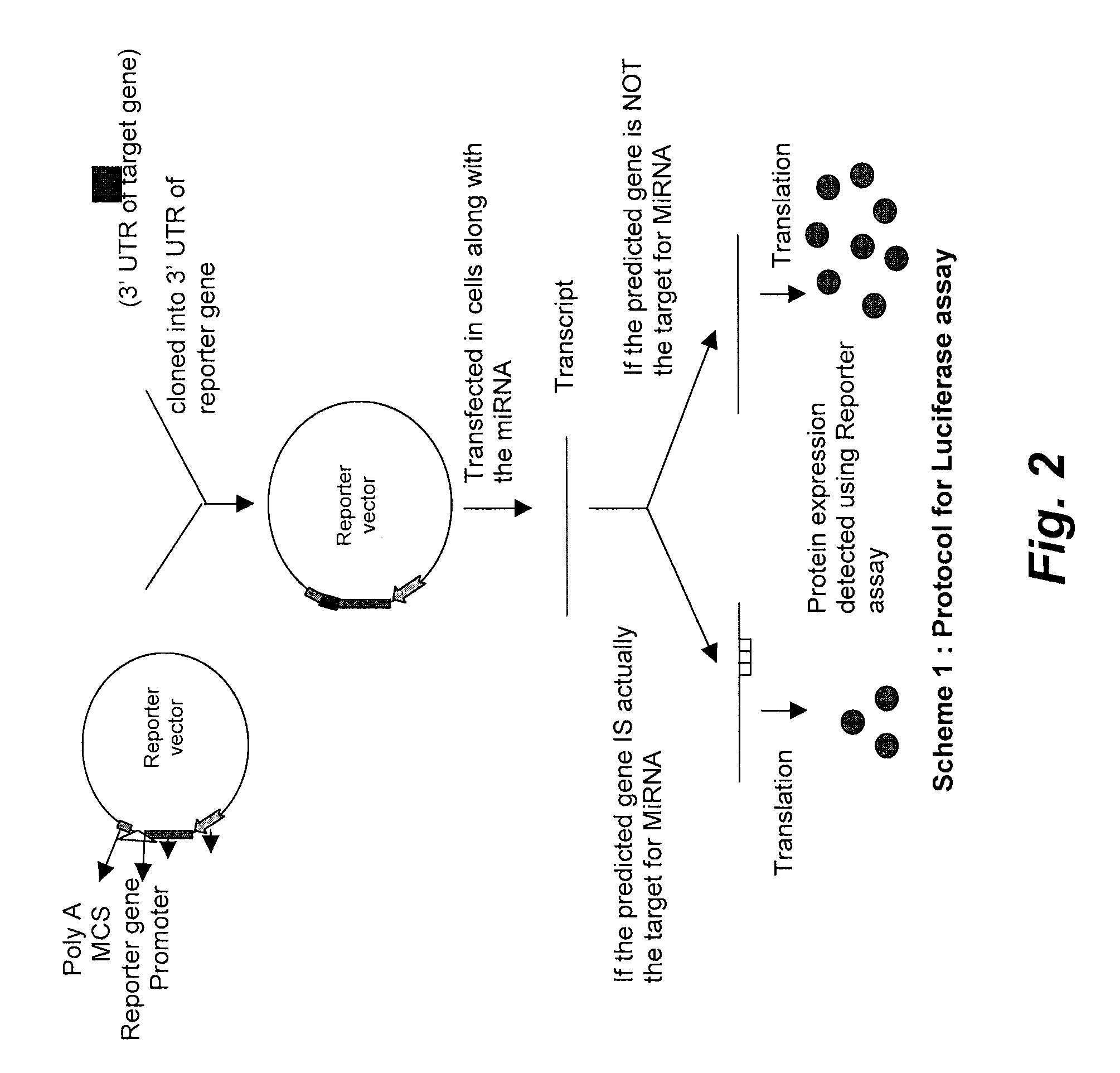
Targets for human micro rnas in avian influenza virus (H5N1) genome
The present invention relates to targets for Human microRNAs in Avian Influenza Virus (H5N1) Genome and provides specific miRNA targets against H5N1 virus. Existing therapies for Avian flu are of limited use primarily due to genetic re-assortment of the viral genome, generating novel proteins, and thus escaping immune response. In animal models, baculovirus-derived recombinant H5 vaccines were immunogenic and protective, but results in humans were disappointing even when using high doses. Currently, two classes of drugs are available with antiviral activity against influenza viruses: inhibitors of the M2 ion channel, amantadine and rimantadine, and inhibitors of neuraminidase, oseltamivir, and zanamivir. There is paucity of information regarding effectiveness of these drugs in H5N1 infection. These drugs are also well known to have side effects like neurotoxicity. Thus there exists a need to develop alternate therapy for targeting the Avian flu virus (H5N1). The present invention addresses this need in the field.
Owner:COUNCIL OF SCI & IND RES
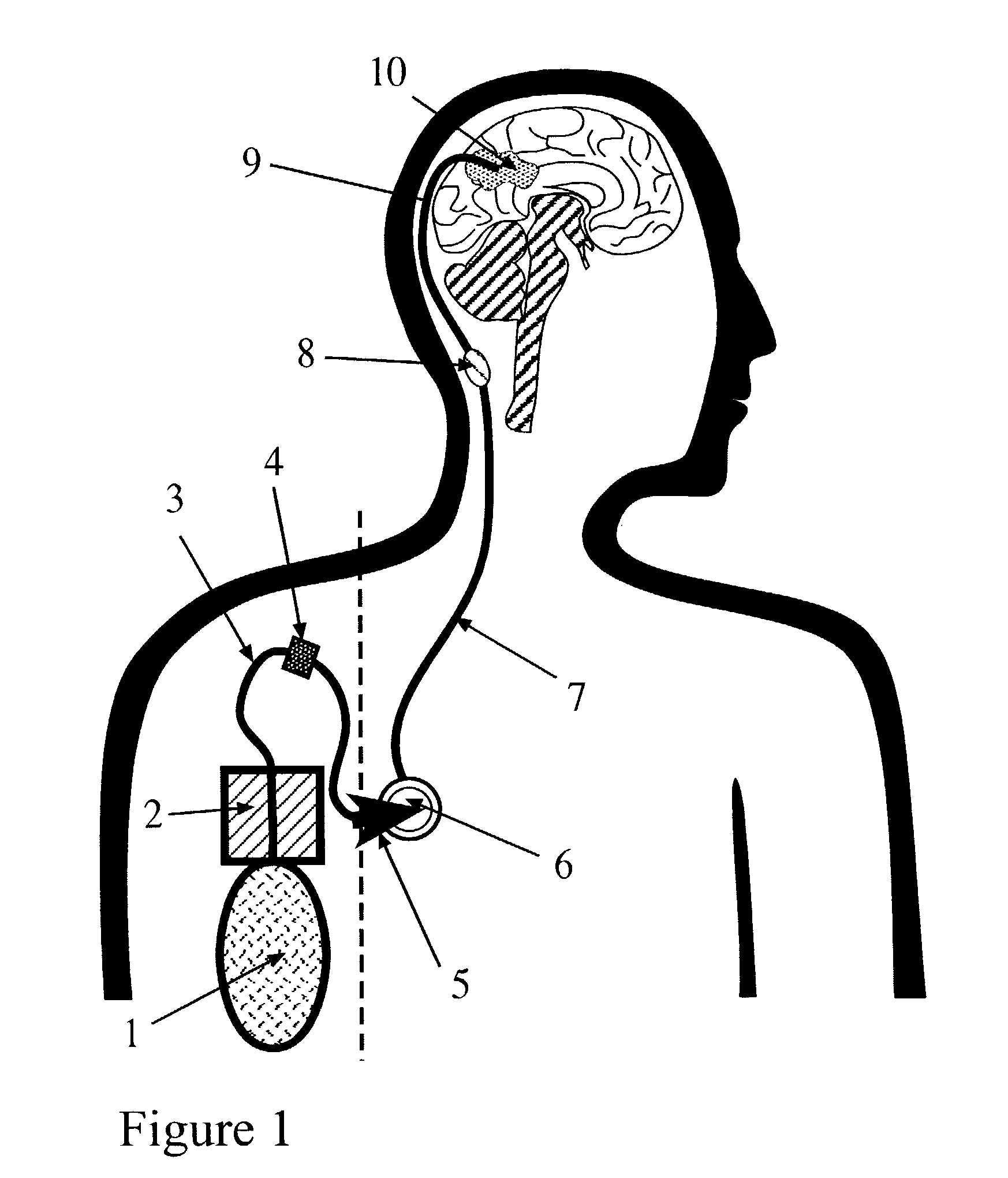
Portable equipment for administration of fluids into tissues and tumors by convection enhanced delivery technique
InactiveUS20110137289A1Efficiently deliversImprove bioavailability and safetyMedical devicesPharmaceutical delivery mechanismOligonucleotideDrug
The present invention is directed to a device for a portable convection enhanced delivery system that allows administering liquids to specific locations within the body, especially tissues and tumors also allowing outsubject treatment. The application system comprises a portable extracorporal pump with a fluid reservoir that is connected via an infusion system to an infusion catheter placeable to any tissue or tumor the fluid should be administered to by high flow microperfusion. The system enables administration of fluids of any kind by convection enhanced delivery also in out-patient treatment. The system can be used for delivering various drugs, proteins, protein toxins, antibodies for treatment or imaging, proteins in enzyme replacement therapy, growth factors and viruses or oligonucleotides in gene therapy etc. The application methods as well as the surgical method to implant this device are enclosed to this invention.
Owner:ANTISENSE PHARMA GMBH
Method and kit for treating illnesses
InactiveUS6242463B1Inhibition effectAvoid expensesBiocideDrug and medicationsDiseasePlacebo treatment
Methods and kits for determining appropriate treatment for illnesses in humans or animals are disclosed. The method includes:providing a test kit containing a random arrangement of drug(s) and placebo(s) and / or alternative treatment(s) along with a questionnaire or other instrument designed to elicit data concerning the safety, efficacy and desirability of a treatment;administering the drug(s) and placebo(s) and / or alternative treatments to each member of the pool in a random, double blind fashion and following up on patient outcomes as appropriate post-study;assembling a database from the completed pool questionnaires and revealing the random schedule to uncover drug and placebo treatment periods;providing the same kit to a patient in need of the same treatment and comparing the results obtained from the single patient trial with those obtained from the pool to determine an optimal treatment for the patient with the drug; andadministering a treatment consistent with the optimal treatment.
Owner:OPT E SCRIP
Gene and protein expression profiles associated with the therapeutic efficacy of irinotecan
InactiveUS20080076134A1Microbiological testing/measurementDisease diagnosisProtein expression profileIrinotecan
The present invention includes gene and protein expression profiles indicative of whether a cancer patient is likely to respond to treatment with irinotecan. By identifying such responsiveness, a treatment provider may determine in advance those patients who would benefit from such treatment, as well as identify alternative therapies for non-responders. The present invention further provide methods of using the gene and / or protein expression profiles and assays for identifying the presence of a gene and / or protein expression profile in a patient sample.
Owner:NUCLEA BIOMARKERS
Gene and protein expression profiles associated with the therapeutic efficacy of egfr-tk inhibitors
InactiveUS20090263819A1Microbiological testing/measurementBiological material analysisProtein expression profileNon responders
The present invention provides protein and gene expression profiles indicative of whether a patient afflicted with non-small cell lung cancer is likely to be responsive to treatment with a therapeutic compound that is a EGFR-TK inhibitor. By identifying such responsiveness, a treatment provider may determine in advance those patients who would benefit from such treatment, as well as identify alternative therapies for non-responders. The present invention further provide methods of using the gene and protein expression profiles, and assays for identifying the presence of a gene or protein expression profile in a patient sample.
Owner:NUCLEA BIOMARKERS
Method and compositions for treatment of cancers
InactiveUS7854717B1Improve inflammationHigh activityHeavy metal active ingredientsPeptide/protein ingredientsSystemic chemotherapyWhole body
A method to treat cancer uses ultrapheresis, refined to remove compounds of less than 120,000 daltons molecular weight, followed by administration of replacement fluid, to stimulate the patient's immune system to attack solid tumors. In the preferred embodiment, the patient is ultrapheresed using a capillary tube ultrafilter having a pore size of 0.02 to 0.05 microns, with a molecular weight cutoff of 120,000 daltons, sufficient to filter one blood volume. The preferred replacement fluid is ultrapheresed normal plasma. The patient is preferably treated daily for three weeks, diagnostic tests conducted to verify that there has been shrinkage of the tumors, then the treatment regime is repeated. The treatment is preferably combined with an alternative therapy, for example, treatment with an anti-angiogenic compound, one or more cytokines such as TNF, gamma interferon, or IL-2, or a procoagulant compound. The treatment increases endogenous, local levels of cytokines, such as TNF. This provides a basis for an improved effect when combined with any treatment that enhances cytokine activity against the tumors, for example, treatments using alkylating agents, doxyrubicin, carboplatinum, cistplatimum, and taxol. Alternatively, the ultrapheresis treatment can be combined with local chemotherapy, systemic chemotherapy, and / or radiation.
Owner:INNATUS CORP
Method and system to remove cytokine inhibitor in patients
InactiveUS20050244371A1Induce inflammationOrganic active ingredientsPeptide/protein ingredientsAbnormal tissue growthSystemic chemotherapy
A method to treat cancer uses ultrapheresis, refined to remove compounds of less than 120,000 daltons molecular weight, followed by administration of replacement fluid, to stimulate the patient's immune system to attack solid tumors. In the preferred embodiment, the patient is ultrapheresed using a capillary tube ultrafilter having a pore size of 0.02 to 0.05 microns, with a molecular weight cutoff of 120,000 daltons, sufficient to filter one blood volume. The preferred replacement fluid is ultrapheresed normal plasma. The patient is preferably treated daily for three weeks, diagnostic tests conducted to verify that there has been shrinkage of the tumors, then the treatment regime is repeated. The treatment is preferably combined with an alternative therapy, for example, treatment with an anti-angiogenic compound, one or more cytokines such as TNF, gamma interferon, or IL-2, or a procoagulant compound. The treatment increases endogenous, local levels of cytokines, such as TNF. This provides a basis for an improved effect when combined with any treatment that enhances cytokine activity against the tumors, for example, treatments using alkylating agents, doxyrubicin, carboplatinum, cisplatinum, and taxol. Alternatively, the ultrapheresis treatment can be combined with local chemotherapy, systemic chemotherapy, and / or radiation.
