Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
37 results about "Mucolytic Agent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Any agent that dissolves mucus, making it less thick and sticky. This makes it easier for the mucus to drain from the upper respiratory system, be removed through coughing, or be transported through the gastrointestinal (GI) tract.
Solution forms of cyclodextrins for nasal or throat delivery of essential oils
This invention further relates to a method for preventing or treating diseases or conditions of the oral cavity, throat or nose of warm-blooded animals including humans. More particularly, the invention pertains to a composition and method for spraying essential oils to the oral cavity, throat or nasal mucosa as cyclodextrin inclusion complexes. The spray composition includes a cyclodextrin in an amount of from about 0.1% w / v to about 20% w / v; at least one essential oil in an amount of from about 0.001% w / v to about 5.0% w / v; an effective amount of an antimicrobial preservative composition; and water. The composition may further comprise an alcohol co-solvent, a thickening agent, a sweetener, an antitussive, an anticholinergic, a decongestant, an antihistamine, an astringent, an anti-inflammatory steroid composition, a vitamin, a respiratory stimulant, a mucolytic agent, a bronchodilator, a beta-antagonist, an antidiarrheal agent, or combinations thereof.
Owner:QPHARMA
Use of inhaled gaseous nitric oxide as a mucolytic agent or expectorant
InactiveUS20070104653A1Increased mucociliary clearanceReduce severityRespiratorsBiocideInhalationCompound (substance)
Methods and devices for treating excess mucus accumulation in mammals by administering gaseous inhaled nitric oxide or nitric oxide releasing compounds as a mucolytic agent or expectorant are provided. Delivery of gaseous nitric oxide can be made nasally or orally and is preferably substantially coincident with inhalation of the mammal or based on a synchronous parameter of the mammal's respiratory cycle. Varying therapeutic profiles may be used for the delivery of gaseous nitric oxide depending on the severity of the excess mucus accumulation. Parameters for the therapeutic profiles may include flow rate of nitric oxide containing gas, duration of administration of nitric oxide containing gas, number of breaths for which nitric oxide containing gas is to be administered, and concentrations of therapeutic NO delivered to the airways.
Owner:BEYOND AIR LTD
Acetylcysteine composition and uses therefor
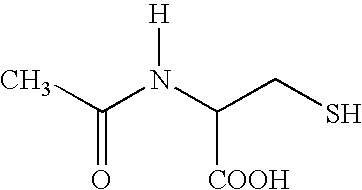
This invention relates to novel acetylcysteine compositions in solution, comprising acetylcysteine and which are substantially free of metal chelating agents, such as EDTA. Further, this invention relates to methods of making and using the acetylcysteine compositions. The present compositions and methods are designed to improve patient tolerance and compliance, while at the same time maintaining the stability of the pharmaceutical formulation. The compositions and methods of this invention are useful in the treatment of acetaminophen overdose, acute liver failure, various cancers, methacrylonitrile poisoning, reperfusion injury during cardio bypass surgery, and radiocontrast-induced nephropathy, and can also be used as a mucolytic agent.
Owner:CUMBERLAND PHARM INC
Acetylcysteine composition and uses therefor
This invention relates to novel acetylcysteine compositions in solution, comprising acetylcysteine and which are substantially free of metal chelating agents, such as EDTA. Further, this invention relates to methods of making and using the acetylcysteine compositions. The present compositions and methods are designed to improve patient tolerance and compliance, while at the same time maintaining the stability of the pharmaceutical formulation. The compositions and methods of this invention are useful in the treatment of acetaminophen overdose, acute liver failure, various cancers, methacrylonitrile poisoning, reperfusion injury during cardio bypass surgery, and radiocontrast-induced nephropathy, and can also be used as a mucolytic agent.
Owner:CUMBERLAND PHARM INC
Dithiol mucolytic agents
ActiveUS20150056305A1Improve toleranceEffectiveAntibacterial agentsPowder deliveryTreatment useDithiol
Owner:PARION SCI DURHAM NC
MAb-based Dot-ELISA method and assay kit for the detection of viruses
A monoclonal antibody-based Dot-ELISA assay for the rapid detection of animal viruses such as avian influenza virus. The assay includes applying a specimen suspected of containing an animal virus on a porous membrane and treating the specimen with a solution of citric acid or lactic acid and a solution containing a mucolytic agent and a detergent. The treated specimen is then contacted with a primary monoclonal antibody for detecting the virus. If present, the primary moncolonal antibody bind with an antigen of the animal virus specimen. The specimen is contacted with an anti-monoclonal antibody conjugate (secondary antibody) and incubated to facilitate binding of the antigen-bound monoclonal antibody to the conjugate. The bound conjugate and antigen-bound monoclonal antibody is contacted with a coloring reagent to allow visual detection of the presence of the animal virus in the specimen.
Owner:PENN STATE RES FOUND
Method for inhibiting bacterial colonisation
InactiveUS20070110758A1Reduce bacterial infectionReduce inflammationAntibacterial agentsBiocideBacteroidesEpithelium
The present invention relates to a method for inhibiting bacterial colonisation of mucous epithelium in a biological system. The method includes the step of administering to the biological system an effective amount of a mucolytic agent and one or more of colostrum, hyperimmune milk, or a component of colostrum and / or hyperimmune milk that is capable of inhibiting bacterial colonisation in combination with the mucolytic agent.
