Antitumor slow-release implantation agent and preparation method thereof
A slow-release implant and anti-tumor technology, applied in the field of biomedicine, can solve the problems of difficult entry into cells, low tissue affinity, short half-life, etc., to achieve the effect of reducing drug concentration, increasing drug concentration, and enhancing drug action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
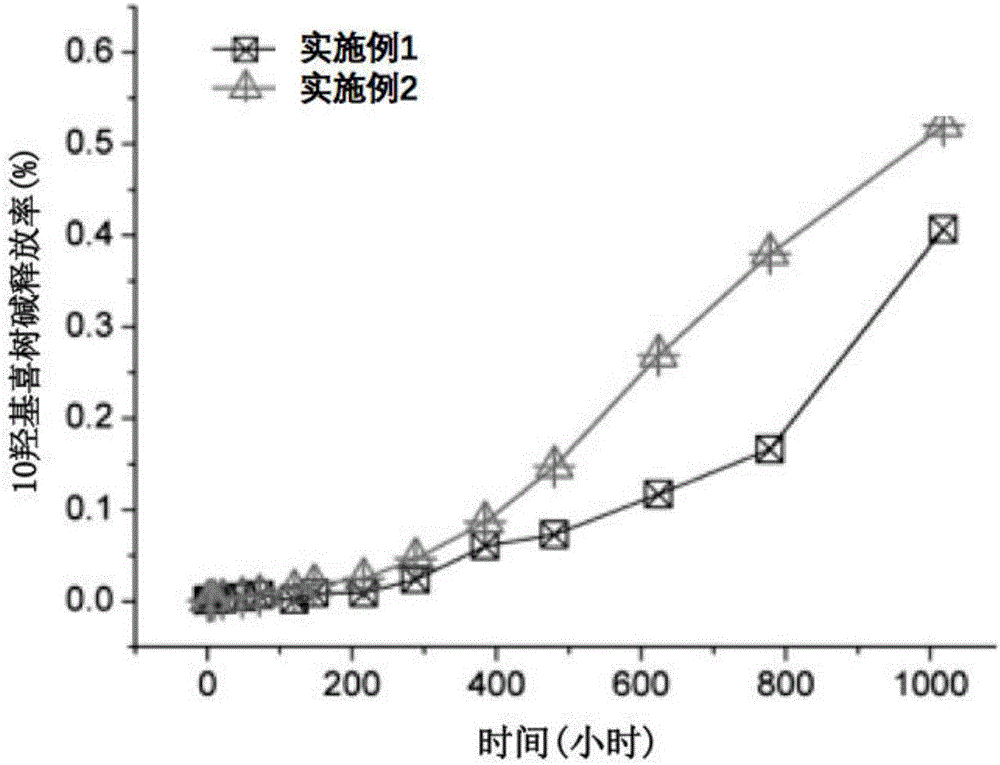
Embodiment 1
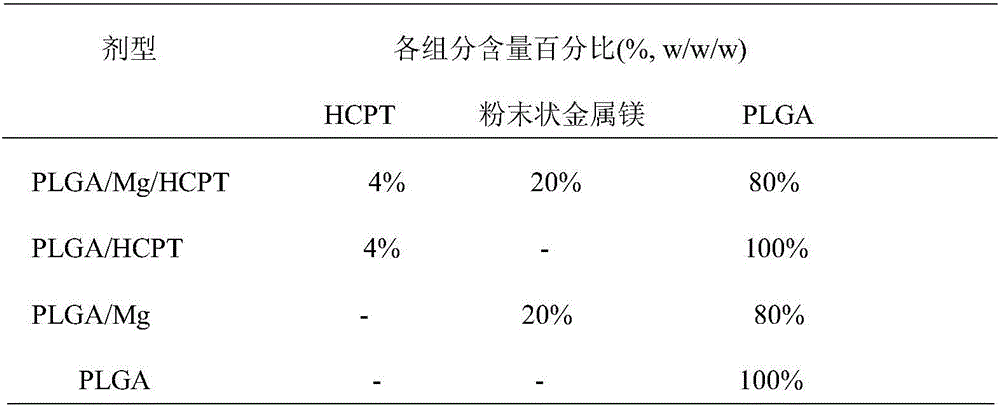
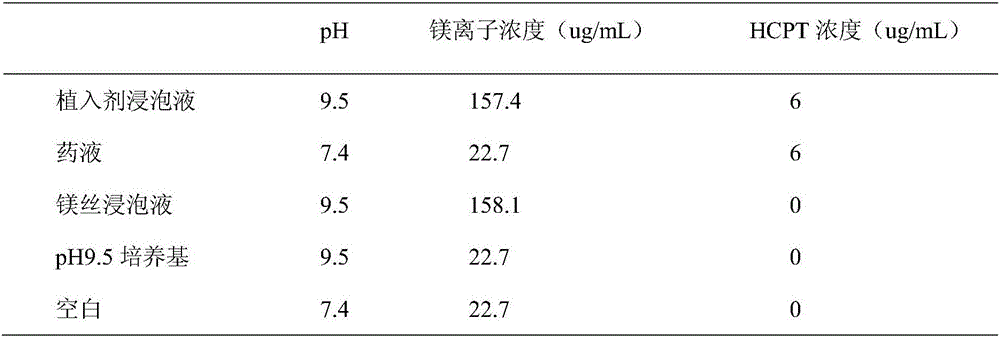
[0022] Under nitrogen protection, 0.2g 10-hydroxycamptothecin, 8.0g lactide-glycolide copolymer (lactide accounted for 50% by mole, weight average molecular weight is 110,000), 2.0g powdered metal Magnesium (average particle size: 74um) was co-melted and extruded at 180°C; the rod-shaped extrudate was cooled and then cut to obtain an anti-tumor implant with a diameter of 0.5mm and a length of 3.0mm.
Embodiment 2
[0024] Under nitrogen protection, 0.7g 10-hydroxycamptothecin, 7.0g polyglycolide (weight-average molecular weight is 10,000), 3.0g powdered magnesium metal (average particle diameter is 300um) are co-melted and extruded, extrusion temperature The temperature is 220°C; after the rod-shaped extrudate is cooled, it is cut to obtain an anti-tumor implant with a diameter of 0.7mm and a length of 2.5mm.
Embodiment 3
[0026] Under nitrogen protection, 0.45g 10-hydroxycamptothecin, 4.5g polylactide (weight average molecular weight 11,000), 4.5g polydioxanone (weight average molecular weight 13,000), 1.0g powdered metal Co-melt extrusion of magnesium (average particle size: 5um) at an extrusion temperature of 180°C; cut the rod-shaped extrudate after cooling to obtain an anti-tumor implant with a diameter of 1.0 mm and a length of 4.0 mm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com