Owner:INNATUS CORP
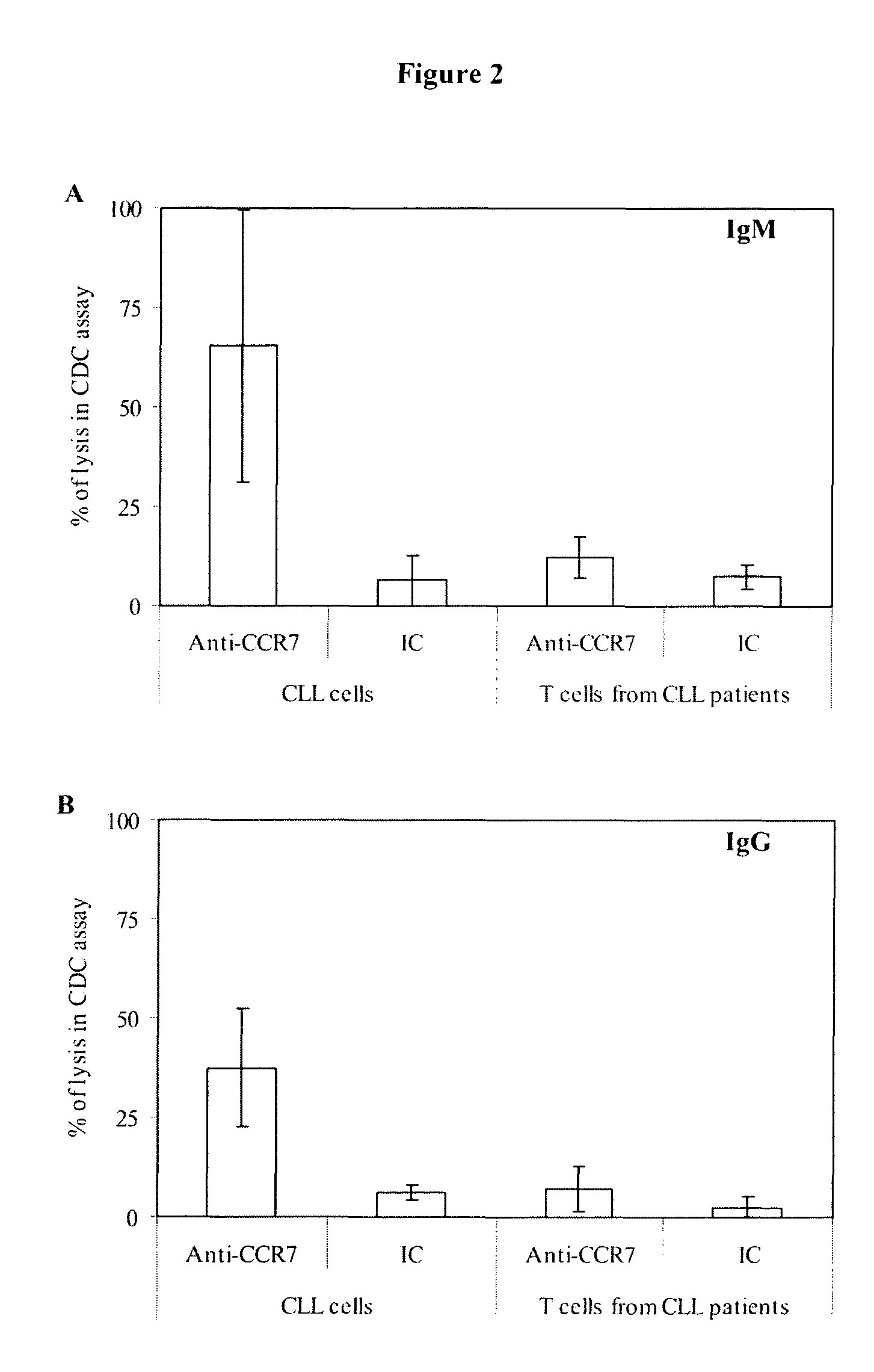
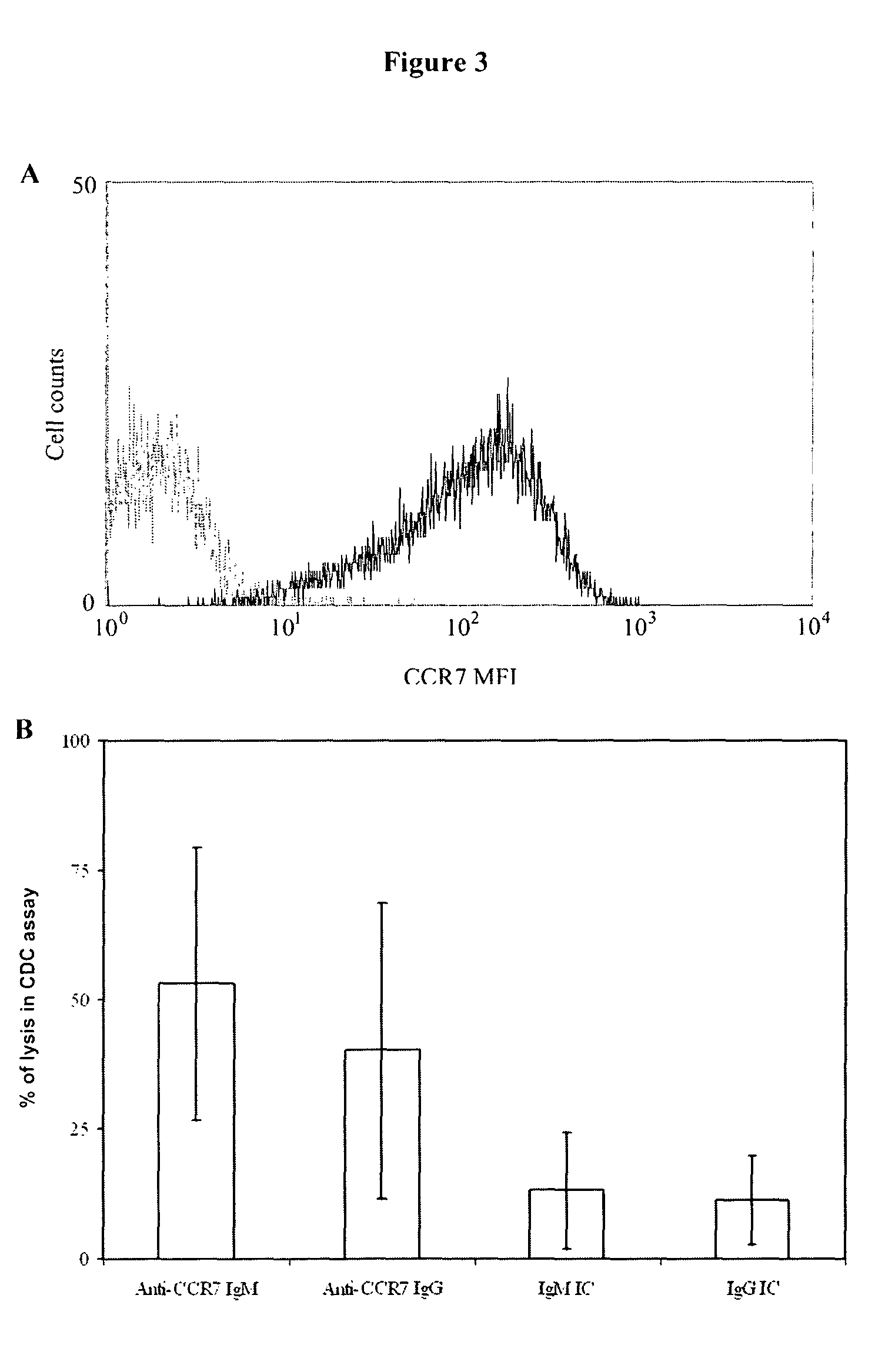
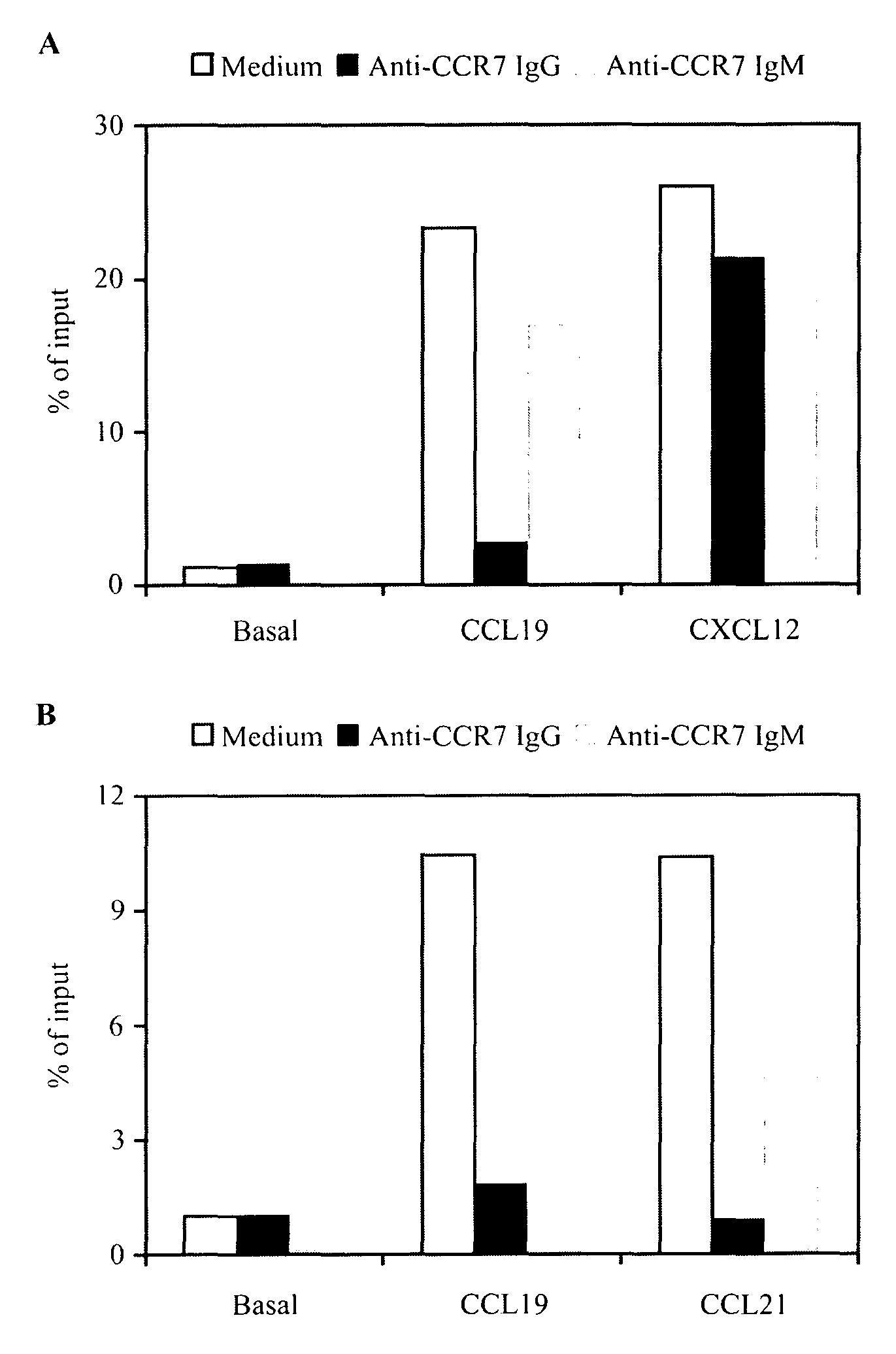
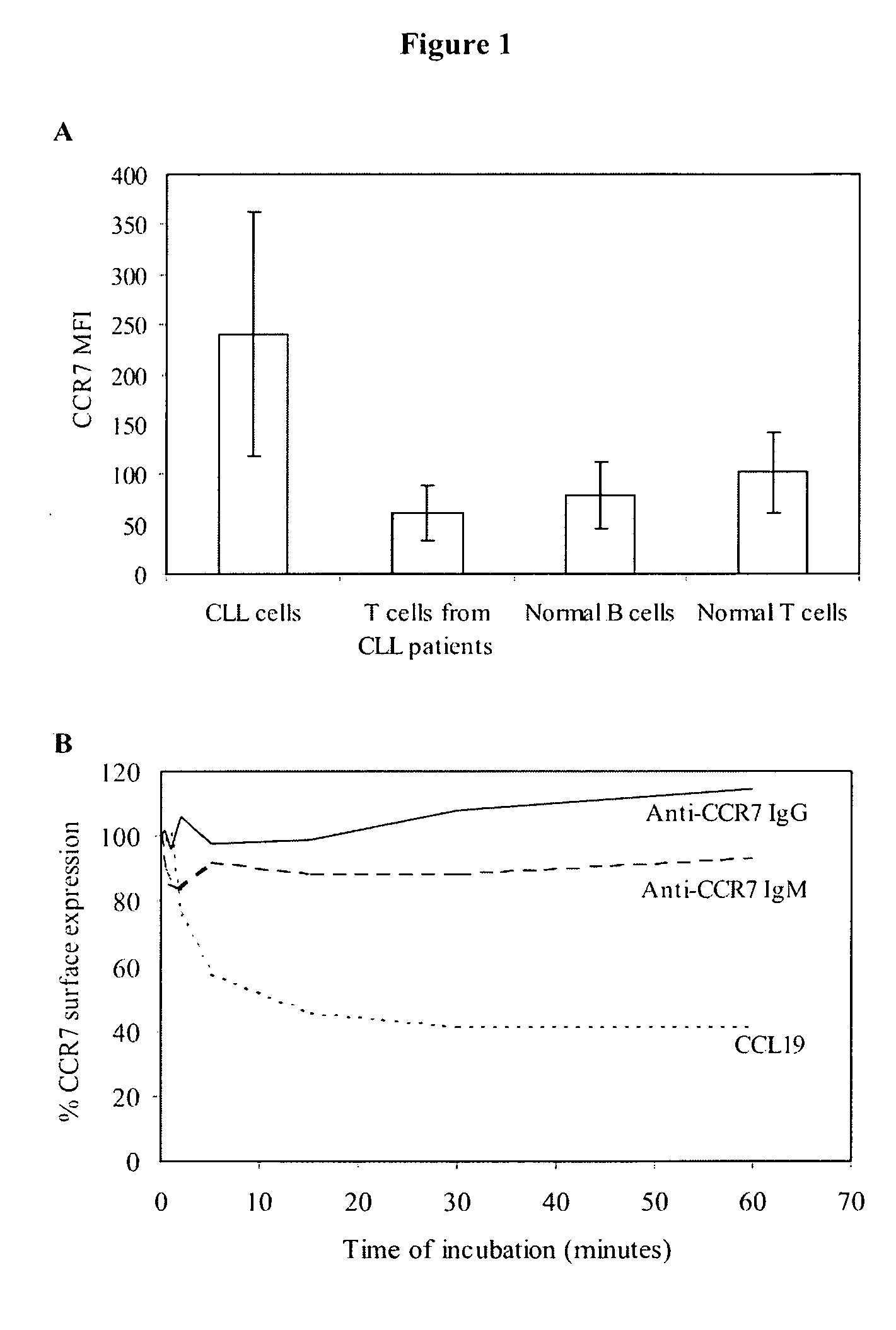
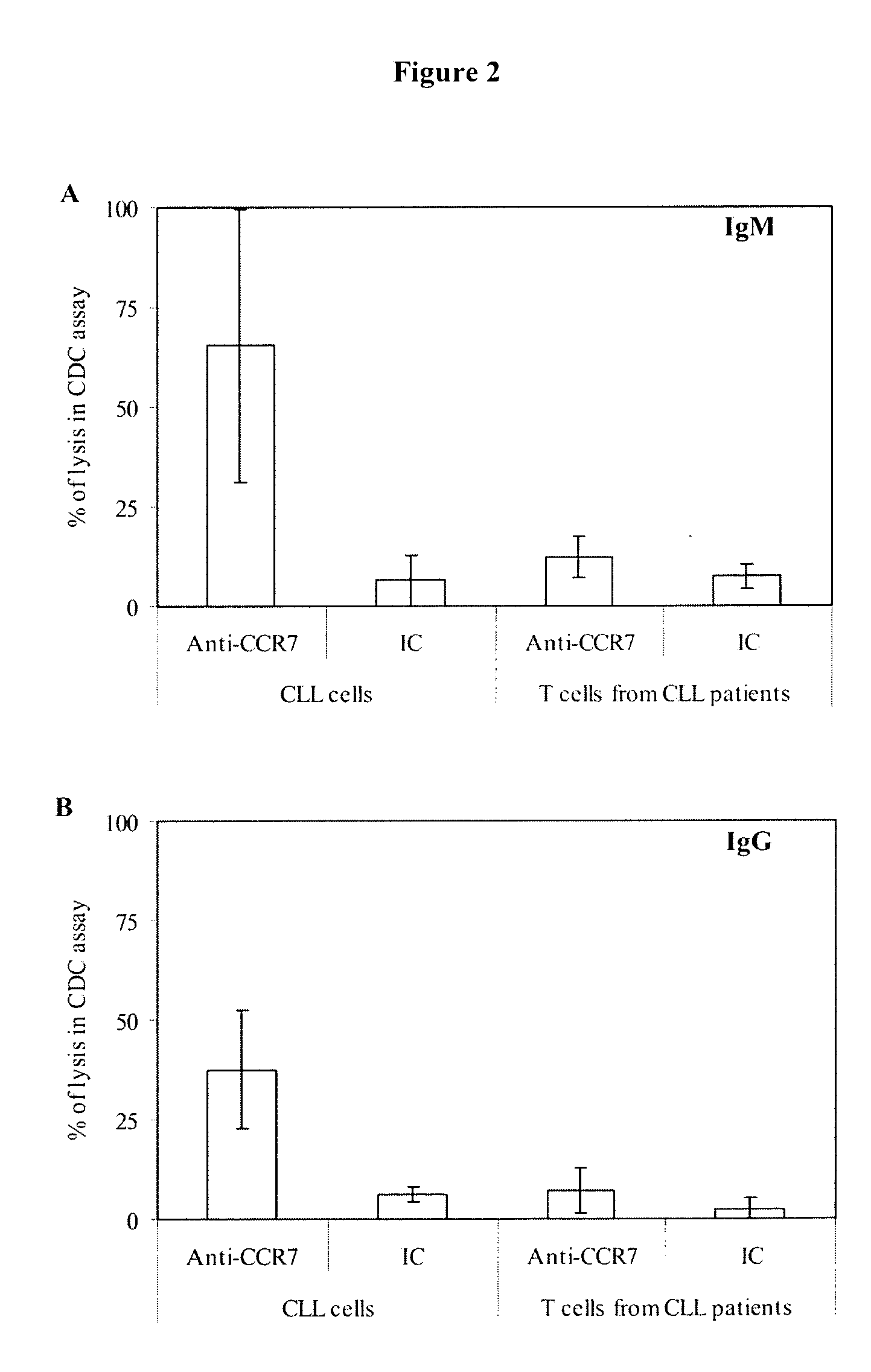
Anti-CCR7 receptor antibodies for the treatment of cancer
ActiveUS8066996B2Reduce expressionEliminate effectiveAntibody ingredientsImmunoglobulinsAntigenAntigen Binding Fragment
Antibodies, or antigen-binding fragment thereof, which bind to a CCR7 receptor are capable of selectively killing, impairing migration and / or blocking dissemination of tumor cells expressing a CCR7 receptor. Use of said antibodies for killing or for inducing apoptosis of said tumor is disclosed, thus providing an alternative therapy for treatment of cancer which tumor cells express a CCR7 receptor.
