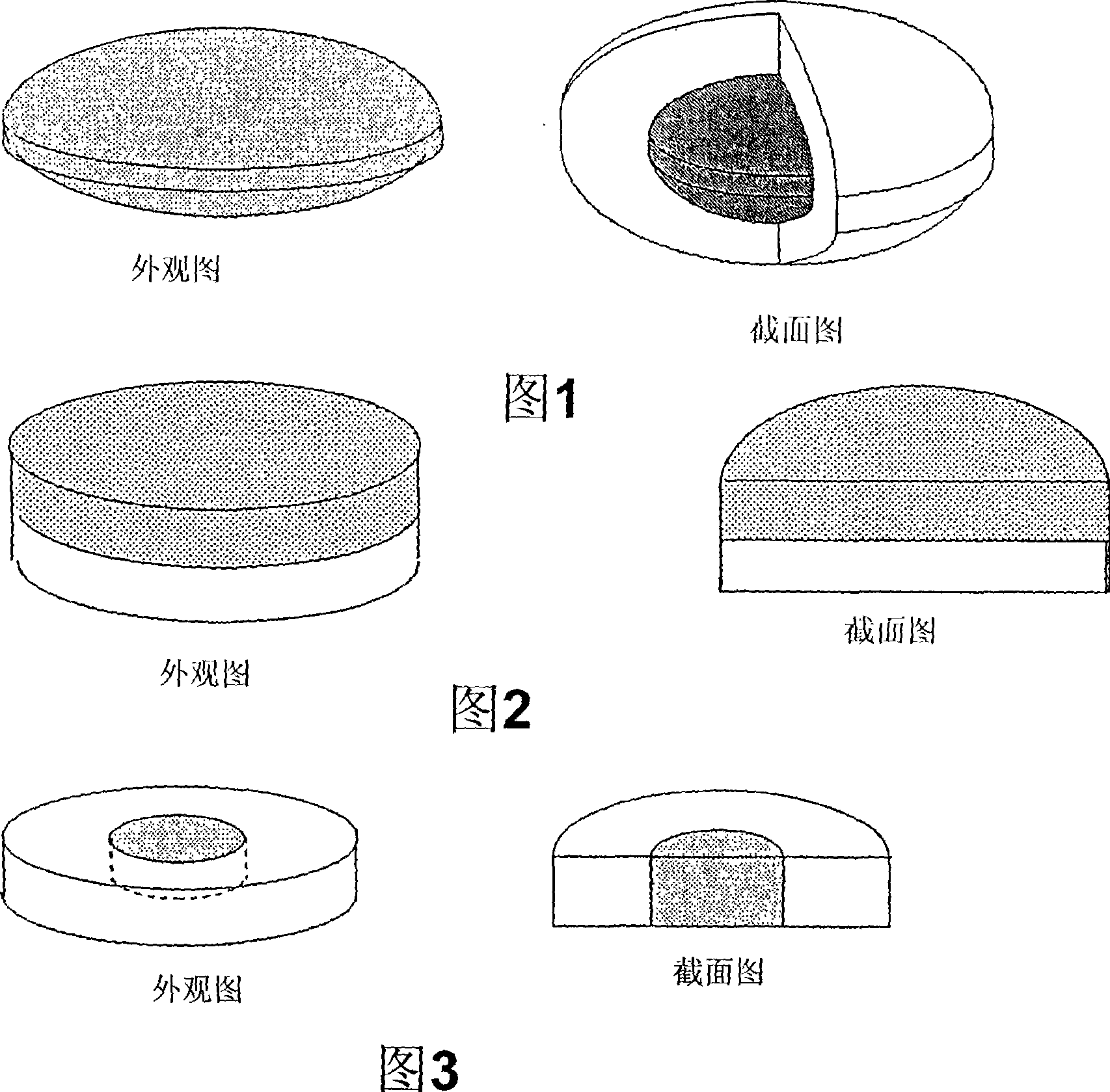
Solid pharmaceutical preparation dissolved in oral cavity
A solid preparation and intraoral technology, applied in the field of solid preparations for intraoral dissolution, can solve problems such as inability to maintain the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Mix 1 g of cetylpyridinium chloride (manufactured by Wako Pure Chemical Industries, Ltd., trade name cetylpyridinium chloride), 183.8 g of sorbitol, and 3 g of sodium saccharin, and use a mixture obtained by dissolving 8 g of hydroxypropyl cellulose in 40 g of ethanol. Wet granulation, the granulated material was dried and then sized to obtain 195.8 g of inner core sized powder. 2 g of magnesium stearate, 2 g of menthol powder, and 0.2 g of citrus flavor were mixed with the obtained sized powder, and uncoated inner core tablets with a diameter of 8 mm and a mass of 200 mg were produced with a rotary tablet machine. Mix tranexamic acid (manufactured by Daiichi Pharmaceutical Co., Ltd., trade name tranexamic acid) 250g, aspartame 30g, dipotassium glycyrrhizinate 13g, mannitol 442.2g, crospovidone 40g, magnesium stearate 8g, peppermint 16 g of alcohol powder and 0.8 g of grapefruit flavor were used to compress and coat the mixed powder with 800 mg per piece of the obtained...
Embodiment 2
[0046] Mix 1 g of cetylpyridinium chloride (manufactured by Wako Pure Chemical Industries: trade name cetylpyridinium chloride), 292.7 g of mannitol, 3 g of magnesium stearate, 3 g of menthol powder, and 0.3 g of citrus flavor, and use direct powder compression method, The mixed powder was made into an uncoated core tablet with a diameter of 9 mm and a mass of 300 mg with a rotary tablet press. Mix tranexamic acid (manufactured by Daiichi Pharmaceutical Co., Ltd.: trade name tranexamic acid) 400g, aspartame 30g, dipotassium glycyrrhizinate 13g, sorbitol 292.2g, crospovidone 40g, stearyl fumaric acid 8 g of sodium, 16 g of menthol powder, and 0.8 g of grapefruit flavor were used to compress and coat the mixed powder with 800 mg per tablet of the uncoated core tablets obtained using a coating tablet press to obtain a solid preparation with a diameter of 13.5 mm and a mass of 1100 mg.
Embodiment 3
[0048] Mix 2 g of cetylpyridinium chloride (manufactured by Wako Pure Chemical Industries: trade name cetylpyridinium chloride), 184.3 g of xylitol, 184.3 g of erythritol, and 5 g of sodium saccharin, and dissolve 16 g of hydroxypropyl cellulose in 80 g of ethanol The mixed solution obtained in the above method was subjected to wet granulation, and the granulated material was dried and then sized to obtain 391.6 g of inner core sized powder. 4 g of magnesium stearate, 4 g of menthol powder, and 0.4 g of citrus flavor were mixed with the obtained sized powder, and an uncoated inner core tablet with a diameter of 10 mm and a mass of 400 mg was produced with a rotary tablet machine. Mix tranexamic acid (manufactured by Daiichi Pharmaceutical Co., Ltd., trade name tranexamic acid) 250g, aspartame 30g, dipotassium glycyrrhizinate 13g, sorbitol 576g, microcrystalline cellulose 100g, magnesium stearate 5g, menthol 25 g of powder and 1 g of grapefruit flavor were used to compress and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com