Application of tanshinone IIA or pharmaceutically acceptable salt in preparation of medicament for treating or inhibiting mucus in wind pipe
A technology of pharmacy and tanshinone, which is applied to the application field of tanshinone IIA or its pharmaceutically acceptable salt in the preparation of medicine for treating or inhibiting airway mucus hypersecretion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Effect of Sodium Tanshinone IIA Sulfonate or a Pharmaceutically Acceptable Salt thereof on LPS-Induced Epithelial Cell MUC1 Expression Level
[0023] one, main experimental materials
[0024] 1. Human alveolar type II epithelial cell line A549 cells: purchased from the American Standard Biological Collection Center (ATCC)
[0025] 2. DMEM medium, fetal bovine serum: American Gibico Company
[0026] 3. Rabbit anti-MUC1 monoclonal antibody: Abcam, USA
[0027] 4. Goat anti-rabbit IgG: Sigma, USA
[0028] 5. Goat anti-mouse IgG: American KPL company
[0029] 6. Lipopolysaccharide (LPS): purchased from Sigma, USA
[0030] 7. Tanshinone Ⅱ A (sulfonate, purity > 99%): purchased from National Institute for the Control of Pharmaceutical and Biological Products (NICPBP)
[0031] 8. Cell lysate:
[0032] 2. Main instruments
[0033] 1. Carbon dioxide incubator: Danish Thermo company
[0034] 2. Inverted phase-contrast microscope: Leica, Germany
[003...
Embodiment 2
[0049] Example 2: Effect of Tanshinone IIA or its pharmaceutically acceptable salt on the expression of MUC1 in lung tissue of LPS-induced mucus hypersecretion mouse model
[0050] 1. Main experimental materials:
[0051] 1. SPF grade C57 mice, weighing 20-25g, Guangdong Experimental Animal Center
[0052] All the other experimental materials are the same as in Example 1
[0053] 2. Main instruments
[0054] 1. Ultrasonic Crusher: SONICS Company of the United States
[0055] 2. Tissue homogenizer: German IKA company
[0056] All the other experimental instruments are the same as in Example 1
[0057] three , experimental method
[0058] 1. Establishment of LPS-induced mucus hypersecretion mouse model
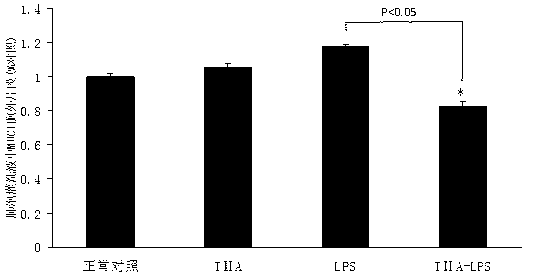
[0059] The C57 mice were randomly divided into four groups: normal control group, TIIA group, LPS group and TIIA+LPS group, each group including 6-8 mice. The dosage of tanshinone IIA sodium sulfonate injection is 25mg / kg body weight, intraperitoneal injection;...
Embodiment 3
[0069] Example 3: Effect of Tanshinone IIA or its pharmaceutically acceptable salt on the expression of MUC5AC in alveolar lavage fluid of LPS-induced mucus hypersecretion mice
[0070] one. Main experimental materials:
[0071] 1. MUC5AC antibody: American RD Company
[0072] All the other experimental materials and instruments are the same as in Example 2
[0073] two , experimental method
[0074] 1. Establishment of LPS-induced acute lung injury model
[0075] Same as Example 2
[0076] 2. Detection of MUC5AC in alveolar lavage fluid
[0077] The collected alveolar lavage fluid from each group of mice was centrifuged at low temperature, and the supernatant protein suspension was collected, and the level of extracellular fragments of MUC1 in the alveolar lavage fluid was detected by enzyme-linked immunosorbent assay (ELISA).
[0078] Experimental results: as Figure 4 As shown, the level of MUC5AC in the alveolar lavage fluid of LPS-induced mucus hypersec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com