Owner:AUTONOMOUS UNIVERSITY OF MADRID
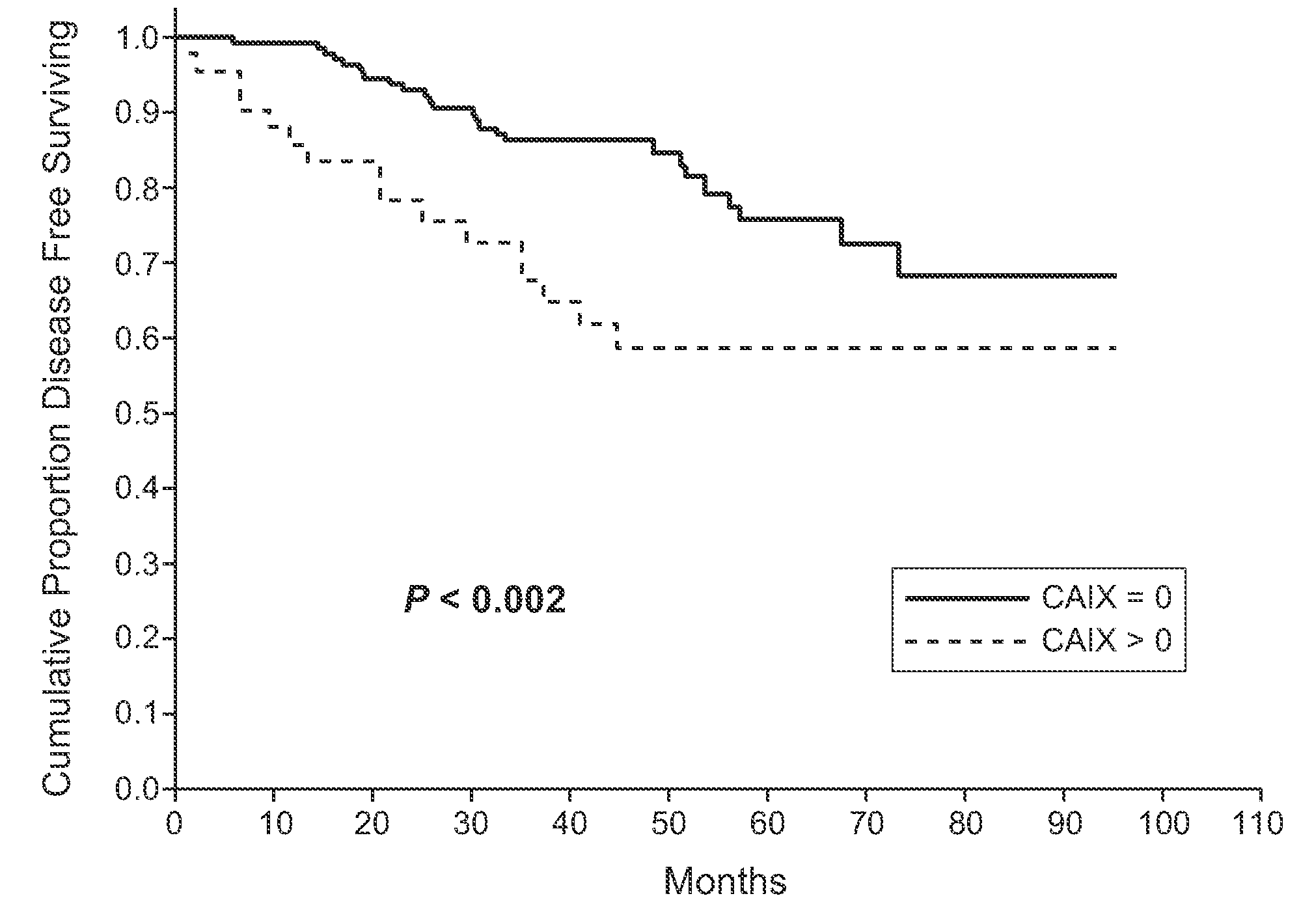
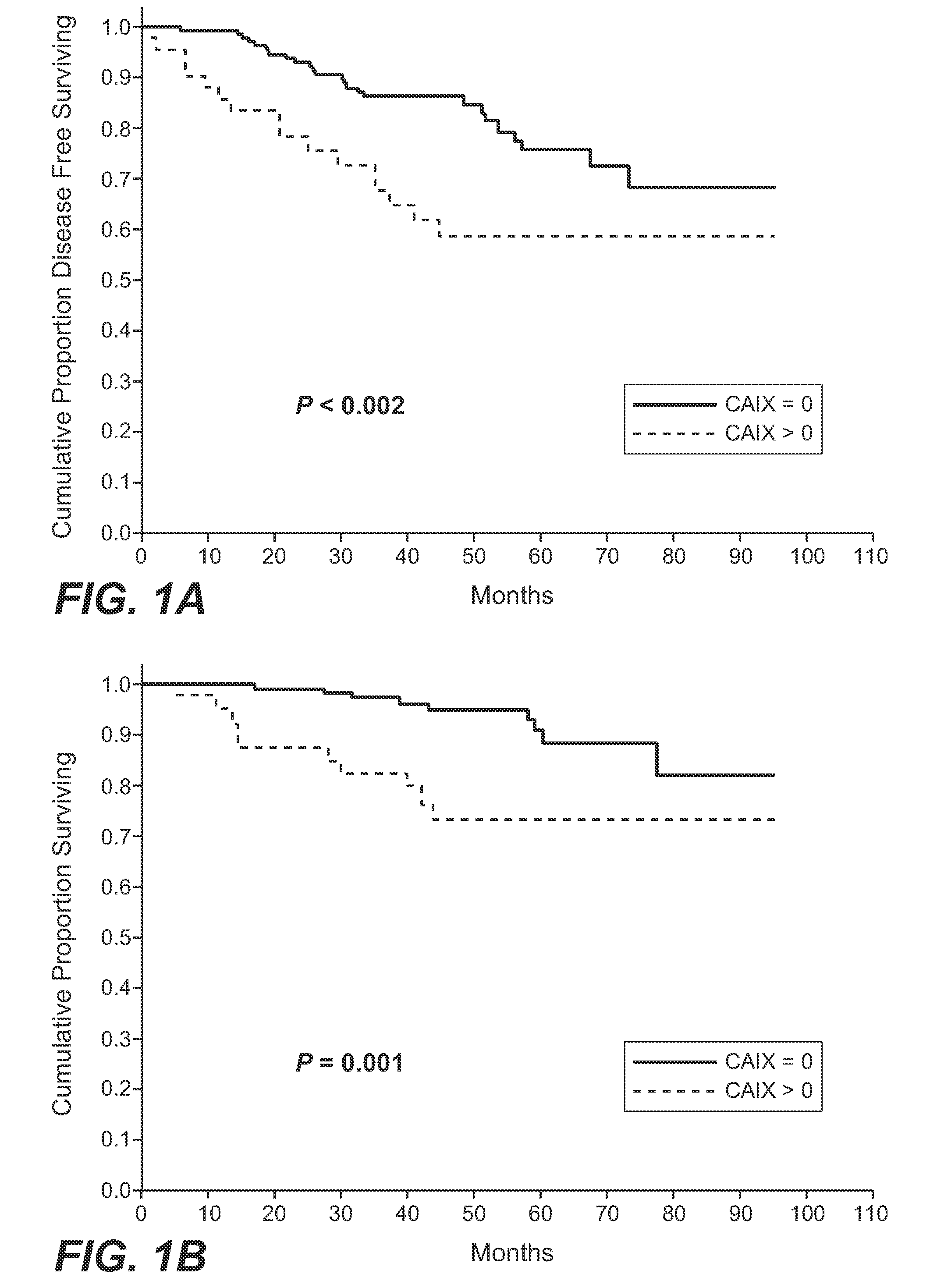
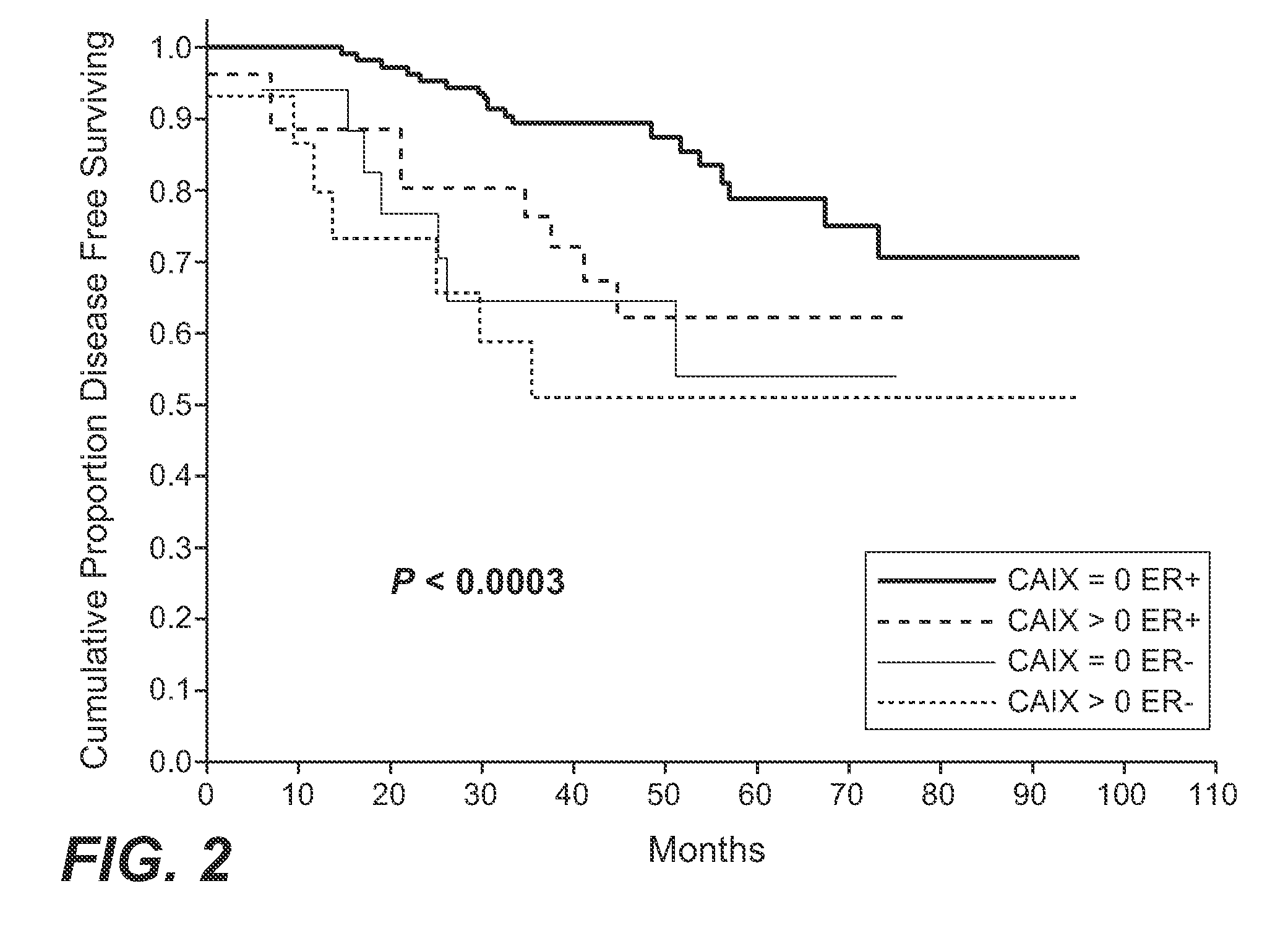
MN/CA IX and Breast Cancer Therapy
InactiveUS20090047215A1Improve the immunityOrganic active ingredientsBiocideAbnormal tissue growthAntigen
Herein disclosed are methods that are predictive of resistance to endocrine therapy in an estrogen receptor-positive (ER-positive) breast cancer patient. An exemplary method comprises detecting the overexpression of MN / CA9 gene expression product(s) in a sample from an affected subject, wherein if MN / CA9 is overexpressed, then the subject is considered to have a greater probability of resistance to endocrine therapy, particularly tamoxifen, and a corresponding poorer prognosis if undergoing endocrine therapy, than if MN / CA9 is not overexpressed. MN / CA9 gene expression products useful in the predictive / prognostic methods include MN / CA IX, MN proteins / polypeptides, MN nucleic acids and soluble MN / CA IX antigen (s-CA IX). The methods are useful as an aid in the selection of treatment for a patient with an ER-positive breast tumor. The methods of the invention can be used, for example, to identify those patients requiring additional / alternative therapies, preferably, but not necessarily, therapies that are not affected by acidic pH. The methods also comprise the use of diagnostic / prognostic imaging to detect MN / CA IX in a patient tumor, wherein the presence of MN / CA IX in one or more tumors is indicative of probable resistance to antiestrogen therapy, particularly to tamoxifen.
