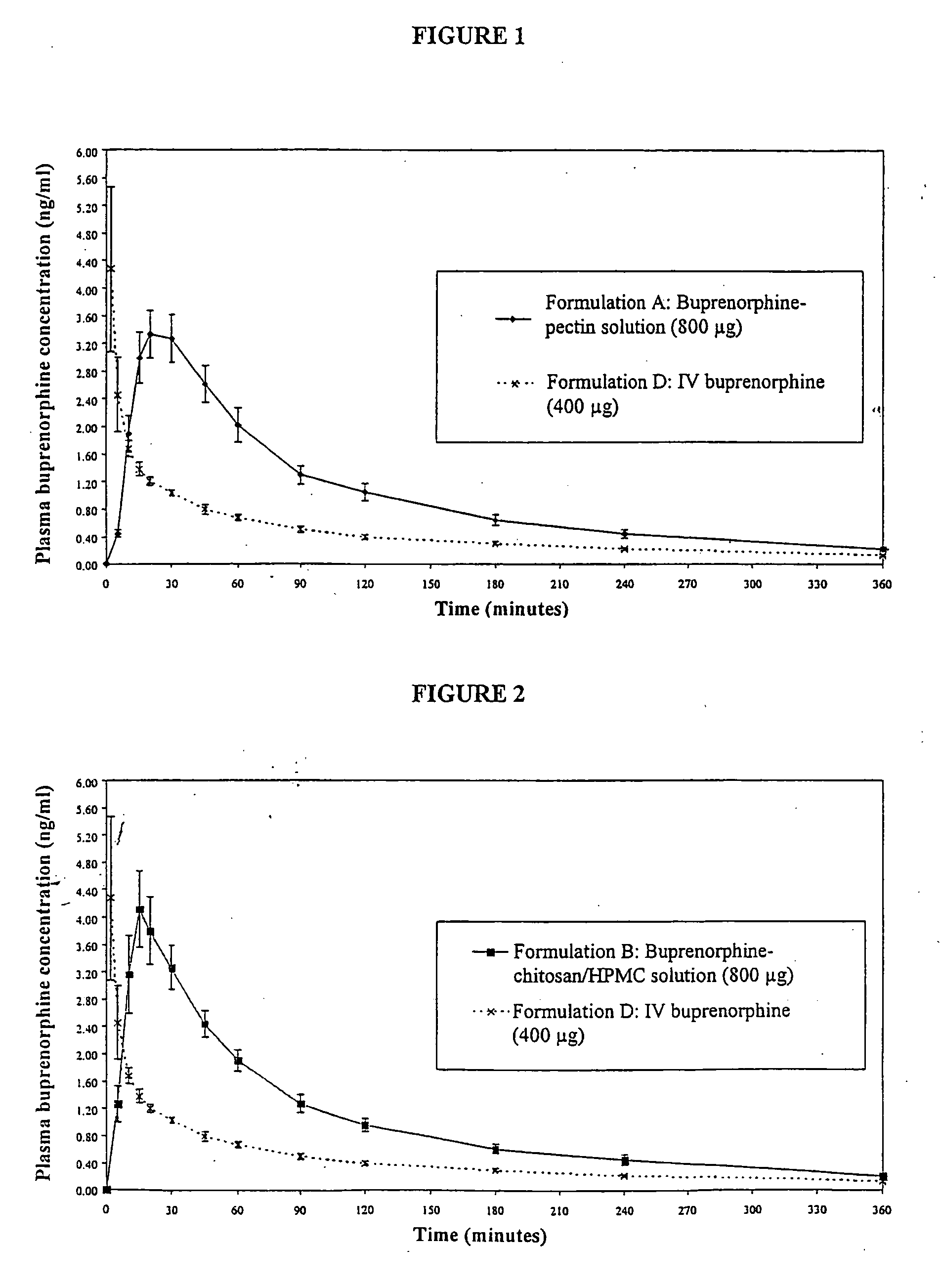
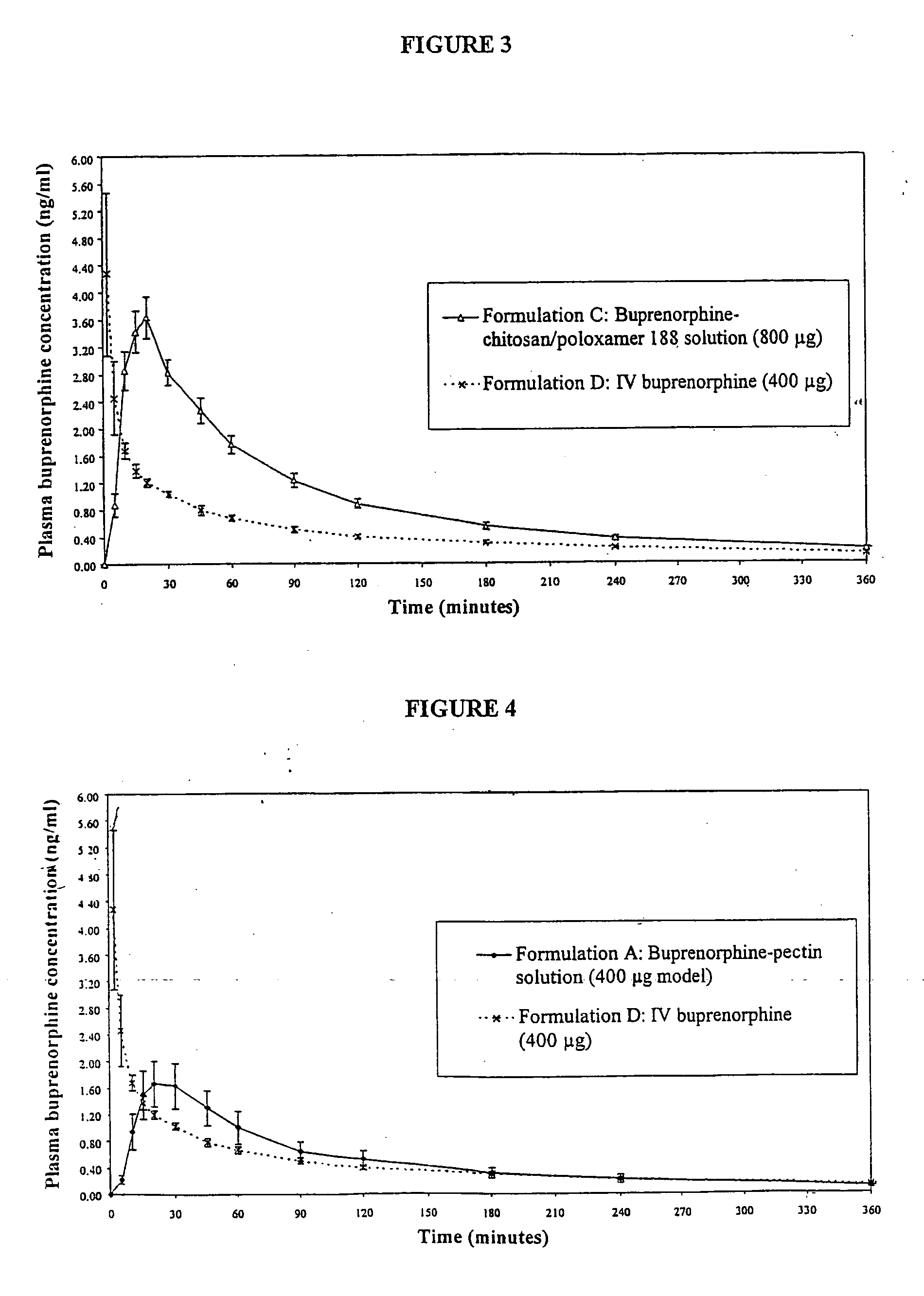
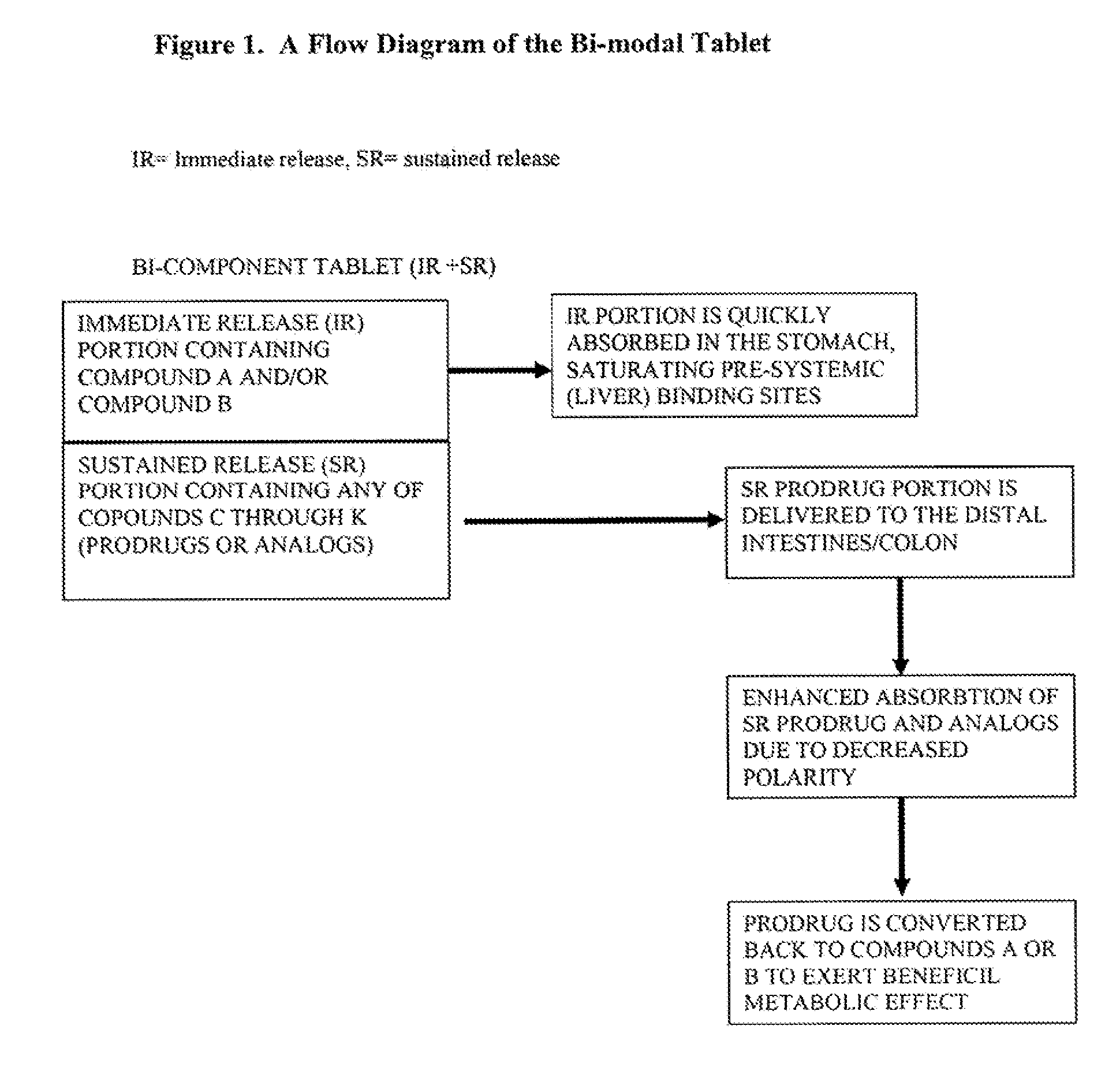
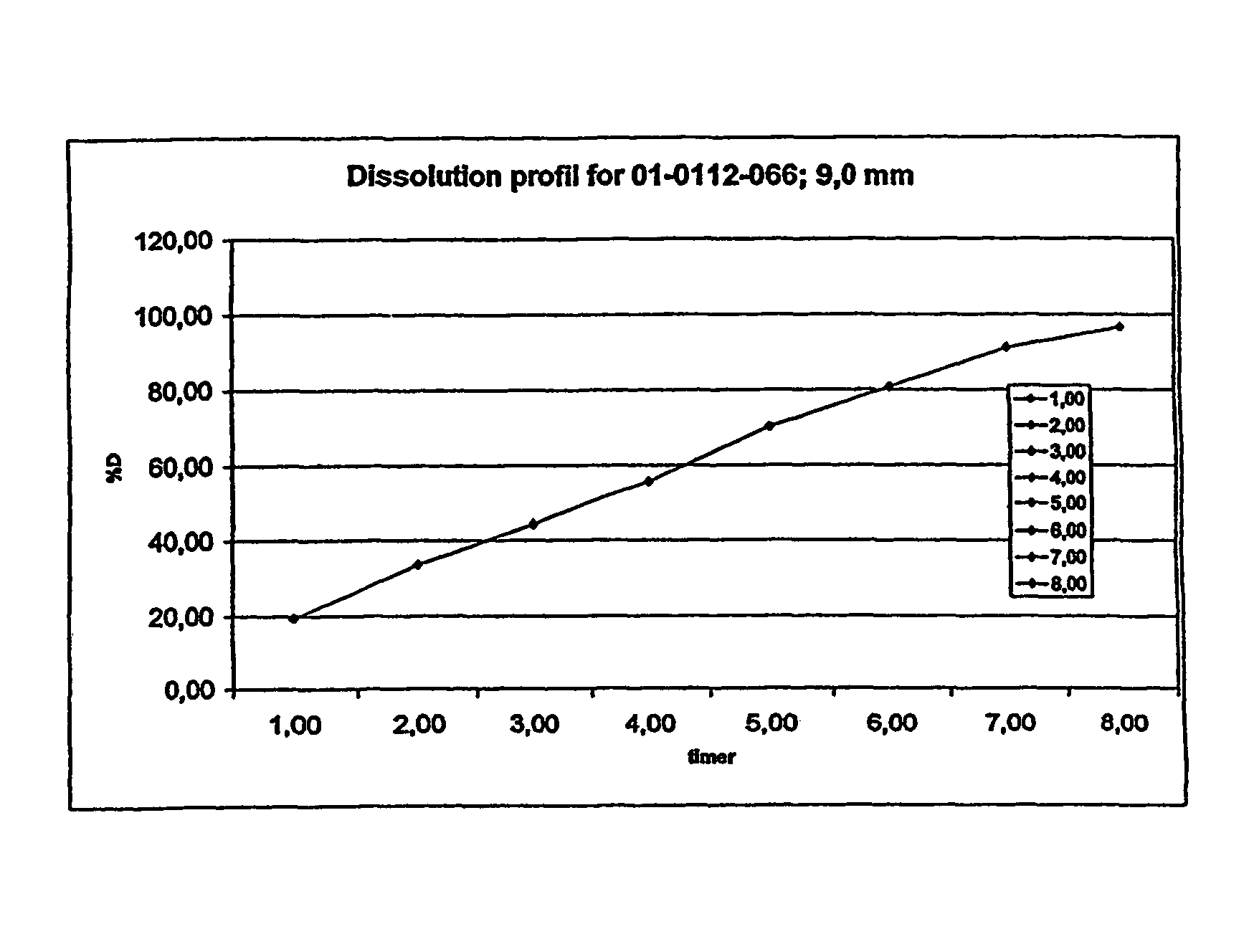
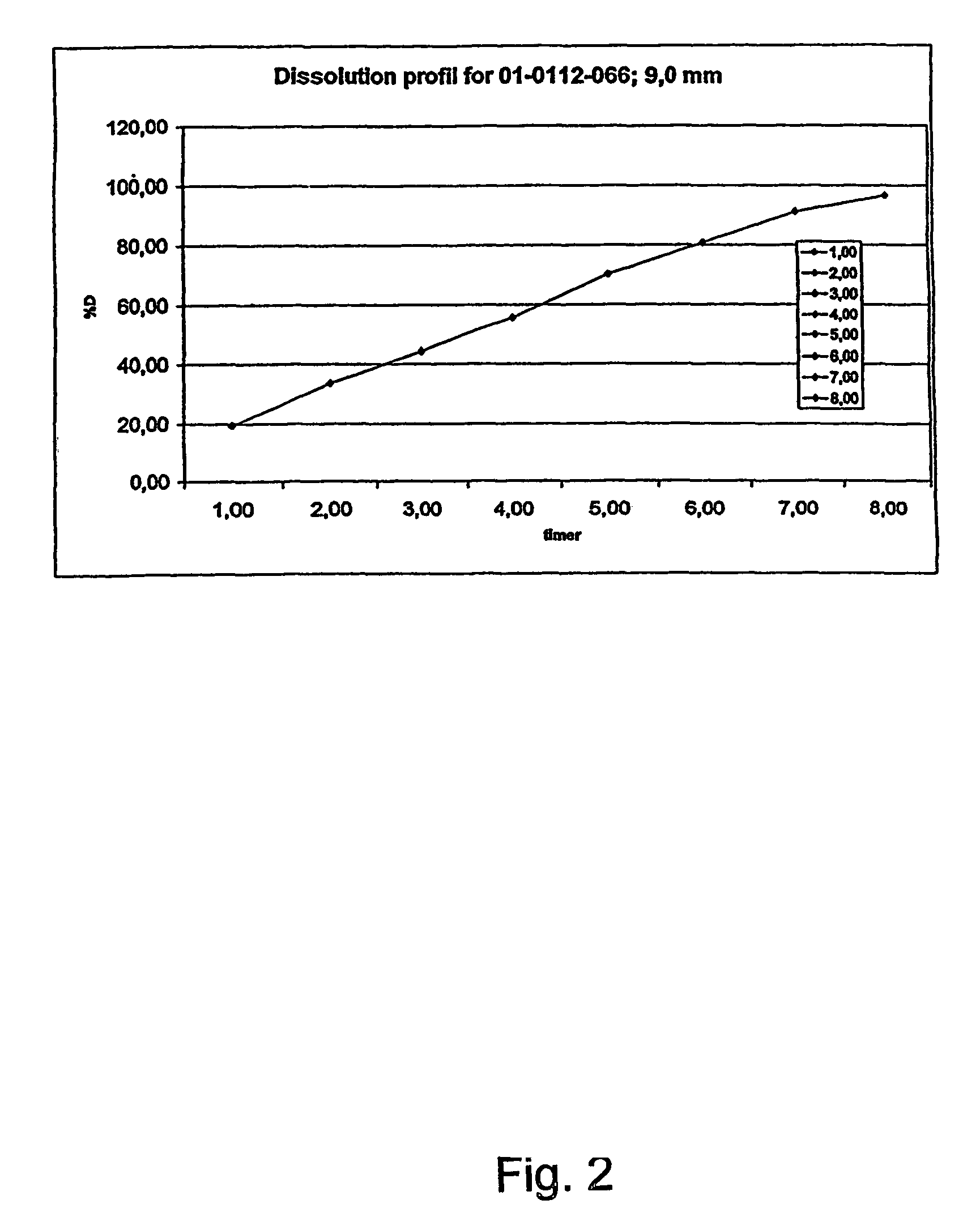
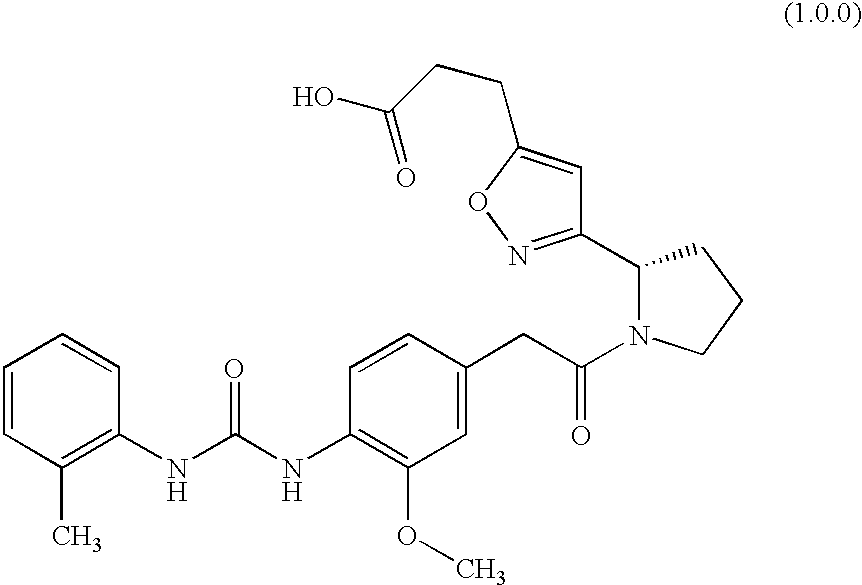
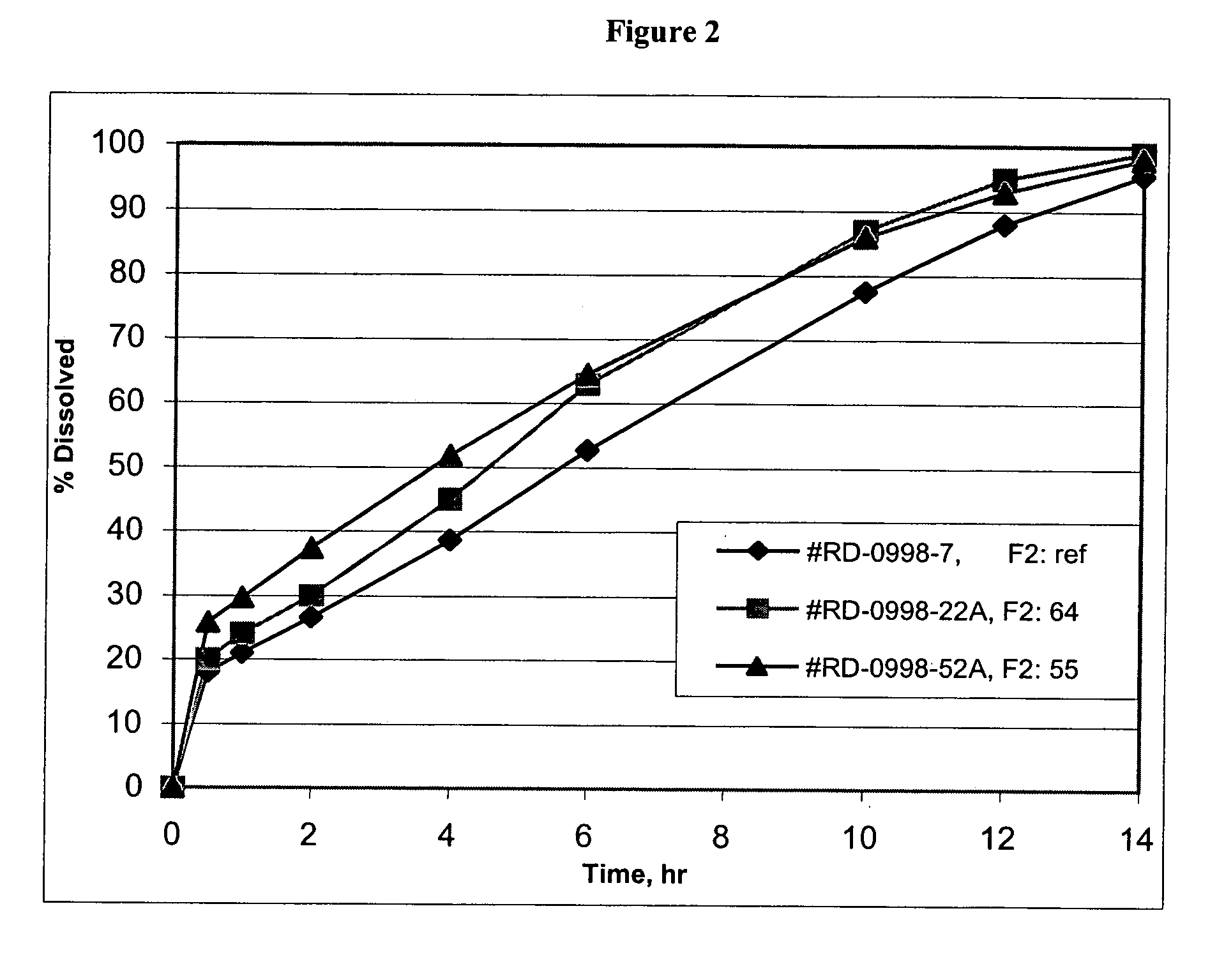
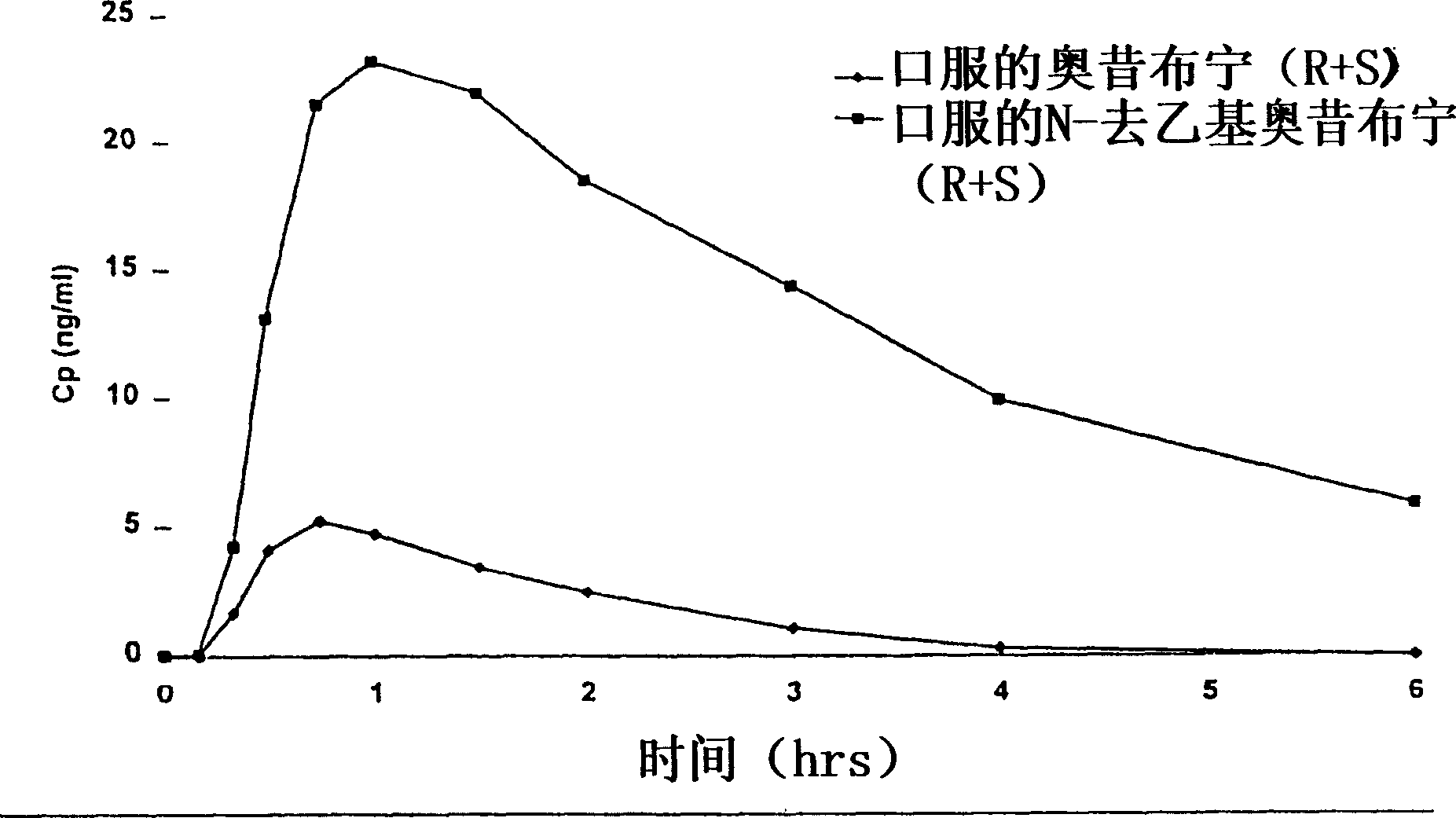
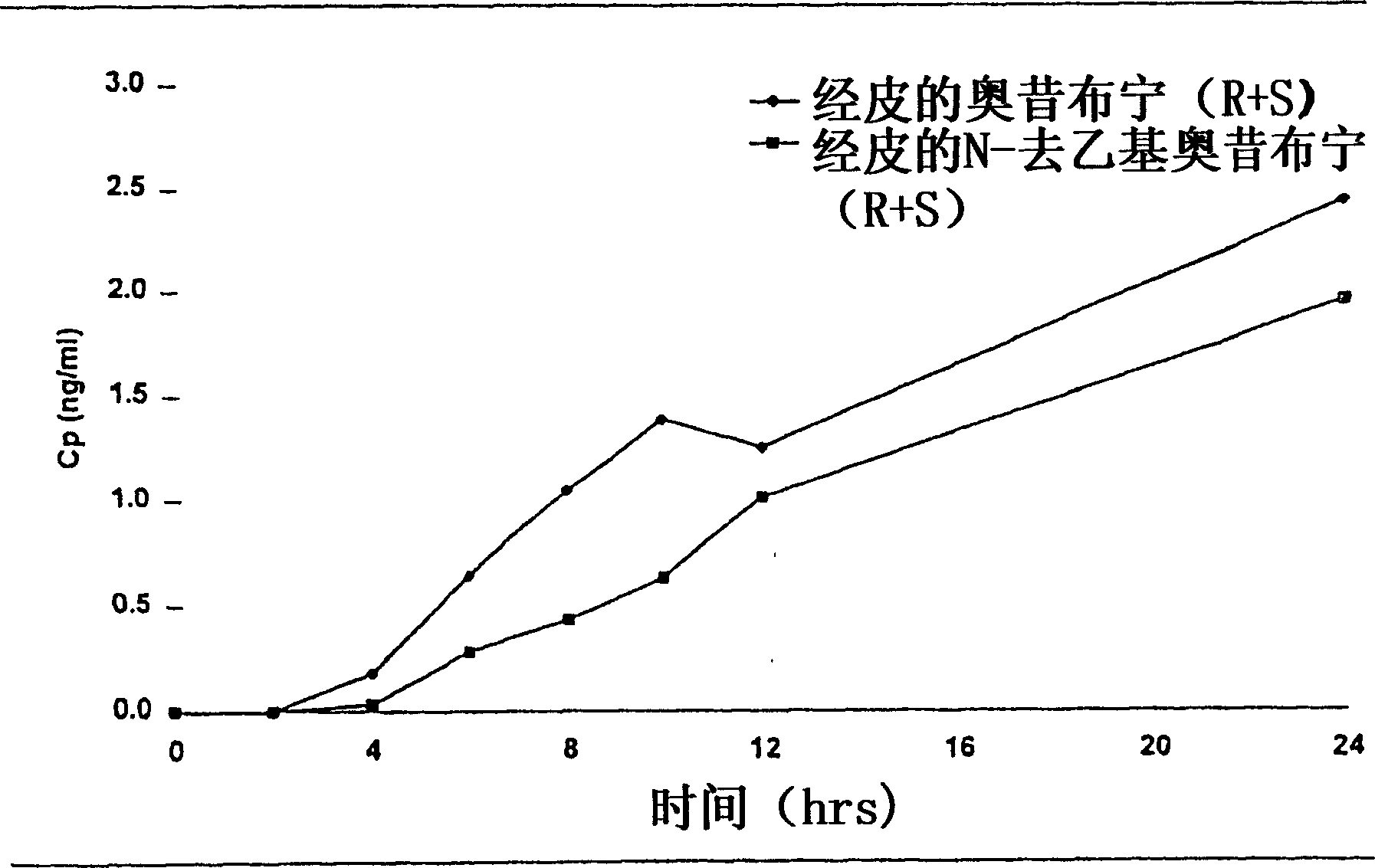
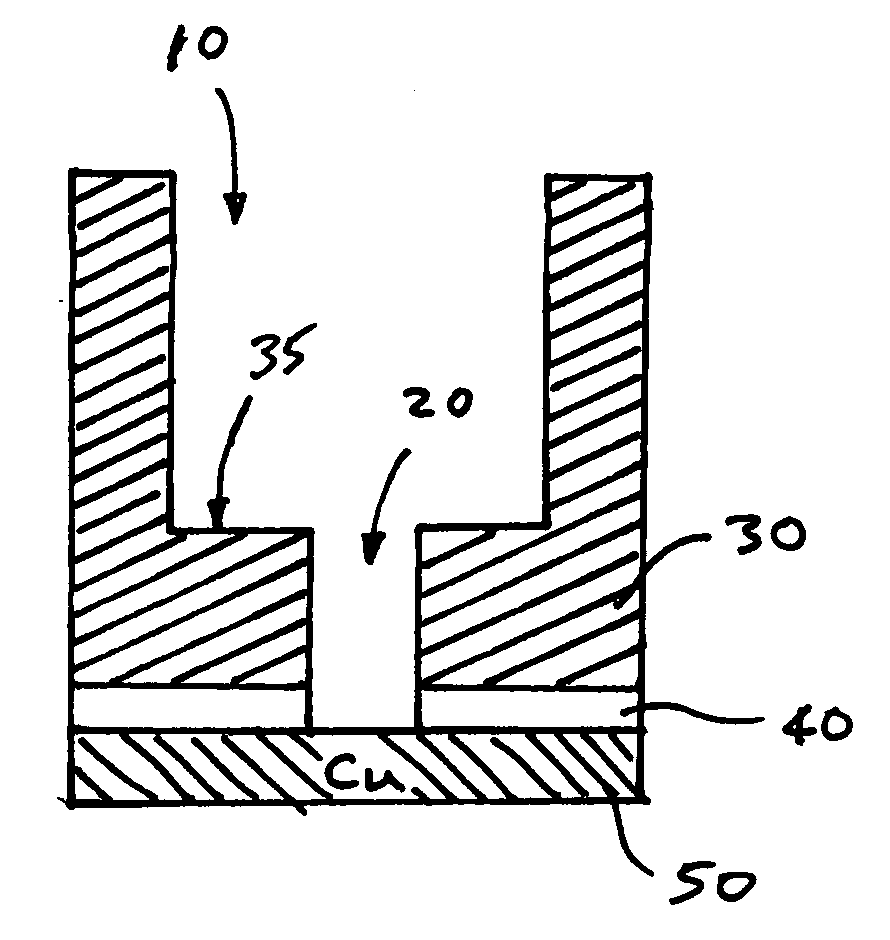
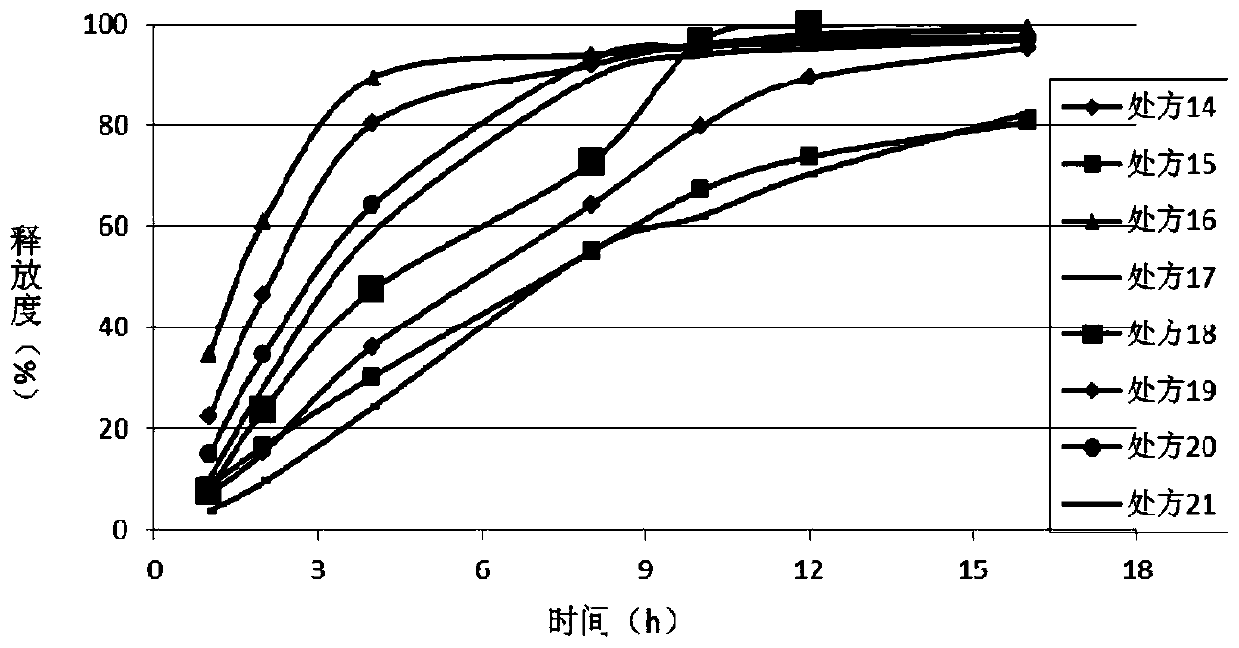
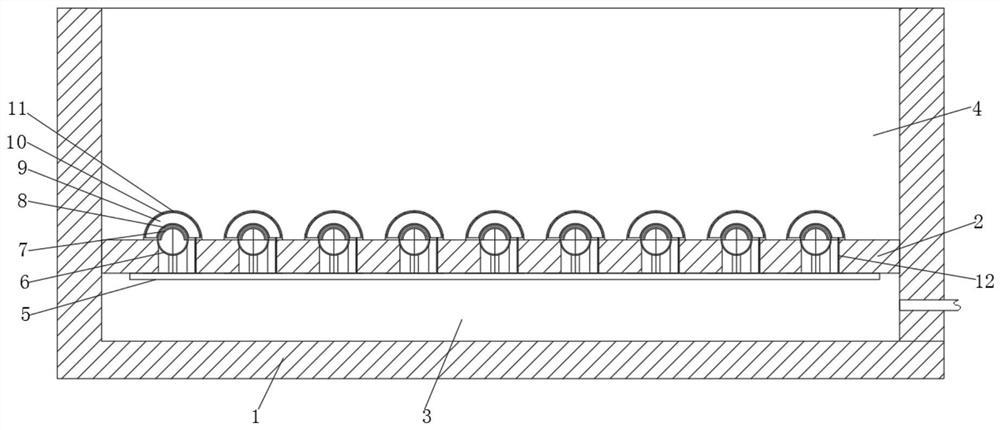
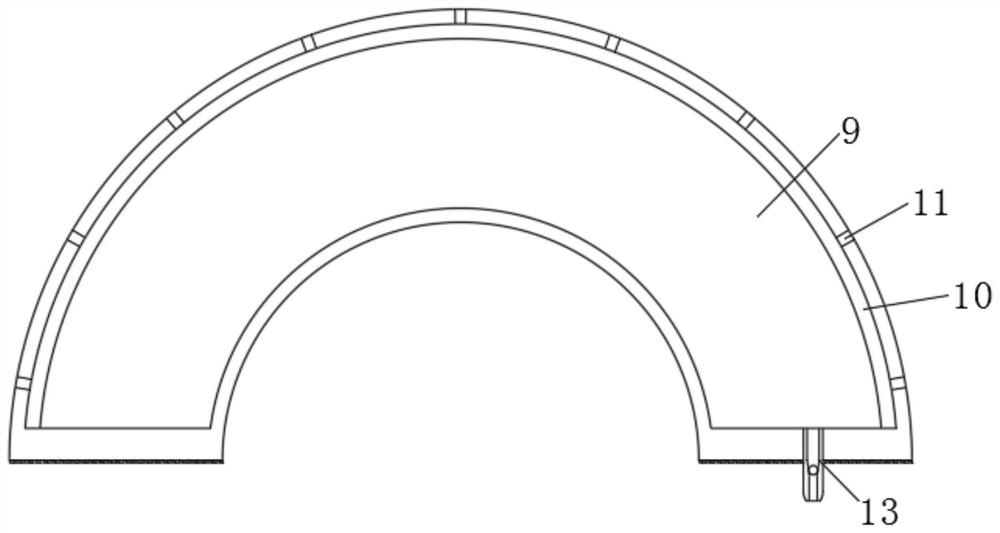
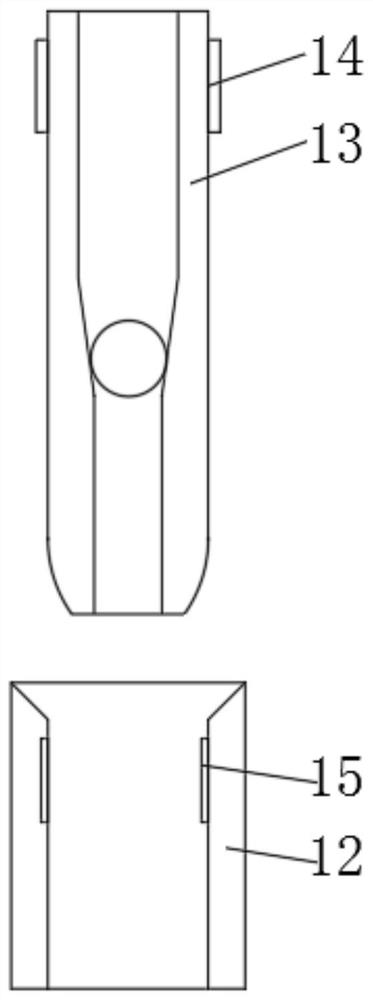
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38 results about "Concentrations plasma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dosage form containing multiple drugs
A pharmaceutical dosage form comprising a first drug and a second drug, both of which are selected from decongestants, antitussives, expectorants, analgesics and antihistamines. The dosage form provides a plasma concentration within a therapeutic range of the second drug over a period which is coextensive with at least about 70% of a period over which the dosage form provides a plasma concentration within a therapeutic range of the first drug. This Abstract is neither intended to define the invention disclosed in this specification nor intended to limit the scope of the invention in any way.
