Oral pharmaceutical compositions in timed-release particle form and fast-disintegrating tablets containing this composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
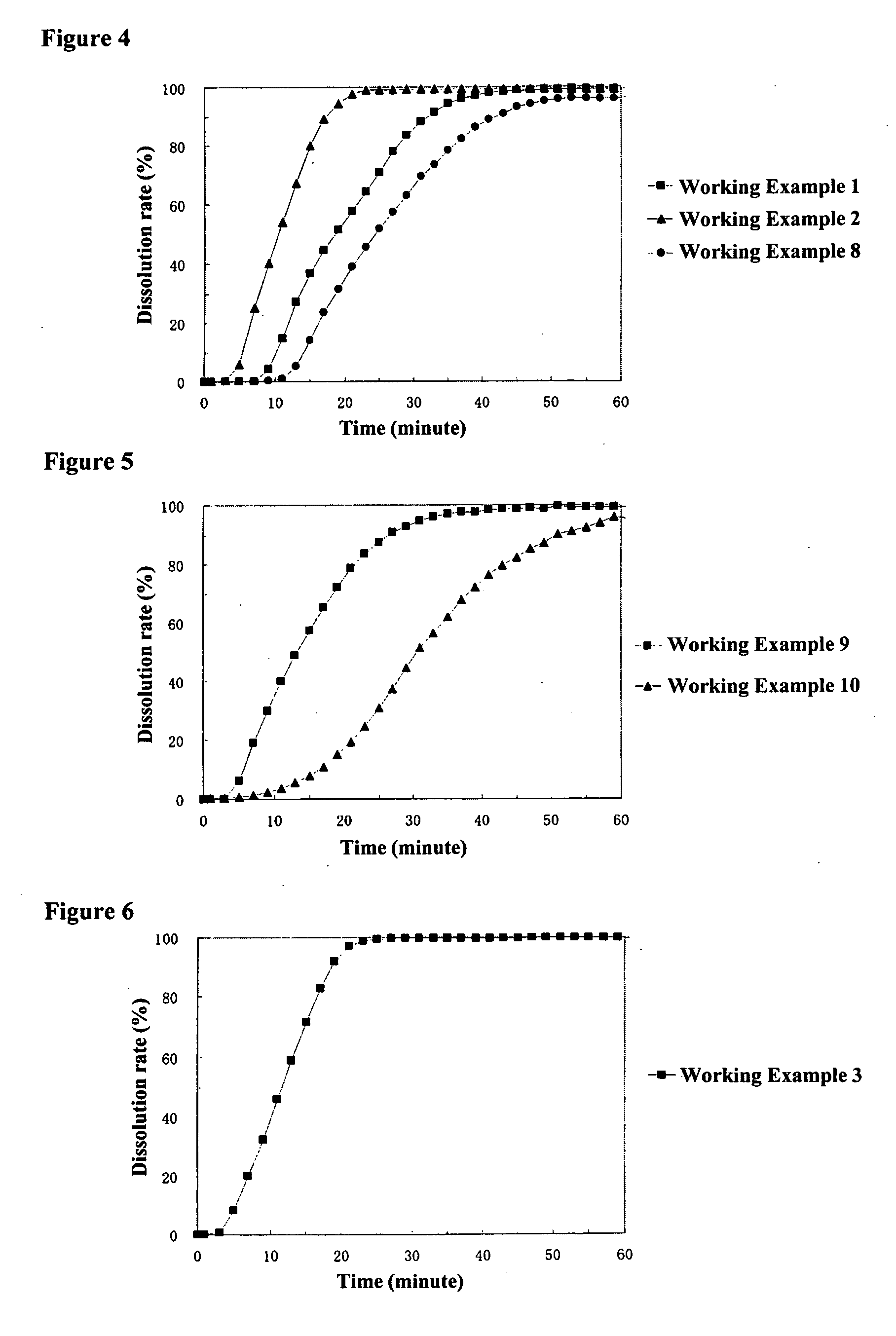
working example 1
[0136] [Preparation of Core Particles Containing a Drug]
[0137] A liquid of 225.0 g of acetaminophen (Yoshitomi Fine Chemicals, Ltd.) and 22.5 g of hydroxypropylmethylcellulose 2910 (Shin-Etsu Chemical Co., Ltd., TC-5E, the same hereafter) dissolved in a mixture of 750.0 g of methanol (Kanto Kagaku, the same hereafter) and 653.0 g of methylene chloride (Kanto Kagaku, the same hereafter) was sprayed from the side onto 750.0 g of sucrose spheres (Freund GmbH; Nonpareil 103 (24-32)) at a product temperature of 30° C., liquid feed rate of 20 g / mL, and spraying air pressure of 2.5 kg / cm using a fluidized bed granulator (Glatt GmbH; GPCG-1, the same hereafter) to obtain core particles containing a drug.