Owner:BIOMEDICAL RES CENT OF THE SLOVAK ACADEMY OF SCI
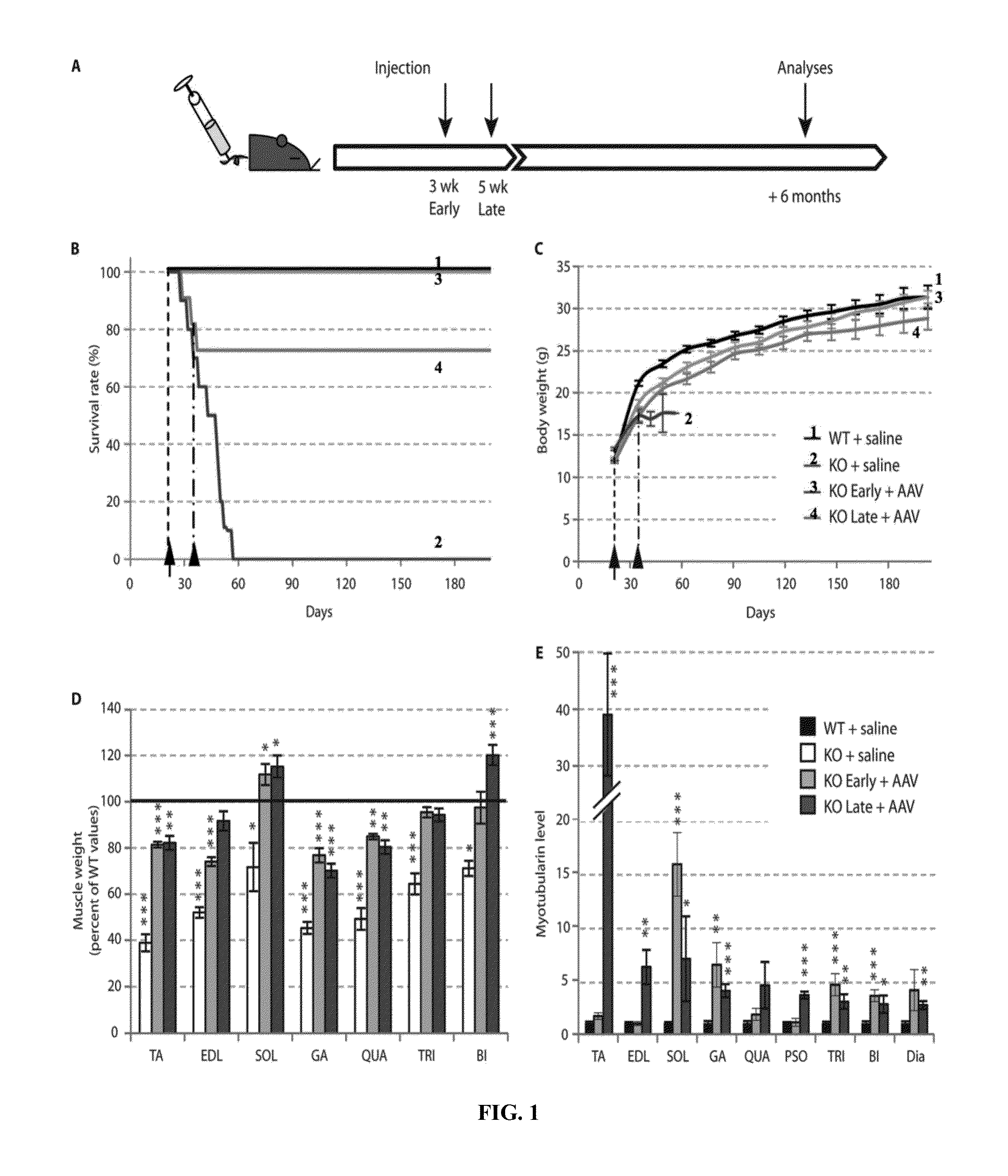
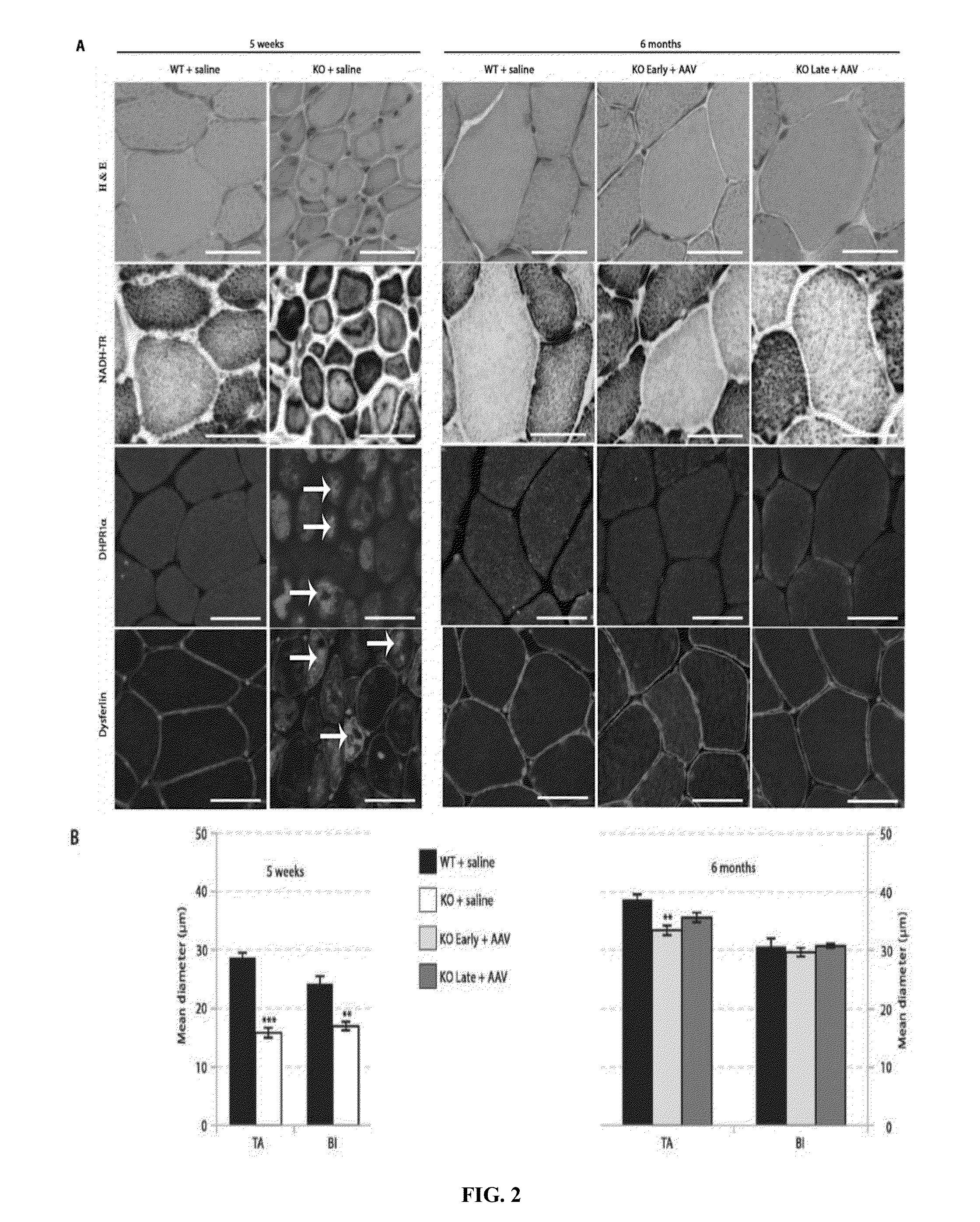
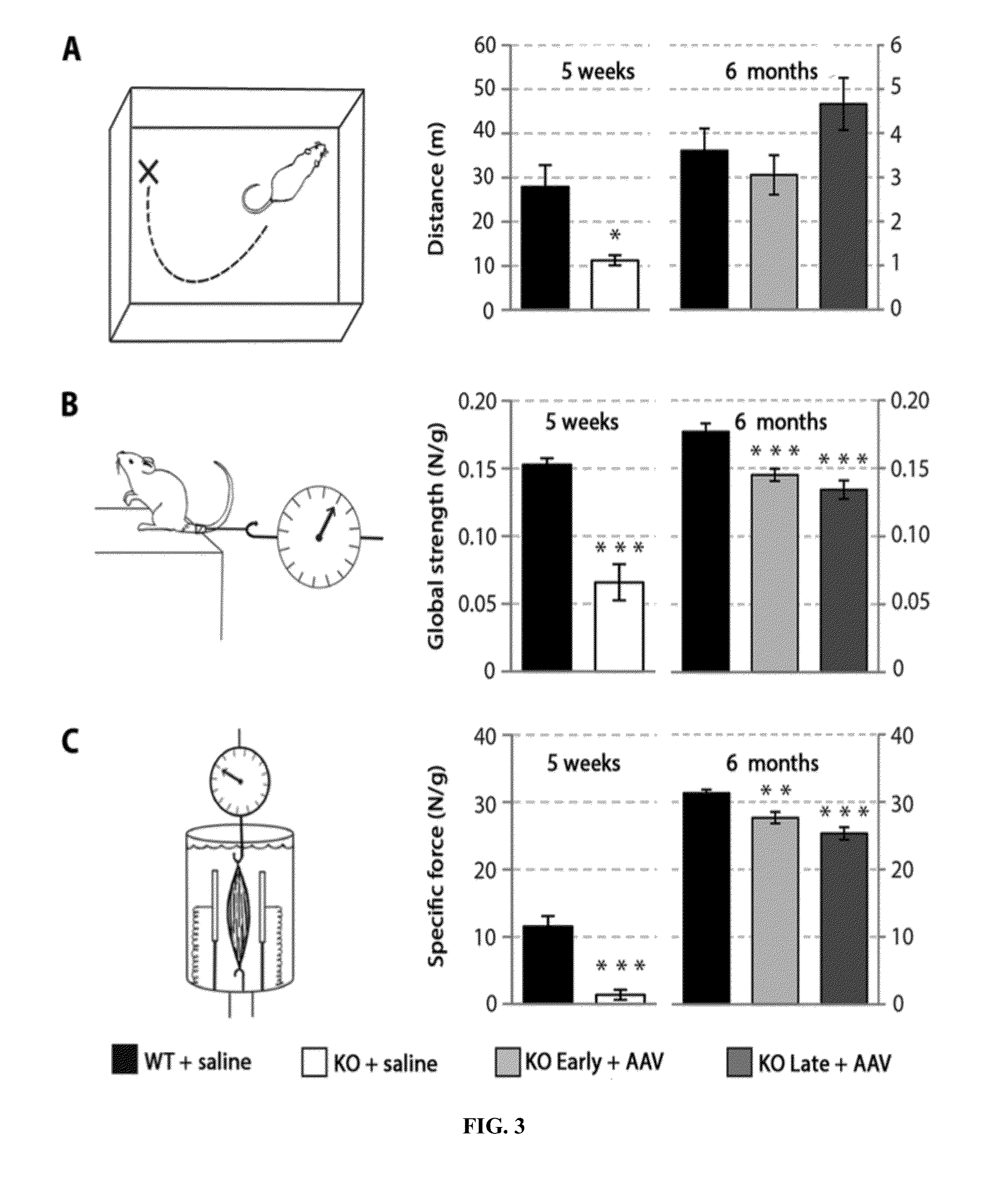
Systemic gene replacement therapy for treatment of X-linked myotubular myopathy (XLMTM)
ActiveUS8957044B2Increase in myotubularin expressionSustained increase in strengthSugar derivativesHydrolasesMyopathyWhole body
The present invention provides compositions and methods for treating a myopathy. In certain embodiments, the invention provides compositions and methods for treating, improving muscle function, and prolonging survival in a subject with X-linked myotubular myopathy (XLMTM). The present invention provides a method comprising systemic administration of a composition that induces the increased expression of myotubularin in the muscle of a subject. The invention provides sustained regional and global increases in muscle function.
Owner:GENETHON +2
Operator independent programmable sample preparation and analysis system
ActiveUS8337755B2Eliminate errorsLow variabilityBioreactor/fermenter combinationsBiological substance pretreatmentsRegimenHandling system
The present invention provides a protocol and apparatus for enriching circulating tumor cells and other rare cells from blood, including debris and other components, from samples with high precision and at high throughput rates. This invention discloses an improved processing system from previously described semi-automated sample processing. The system further reduces operator intervention and hands-on time from prior systems. While this system has general utility in processing diverse materials, the system is configured for sample processing of biological specimens to provide an enriched fraction suitable for detection, enumeration and identification of target cells by appropriate analytical methodologies. The presence and quantities of such target cells in a sample specimen can utilized for screening and detection in disease such as cancer, assessing early stage pre-metastatic cancer, monitoring for disease remission in response to therapy and selection of more effective dose regimens or alternative therapies in case of relapse.
Owner:MENARINI SILICON BIOSYSTEMS SPA
Method and system to remove cytokine inhibitor in patients
A method to treat cancer uses ultrapheresis, refined to remove compounds of less than 120,000 daltons molecular weight, followed by administration of replacement fluid, to stimulate the patient's immune system to attack solid tumors. In the preferred embodiment, the patient is ultrapheresed using a capillary tube ultrafilter having a pore size of 0.02 to 0.05 microns, with a molecular weight cutoff of 120,000 daltons, sufficient to filter one blood volume. The preferred replacement fluid is ultrapheresed normal plasma. The patient is preferably treated daily for three weeks, diagnostic tests conducted to verify that there has been shrinkage of the tumors, then the treatment regime is repeated. The treatment is preferably combined with an alternative therapy, for example, treatment with an anti-angiogenic compound, one or more cytokines such as TNF, gamma interferon, or IL-2, or a procoagulant compound. The treatment increases endogenous, local levels of cytokines, such as TNF. This provides a basis for an improved effect when combined with any treatment that enhances cytokine activity against the tumors, for example, treatments using alkylating agents, doxyrubicin, carboplatinum, cisplatinum, and taxol. Alternatively, the ultrapheresis treatment can be combined with local chemotherapy, systemic chemotherapy, and / or radiation.