Owner:CHILDREN YOUTH & WOMENS HEALTH SERVICE +1
Nasal passage cleaning composition
InactiveUS6929800B2Avoid discomfortAvoids significant side effectBiocidePharmaceutical delivery mechanismNasal passageNasal passages
A nasal passage cleaning composition according to the invention comprises water and salt as a base, and contains a mucolytic agent such as alum and / or zinc sulphate to shrink the mucosa and allow sinus passage drainage, decreasing pressure in the infected sinus an alleviates sinus headache and face ache. A preferred mucolytic agent is n-acetyl-L-cystine, which is used to dissolve or soften mucus in the nasal passages, though methyl salicylate may also be used to disintegrate crusted mucus, acting also as a topical anti-inflammatory agent and as a pain relieving agent to reduce pain and discomfort.
Owner:SALMAN ABDUL RASOUL
Exfoliated cell preservation solution and preparation method thereof
InactiveCN102113481AHigh retention rateScatter tilingDead animal preservationBenzoic acidHeparin sodium
The invention relates to exfoliated cell preservation solution and a preparation method thereof. The preservation solution comprises the following components in percentage by weight: 0.6 percent of sodium chloride, 0.06 percent of potassium chloride, 2 percent of glucose, 20 percent of ethanol, 3 percent of 1-3 propylene glycol, 2 percent of glycerin, 0.15 percent of glycine, 0.1 percent of mucolytic agent, 0.2 percent of cell protective agent, 0.1 percent of heparin sodium and 0.05N of sodium benzoate-benzoic acid buffer solution. A pH value is adjusted by 1 percent sodium hydroxide or hydrochloric acid solution to ensure that the pH of the preservation solution is 5.8. By the exfoliated cell preservation solution, cells can be well preserved and the preservation time is more than 10 days; cell nucleuses are clear, and cytoplasm is stretched; red blood cells and mucus are effectively removed; the cells are dispersed and flatly laid and an acid phosphatase (ACP) enzyme is well preserved simultaneously; and the exfoliated cell preservation solution is suitable for ACP enzyme staining.
Owner:XIAMEN MAIWEI BIOTECH
Dithiol mucolytic agents
ActiveUS9346753B2Improve toleranceEffectiveAntibacterial agentsPowder deliveryBiomedical engineeringPharmacology
Owner:PARION SCI DURHAM NC
Cell preservation solution and application thereof
PendingCN107410287AEasily brokenAvoid destructionDead animal preservationTreatment effectAnticoagulant
The invention provides a cell preservation solution and application thereof. The cell preservation solution is prepared from the following components in parts by weight: 60 to 1000 parts of pH buffer, 10 to 150 parts of osmotic pressure maintenance agent, 4 to 55 parts of anticoagulant, 1 to 30 parts of mucolytic agent, 800 to 5000 parts of fixing agent, 1 to 150 parts of component A, 10 to 30 parts of component B and 10000 to 20000 parts of water, wherein the component A is prepared from sodium azide and lauryl sodium sulfate according to a weight ratio of (1 to 10) to (2 to 110); the component B is carbon disulfide. The cell preservation solution of the invention has a better impurity treatment effect, and reduces the interference factors in cell samples; at the same time, target cells therein can retain the characteristics of structural morphology for a long time, thereby facilitating the medical detection and analysis of the target cells, and the cost is low.
Owner:GUANGZHOU HONGQI OPTICAL INSTR TECH
Isolating fetal trophoblasts
InactiveUS20070224597A1Fast and accurate analysisEfficient captureCell dissociation methodsMicrobiological testing/measurementTrophoblastHydrolysis
Methods for isolating and purifying fetal trophoblasts from a mucus sample obtained from the uterine cavity of a pregnant female. The mucus sample is transported from a clinical collection facility to a laboratory in a transportation medium so the cells remain viable. The mucus sample is then subjected to precise processing steps, including treatment with mucolytic agents or mucinases, sugar hydrolysis enzymes, nucleases, and proteases to provide fetal cells, the outer surfaces of which are so essentially completely devoid of attached mucosal biological material that they are then isolated in greater numbers than previously had been possible. The isolated cells are in appropriate condition to immediately be effectively subjected to FISH or to other molecular diagnostics.
Owner:NOVARTIS AG
Methods of treating pulmonary sarcoidosis
ActiveUS9402884B2Pulmonary cellular antioxidant defense is significantly reducedImprove the level ofDispersion deliveryHydrolasesRecombinant human dnasePulmonary sarcoidosis
Methods of treating pulmonary sarcoidosis are described herein. Patients in need of treatment for pulmonary sarcoidosis are administered a therapeutically effective amount of a mucolytic agent such as DNase I. In some embodiments, the DNase I is a recombinant human DNase I such as dornase alfa.