[0138] [Preparation of Middle Layer Coating Liquid]
[0139] 44.2 g of hydroxypropylmethylcellulose 2910 were dissolved in a mixture of 1052.0 g of methanol and 450.8 g of methylene chloride, 221.0 g of sodium carbonate (Kanto Kagaku, the same hereafter) pulverized with a jet mill pulverizer (...
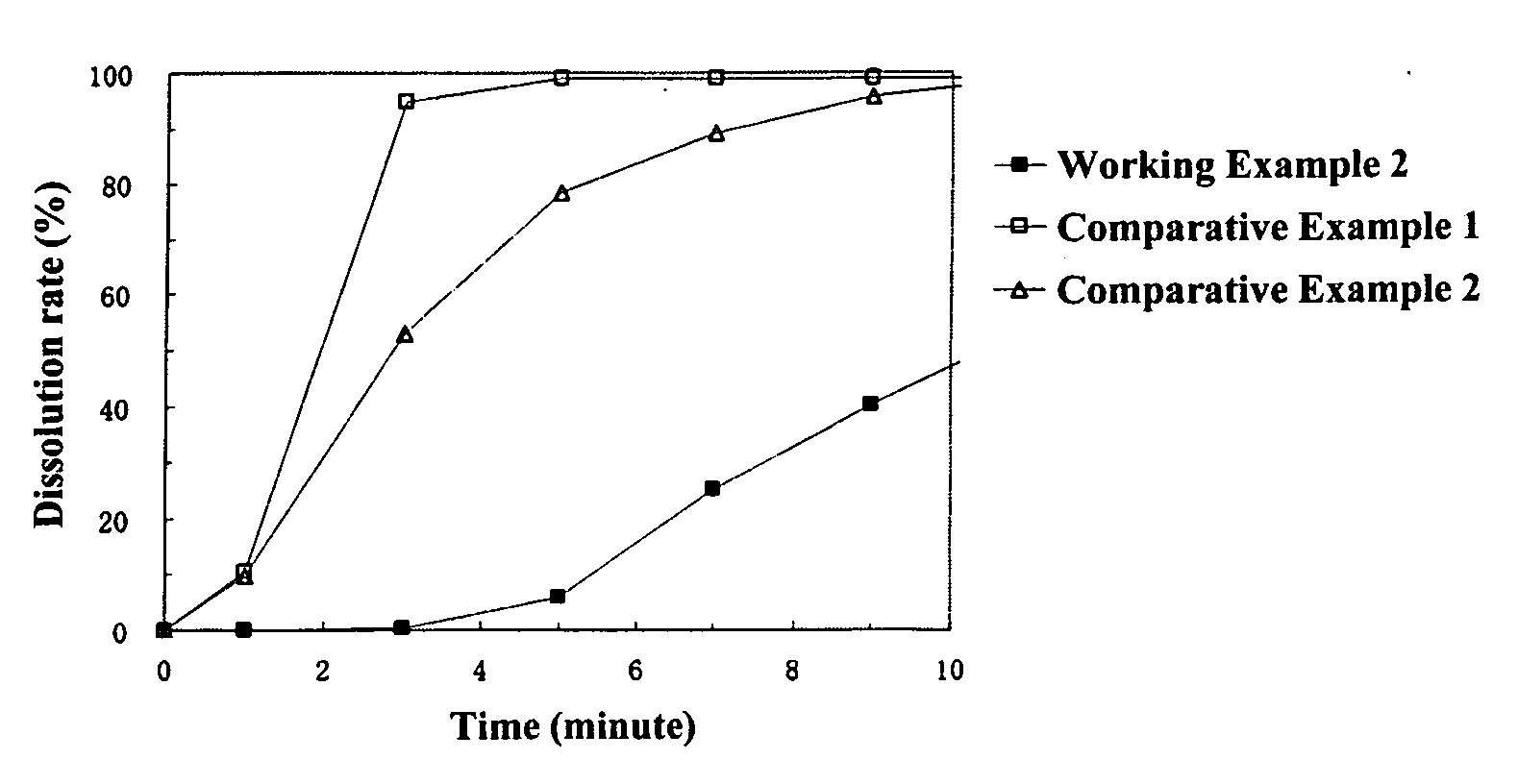
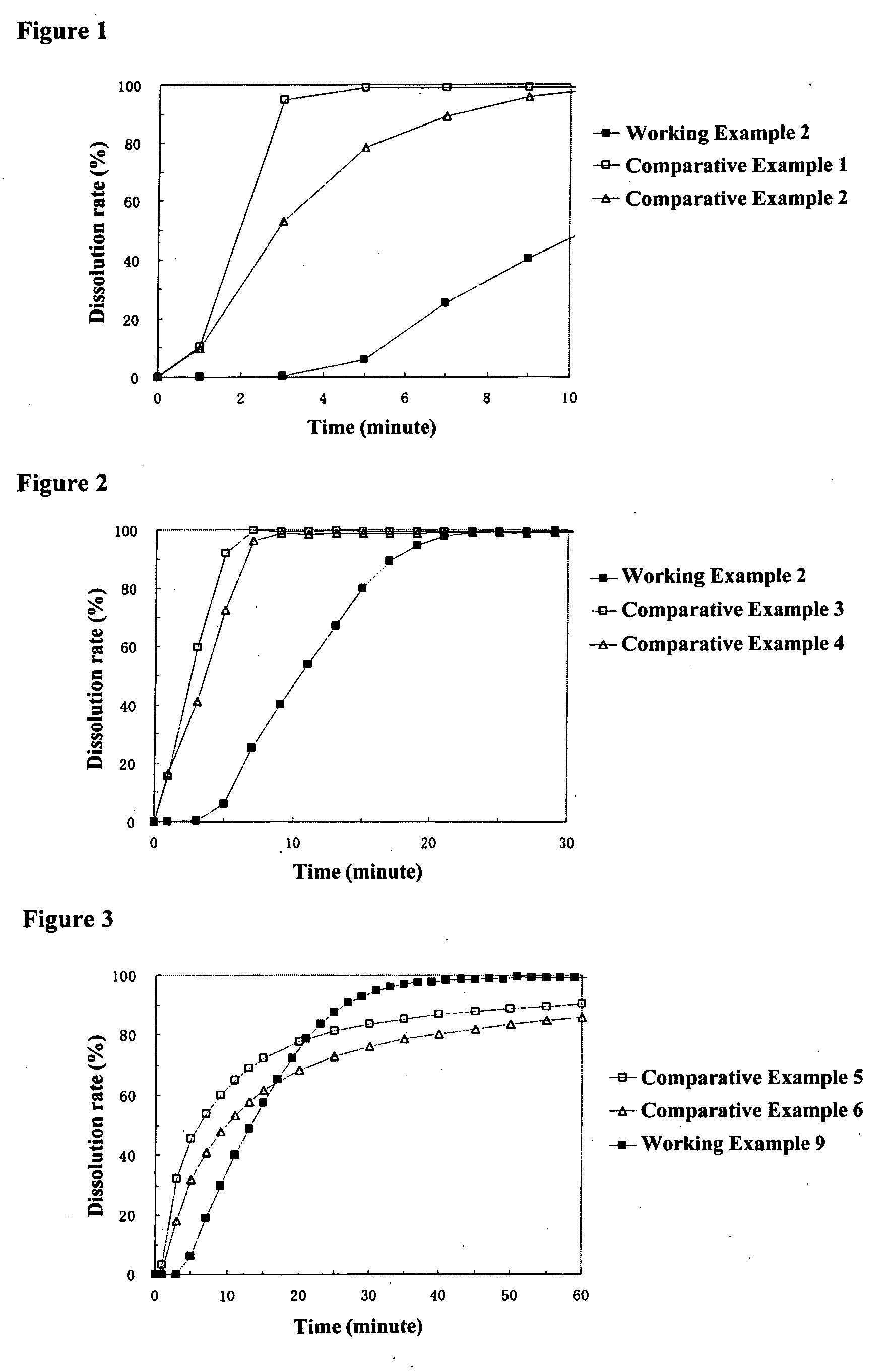
working example 2
[0146] [Preparation of Coating Liquid for Controlling Water Penetration]
[0147] 25.0 g of cetanol were dissolved in 975.0 g of methylene chloride to prepare the coating liquid for the layer for controlling water penetration.
[0148] [Preparation of Particles Coated with Middle Layer and Layer for Controlling Water Penetration]
[0149] The above-mentioned coating liquid for a layer for controlling water penetration was sprayed from the side onto 500.0 g of particles coated with a middle layer obtained in Working Example 1 at a product temperature of 30° C., liquid feed rate of 15 g / min, and a spraying air pressure of 2.0 kg / cm2 using a fluidized bed granulator to obtain particles coated with a middle layer and a layer for controlling water penetration wherein the particles coated with a middle layer were coated with a 5 wt % layer for controlling water penetration. The average particle diameter of the resulting particles coated with a middle layer and( a layer for controlling water penet...
working example 3
[0150] [Preparation of Middle Layer Coating Liquid]
[0151] 39.8 g of hydroxypropylmethylcellulose 2910 were dissolved in a mixture of 946.8 g of methanol and 405.8 g of methylene chloride, 198.9 g of sodium dihydrogen phosphate dihydrate (Kanto Kagaku, the same hereafter) pulverized with a jet mill pulverizer were added, and the product was stirred to prepare the middle layer coating liquid.
[0152] [Preparation of Particles Coated with Middle Layer]
[0153] The above-mentioned middle layer coating liquid was sprayed from the side onto 450.0 g of core particles containing a drug obtained in Working Example 1 at a product temperature of 30° C., liquid feed rate of 22.0 g / min, and a spraying air pressure of 4.5 kg / cm2 using a fluidized bed granulator to obtain particles coated with a middle layer wherein core particles containing a drug were coated with a 53 wt % middle layer. The average particle diameter of the resulting particles coated with a middle layer was 889 μm.
[0154] [Preparati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Acidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com