Owner:INNATUS CORP
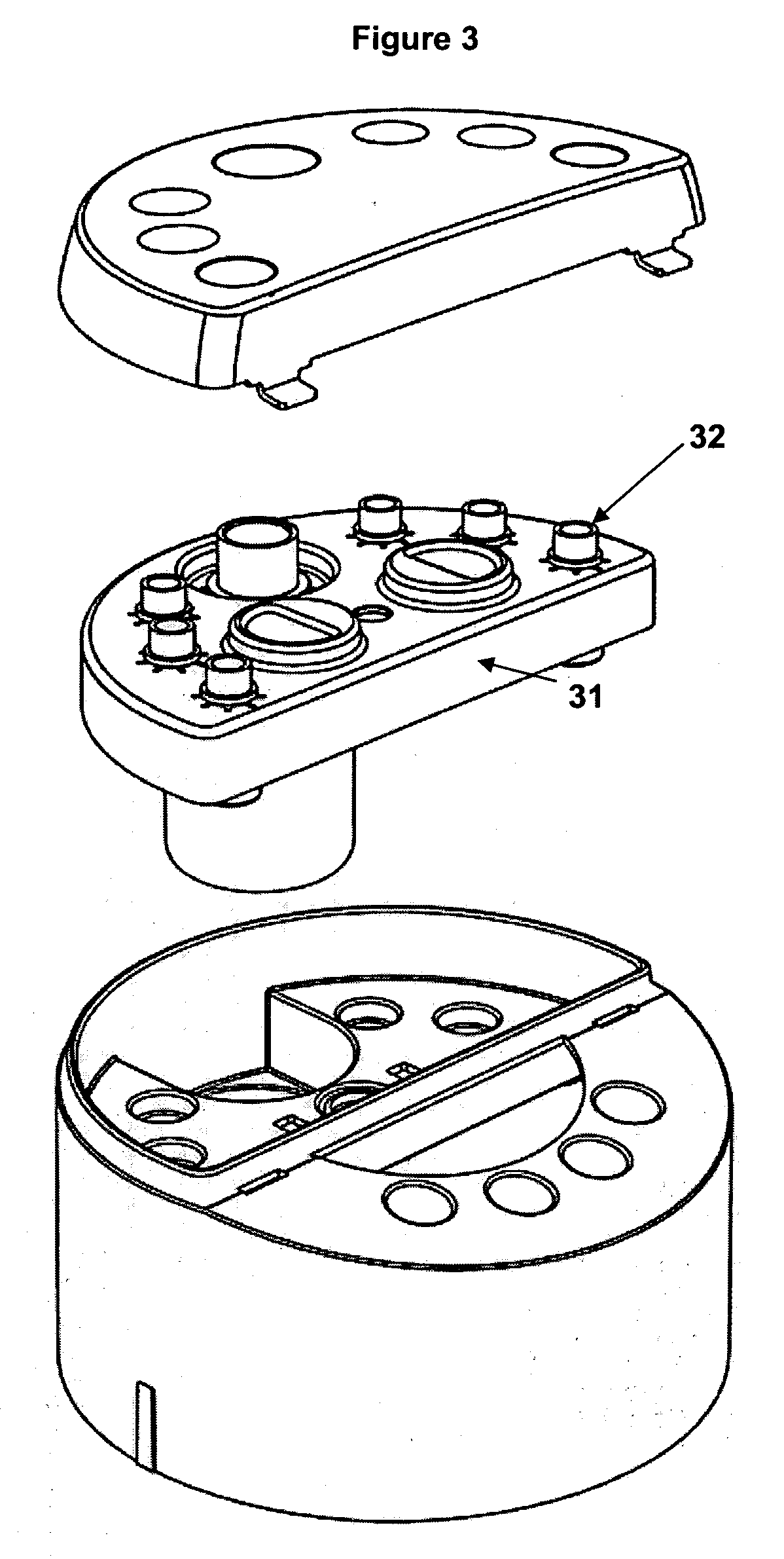
Diagnostic imaging device for the analysis of circulating rare cells
ActiveUS7777885B2Minimal spaceEasy accessMicrobiological testing/measurementDead animal preservationDiseaseRegimen
Owner:MENARINI SILICON BIOSYSTEMS SPA
Predictors for patients at risk for glaucoma from steroid therapy
InactiveUS20050192257A1Increased riskEvaluate benefitBiocideOrganic active ingredientsIntraocular pressureAnti angiogenic
A patient's risk for increased intraocular pressure after being treated with a steroid for an ocular disease, such as macular edema or age related macular degeneration, is assessed. The patient receives an intraocular challenge dose of triamcinolone, and the patient's intraocular pressure