Owner:SOVEREIGN PHARMA
Dosage form containing a morphine derivative and another drug
InactiveUS20050232987A1Extended maintenance periodBiocidePill deliveryPharmaceutical drugBlood plasma
A pharmaceutical dosage form which comprises a first drug which comprises at least one morphine derivative with antitussive activity and at least one second drug. The dosage form provides a plasma concentration within the therapeutic range of the at least one second drug over a period which is coextensive with at least about 70% of the period over which the dosage form provides a plasma concentration within the therapeutic range of the first drug. This abstract is neither intended to define the invention disclosed in this specification nor intended to limit the scope of the invention in any way.
Owner:SOVEREIGN PHARMA
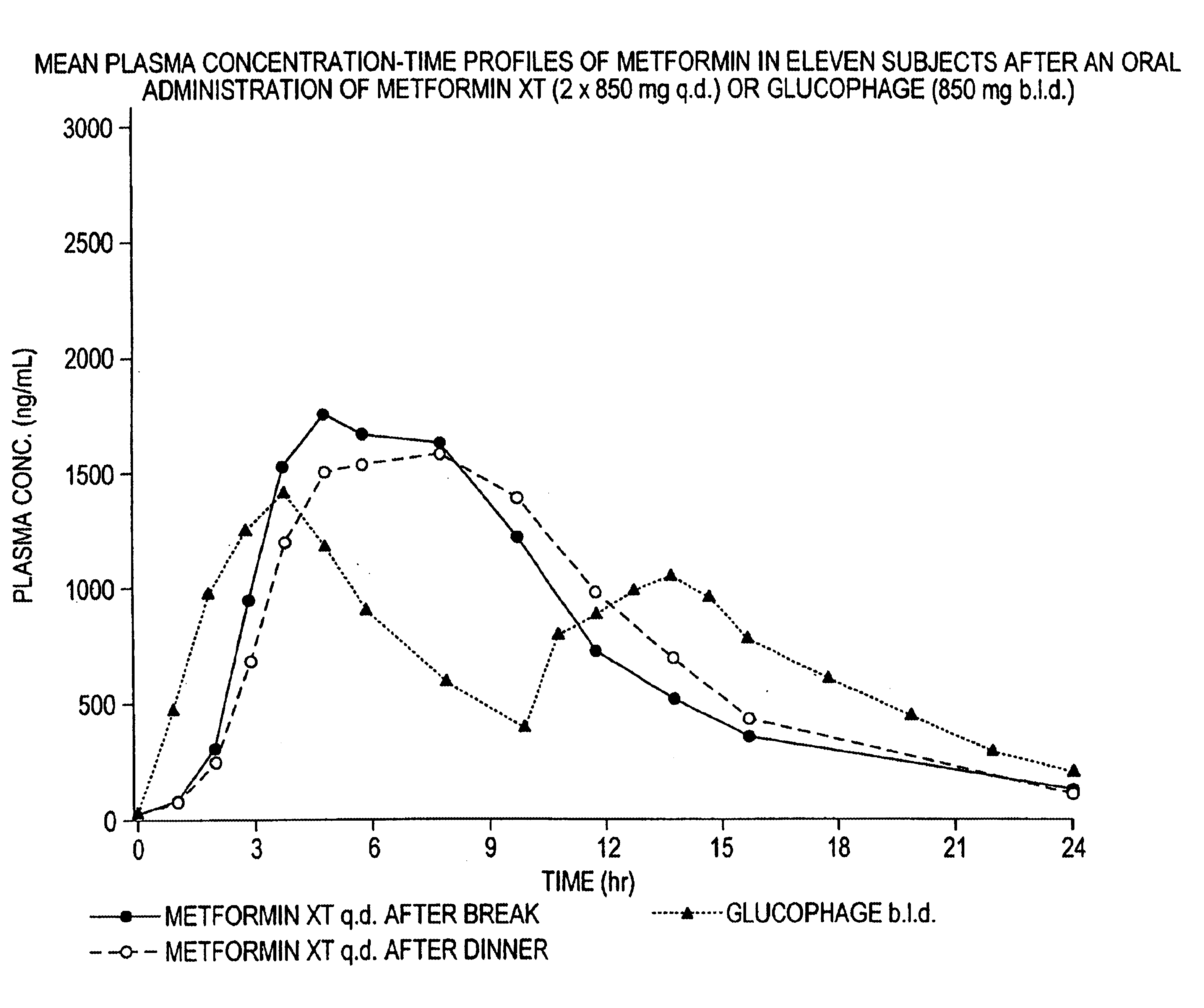
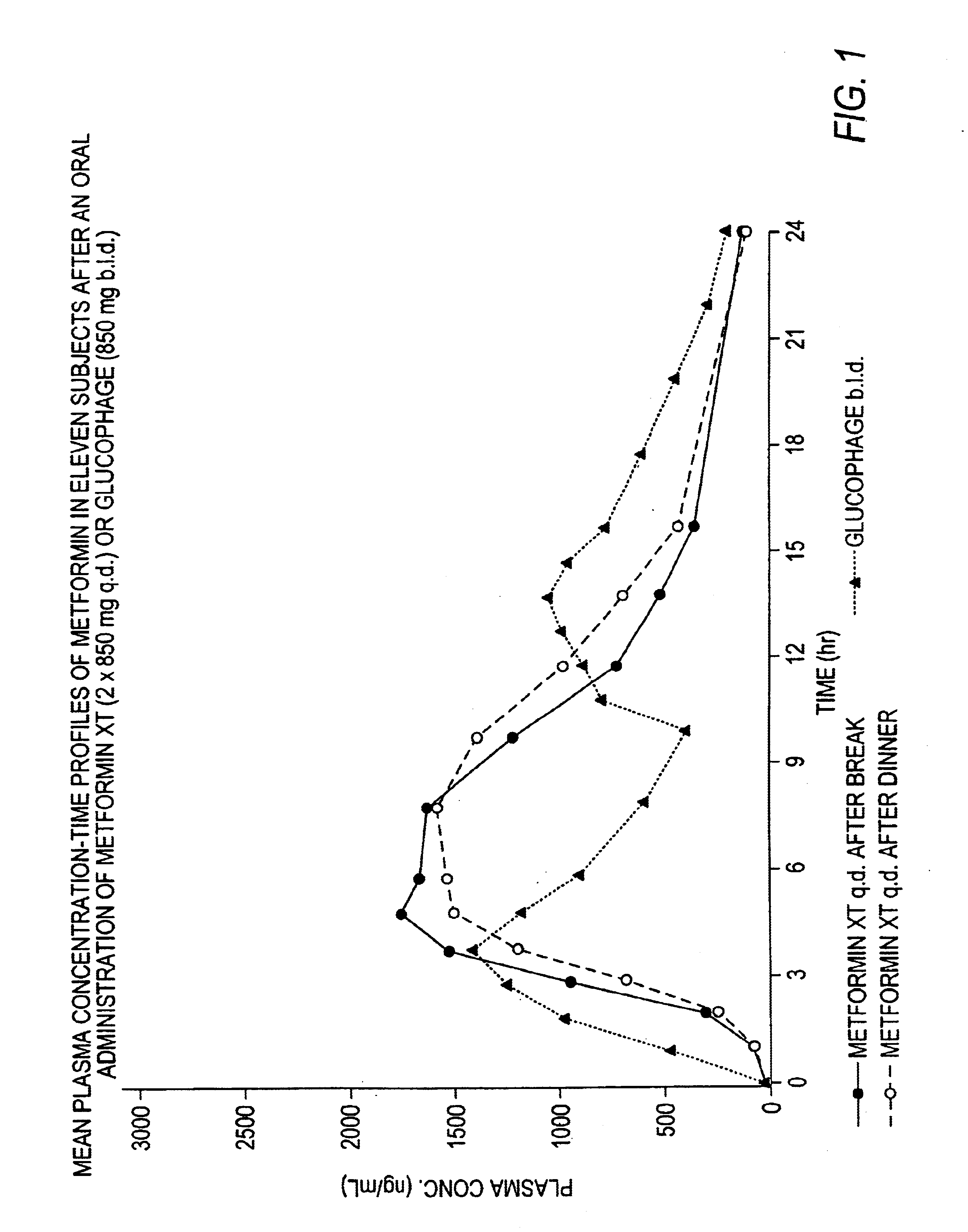
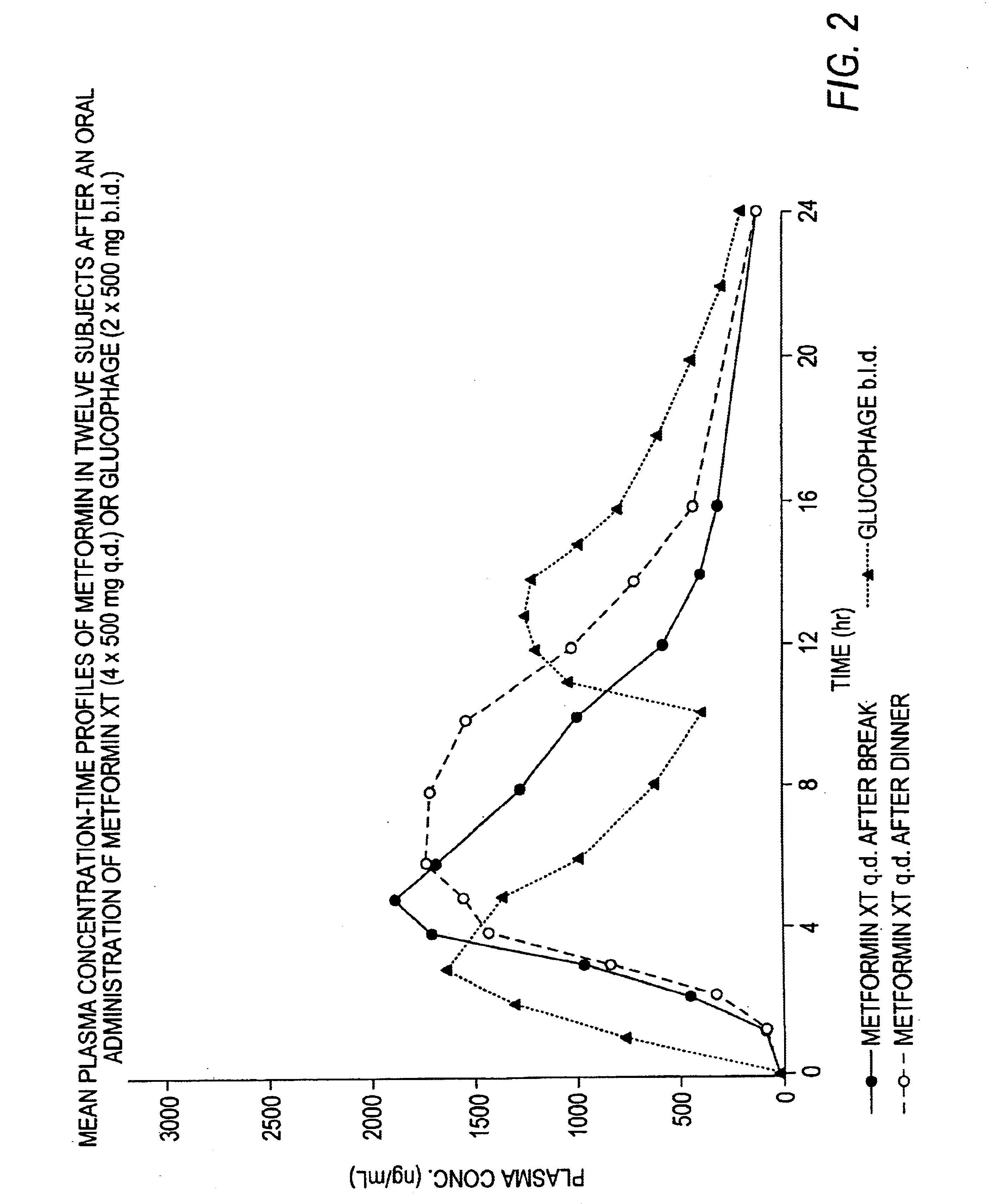
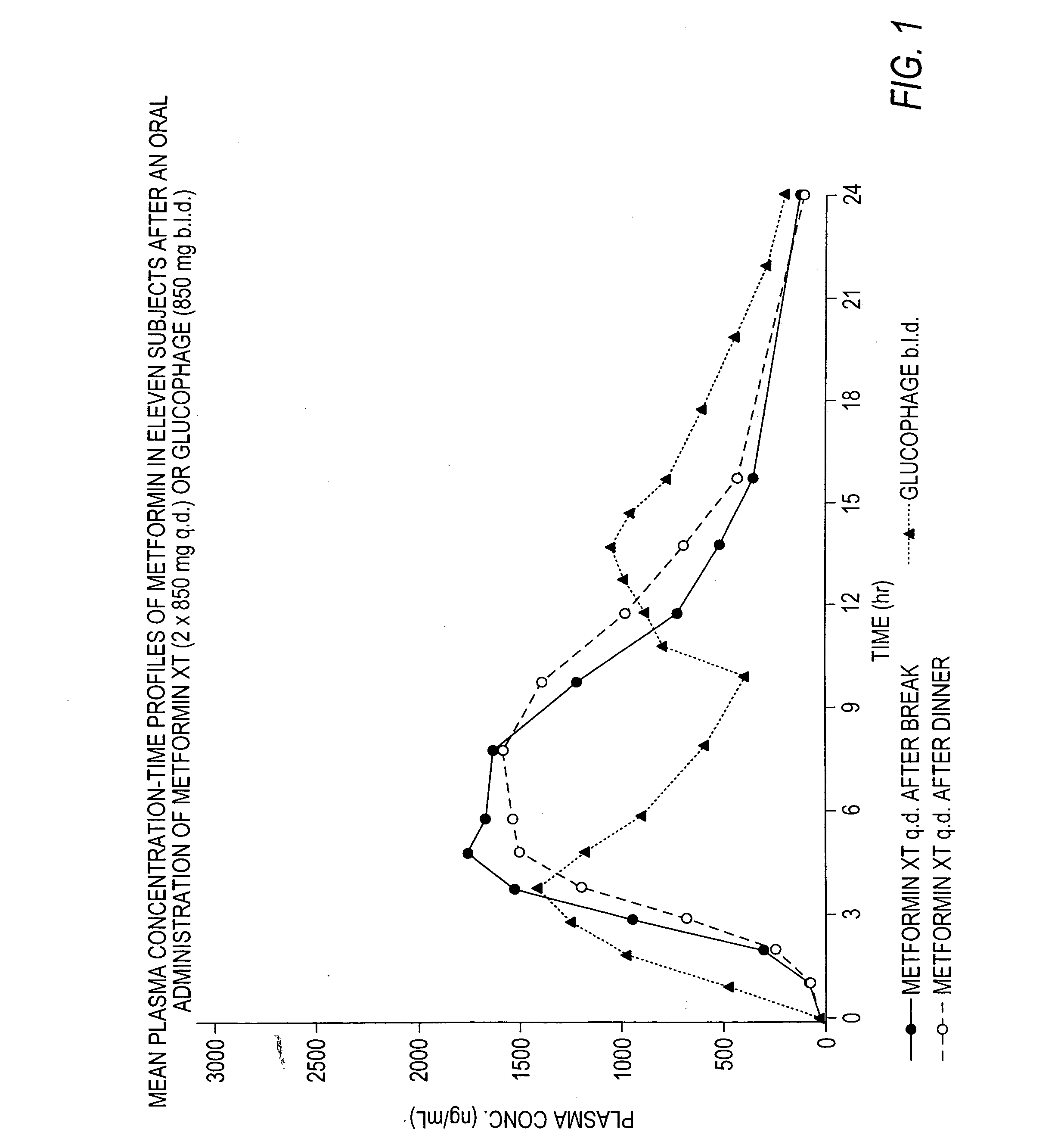
Controlled release metformin compositions
InactiveUS6866866B1Effective controlImprove bioavailabilityOrganic active ingredientsCoatingsCo administrationBlood plasma
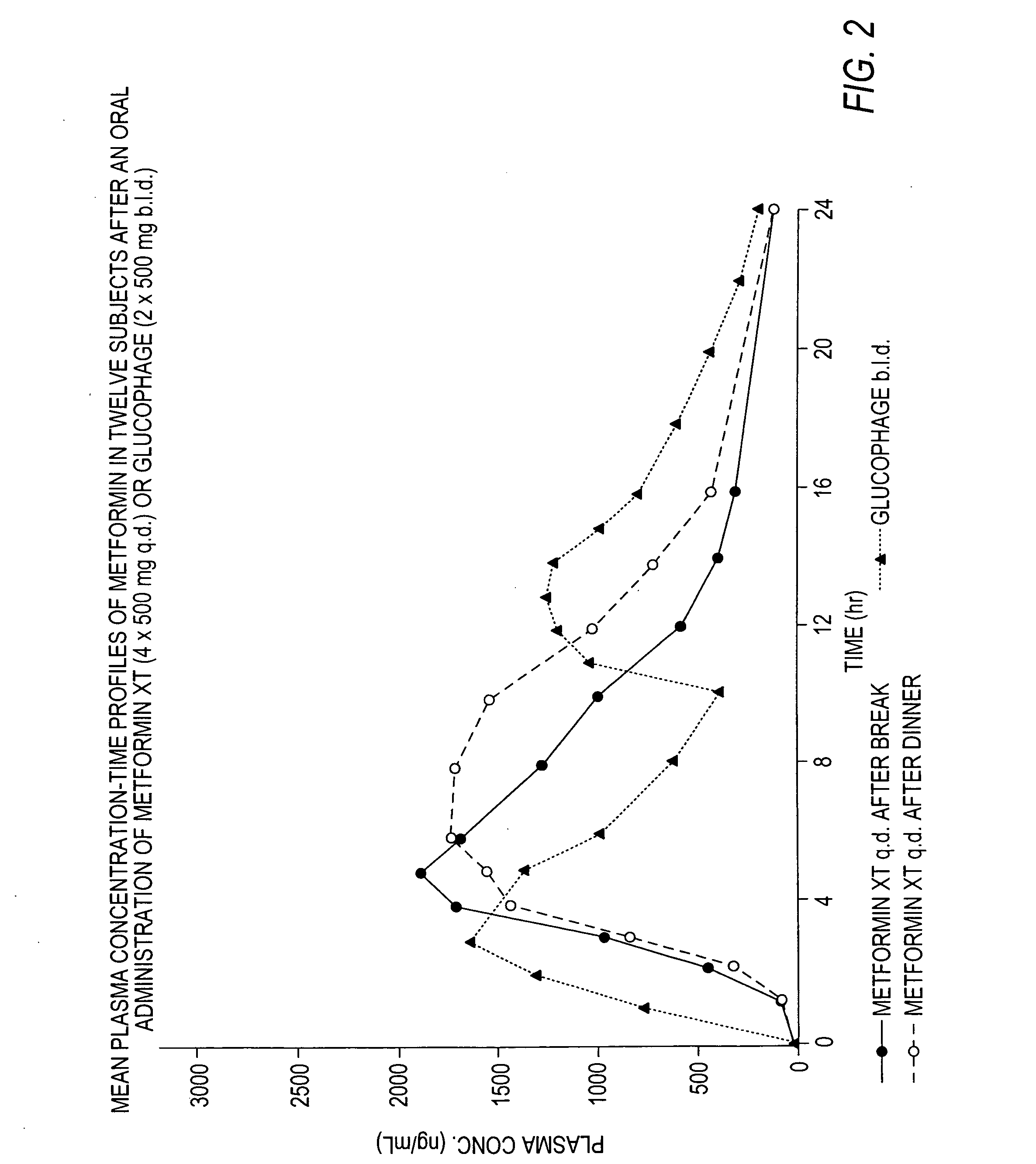
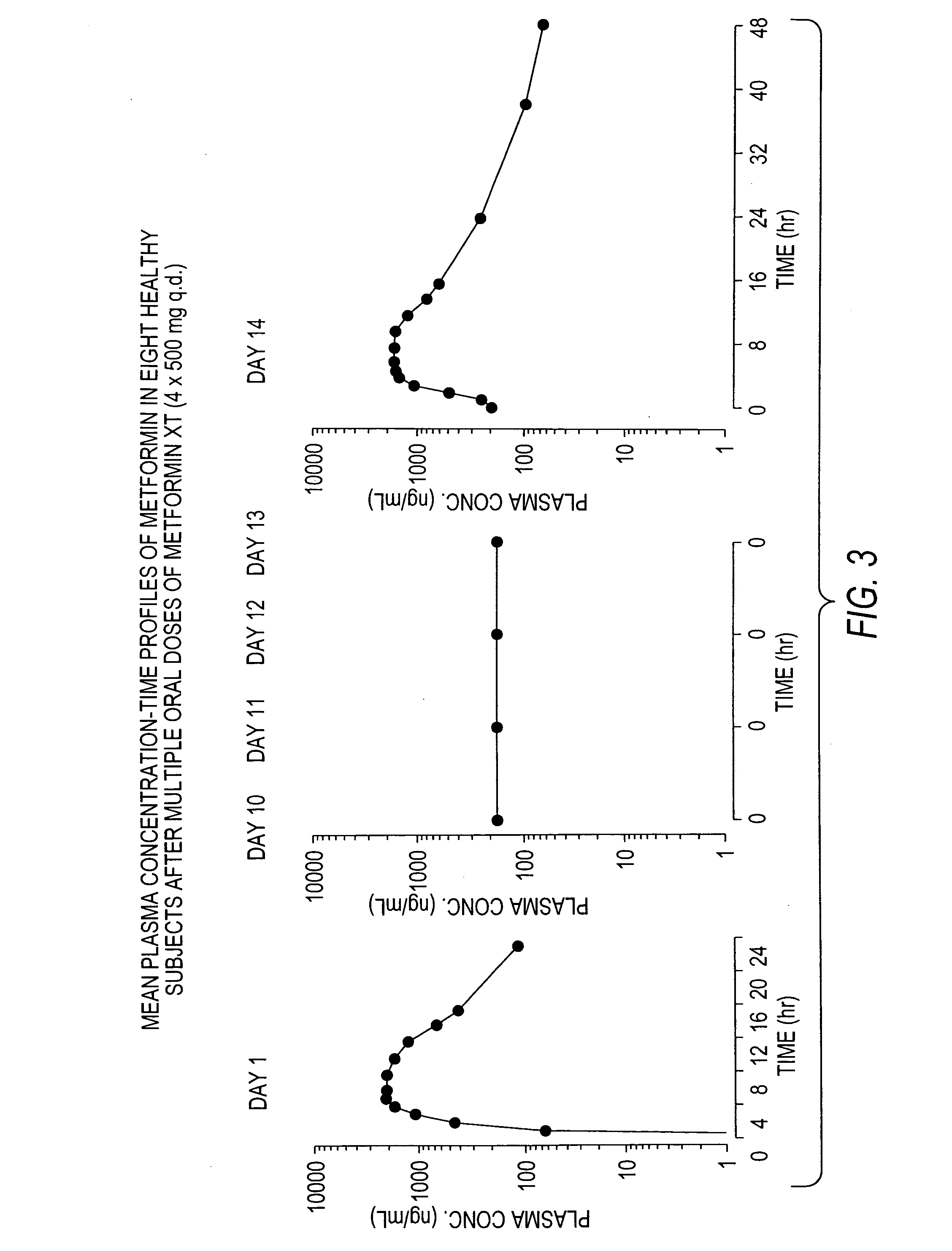
A composition for treating patients having non-insulin-dependent diabetes mellitus (NIDDM) by administering a controlled release oral solid dosage form containing preferably a biguanide drug such as metformin, on a once-a-day basis. The dosage form provides a mean time to maximum plasma concentration (Tmax) of the drug which occurs at 5.5 to 7.5 hours after oral administration on a once-a-day basis to human patients. Preferably, the dose of drug is administered at dinnertime to a patient in the fed state.
