Hsp therapy in conjunction with a low antigenicity diet
a low-antigenicity, hydrolyzed protein technology, applied in animal/human proteins, metabolism disorders, non-active ingredients of pharmaceuticals, etc., can solve the problems of difficult to distinguish clinically between type 1 and type 2 diabetes, increase the risk of type 1 diabetes in finnish children, and the immune destruction process is much slower. , to achieve the effect of improving the protective effect of hydrolyzed protein/low-antigenic di
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Neonatal Oral Administration of DiaPep277 in Combination with the Protective Hydrolyzed Casein Diet
Materials and Methods:
[0090] Experimental Set-Up:
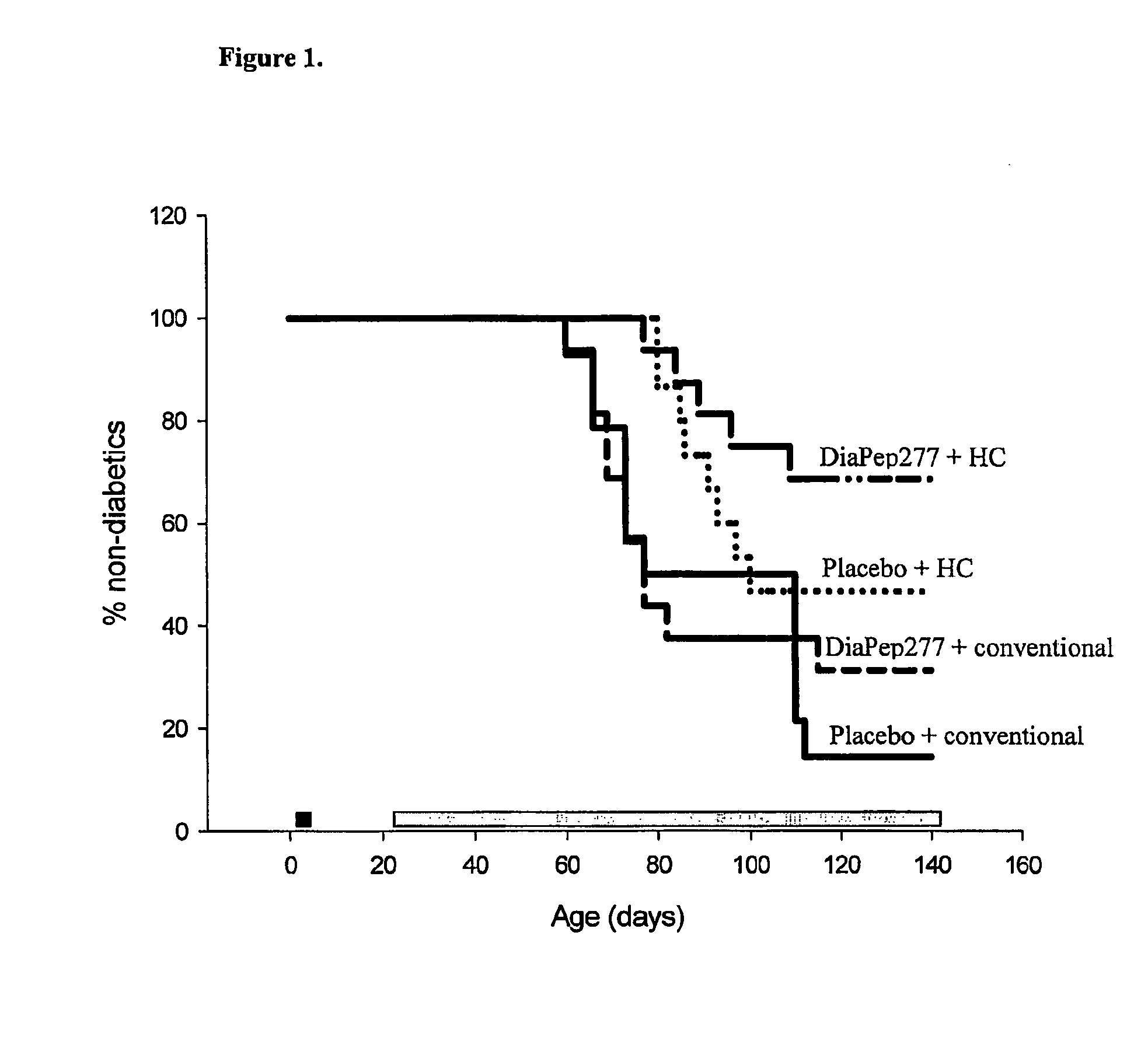
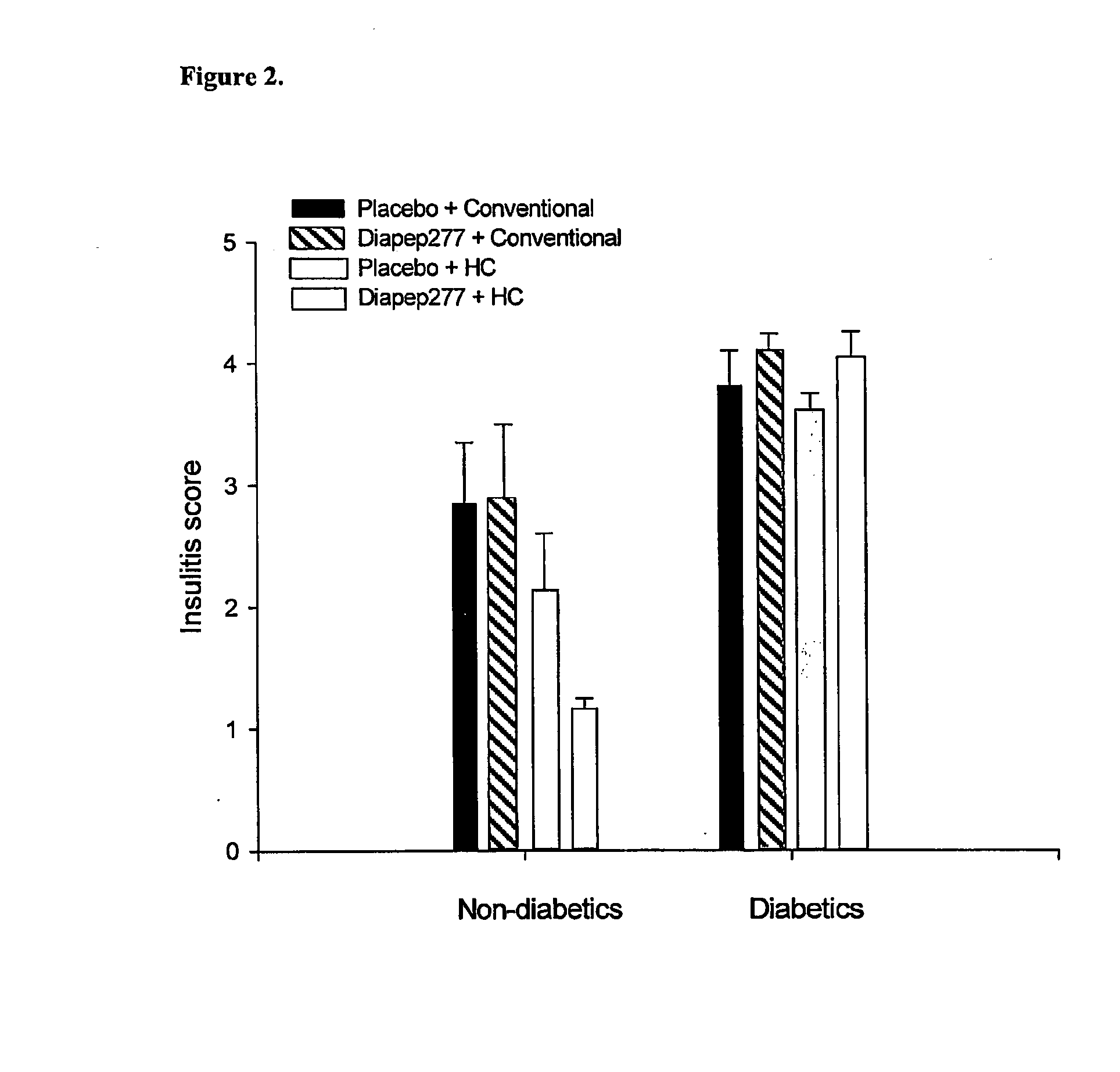
[0091] Group-housed BB-DP rats (breeding colony, Groningen, the Netherlands) were orally inoculated once per day with either placebo (aqua dist.) or Diapep277 at days 4, 5, 6 and 7 of life (black box in FIG. 1). Animals were treated in compliance with the principles of laboratory care (NIH publication no. 85-23, revised 1985) and the Dutch law on experimental animal care. Inoculation was done via a silicon-tube swallowed by the neonate and fluid was inoculated directly into the stomach. DiaPep277 was supplied by Peptor Ltd., Rehovot, Israel. It is an analog of the native 437-460 sequence of human hsp60, in which the existing cystein residues at positions 442 and 447 were replaced by valine, for better chemical stabilization. Per inoculation 300 μg / rat of Diapep277 in a volume of 300 μl was administered. At the age of 21 days (gray bo...
example 2
Dose Response Effect of Oral Administration of DiaPep277 on the Development of Diabetes Type 1 in the BB-DP Rat
[0097] It was shown that neonatal oral administration of DiaPep277 in combination with the protective HC diet significantly delayed the onset of diabetes type 1 in the BB-DP rat and decreased the incidence by 64% compared to placebo controls on a conventional diet. Administration of DiaPep277 in combination with a conventional diet tended to lower the incidence. Instead of 85% only 69% of the animals became diabetic. In this previous experiment orally DiaPep277 was administered on four consecutive days (day 4, 5, 6, and 7 of life) in a concentration of 300 μg / rat / day. Since this dose by itself tended to lower the diabetes incidence a dose response experiments are performed to check whether the effect of DiaPep277 becomes more pronounced at higher doses and whether a prolongation of the administration period (longer than four consecutive days) also increases the effect of D...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Dimensionless property | aaaaa | aaaaa |
| Dimensionless property | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com