Owner:GOTHAM BIOPHARM INC
Nucleic acid preservation solution and methods of manufacture and use
ActiveUS20180201977A1Avoid problemsSuitable for useMicrobiological testing/measurementDNA preparationBuffering agentEXPECTORANT AGENTS
Disclosed is nucleic acid preserving compositions and methods of manufacturing and using the same. Compositions include a carrier, a chaotropic agent, a buffering agent, a chelating agent, a surfactant, an alcohol, an acid, and a mucolytic agent. Compositions as aqueous solutions can include water as a carrier. Preferred embodiments include water, guanidine thiocyanate, Tris, EDTA, SLS, SDA 3C, HCl, and N-acetyl-L-cysteine. Some embodiments include a colored dye as a visual indicator. Methods of manufacturing include combining the components into a mixture, such as an aqueous solution. Methods of use include providing a biological sample that includes nucleic acid and contacting the biological sample with the composition. Kits include the composition disposed in a portion of a biological sample collection apparatus.
Owner:SPECTRUM SOLUTIONS LLC
Mab-based dot-elisa method and assay kit for the detection of viruses
InactiveUS20060105328A1Rapid and less-costly methodMicrobiological testing/measurementEnzymologyAnimal virusAntigen binding
A monoclonal antibody-based Dot-ELISA assay for the rapid detection of animal viruses such as avian influenza virus. The assay includes applying a specimen suspected of containing an animal virus on a porous membrane and treating the specimen with a solution of citric acid or lactic acid and a solution containing a mucolytic agent and a detergent. The treated specimen is then contacted with a primary monoclonal antibody for detecting the virus. If present, the primary moncolonal antibody bind with an antigen of the animal virus specimen. The specimen is contacted with an anti-monoclonal antibody conjugate (secondary antibody) and incubated to facilitate binding of the antigen-bound monoclonal antibody to the conjugate. The bound conjugate and antigen-bound monoclonal antibody is contacted with a coloring reagent to allow visual detection of the presence of the animal virus in the specimen.
Owner:PENN STATE RES FOUND
Methods And Devices For Using Mucolytic Agents Including N-Acetyl Cysteine (NAC)
ActiveUS20120258469A1Bioreactor/fermenter combinationsBiological substance pretreatmentsEngineeringBiochemistry
Devices and methods incorporate mucolytic agents into a point-of-care testing device. The sample is loaded, and then the sample travels until it encounters one or more lysis agents and / or mucolytic agents. The mucolytic agent is preferably pre-loaded onto the collection device. In a preferred embodiment, the mucolytic agent is localized between the sample