Owner:ANDRX LABS
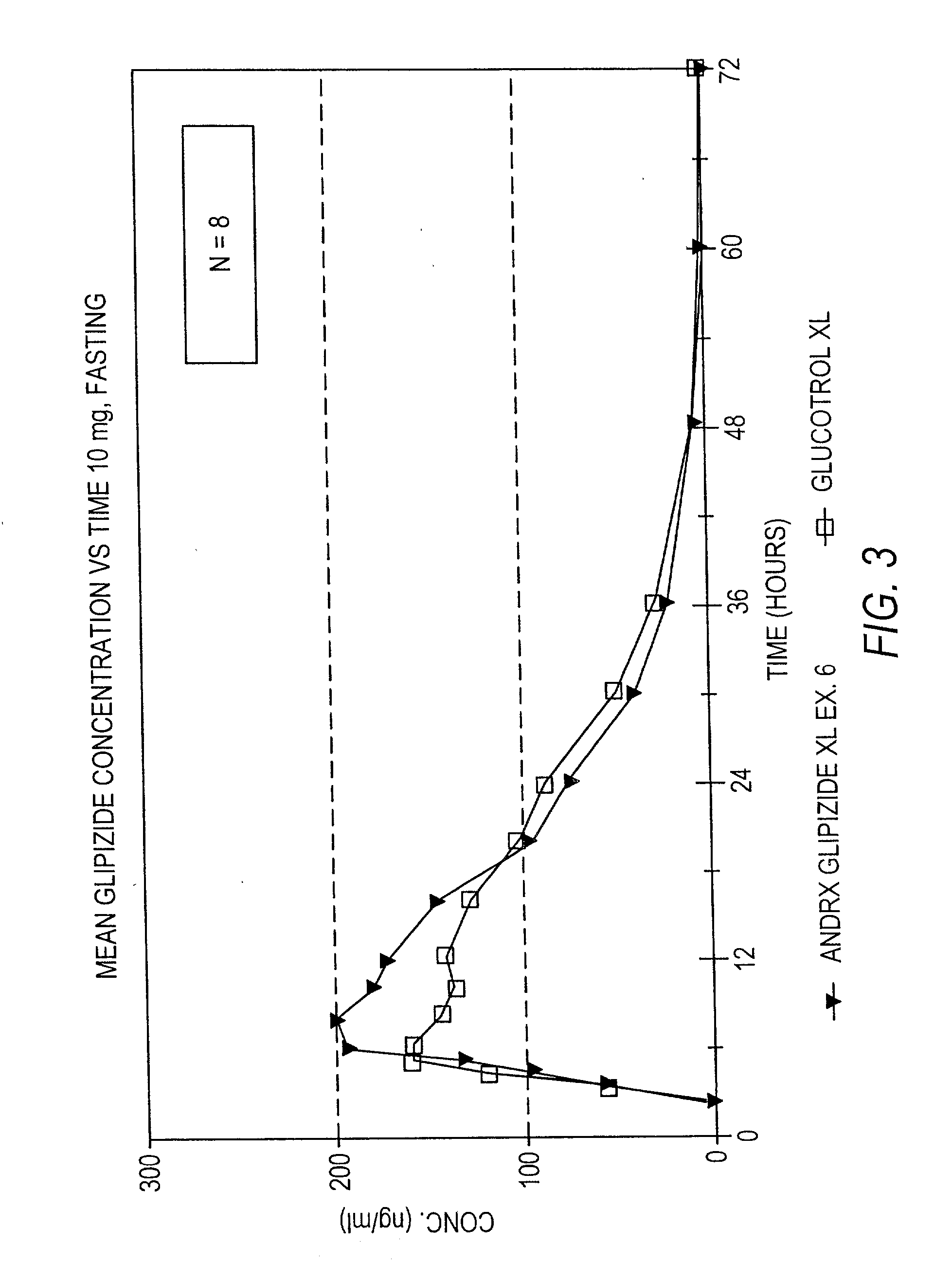
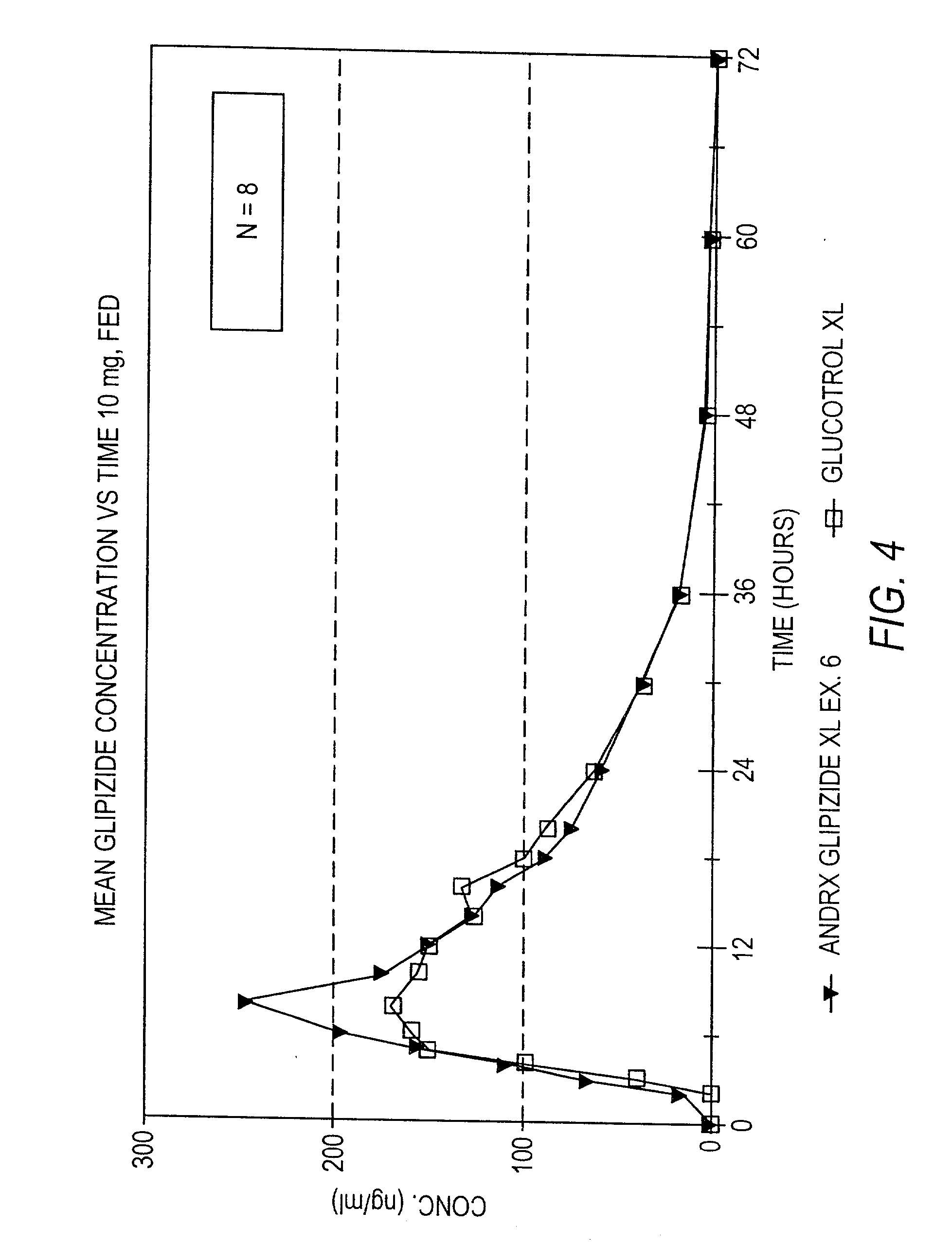
Controlled release sulfonylurea formulation
InactiveUS20030157166A1Effective controlBiocideSulfonylurea active ingredientsControl releaseSulfonylurea
Disclosed in a controlled release sulfonylurea formulation. In certain embodiments, the invention comprises: (a) a core comprising: (i) a sulfonylurea or a pharmaceutically acceptable salt thereof; (ii) a pharmaceutically acceptable polymer; (b) a membrane surrounding the core which is permeable to the sulfonylurea and gastrointestinal fluid, wherein said dosage form provides a mean time to maximum plasma concentration (Tmax) of said sulfonylurea.
Owner:ANDRX PHARMA INC
Novel clonidine formulation
Owner:TRIS PHARMA
Analgesics for nasal administration
InactiveUS20050142072A1Rapid uptakeRapid onsetPowder deliveryBiocideNasal Cavity EpitheliumBlood plasma
An analgesic and a delivery agent are combined in a pharmaceutical composition such that, on introduction into the nasal cavity of a patient to be treated, the analgesic may be delivered to the bloodstream to produce within 30 minutes a therapeutic plasma concentration, Cther, of 0.2 ng / ml or greater which is maintained for a duration Tmaint of at least 2 hours. The analgesic may be an opioid analgesic or a non-steroidal anti-inflammatory drug.
Owner:IONIX PHARMA +1
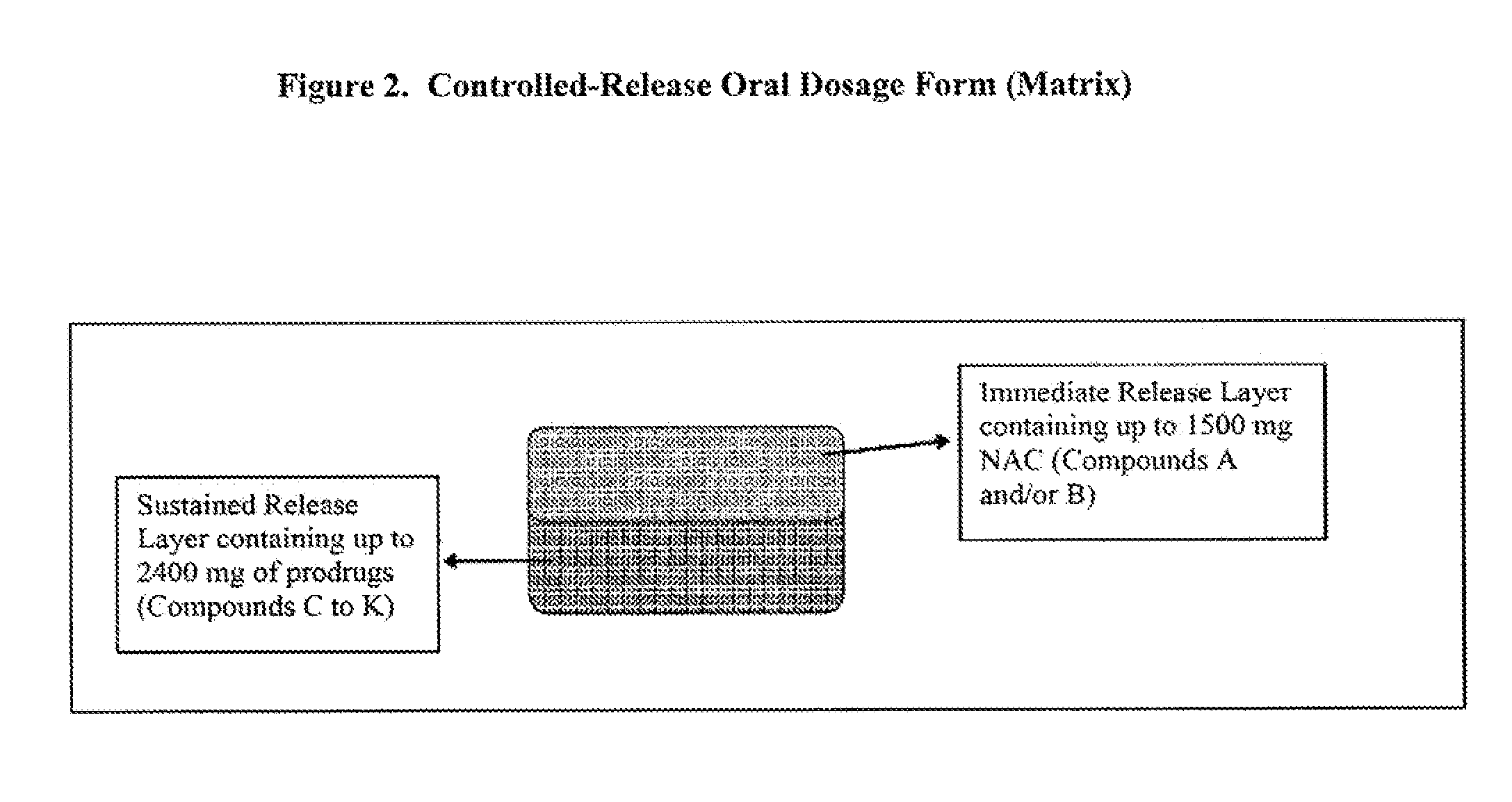
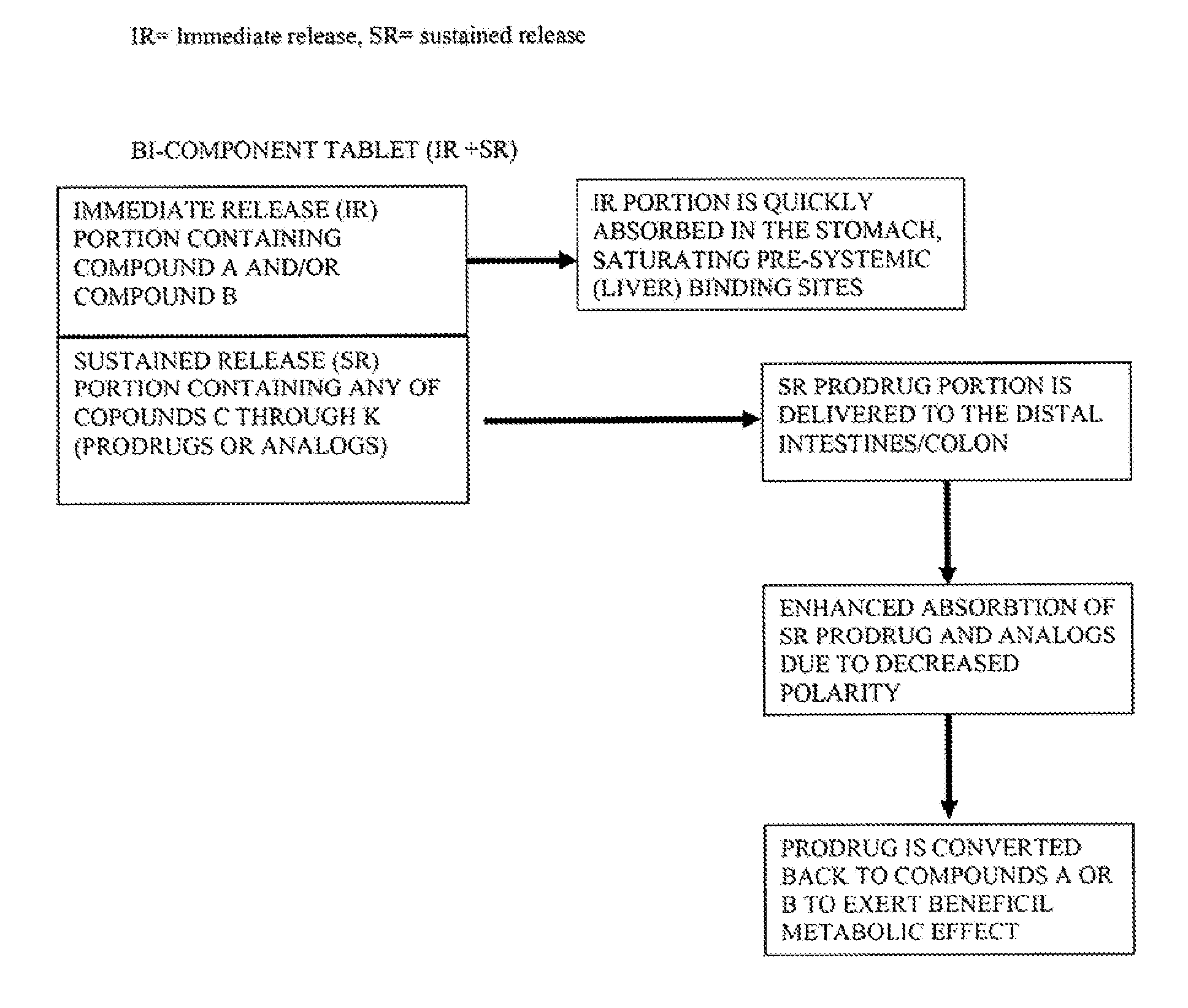
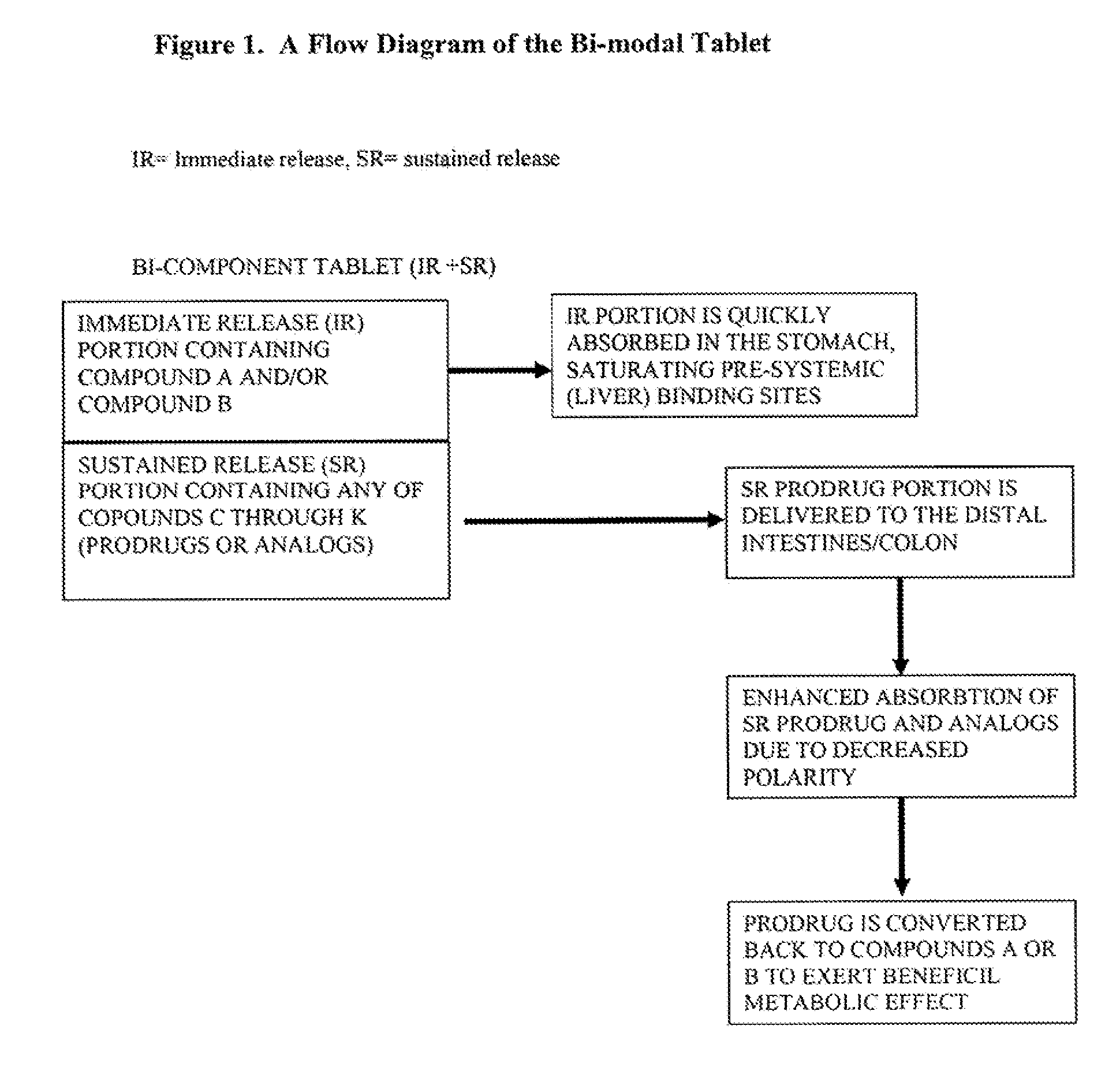
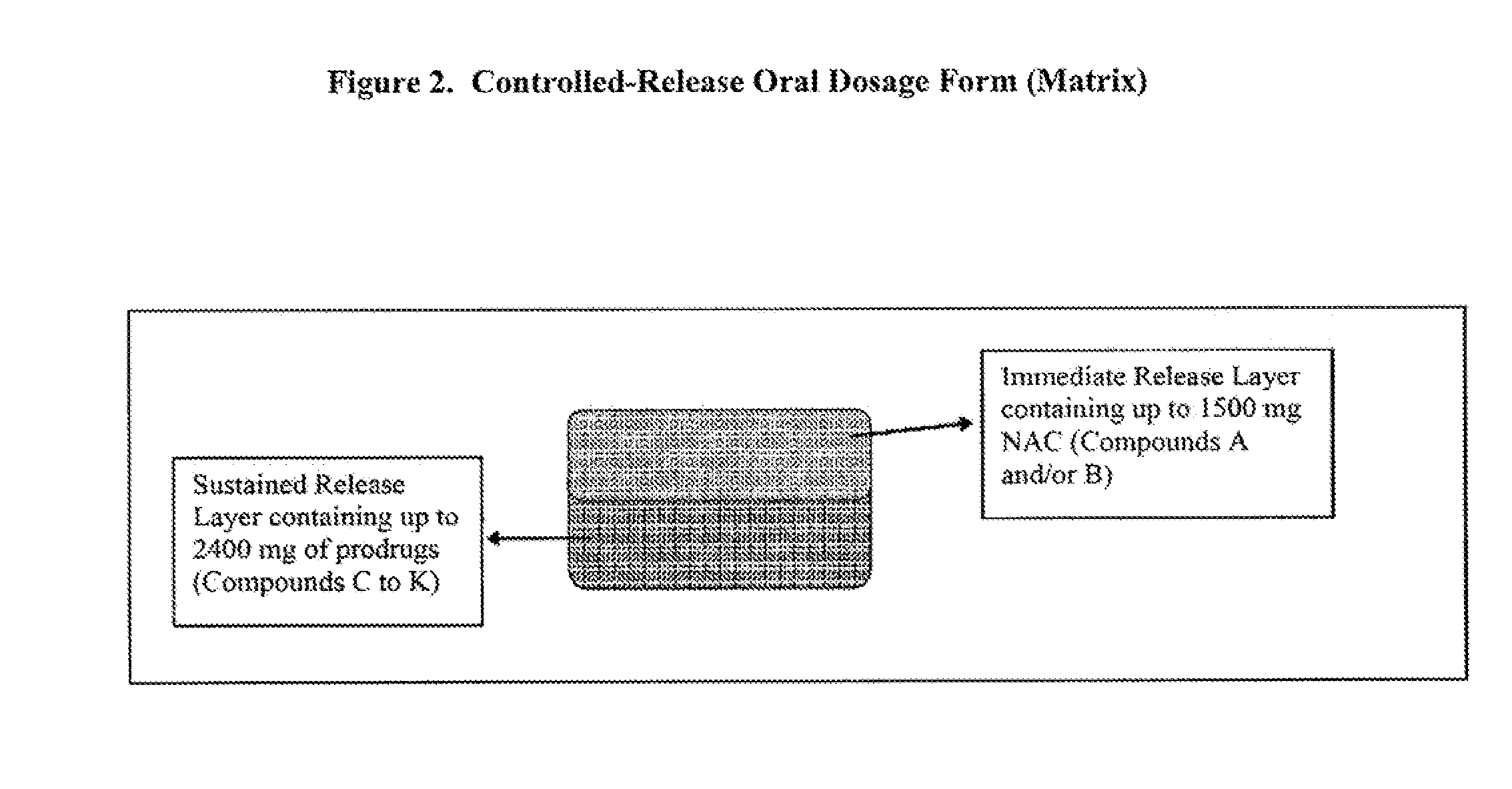
Controlled release of N-acetylcysteine (NAC) for reduction of systemic and/or vascular inflammation