before and after the challenge dose is compared. If the intraocular pressure after the challenge is increased by at least 5 mm Hg, the patient is at risk for glaucoma if a therapeutic dose of a steroid is administered to treat the disease. This allows the physician to better manage the risk and / or provide an alternative therapy. The challenge composition may also contain an anti-angiogenic agent that will beneficially reduce the risk of new blood vessel growth in the eye.
Owner:MINU
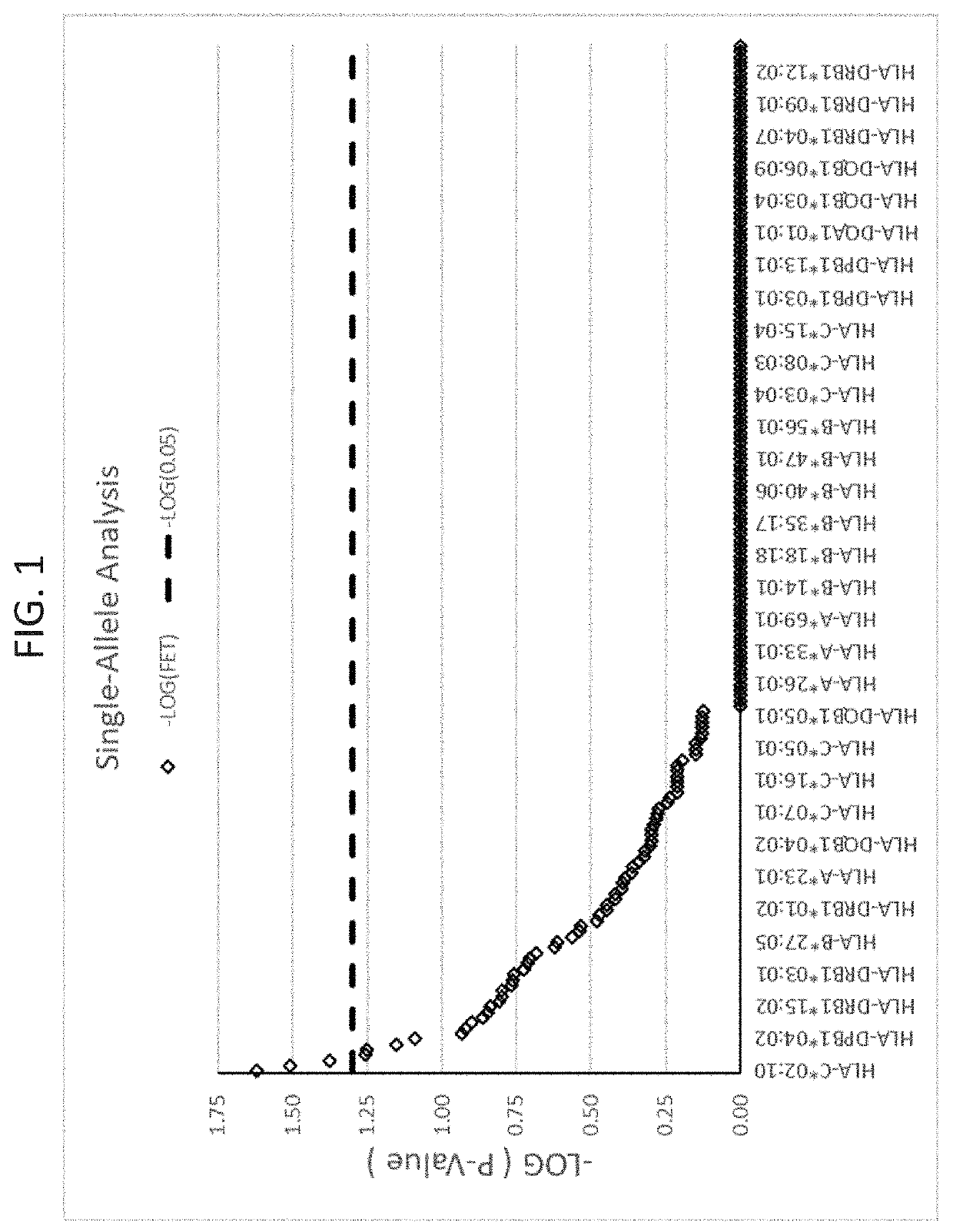
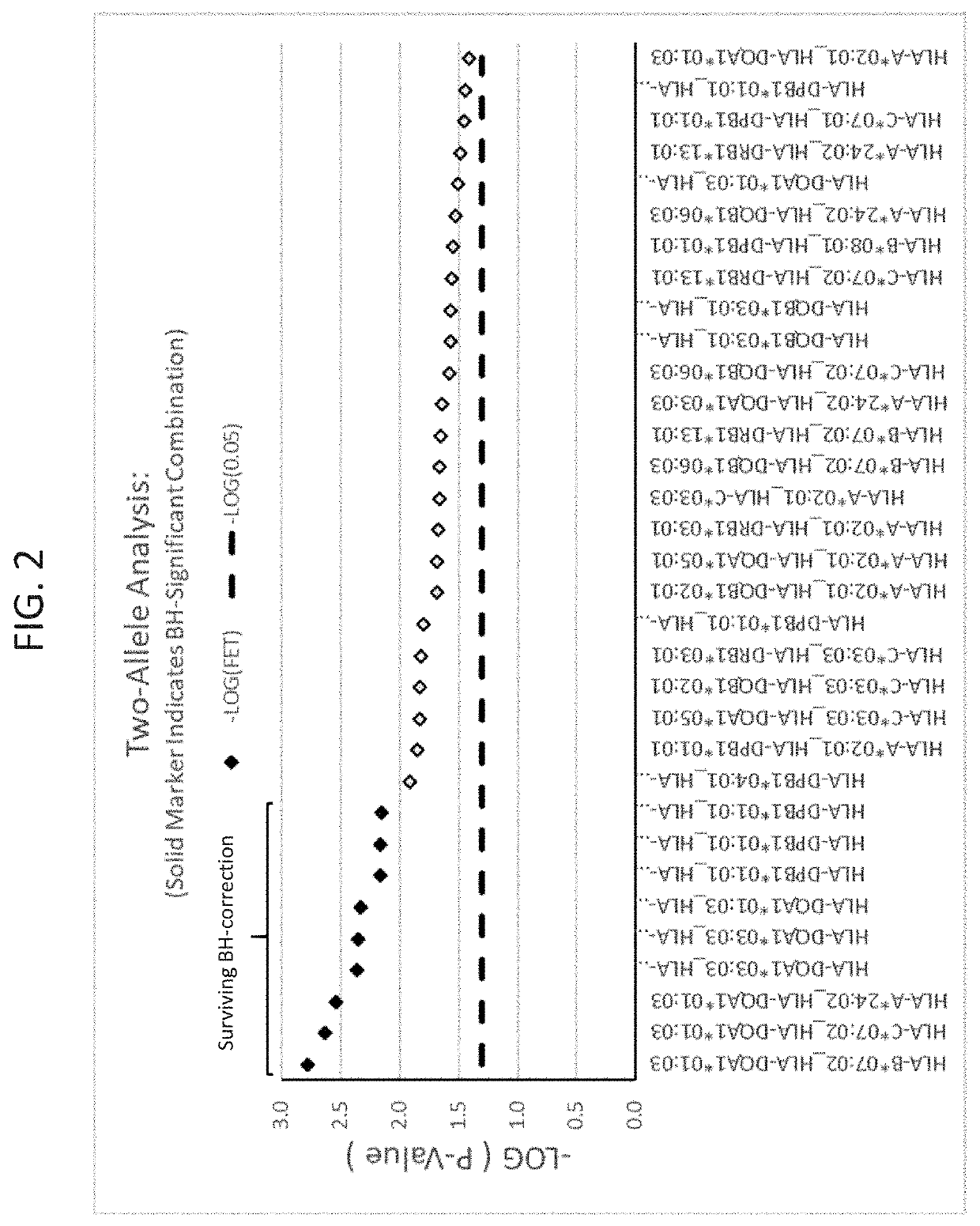
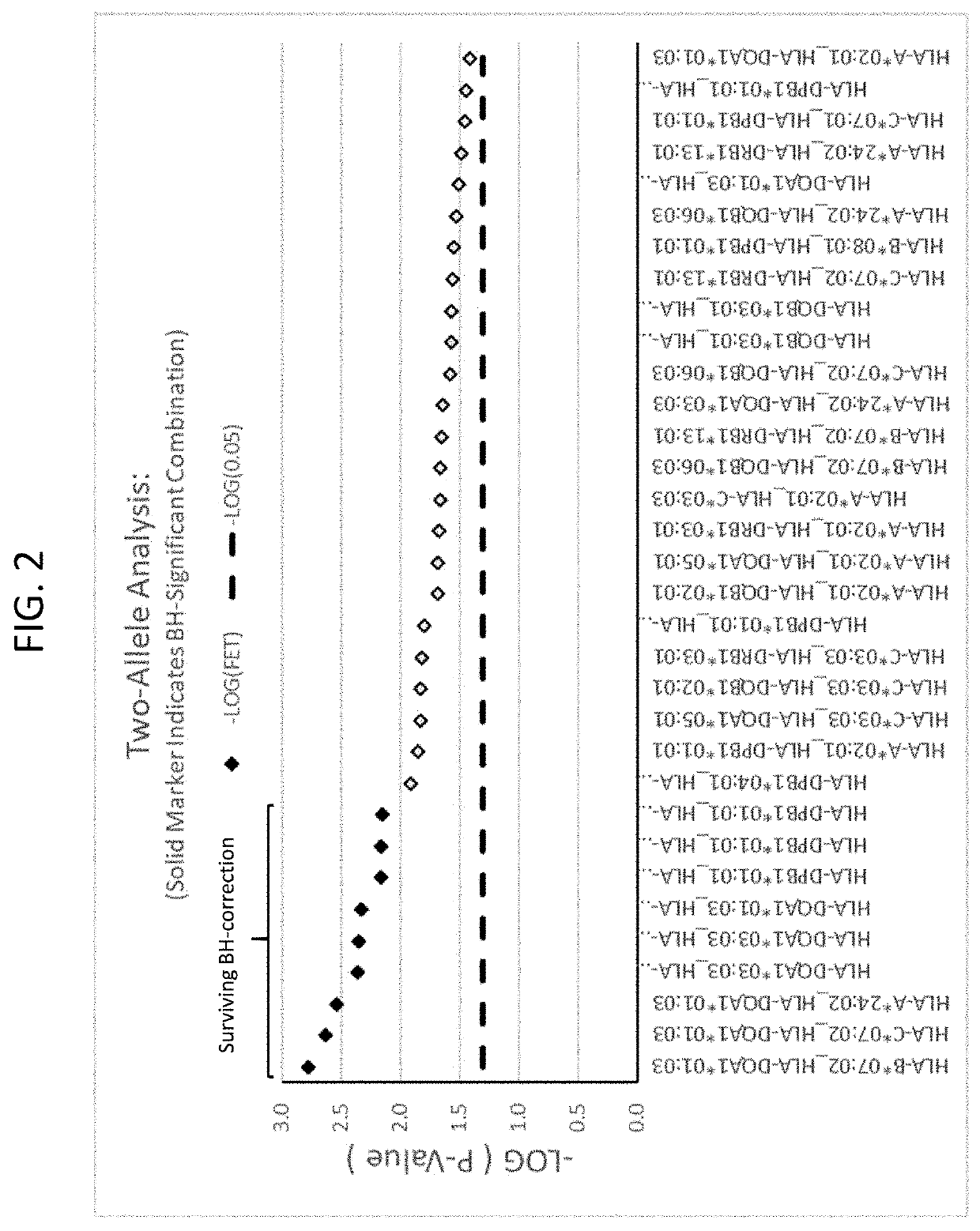
Methods for reducing drug-induced liver injury
ActiveUS20190345242A1Reduce riskReduce severityMicrobiological testing/measurementDigestive systemAlleleDrug reaction