application zone and the conjugate zone. In embodiments with a sample compressor, one or more mucolytic agents may be pre-loaded and dried on the sample compressor, the sample collector, in various locations on the test strip, or in the running buffer.
Owner:RAPID PATHOGEN SCREENING INC
Powdery preparation for nasal administration
InactiveUS7591999B2Substance may accumulatePromote absorptionPowder deliveryOrganic active ingredientsNasal cavityWater soluble
According to the present invention, a powdery preparation for nasal administration comprising a physiologically active substance, a non-water-absorbing and hardly water-soluble powder(s) and one or two selected from the group consisting of a mucolytic agent and a nonionic surfactant is provided.
Owner:MITSUBISHI TANABE PHARMA CORP
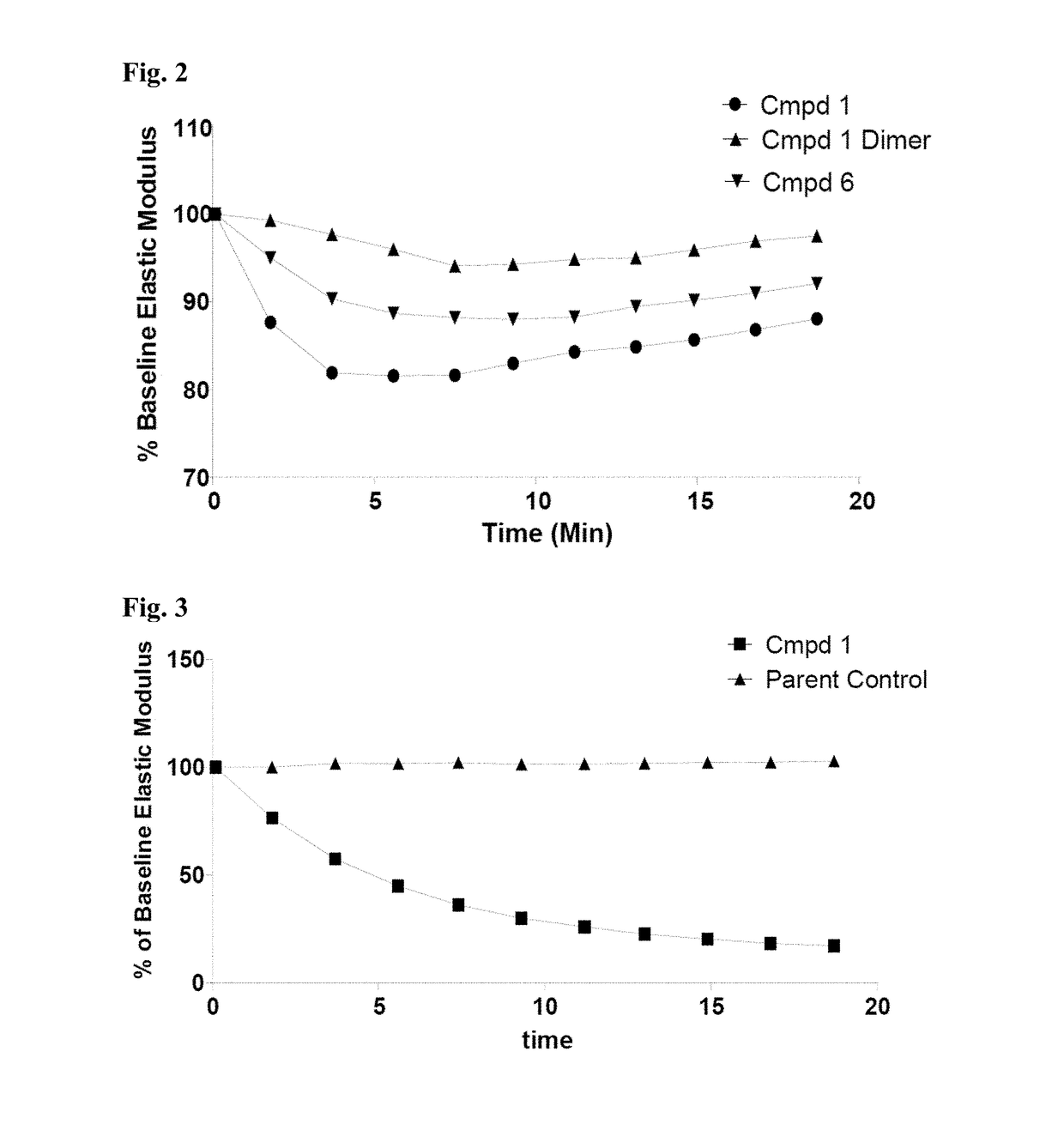
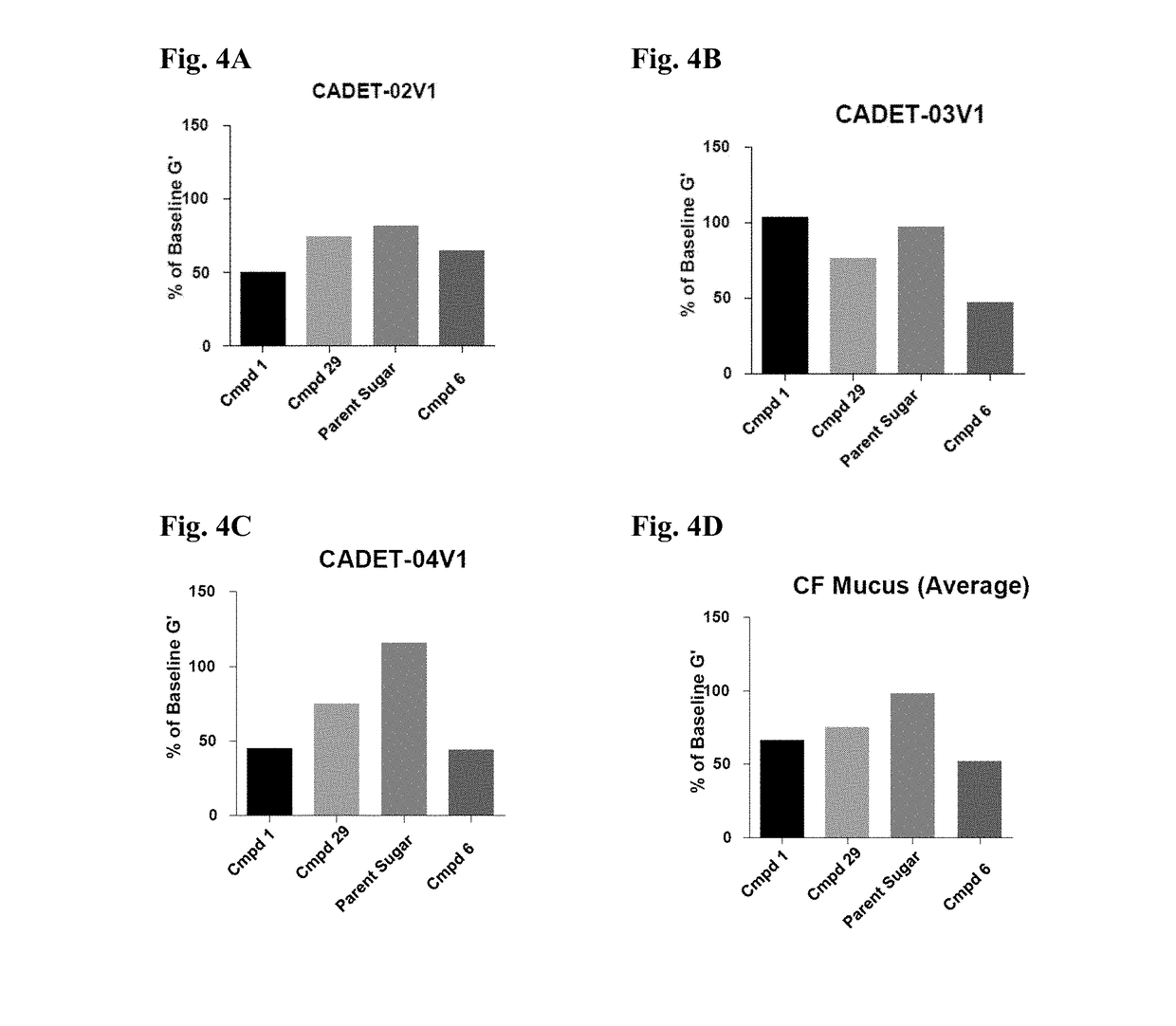
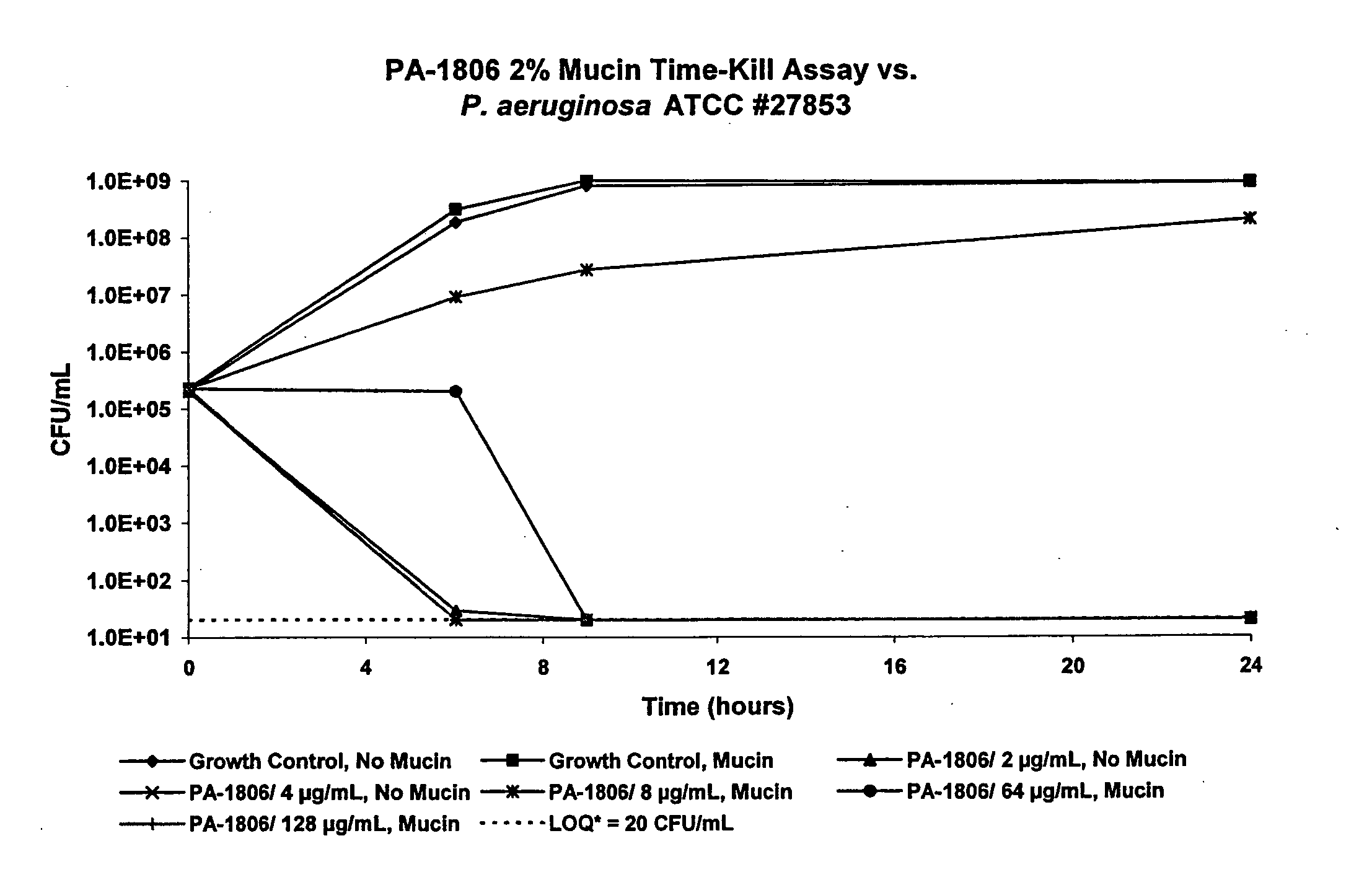
Thiosaccharide mucolytic agents
ActiveUS20160060284A1Decreasing mucus elasticityDecreasing mucus viscosityEsterified saccharide compoundsBiocideMedicineViscosity
There are provided, inter alia, methods for decreasing mucus elasticity or decreasing mucus viscosity in a subject in need thereof, the methods including administering to the subject an effective amount of a thiosaccharide mucolytic agent, and compounds and pharmaceutical compositions useful for the methods.