The present invention provides a controlled-release composition which provides a therapeutically effective plasma concentration of N-acetylcysteine over prolonged period of time. The present invention also includes the use of the controlled-release composition, either alone or in combination with at least one additional active agent, for reduction of vascular inflammation marker and treatment of diseases, conditions, and / or symptoms associated with systemic and / or vascular inflammation in a patient. Furthermore, the present invention provides a process of making granules comprising N-acetylcysteine, or a salt, solvate, prodrug, and / or analog thereof.
Owner:TIARA PHARMA
Method of providing customized drug delivery systems
InactiveUS20060078621A1Promote absorptionMaximum therapeutic effectivenessPowder deliveryPill deliveryAdditive ingredientBlood plasma
A novel method of correlating the disposition of a specific drug in an individual patient to a controlled and modulated delivery system for optimizing therapeutic response of orally ingested dosage forms is provided. Such a method broadly encompasses a first determination of an individual's metabolic rate in terms of absorption of pharmaceutical materials from within the gastrointestinal tract measured as blood plasma concentration over a specific period of time after ingestion or by other commercially available methods and subsequent determination: 1) predicting a proper pharmaceutical compositions, in terms of amount of active available for absorption by the target patient; and 2) amount of such active pharmaceutical ingredient (API) to be formulated within a drug-delivery device that will take into account the unique metabolic profile of the drug (or drugs) in a specific patient. As a result, the API may be formulated as beads, pellets, minitablets, powders, granules, suspensions, and / or emulsions present within the drug-delivery source. As one potentially preferred embodiment, such beads and / or pellets, which may be coated with different polymers and differing levels of coatings, are selected in response to the initial determination of the patient's metabolic profile in order to ensure the specific targeted patient receives the most efficient dosage of the active drug at a rate unique to that individual.