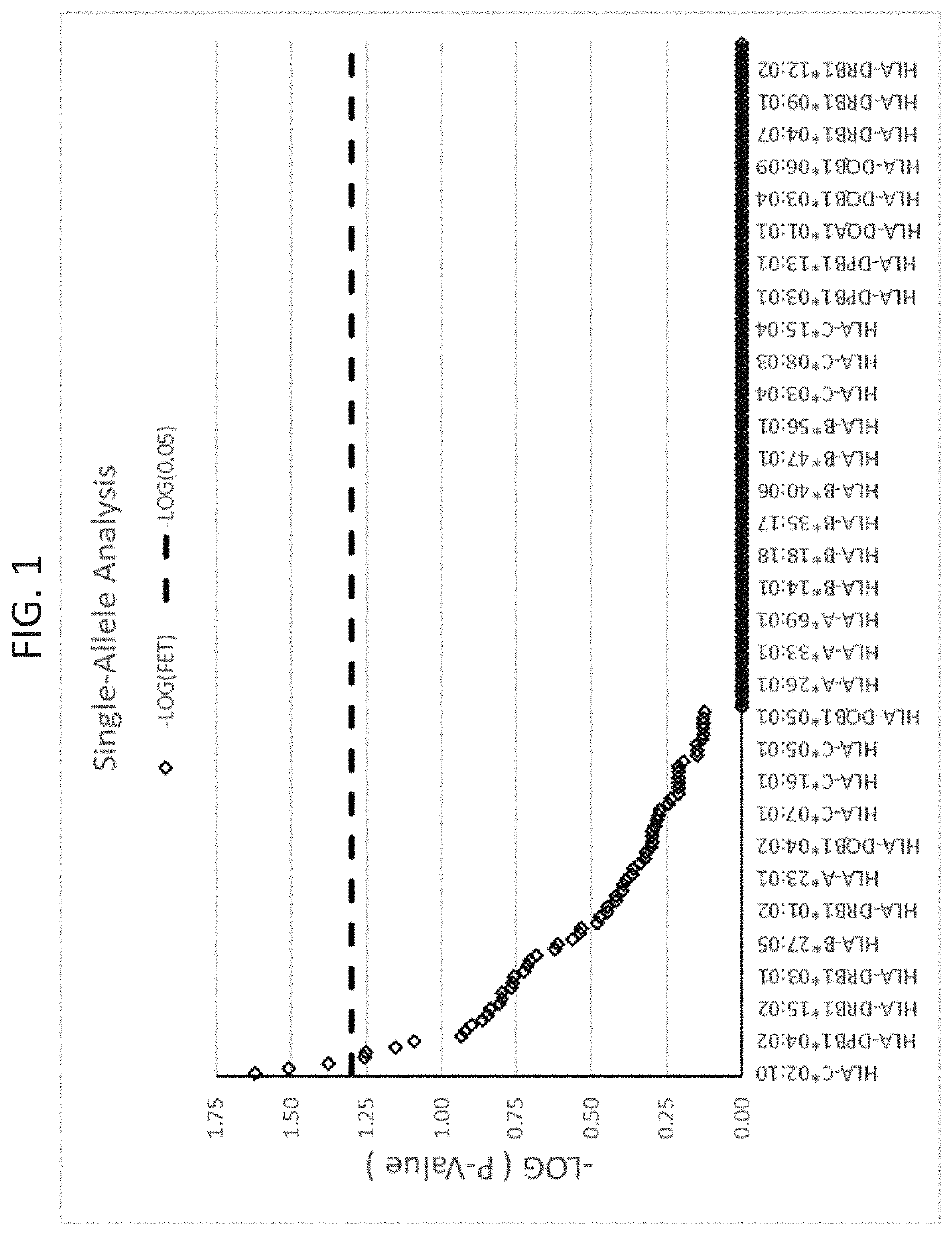
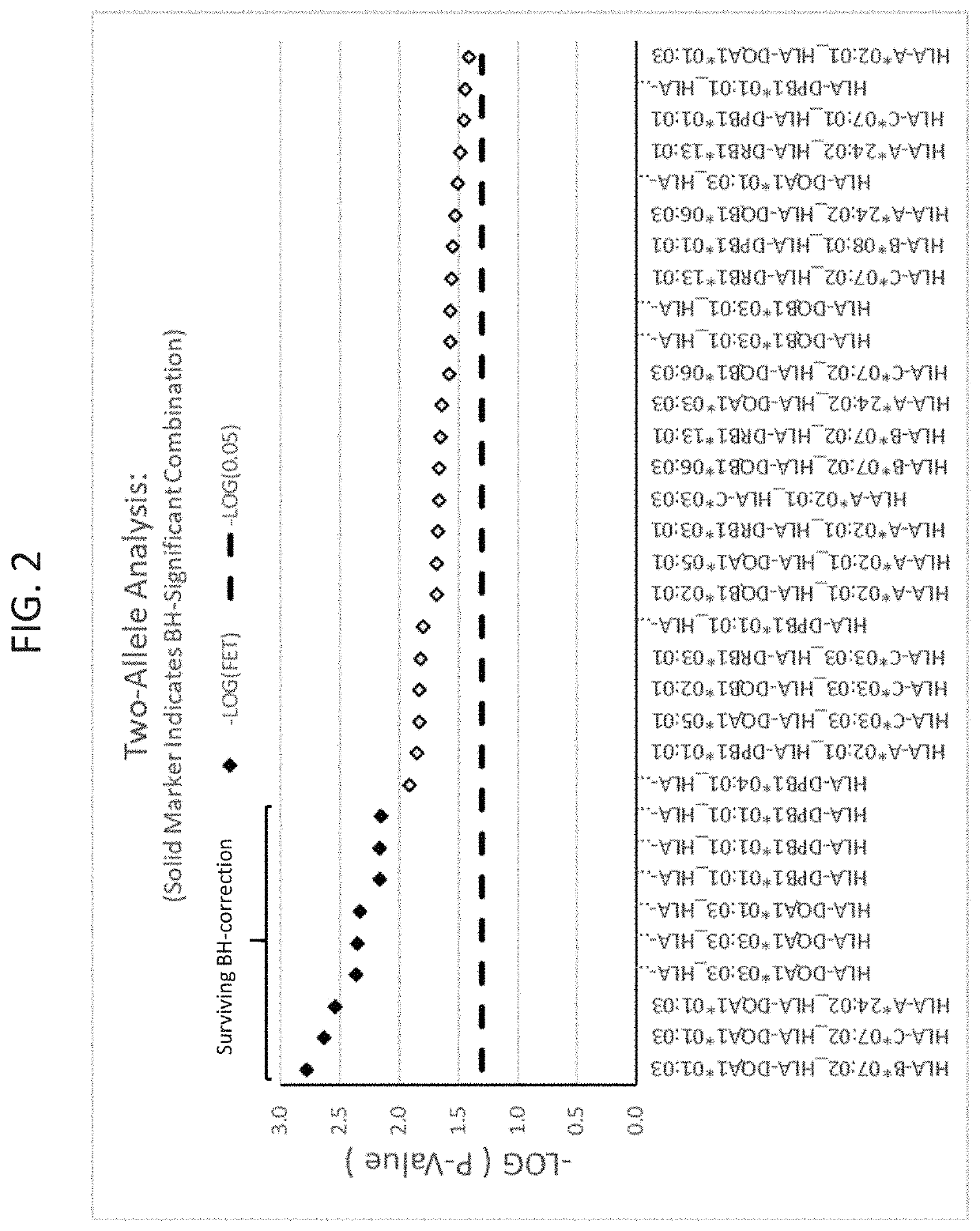
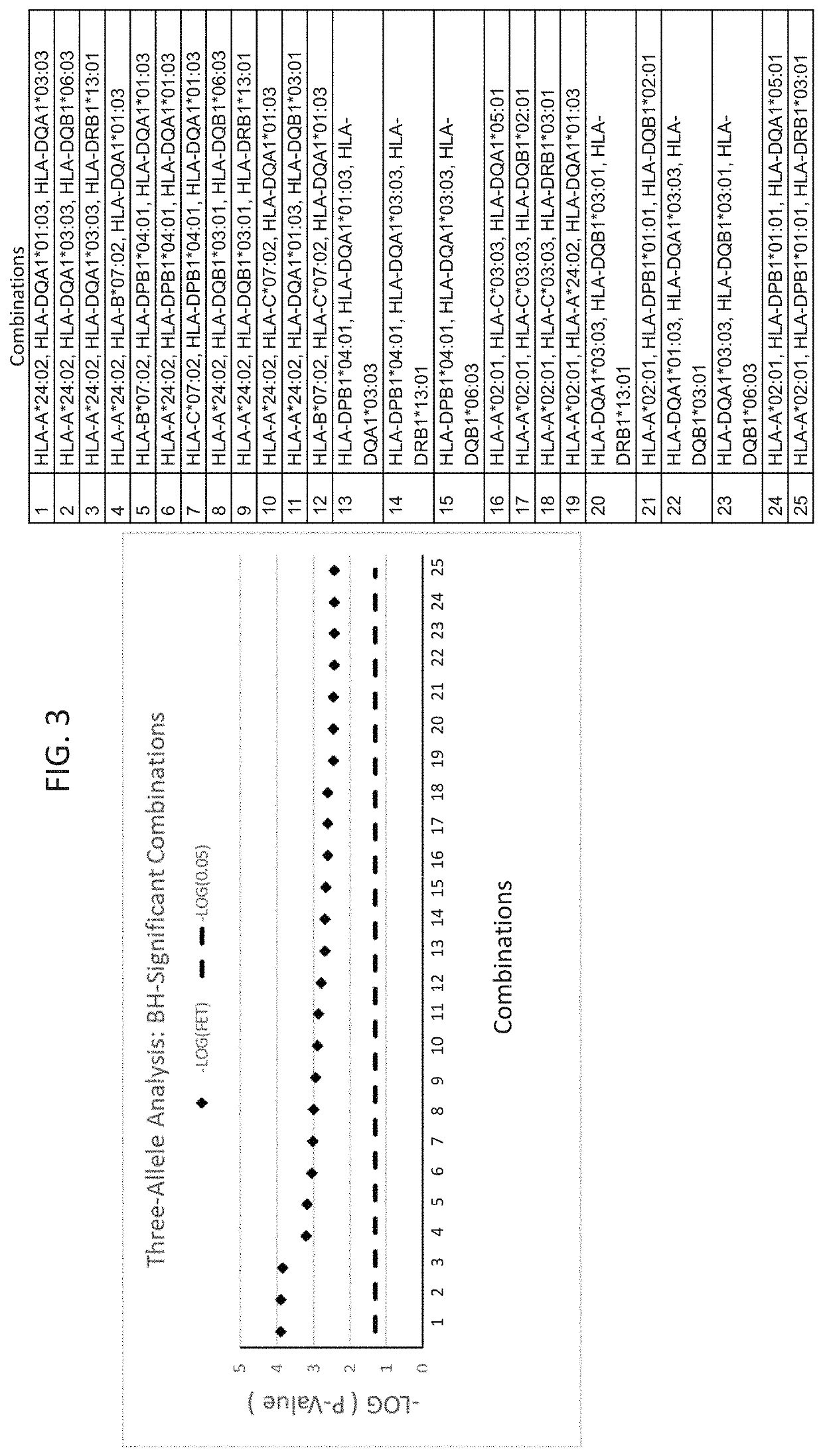
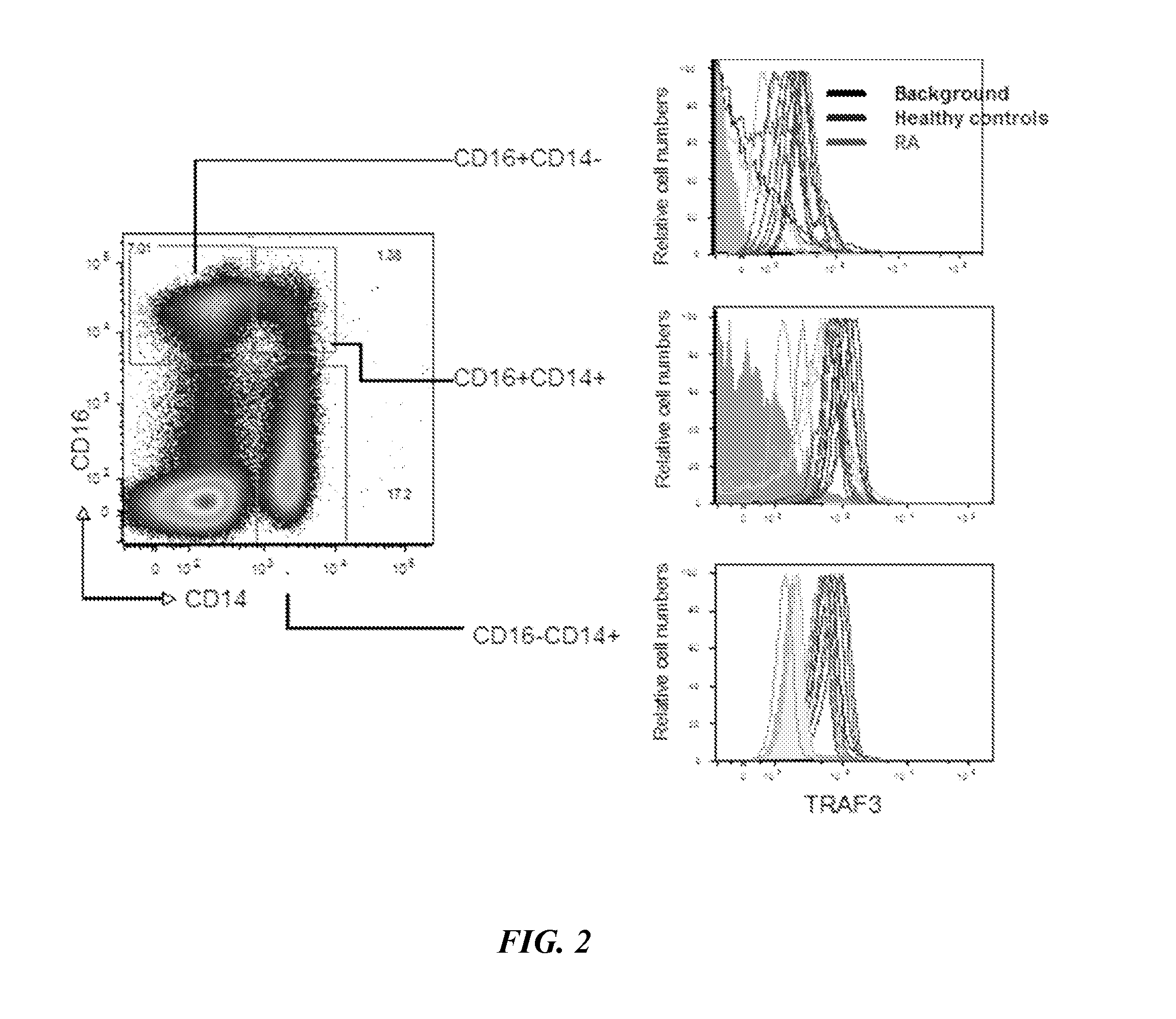
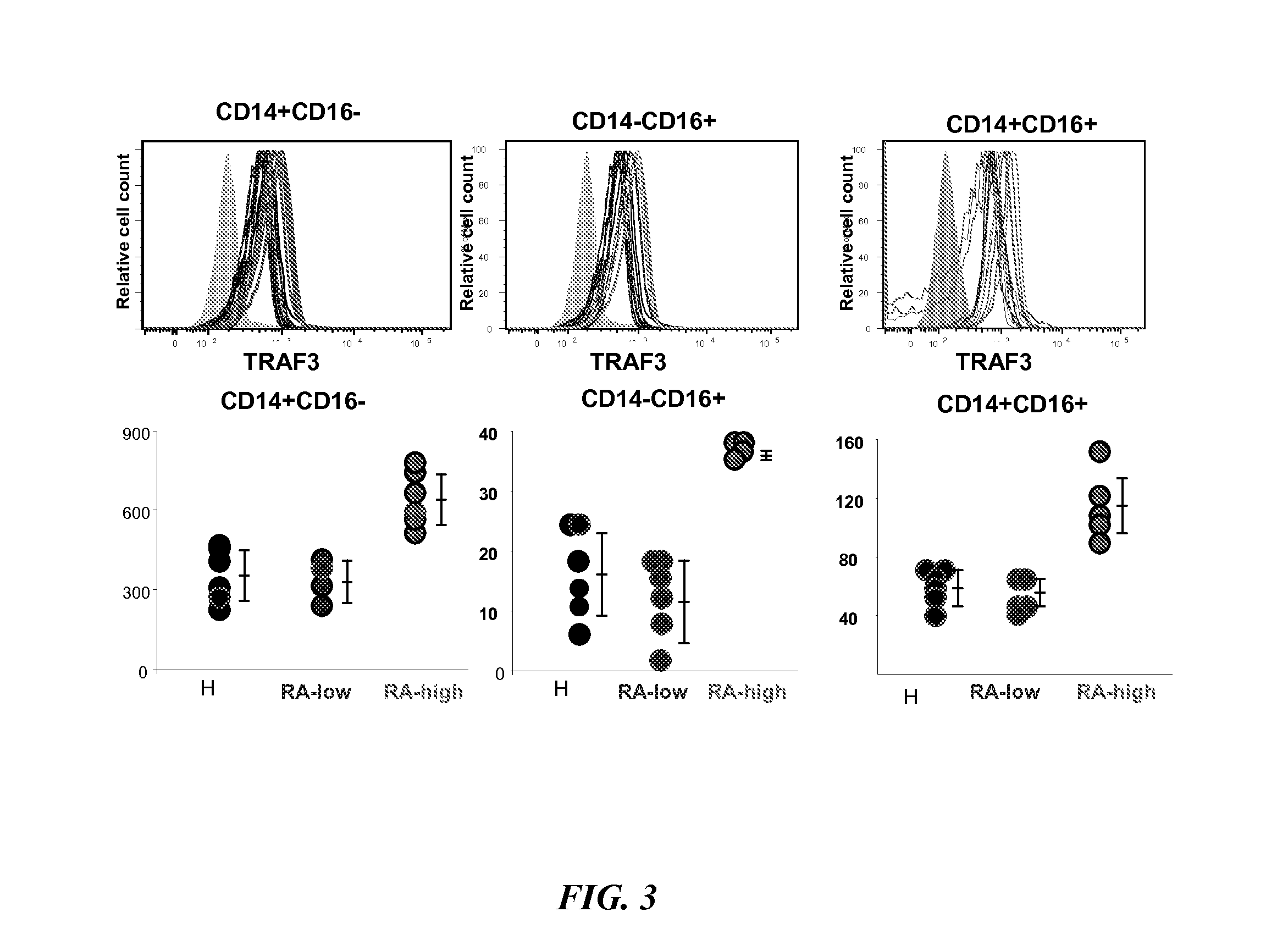
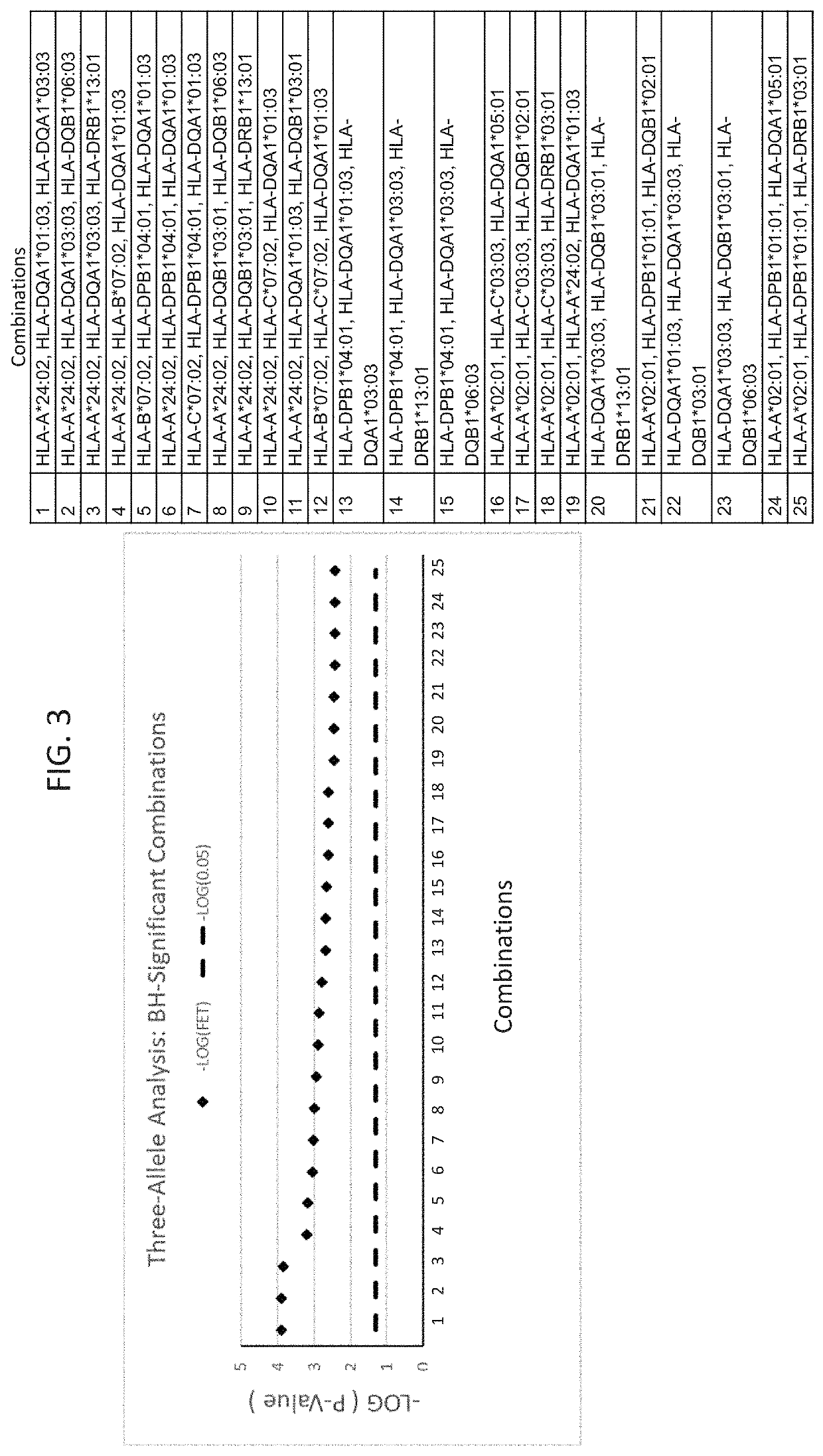
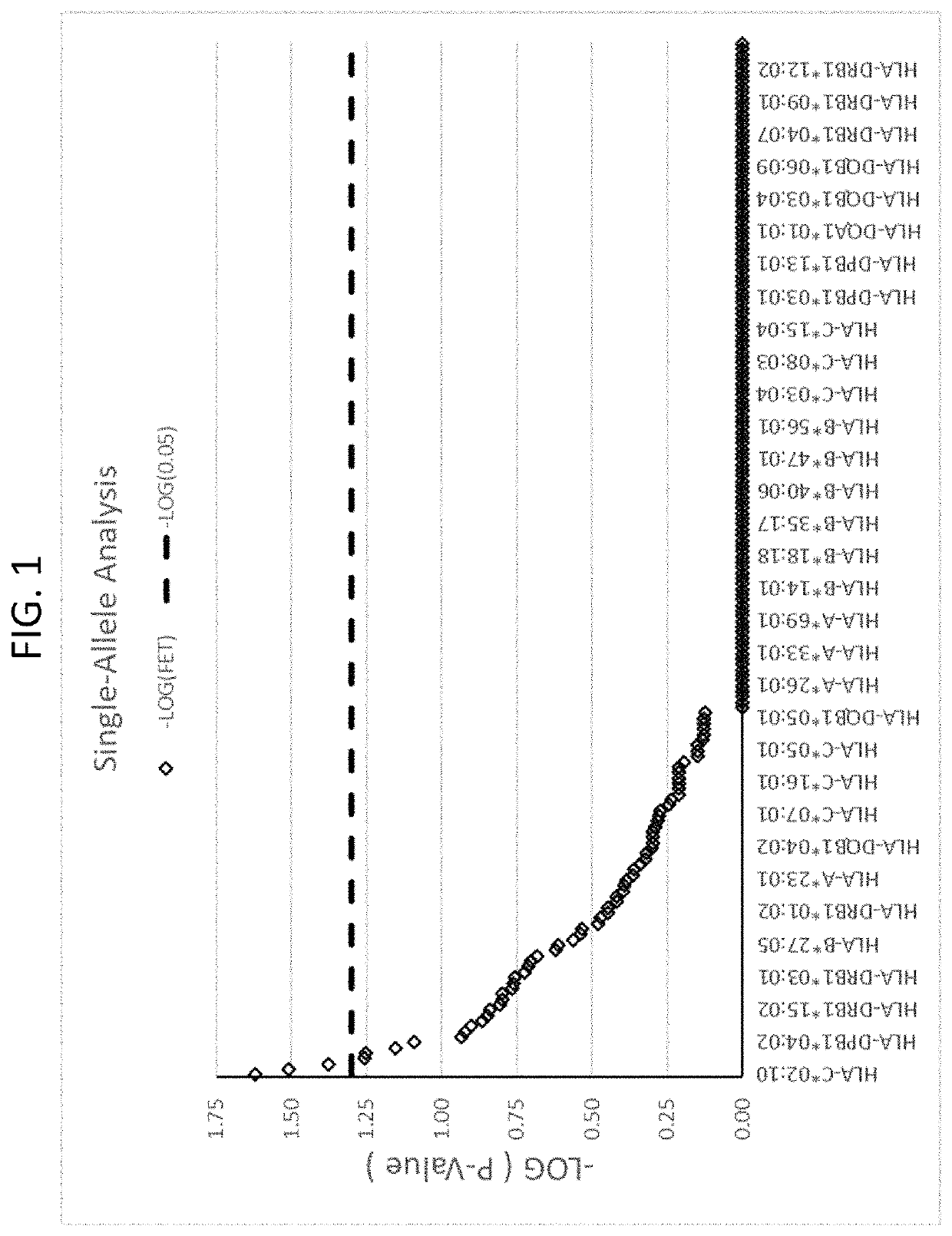
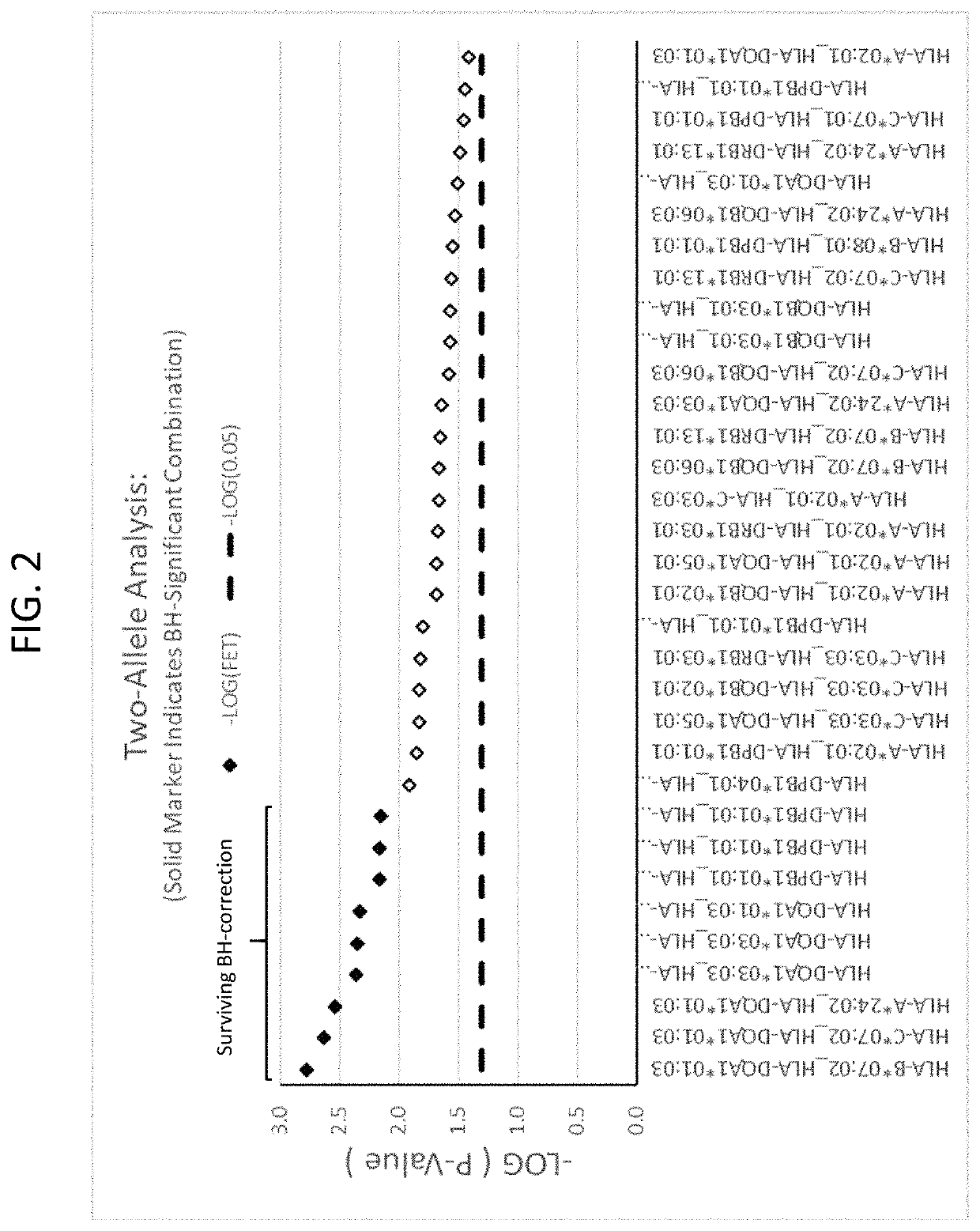
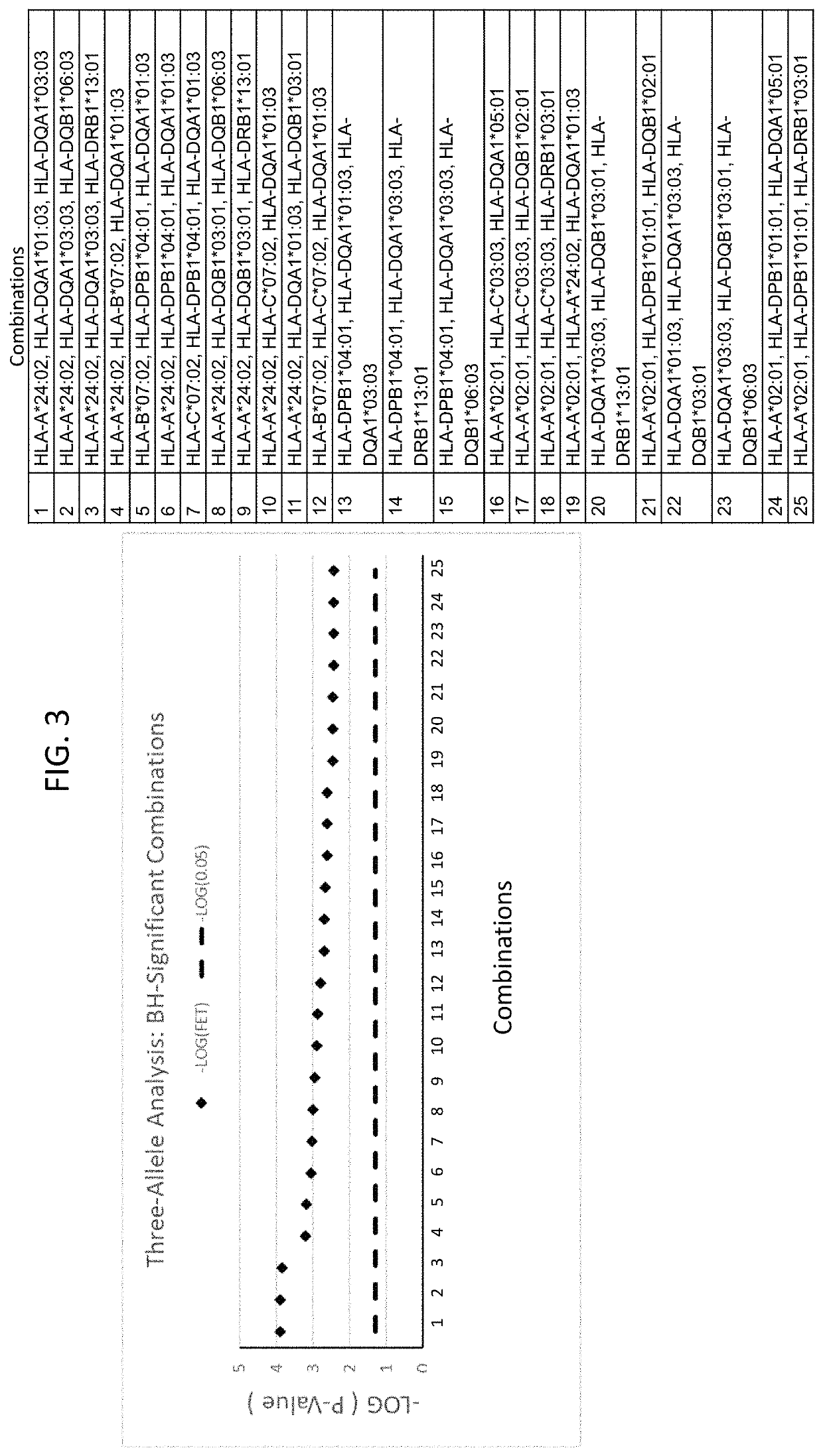
The present disclosure provides methods of treating a patient with infliximab or alternative therapies to reduce the risk of developing, and / or severity of, an adverse drug reaction such as drug-induced liver injury. The methods include identifying patients at risk for developing DILI by determining the presence or absence of one or more HLA alleles in the patients.