Owner:UNIV COLLEGE DUBLIN NAT UNIV OF IRELAND DUBLIN +1
Isolating fetal trophoblasts
InactiveCN101432441AUnderstand the implementationCell dissociation methodsMicrobiological testing/measurementTrophoblastMolecular diagnostics
Methods for isolating and purifying fetal trophoblasts from a mucus sample obtained from the uterine cavity of a pregnant female. The mucus sample is transported from a clinical collection facility to a laboratory in a transportation medium so the cells remain viable. The mucus sample is then subjected to precise processing steps, including treatment with mucolytic agents or mucinases, sugar hydrolysis enzymes, nucleases, and proteases to provide fetal cells, the outer surfaces of which are so essentially completely devoid of attached mucosal biological material that they are then isolated in greater numbers than previously had been possible. The isolated cells are in appropriate condition to immediately be effectively subjected to FISH or to other molecular diagnostics.
Owner:BIOCEPT INC
Subglottic suctioning system
A subglottic suctioning system with a tracheal tube (10) having a ventilation lumen, a cuff inflation lumen (14), and a suction lumen (16) are disclosed which may help reduce the incidence of ventilator associated (or acquired) pneumonia. The suction lumen communicates with the space in the trachea above the cuff (22) where secretions accumulate. The suction lumen has a valve (36) on the proximal end for connection to a source of vacuum. The valve is adapted to interrupt the supply of vacuum to the suction lumen to allow for the introduction of a rinsing fluid in its place and to automatically re-establish the connection to the source of vacuum upon completion of rinsing. The rinsing fluid aids in maintaining an open suction lumen and may include medicaments and mucolytic agents to enhance or promote healing or to alter the properties of the mucus to make removal easier. The user may easily and repeatedly alternate suction and rinsing fluid through the suction lumen, i.e., the user may 'pulse' the line to loosen, break up and remove secretions and deposits that may partially or completely block or clog the suction lumen.
Owner:AVENT INC
Treatment and diagnosis of ocular surface disorders
The present invention provides an ophthalmic formulation consisting essentially of one or more pharmaceutically acceptable excipients; a pharmaceutically active compound that is capable of reducing the amount of inflammatory neutrophil product on the ocular surface; and optionally a second pharmaceutically active compound selected from the group consisting of a steroid, an anti-inflammatory agent, a mucolytic agent and a combination thereof. In particular, the present invention provides an ophthalmic formulation where the pharmaceutically active compound is capable of treating a clinical condition selected from the group consisting of inflammatory and immunological ocular surface disease that can cause symptoms of ocular discomfort, mucocellular aggregates / debris in tear film, symblepheron formation, fornix foreshortening, eyelid margin / conjunctival keratinization, corneal neovascularization / pannus, subconjunctival fibrosis, and herpetic eye disease. The present invention also provides a method for diagnosing or monitoring an ocular surface disease.
Owner:ADVAITE LLC
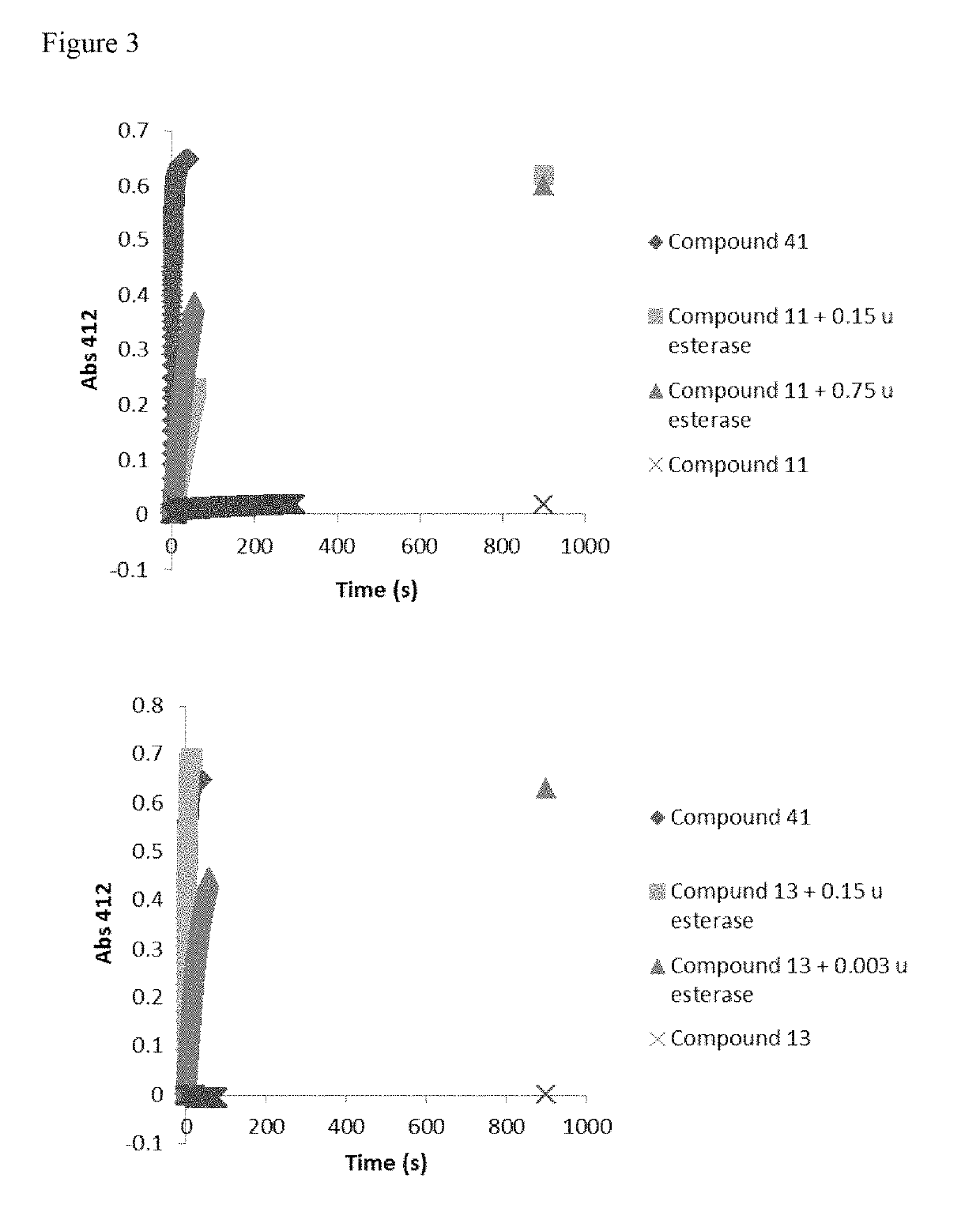
Monothiol mucolytic agents