Owner:J M HUBER CORP
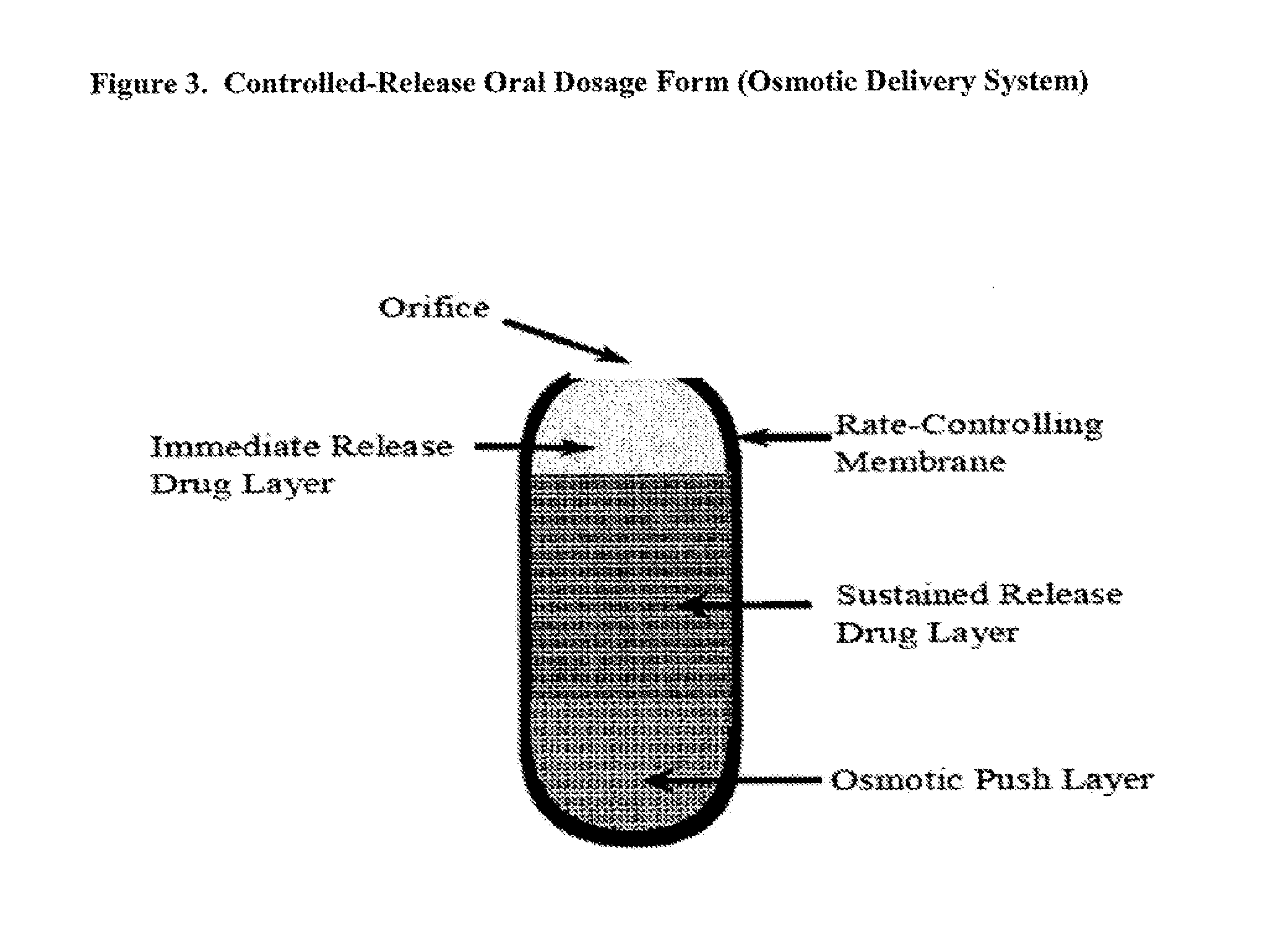
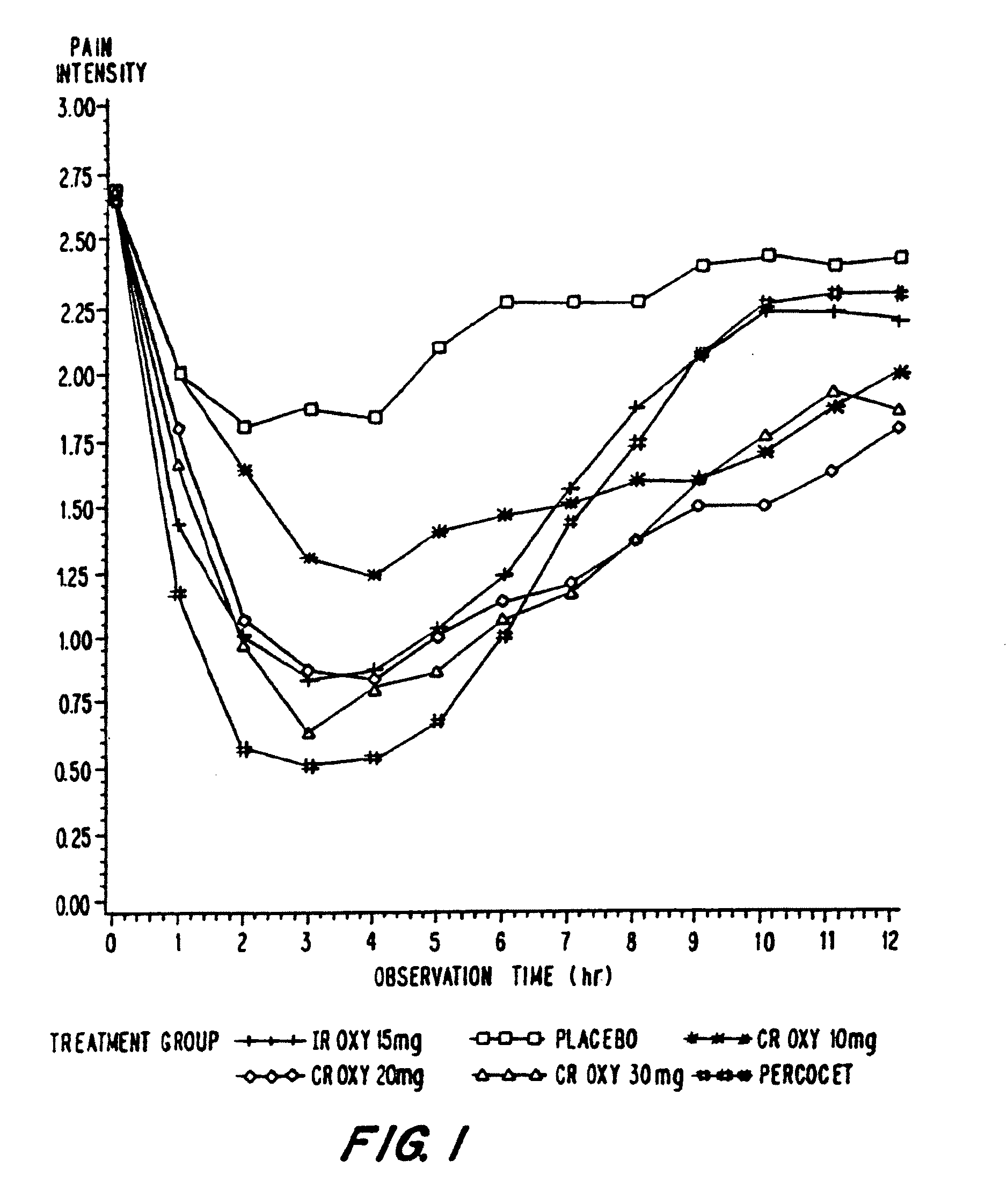
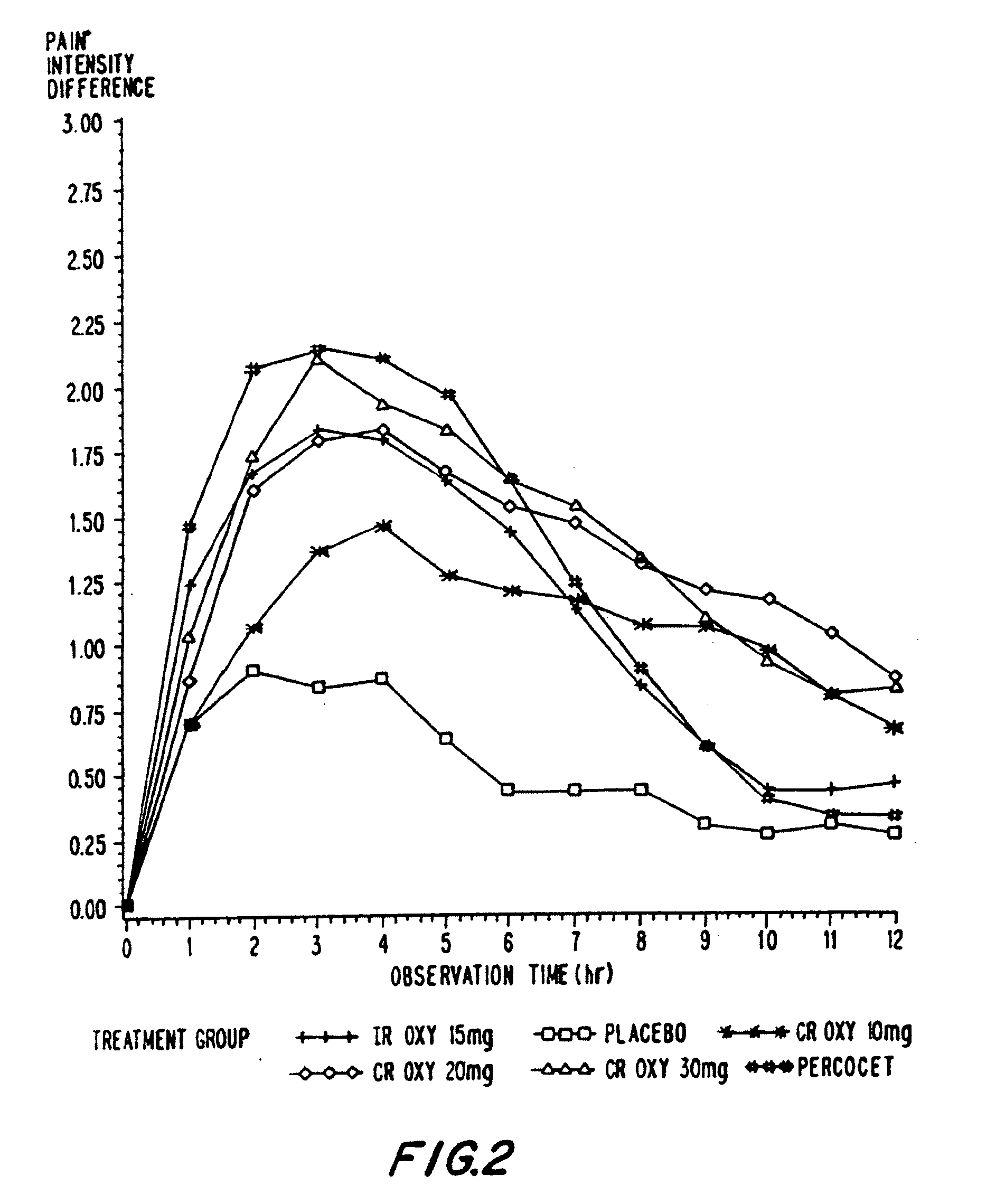
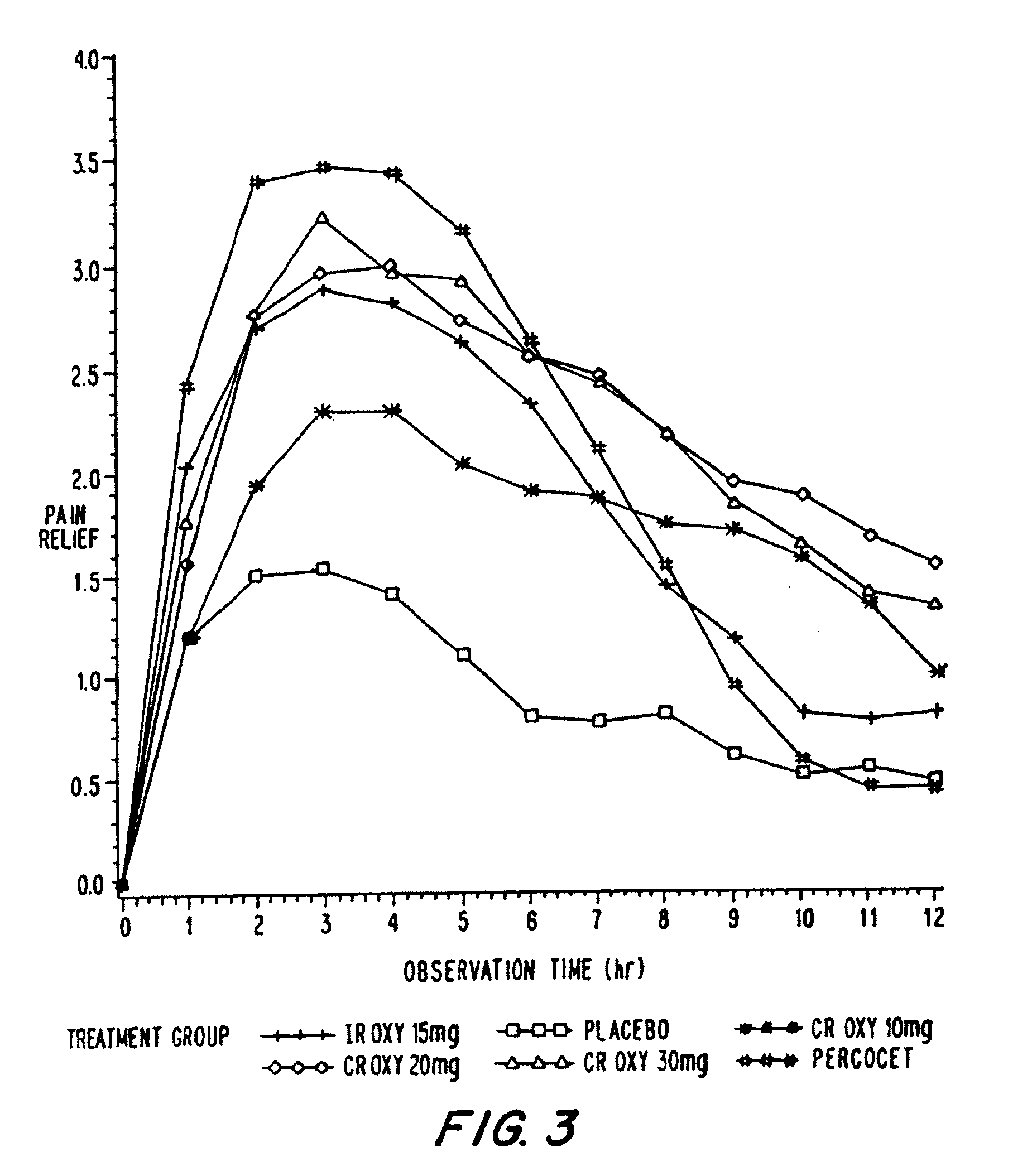
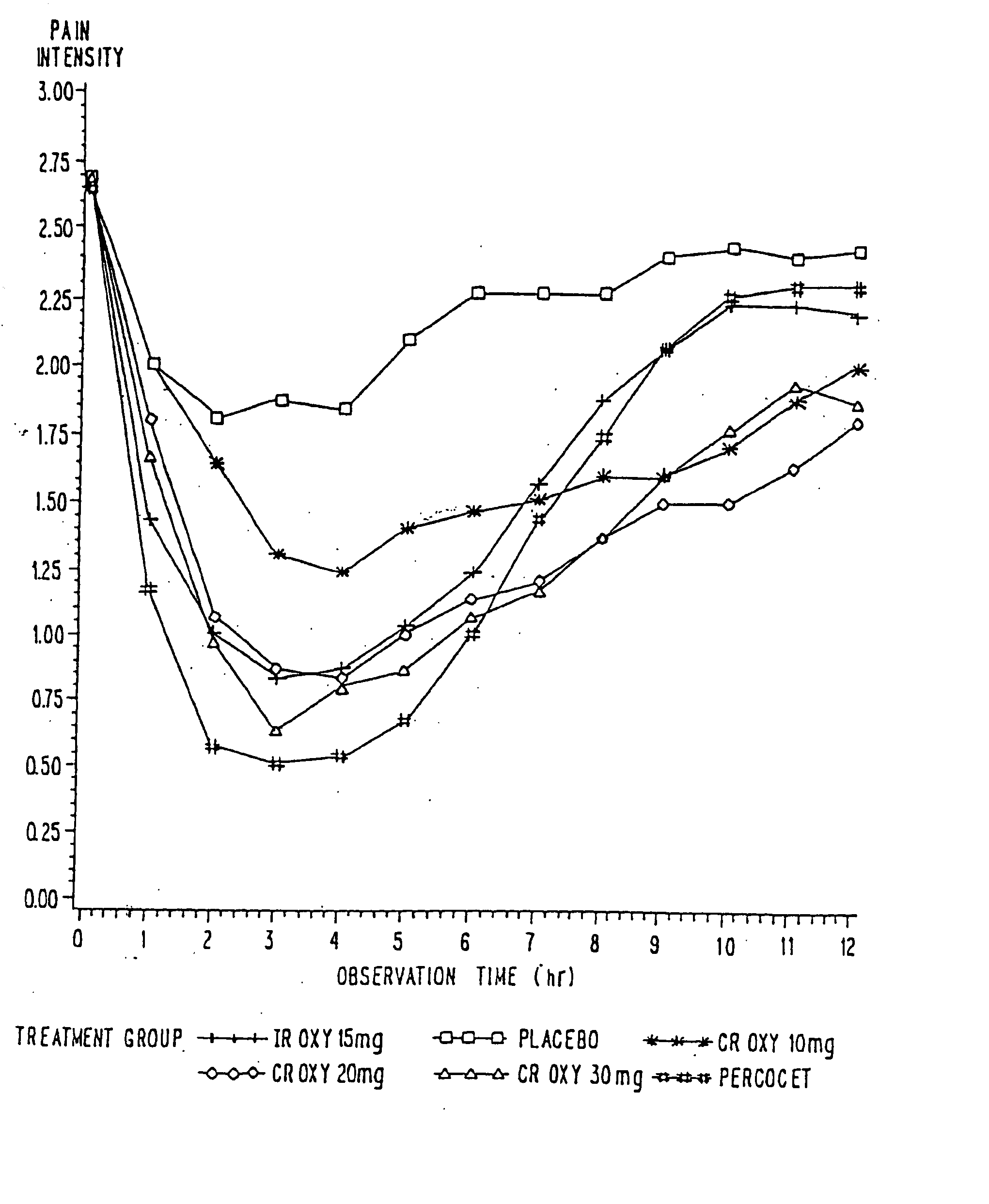
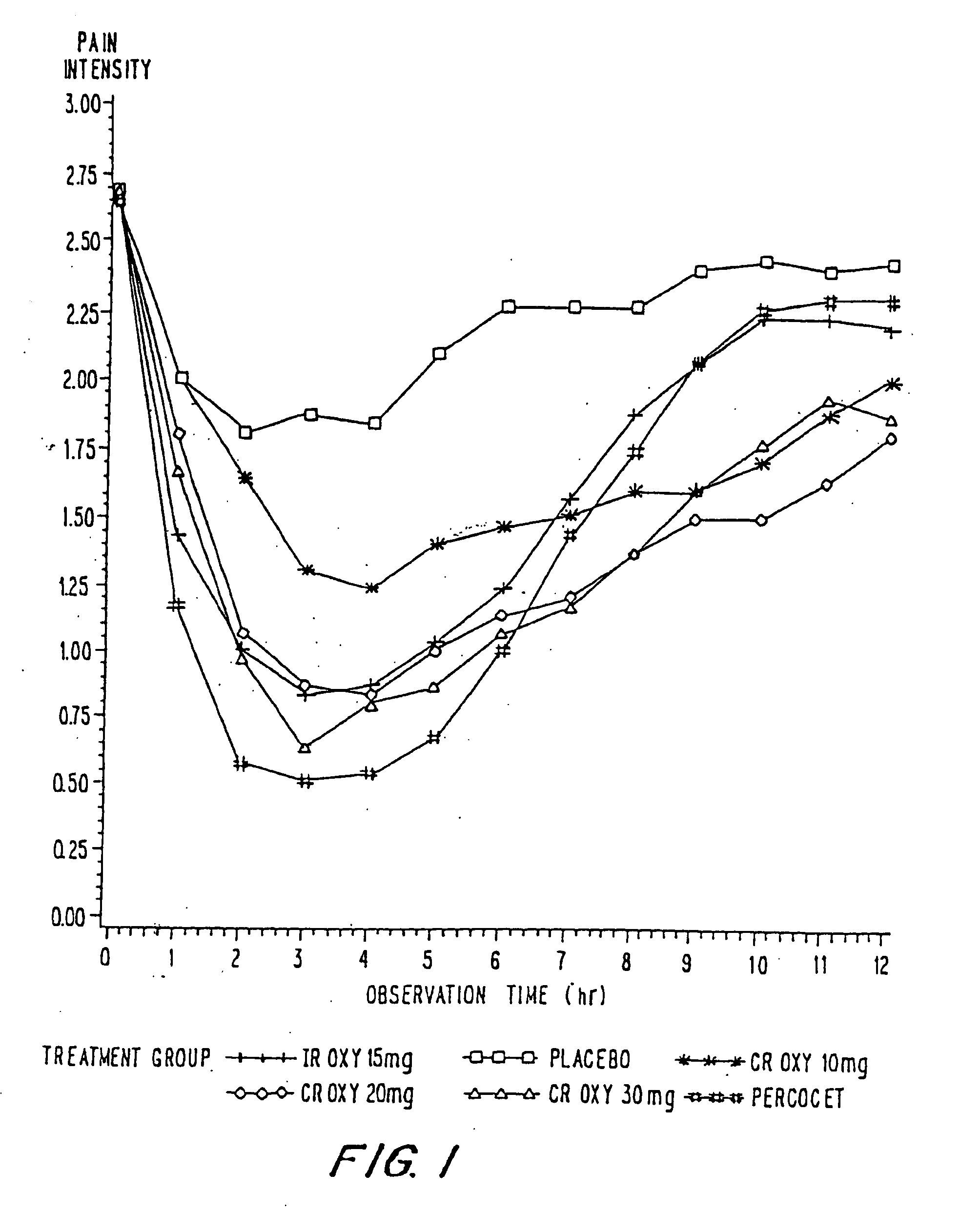
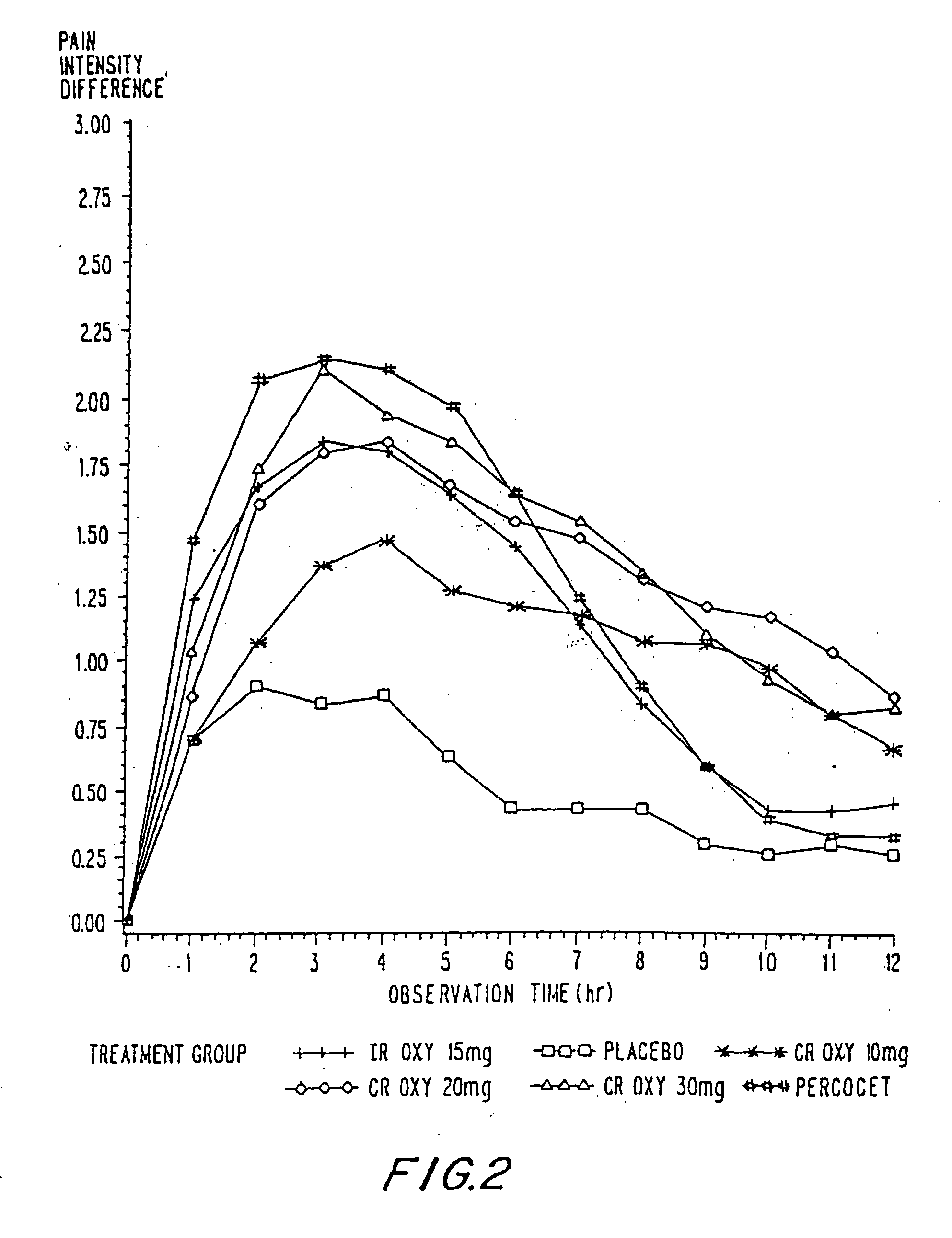
Controlled release oxycodone compositions
InactiveUS20100092570A1Improve efficiency and qualityLow variabilityBiocidePowder deliveryControl releaseBlood plasma
A method for substantially reducing the range in daily dosages required to control pain in approximately 90% of patients is disclosed whereby an oral solid controlled release dosage formulation having from about 10 to about 40 mg of oxycodone or a salt thereof is administered to a patient. The formulation provides a mean maximum plasma concentration of oxycodone from about 6 to about 60 ng / ml from a mean of about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration from about 3 to about 30 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions. Another embodiment is directed to a method for substantially reducing the range in daily dosages required to control pain in substantially all patients by administering an oral solid controlled release dosage formulation comprising up to about 160 mg of oxycodone or a salt thereof, such that a mean maximum plasma concentration of oxycodone up to about 240 ng / ml from a mean of up to about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration up to about 120 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions are achieved. Controlled release oxycodone formulations for achieving the above are also disclosed.