Owner:TEN PEAKS LLC
Androgen receptor ligands
Non ligand binding pocket antagonists for the human androgen receptor. The androgen receptor (AR) is a member of the Nuclear Receptor (NR) family and its role is to modulate the biological effects of the endogenous androgens, testosterone (tes) and dihydrotestosterone (DHT). Synthetic androgens and anti-androgens have therapeutic value in the treatment of various androgen dependent conditions, from regulation of male fertility to prostate cancer. Current treatment of prostate cancer (PCa) typically involves administration of ‘classical’ antiandrogens, competitive inhibitors of natural AR ligands, DHT and tes, for the ligand binding pocket (LBP) in the C-terminal ligand binding domain (LBD) of the AR. However, prolonged LBP-targeting can often lead to androgen resistance and alternative therapies and therapeutic strategies are urgently required. Disclosed herein are a class of non-steroidal, small molecule AR antagonists which inhibit the transcriptional activity of the AR by non LBP-mediated modulation. The novel class reported demonstrates full (‘true’) antagonism in AR with low micromolar potency, high selectivity over both the Estrogen Receptors alpha and beta (ERα and ERβ) and the Glucocorticoid Receptor (GR) and only micromolar partial antagonism in the Progesterone Receptor (PR). Data provide compelling evidence for such non-LBP intervention as an alternative approach to classical PCa therapy. (Formula I).
Owner:TRINITY COLLEGE DUBLIN
Methods for reducing drug-induced liver injury
ActiveUS10519228B2Lessen risk of and severityReduce development riskSugar derivativesMicrobiological testing/measurementAlleleCvd risk
The present disclosure provides methods of treating a patient with infliximab or alternative therapies to reduce the risk of developing, and / or severity of, an adverse drug reaction such as drug-induced liver injury. The methods include identifying patients at risk for developing DILI by determining the presence or absence of one or more HLA alleles in the patients.
Owner:TEN PEAKS LLC
Method to identify patients that will likely respond to Anti-tnf therapy
InactiveUS20150369823A1Improve the level ofMicrobiological testing/measurementLibrary screeningHigh rateAnti-TNF therapy
The present invention identifies various methods for the identification of patients that suffer from an immune system disorder and are likely to benefit from anti-TNF therapy. Because of the significant cost of anti-TNF therapy and the high rate of ineffectiveness of anti-TNF therapy for treating immune disorders such as rheumatoid and psoriatic arthritis, the present invention will improve the delivery of effective therapies to patients in need of either anti-TNF therapy or alternative therapies.
Owner:UNIVERSITY OF ROCHESTER
Methods for reducing drug-induced liver injury
ActiveUS20190345243A1Reduce riskReduce severityMicrobiological testing/measurementDigestive systemInfliximab AntibodyAlternative therapy
The present disclosure provides methods of treating a patient with infliximab or alternative therapies to reduce the risk of developing, and / or severity of, an adverse drug reaction such as drug-induced liver injury. The methods include identifying patients at risk for developing DILI by determining the presence or absence of one or more HLA alleles in the patients.
Owner:TEN PEAKS LLC
Methods for reducing drug-induced liver injury
ActiveUS10519227B2Lessen risk of and severityReduce development riskSugar derivativesMicrobiological testing/measurementAlleleDrug reaction
The present disclosure provides methods of treating a patient with infliximab or alternative therapies to reduce the risk of developing, and / or severity of, an adverse drug reaction such as drug-induced liver injury. The methods include identifying patients at risk for developing DILI by determining the presence or absence of one or more HLA alleles in the patients.
Owner:TEN PEAKS LLC
Anti-ccr7 receptor antibodies for the treatment of cancer
ActiveUS20090123483A1Reduce expressionEliminate effectiveImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigenAntigen Binding Fragment
Antibodies, or antigen-binding fragment thereof, which bind to a CCR7 receptor are capable of selectively killing, impairing migration and / or blocking dissemination of tumour cells expressing a CCR7 receptor. Use of said antibodies for killing or for inducing apoptosis of said tumour is disclosed, thus providing an alternative therapy for treatment of cancer which tumour cells express a CCR7 receptor.
Owner:AUTONOMOUS UNIVERSITY OF MADRID
Gene and protein expression profiles associated with the therapeutic efficacy of irinotecan
InactiveUS20090047684A1Microbiological testing/measurementDepsipeptidesIrinotecanProtein expression profile
The present invention includes gene and protein expression profiles indicative of whether a cancer patient is likely to respond to treatment with irinotecan. By identifying such responsiveness, a treatment provider may determine in advance those patients who would benefit from such treatment, as well as identify alternative therapies for non-responders. The present invention further provide methods of using the gene and / or protein expression profiles and assays for identifying the presence of a gene and / or protein expression profile in a patient sample.
Owner:NMDX LLC
Methods for reducing drug-induced liver injury
InactiveUS20190345240A1Reduce riskReduce severityMicrobiological testing/measurementDigestive systemAlleleCvd risk
The present disclosure provides methods of treating a patient with infliximab or alternative therapies to reduce the risk of developing, and / or severity of, an adverse drug reaction such as drug-induced liver injury. The methods include identifying patients at risk for developing DILI by determining the presence or absence of one or more HLA alleles in the patients.
Owner:TEN PEAKS LLC
Method and compositions for treatment of cancers
InactiveUS20100285044A1Improve inflammationEnhances cytokine activityOrganic active ingredientsPeptide/protein ingredientsSystemic chemotherapyWhole body
A method to treat cancer uses ultrapheresis, refined to remove compounds of less than 120,000 daltons molecular weight, followed by administration of replacement fluid, to stimulate the patient's immune system to attack solid tumors. In the preferred embodiment, the patient is ultrapheresed using a capillary tube ultrafilter having a pore size of 0.02 to 0.05 microns, with a molecular weight cutoff of 120,000 daltons, sufficient to filter one blood volume. The preferred replacement fluid is ultrapheresed normal plasma. The patient is preferably treated daily for three weeks, diagnostic tests conducted to verify that there has been shrinkage of the tumors, then the treatment regime is repeated. The treatment is preferably combined with an alternative therapy, for example, treatment with an anti-angiogenic compound, one or more cytokines such as TNF, gamma interferon, or IL-2, or a procoagulant compound. The treatment increases endogenous, local levels of cytokines, such as TNF. This provides a basis for an improved effect when combined with any treatment that enhances cytokine activity against the tumors, for example, treatments using alkylating agents, doxyrubicin, carboplatinum, cisplatinum, and taxol. Alternatively, the ultrapheresis treatment can be combined with local chemotherapy, systemic chemotherapy, and / or radiation.
Owner:INNATUS CORP
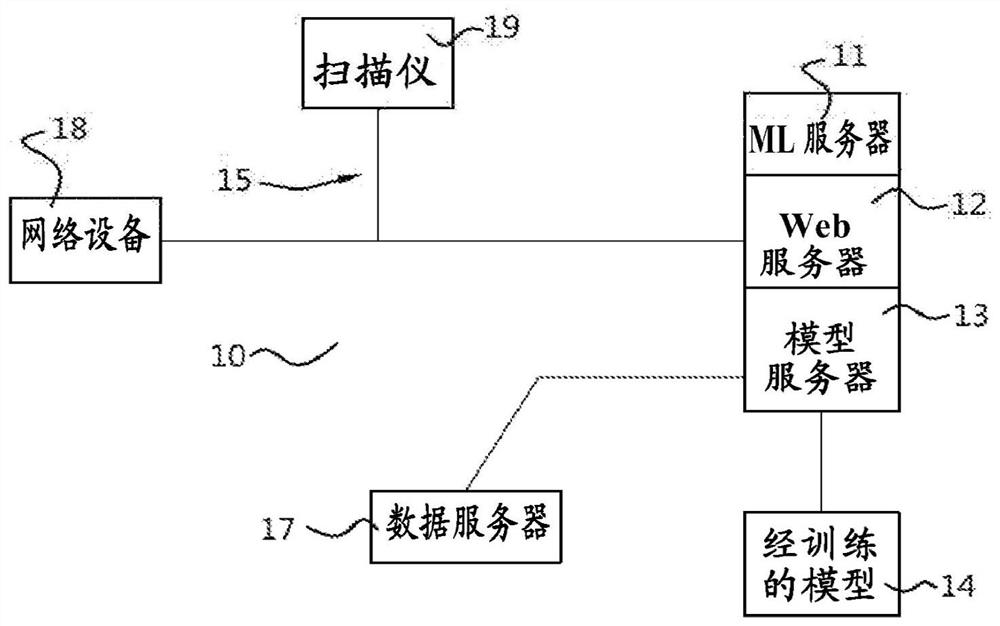
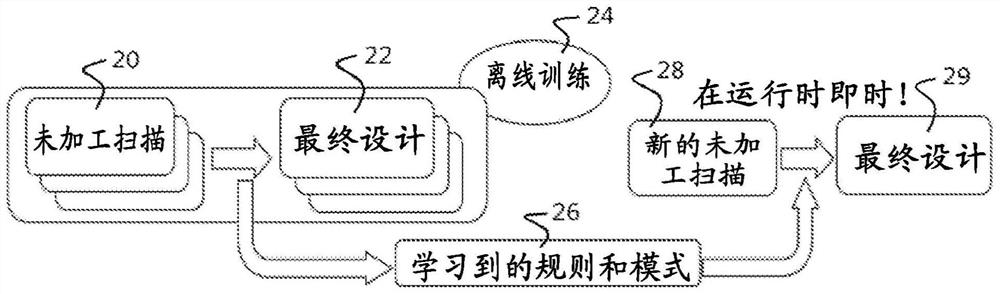
Method, system and apparatus for customizing immediate automated design of dental object
Embodiments of the present invention provide methods, systems, devices, and software for providing customized, clinically related designs in real time as needed, such as treatment plans / solutions for at least a single tooth replacement therapy, to enable immediate confirmation of the effectiveness and execution of patient-specific treatment solutions. Machine learning algorithms are used in computer-implemented methods or systems for automated prosthesis design. The prosthetic designs include designs of prosthetic elements such as dental crowns and abutments. Software for performing a method when executed on a digital processor is provided. Also included is fabrication of the repairable element.
Owner:DENTSPLY SIRONA INC
Diagnostic imaging device for the analysis of circulating rare cells
ActiveUS20070153280A1Minimal spaceEasy accessMicrobiological testing/measurementDead animal preservationDiseaseRegimen
The present invention provides a system for imaging circulating tumor cells from blood after enrichment. The system is designed to provide optimum use in a clinical laboratory setting with minimum laboratory bench top space. Operator intervention is minimized compared to other analytical methodologies. The system is useful in the enumeration and identification of target cells in a sample specimen for screening and detection of early stage pre-metastatic cancer, monitoring for disease remission in response to therapy and selection of more effective dose regimens or alternative therapies for individual patients.
Owner:MENARINI SILICON BIOSYSTEMS SPA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com