ActiveUS10106551B2Improve clearancePromote hydrationAntibacterial agentsOrganic active ingredientsMedicinal chemistryBiomedical engineering
Provided are mucolytic agents represented by formula (Ia)-(Id):where the structural variables R1, R2, R5 and R6 are as defined herein. Also provided are a variety of methods of treatment which take advantage of the mucolytic properties of the compounds represented by formula (Ia)-(Id).
Owner:PARION SCI DURHAM NC
Treatment of diseases involving mucin
The present invention relates to compositions containing one or more of the compounds contained in bromelain, or salts, solvates or prodrugs thereof, and one or more mucolytic agents, or salts, solvates or prodrugs thereof, for treatment of diseases involving mucin, especially mucin secreting cancers, or diseases involving blood clots (thrombi).
Owner:NEWSOUTH INNOVATIONS PTY LTD
Application of tanshinone IIA or pharmaceutically acceptable salt in preparation of medicament for treating or inhibiting mucus in wind pipe
InactiveCN103006667AInhibition of hypersecretionInhibit expressionOrganic active ingredientsRespiratory disorderPharmaceutical drugTanshinone IIA
The invention discloses an application of tanshinone IIA or pharmaceutically acceptable salt in preparation of medicament for treating or inhibiting mucus in a wind pipe. The tanshinone IIA or pharmaceutically acceptable salt can obviously inhibit the hypersecretion of mucoprotein in the wind pipe of the lung tissue. Compared with the commonly used mucolytic agents, the tanshinone IIA or pharmaceutically acceptable salt can obviously inhibit the expression and the secretion of mucoprotein, and the obvious side and toxic effects can not be found.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV (GUANGZHOU RESPIRATORY CENT) +1
Thiosaccharide mucolytic agents
There are provided, inter alia, methods for decreasing mucus elasticity or decreasing mucus viscosity in a subject in need thereof, the methods including administering to the subject an effective amount of a thiosaccharide mucolytic agent, and compounds and pharmaceutical compositions useful for the methods.
Owner:UNIV COLLEGE DUBLIN NAT UNIV OF IRELAND DUBLIN +1
Methods and Devices for Using Mucolytic Agents Including N-Acetyl Cysteine (NAC)
ActiveUS20150323527A1Bioreactor/fermenter combinationsBiological substance pretreatmentsPoint of carePoint-of-care testing
Devices and methods incorporate mucolytic agents into a point-of-care testing device. The sample is loaded, and then the sample travels until it encounters one or more lysis agents and / or mucolytic agents. The mucolytic agent is preferably pre-loaded onto the collection device. In a preferred embodiment, the mucolytic agent is localized between the sample application zone and the conjugate zone. In embodiments with a sample compressor, one or more mucolytic agents may be pre-loaded and dried on the sample compressor, the sample collector, in various locations on the test strip, or in the running buffer.
Owner:RAPID PATHOGEN SCREENING INC
Monobactam compositions and methods of use thereof
InactiveUS20060003984A1High antibacterial activityAntibacterial agentsBiocideMicroorganismCompound (substance)
Methods, compounds and compositions are provided for inhibiting the growth of pathogenic microbes in vitro and of treatment of pathogenic bacterial infections in vivo using an antibacterial monobactam compound and a mucolytic agent.