Owner:PURDUE PHARMA LP +1
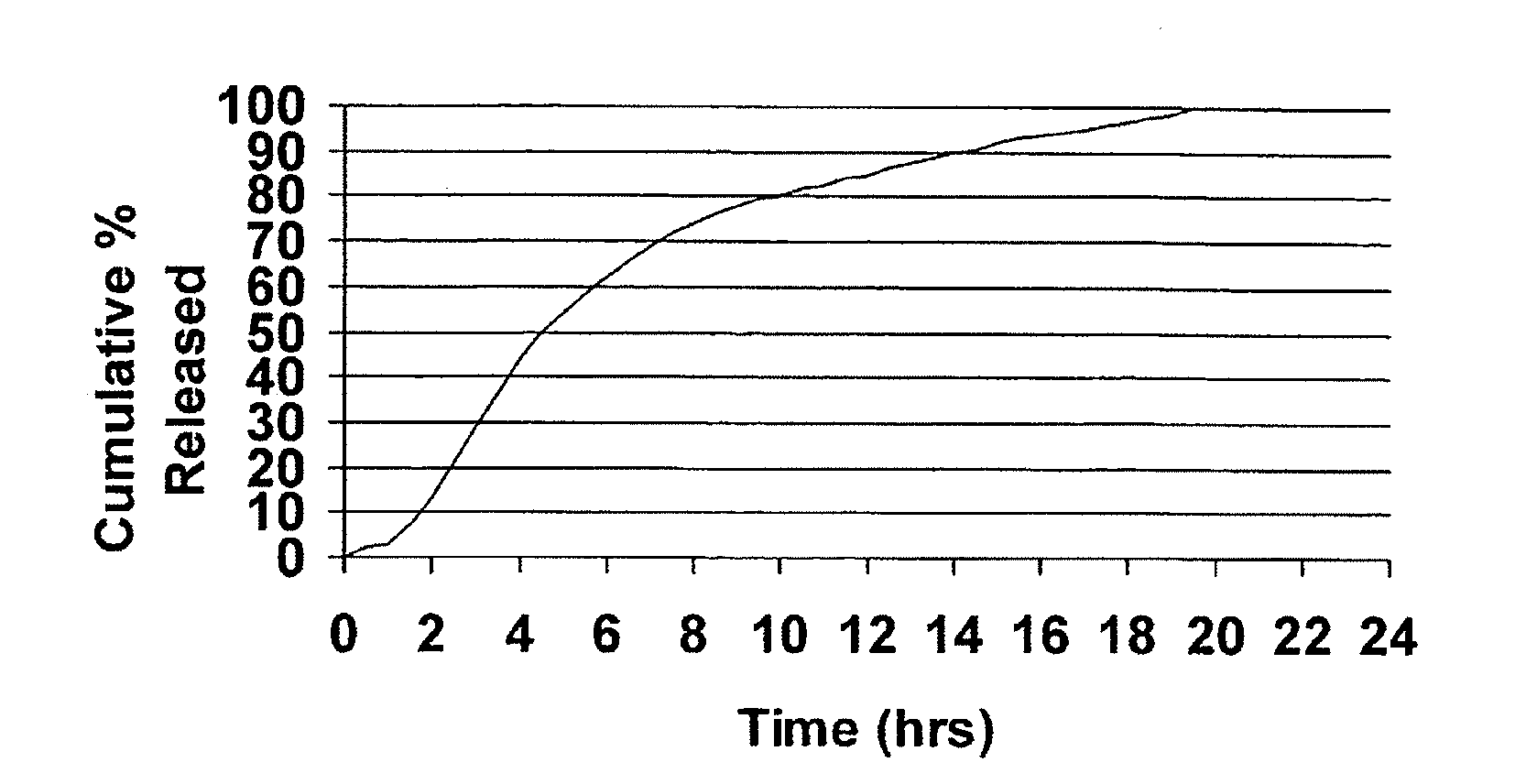
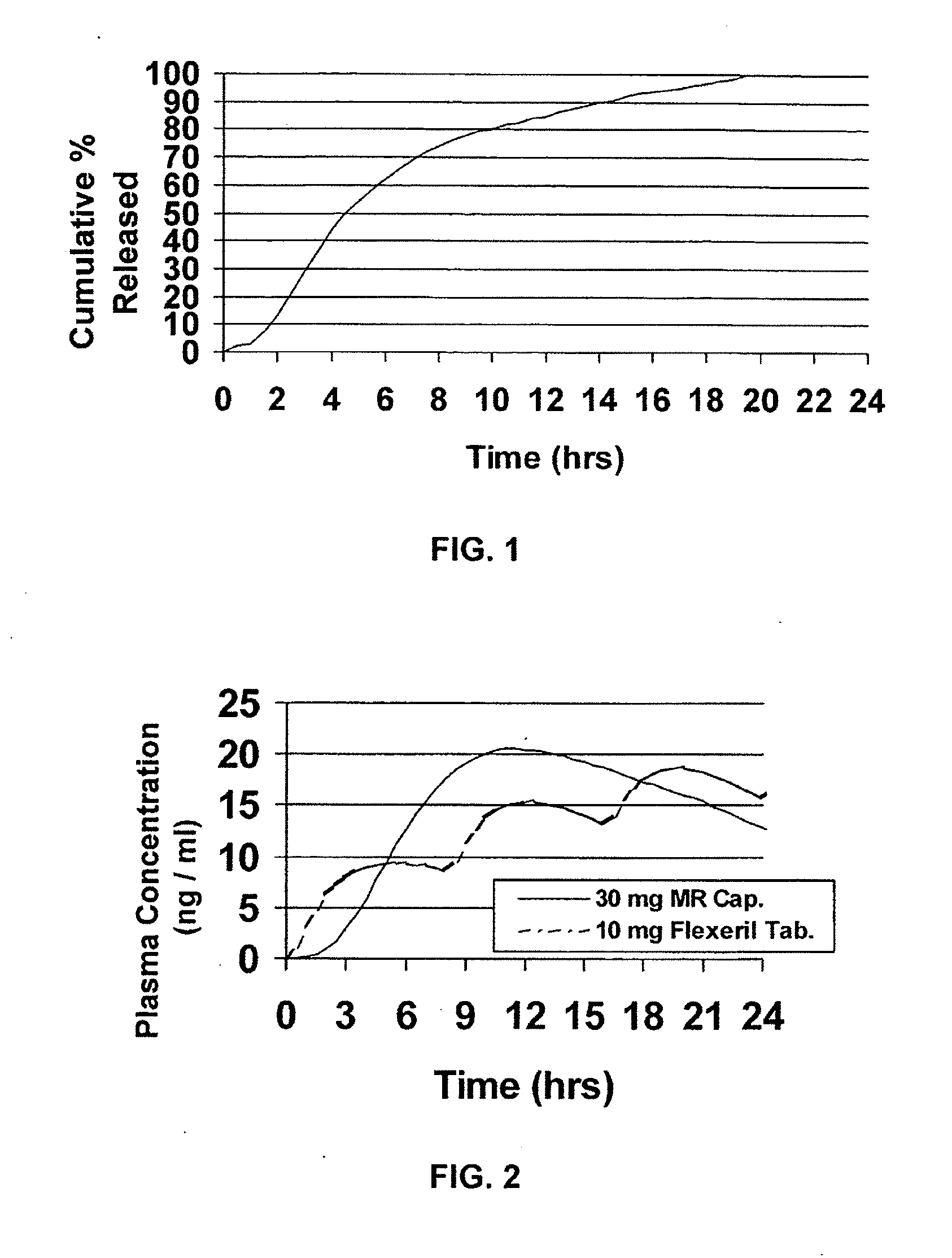
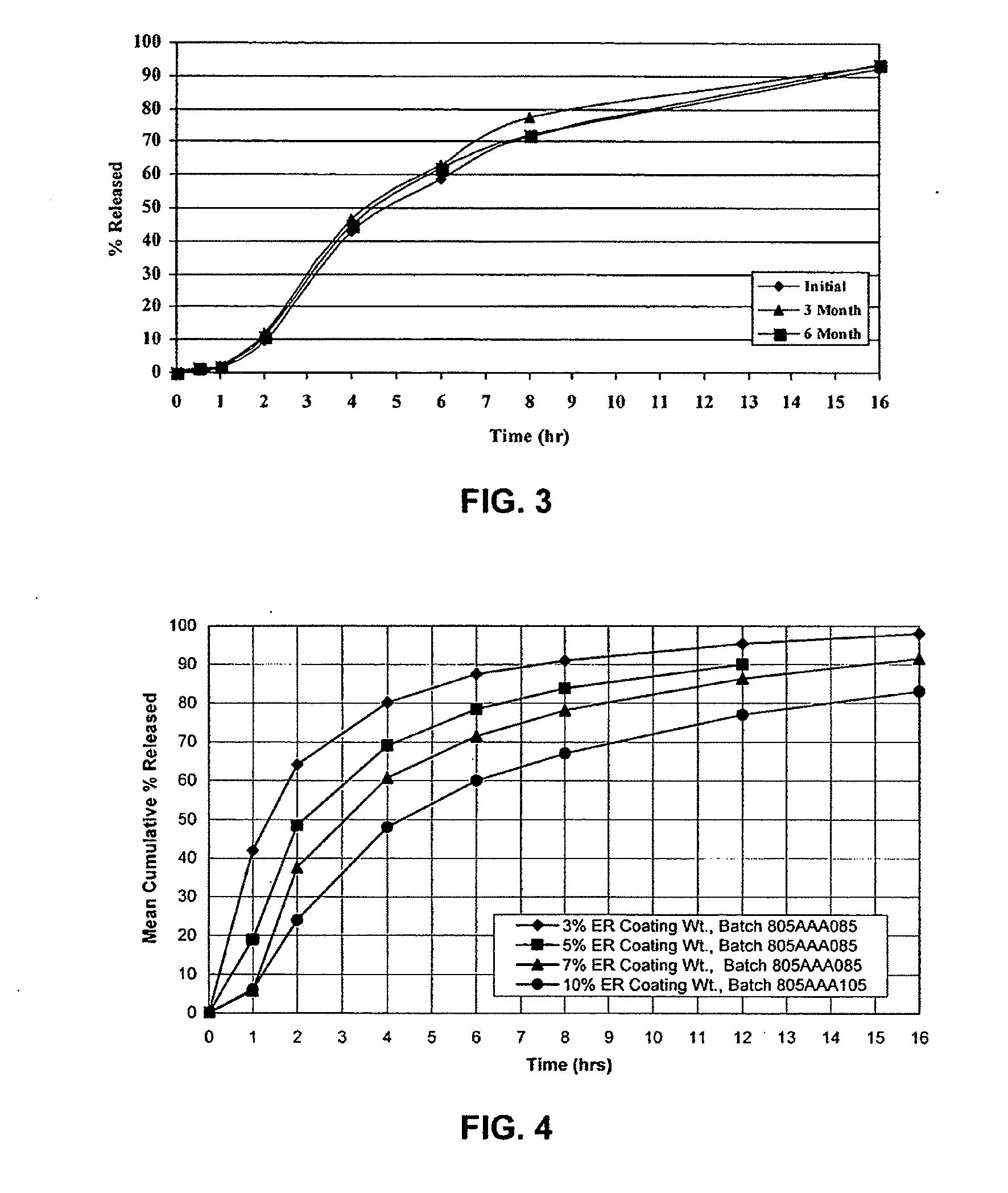
Modified release dosage forms of skeletal muscle relaxants
InactiveUS20080124398A1Patient compliance is goodEfficient ConcentrationPowder deliveryOrganic active ingredientsDiseaseModified Release Dosage Form
A unit dosage form, such as a capsule or the like, for delivering a skeletal muscle relaxant, such as cyclobenzaprine hydrochloride, into the body in an extended or sustained release fashion comprising one or more populations of drug-containing particles (beads, pellets, granules, etc.) is disclosed. At least one bead population exhibits a pre-designed sustained release profile. Such a drug delivery system is designed for once-daily oral administration to maintain an adequate plasma concentration—time profile, thereby providing relief of muscle spasm associated with painful musculoskeletal conditions over a 24 hour period.
Owner:ADARE PHARM INC
Morphine controlled release system
InactiveUS8877241B2Affecting extent of drug bioavailabilityReduce frequencyBiocideNervous disorderMorphineDissolution
A composition for controlled release of an opioid from a pharmaceutical composition, the method comprises controlling the release of at least one opioid into an aqueous medium by erosion of at least one surface of a pharmaceutical composition comprising I) a matrix composition comprising a) polymer or a mixture of polymers, b) an opioid and, optionally, c) one or more pharmaceutically acceptable excipients, and (i) a coating. The matrix composition has a conus-like shape so the surface area exposed to the aqueous medium increases at least during initial erosion of the matrix composition, and the dissolution of the opioid—when tested in a Dissolution Test as described herein with or without application of sinkers-results in a zero order release of at least 80% of the opioid contained in the composition. Such compositions are especially suitable for controlled release of an opioid to obtain a delayed peak concentration and a prolonged therapeutically effective plasma concentration upon oral administration. Once or twice daily administration is possible. The matrix typically comprises PEO and the active substance is typically an opioid such as morphine or a glucuronide thereof.
Owner:EGALET LTD
Method of selecting nonsedating H1-antagonists
A method is described in accordance with which one may select a nonsedating histamine H.sub.1-antagonist; comprising the step of determining whether a candidate H.sub.1-antagonist is a substrate for P-gp, especially P-gp expressed by MDR1 or mdr1a / 1b, comprising the step of determining the brain-to-plasma AUC ratio in mdr1a / 1b KO mice, and in WT mice, and selecting said candidate where the brain-to-plasma AUC ratio for said KO mice to the brain-to-plasma concentration ratio for said WT mice is 1.5 or greater.
Owner:PFIZER INC +1
Controlled release oxycodone compositions
InactiveUS20070275065A1Improve efficiencyQuality improvementBiocidePill deliveryControlled releaseBlood plasma
A method for substantially reducing the range in daily dosages required to control pain in approximately. 90% of patients is disclosed whereby an oral solid controlled release dosage formulation having from about 10 to about 40 mg of oxycodone or a salt thereof is administered to a patient. The formulation provides a mean maximum plasma concentration of oxycodone from about 6 to about 60 ng / ml from a mean,of about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration from about 3 to about 30 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour): administration through steady-state conditions. Another embodiment is directed to a method for substantially reducing the range in daily dosages required to control pain in substantially all patients by administering an oral solid controlled release dosage formulation comprising up to about 160 mg of oxycodone or a salt thereof, such that a mean maximum plasma concentration of oxycodone up to about 240 ng / ml from a mean of up to about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration up to about 120 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions are achieved. Controlled release oxycodone formulations for achieving the above are also disclosed.
Owner:PURDUE PHARMA LP +2
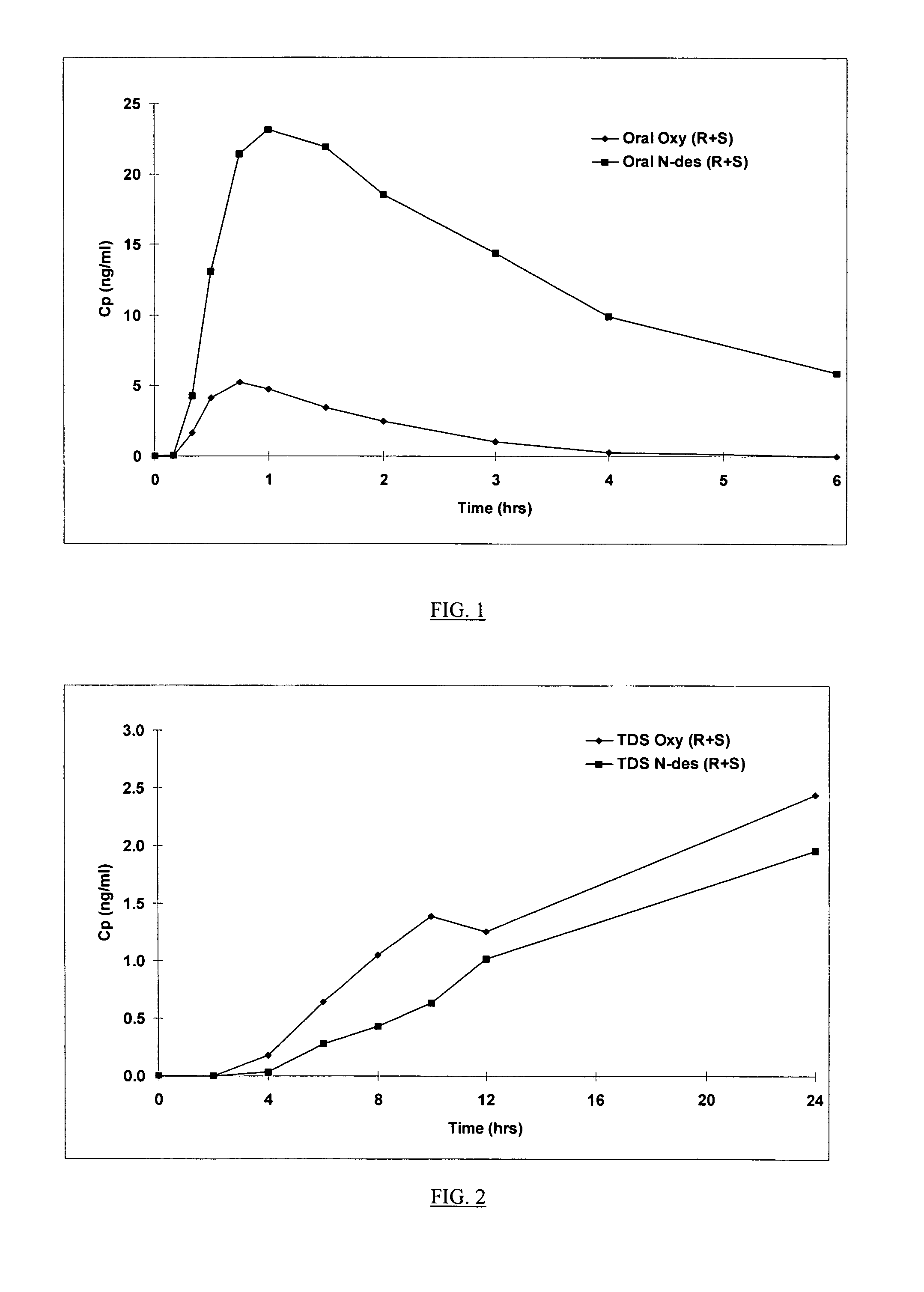
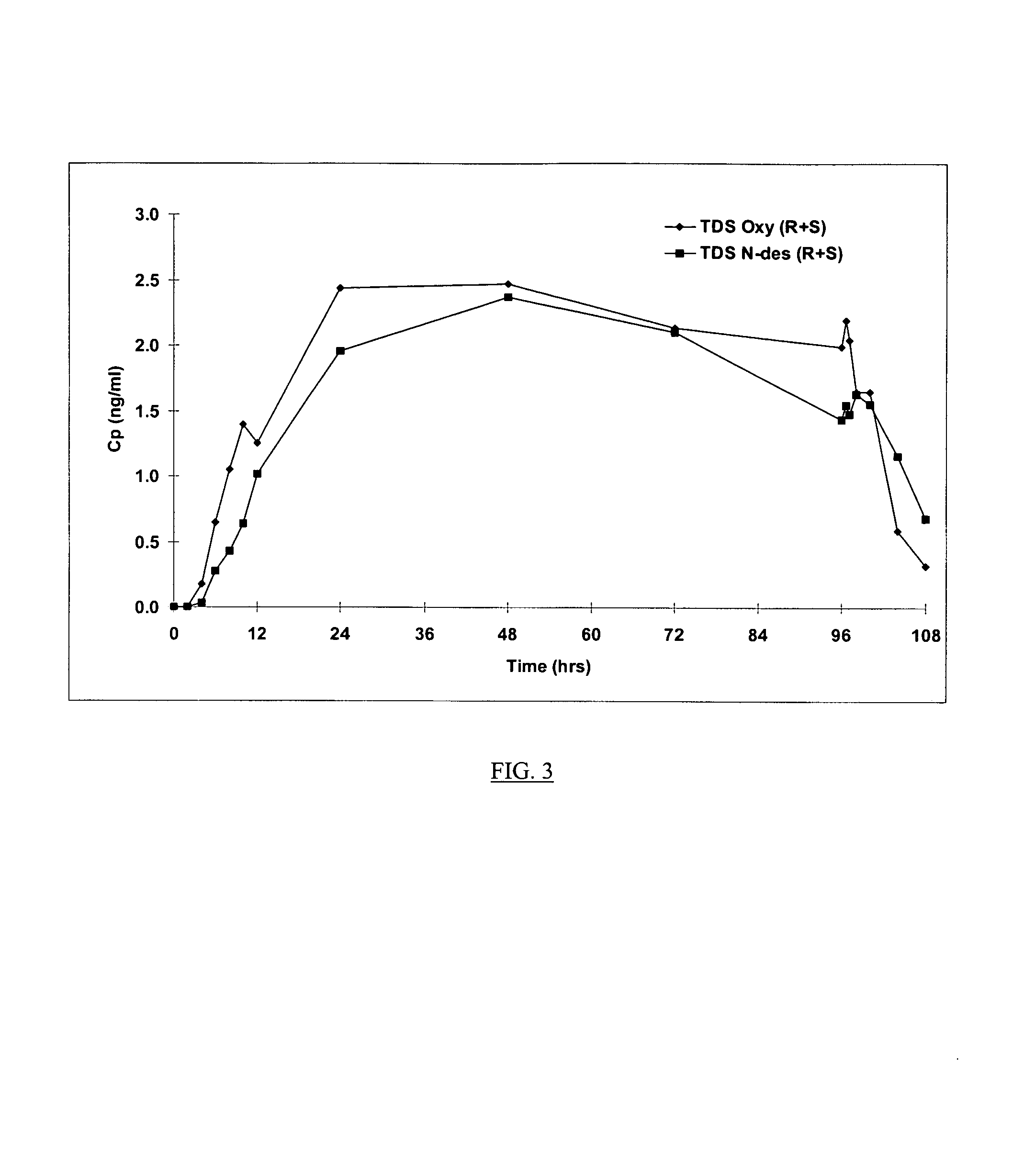
Compositions and methods for transdermal oxybutynin therapy
InactiveUS7179483B2Minimizing adverse side effectOrganic active ingredientsPlastersMetaboliteOxybutynine
The present invention provides compositions and methods for administering oxybutynin while minimizing the incidence and or severity of adverse drug experiences associated with oxybutynin therapy. In one aspect, these compositions and methods provide a lower plasma concentration of oxybutynin metabolites, such as N-desethyloxybutynin, which is presumed to be contributing at least in part to some of the adverse drug experiences, while maintaining sufficient oxybutynin plasma concentration to benefit a subject with oxybutynin therapy. The invention also provides isomers of oxybutynin and its metabolites that meet these characteristics of minimized incidence and / or severity of adverse drug experiences, and maintenance of beneficial and effective therapy for overactive bladder. In some aspects, the composition may be presented in the form of an unoccluded or free form topically administered gel.
Owner:ALLERGAN SALES LLC +1
Controlled release metformin compositions
InactiveUS20060008523A1Effective controlImprove bioavailabilityOrganic active ingredientsCoatingsPancreatic hormoneBlood plasma
A composition for treating patients having non-insulin-dependent diabetes mellitus (NIDDM) by administering a controlled release oral solid dosage form containing preferably a biguanide drug such as metformin, on a once-a-day basis. The dosage form provides a mean time to maximum plasma concentration (Tmax) of the drug which occurs at 5.5 to 7.5 hours after oral administration on a once-a-day basis to human patients. Preferably, the dose of drug is administered at dinnertime to a patient in the fed state.