Owner:CHIRON CORP
Prodrugs of dithiol mucolytic agents
ActiveUS10526283B2Improve toleranceEffectiveAntibacterial agentsPowder deliveryAcetylcysteineMedicinal chemistry
Owner:PARION SCI DURHAM NC
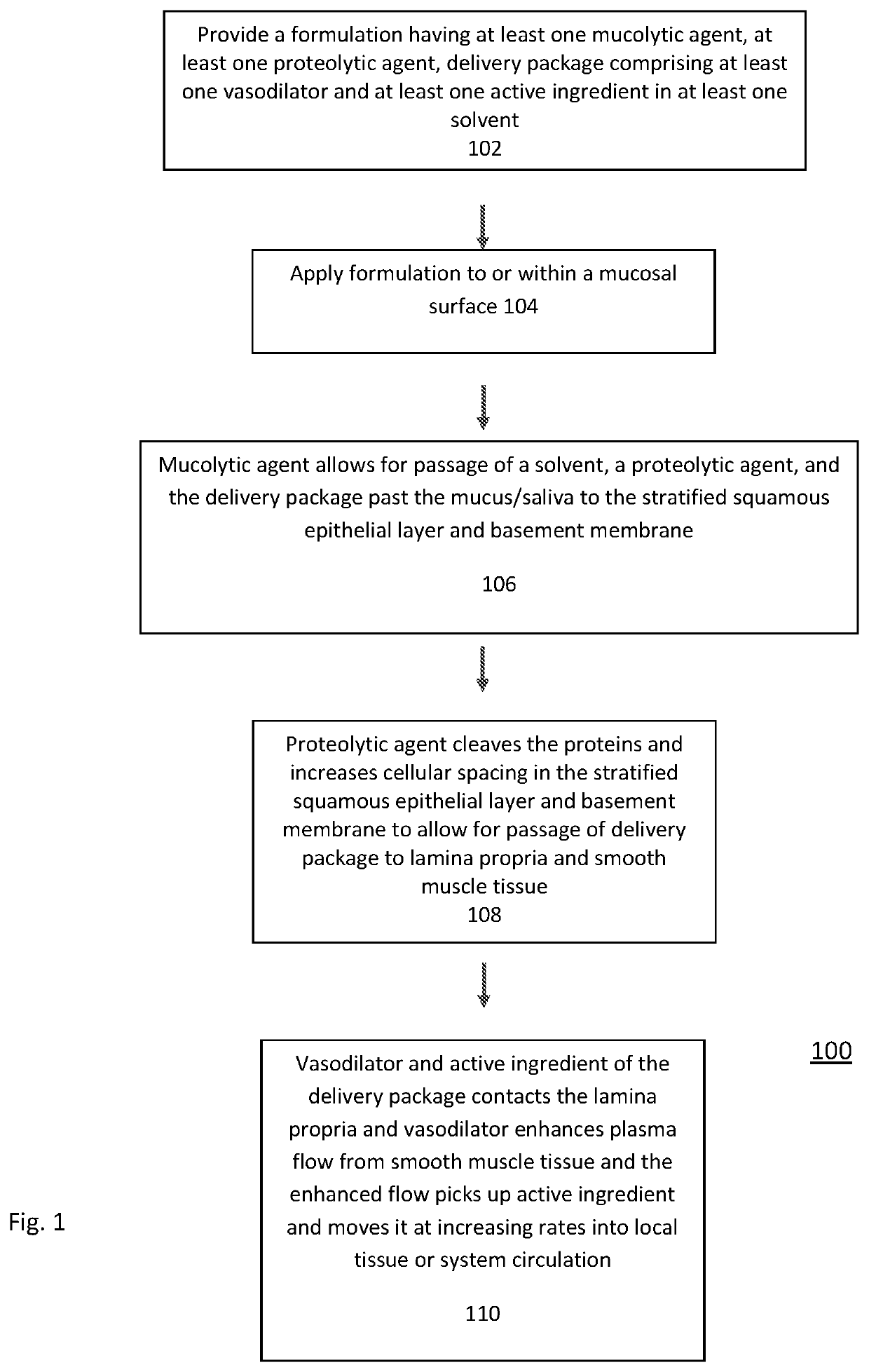
Transmucosal Drug Delivery System
PendingUS20210259951A1Faster and efficient onset of actionIncrease rangeInorganic non-active ingredientsPharmaceutical delivery mechanismLamina epithelialisActive agent
The present invention relates to transmucosal delivery systems, methods and kits that include agents to penetrate the mucus, stratified squamous epithelial layer, and basement membrane, to deliver a vasodilatory agent and active agent to the lamina propria and smooth muscle. The formulation of the present invention further includes having a mucolytic agent for thinning or decreasing viscosity of mucus; a solvent; a proteolytic agent that cleaves or fragments long chain proteins to create cellular spacing of the stratified squamous epithelial layer and / or basement membrane, a vasodilatory agent; and at least one active ingredient. The formulation allows for penetration of the active ingredient at the mucosal surface to the smooth muscle. The active ingredient, once at the smooth muscle, is delivered locally to the tissue or systemically to the blood stream.
Owner:NORTH ATLANTIC HLDG LLC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com