Owner:ANDRX
Lercanidipine capsules
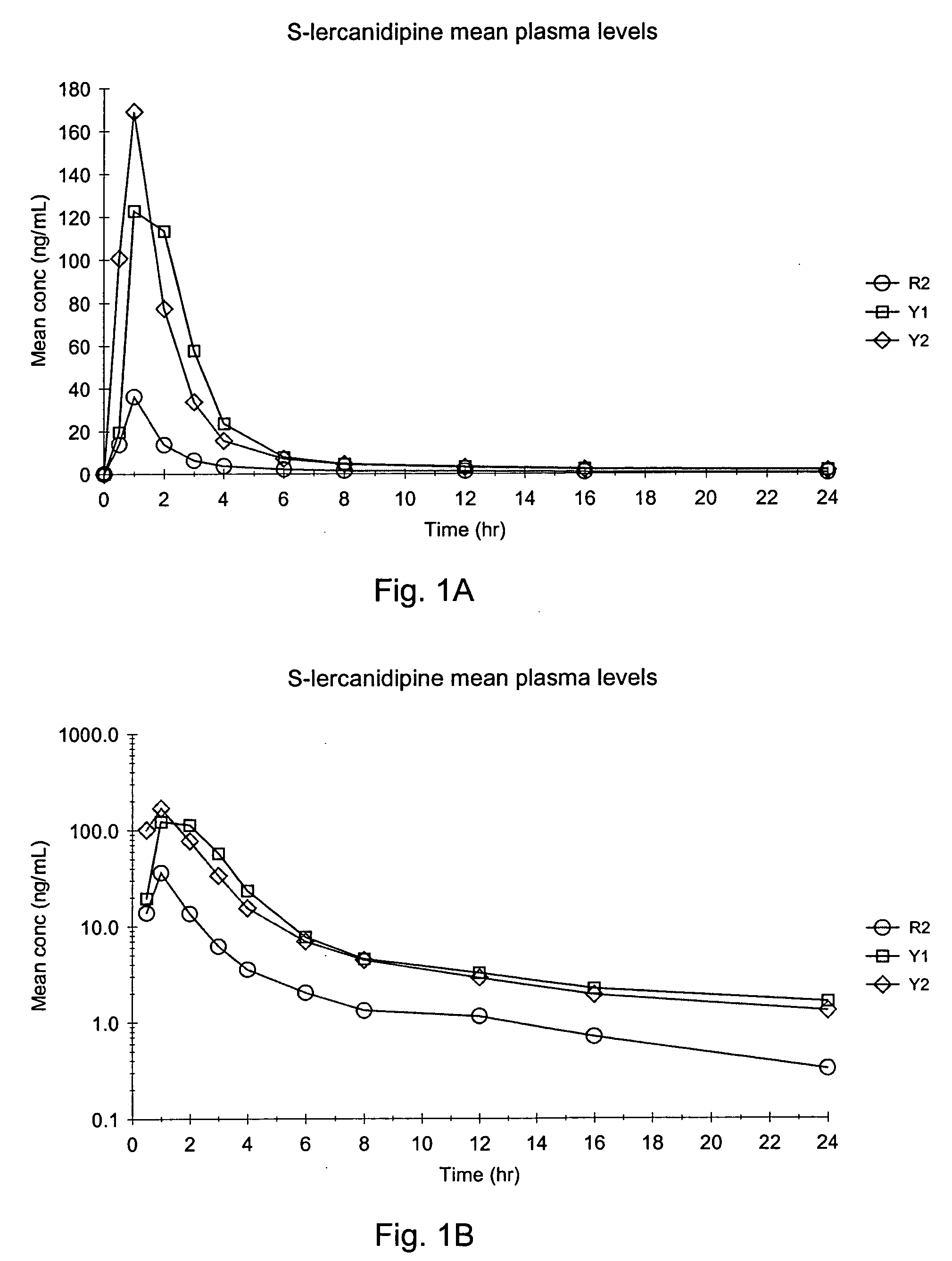
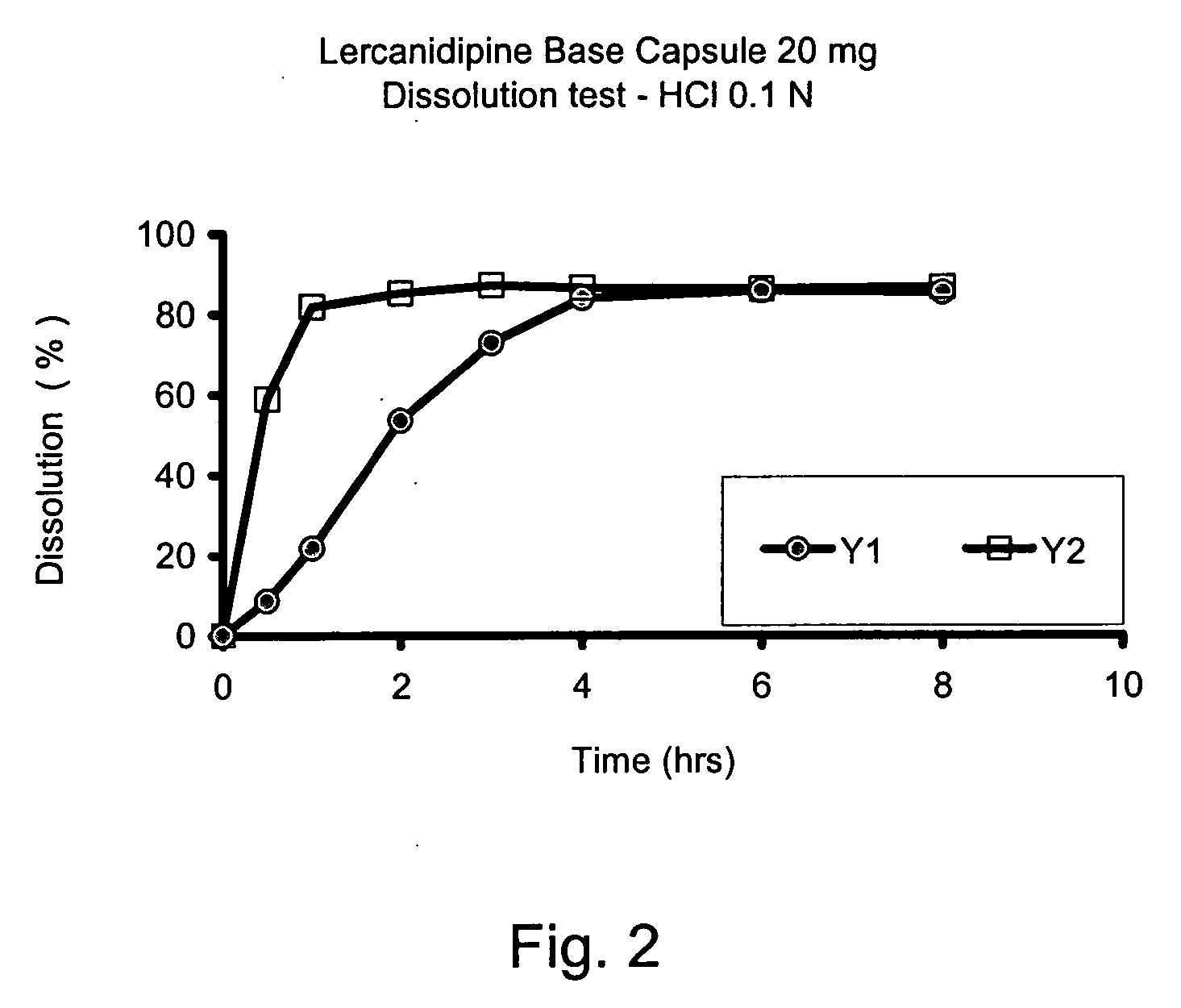
The invention provides a modified release lercanidipine pharmaceutical composition comprising at least one waxy substance and a therapeutically effective amount of lercanidipine, wherein oral administration of the modified release lercanidipine pharmaceutical composition to a patient results in a mean lercanidipine plasma concentration of greater than 0.5 ng / ml for the full time period of about 24 hours after administration of the composition to the patient.
Owner:RECORDATIE IRELAND LTD
Lercanidipine pH dependent pulsatile release compositions
Pursuant to the present invention it has been found that a modified release composition containing the low solubility and permeability drug, lercanidipine may be prepared that provides for therapeutically effective plasma concentrations of lercanidipine for 24 hours. The modified release composition of the present invention release pulses of lercanidipine based on the pH of the use environment. An effective quantity of dissolved lercanidipine is released throughout the GI Tract.
Owner:FOREST LAB HLDG LTD
Lercanidipine modified release compositions
InactiveUS20060165789A1Reduce penetrationReduce solubilityBiocidePill deliverySolubilityGastric fluid
Pursuant to the present invention, it has been found that a modified release composition containing the low permeability and poor solubility drug, lercanidipine, may be prepared which provides for therapeutically effective plasma concentrations of lercanidipine for a period of about 20 to about 25 hours. The modified release composition of the present invention provides modified release of lercanidipine independent of pH and therefore provides release of lercanidipine even upon exposure to the low pH use environments, such as gastric fluid.
Owner:FOREST LAB HLDG LTD
Compositions and methods for transdermal oxybutynin therapy
The present invention provides compositions and methods for administering oxybutynin while minimizing the incidence and or severity of adverse drug experiences associated with oxybutynin therapy. In one aspect, these compositions and methods provide a lower plasma concentration of oxybutynin metabolites, such as N-desethyloxybutynin, which is presumed to be contributing at least in part to some of the adverse drug experiences, while maintaining sufficient oxybutynin plasma concentration to benefit a subject with oxybutynin therapy. The invention also provides isomers of oxybutynin and its metabolites that meet these characteristics of minimized incidence and / or severity of adverse drug experiences, and maintenance of beneficial and effective therapy for overactive bladder. In some aspects, the composition may be presented in the form of an unoccluded or free form topically administered gel.
Owner:WATSON LAB INC
Low K dielectric surface damage control
A method of removing a silicon nitride or a nitride-based bottom etch stop layer in a copper damascene structure by etching the bottom etch stop layer using a high density, high radical concentration plasma containing fluorine and oxygen to minimize back sputtering of copper underlying the bottom etch stop layer and surface roughening of the low-k interlayer dielectric caused by the plasma.
Owner:IONIS PHARMA INC +1
Controlled release of n-acetylcysteine (NAC) for reduction of systemic and/or vascular inflammation
ActiveUS20110244045A1Reduce polarityPromote absorptionPowder deliveryBiocideDiseaseVascular inflammation
The present invention provides a controlled-release composition which provides a therapeutically effective plasma concentration of N-acetylcysteine over prolonged period of time. The present invention also includes the use of the controlled-release composition, either alone or in combination with at least one additional active agent, for reduction of vascular inflammation marker and treatment of diseases, conditions, and / or symptoms associated with systemic and / or vascular inflammation in a patient. Furthermore, the present invention provides a process of making granules comprising N-acetylcysteine, or a salt, solvate, prodrug, and / or analog thereof.
Owner:TIARA PHARMA
Pharmaceutical formulations for the sustained release of interleukins and therapeutic applications thereof
InactiveCN1925867AExtended release timeWell tolerated locallyPowder deliveryPeptide/protein ingredientsWhite blood cellBlood plasma
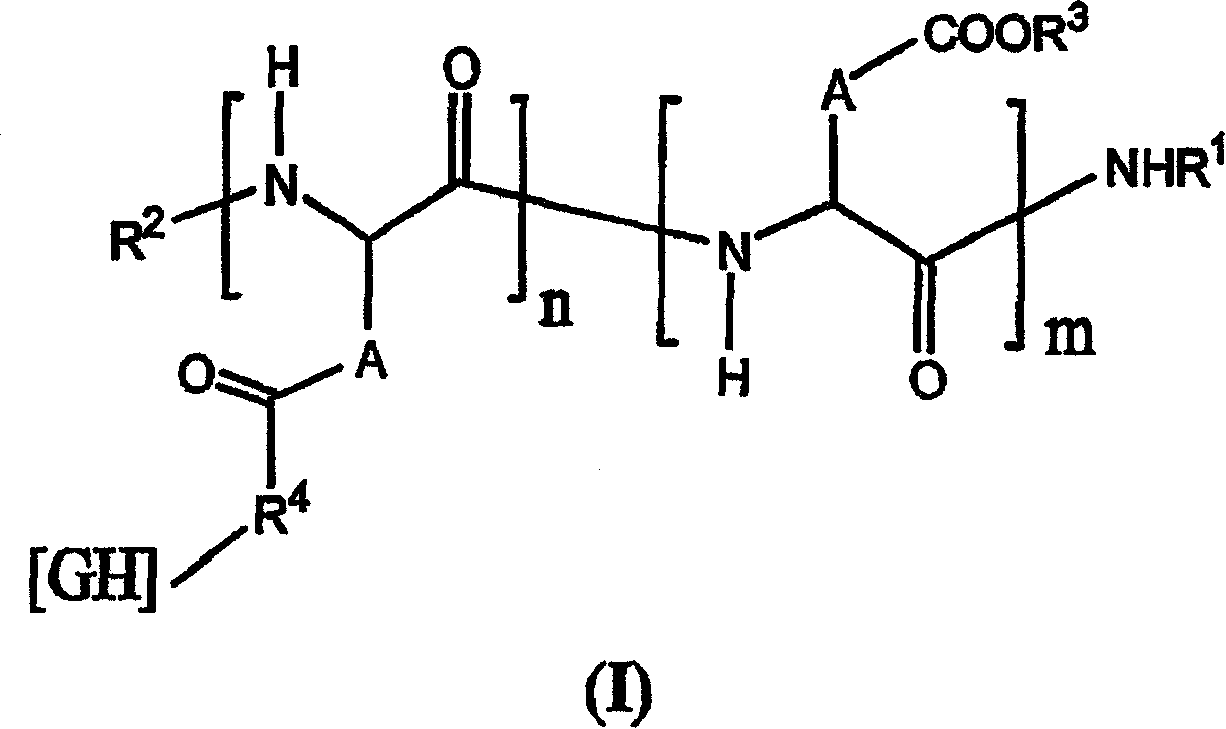
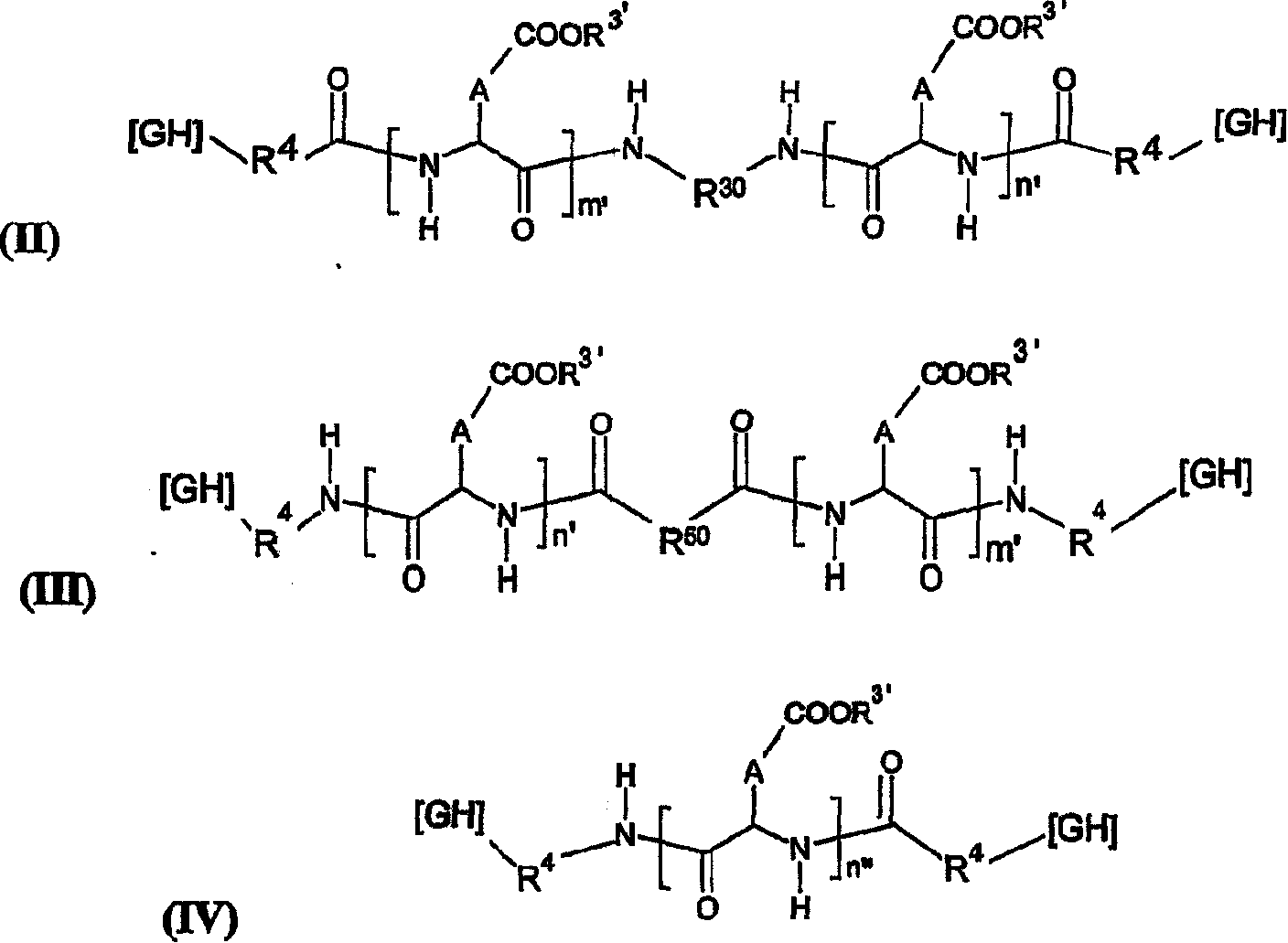
The invention relates to novel pharmaceutical formulations based on fluids and stable aqueous colloidal suspensions for the sustained release of an interleukin IL- (and one or more other optional active principles), and to the applications, particularly the therapeutic applications, of said formulations. The invention aims to provide a fluid pharmaceutical formulation for the sustained release ofinterleukin(s) (and one or more other optional active principles), such that, following parenteral injection, the in vivo IL release time is increased significantly, while the plasma concentration peak thereof is lowered. Moreover, said formulation must be storage stable and, in addition, biocompatible, non-toxic biodegradable, non-immunogenic and well tolerated locally. According to the invention, the formulation is a low-viscosity aqueous colloidal suspension of submicronic particles of water-soluble, biodegradable polymer PO bearing hydrophobic groups (GH). The aforementioned particles arenoncovalently associated with at least one interleukin (and one or more other optional active principles) and form a gelled deposit on the injection site, said gelling being caused by a protein present in the physiological medium.
Owner:FLAMEL TECHNOLOGIES
Large-air-volume low-concentration plasma exhaust gas treatment device
InactiveCN103111168AGuaranteed parallelHigh densityDispersed particle separationPlasma techniqueAir volumePlasma generator
The invention discloses a large-air-volume low-concentration plasma exhaust gas treatment device comprising a plasma shell, wherein an air inlet and an air outlet are arranged on the plasma shell; a plurality of plasma generators are arranged in at least one cross section of the cavity of the plasma shell; the plasma generators in the same cross section are connected with each other; the plasma generators are vertical to the air inlet and comprise insulating bases; air flow uniform grids are connected at the front and rear parts of the bases; a plurality layers of wire electrodes and a plurality of plate electrodes are vertically connected between the left and right plates in the cavities of the bases from top to bottom; the plate electrodes as well as the plate electrodes and the layers of wire electrodes are parallel to each other; each plate electrode is in interleave arrangement with each layer of wire electrodes and has equal space with adjacent wire electrodes; each layer of wire electrodes are uniformly arranged at intervals; each plate electrode and wire electrode are respectively connected with an outgoing wire; and the outgoing wires are respectively connected with the ground terminal and the high voltage terminal of a high voltage alternating current power supply. The large-air-volume low-concentration plasma exhaust gas treatment device disclosed by the invention is capable of obtaining relatively high gas treatment flow, maintaining high-density large-area plasma, discharging in a stable state and improving the treatment efficiency.
Owner:中维环保科技有限公司
Nanomaterials with enhanced drug delivery efficiency
ActiveUS20200214989A1Improve bioavailabilityImprove targeting and therapeutic efficacyOrganic active ingredientsPowder deliveryBlood plasmaPharmaceutical Substances
Supramolecular particle compositions based on medicinal natural products (MNPs), their synthetic analogs and derivatives, and methods to prepare and use them are provided. Five classes of MNPs and their derivatives including diterpene resin acid, phytosterol, lupane-type pentacyclic triterpene, oleanane-type pentacyclic tritepene, and lanostane-type triterpene form functional nano- or micro-structures that are stable to strong acidic environment and effectively penetrate the gastrointestinal tract. Therapeutic, prophylactic, or diagnostic agents that generally have poor intestinal permeability are converted to bioavailable forms when delivered with these supramolecular particles. Among many others, small compound chemotherapeutic agents and peptide therapeutics encapsulated therein have a much greater plasma concentration following oral administration, and effectively controls and treat symptoms associated with tumors or diabetes.
Owner:YALE UNIV
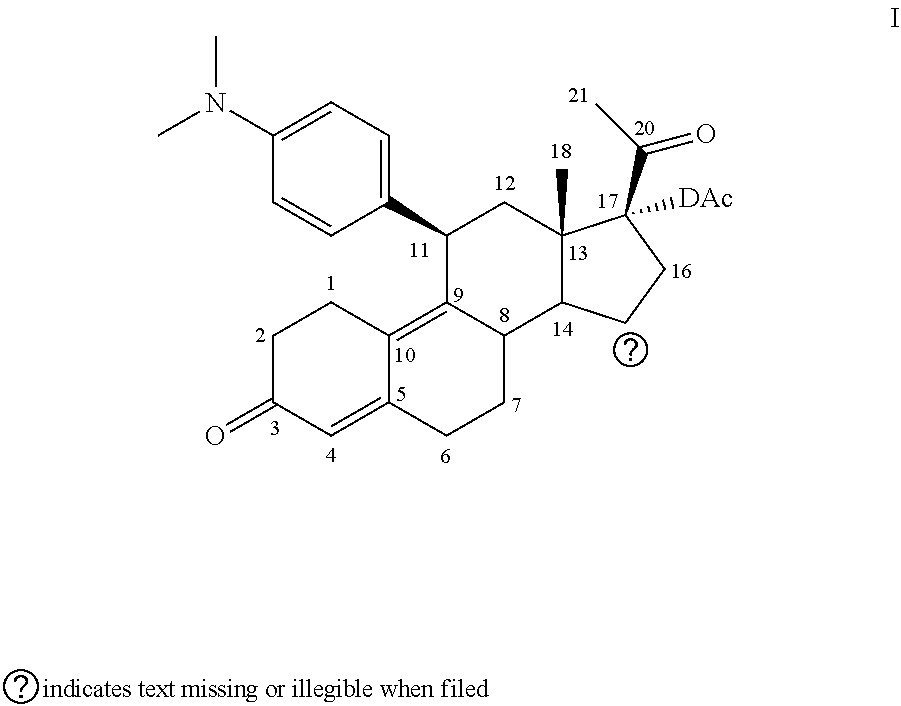
Method for using ulipristal acetate with cytochrome isozyme modulators
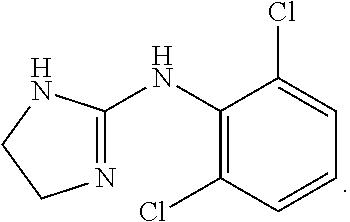
InactiveUS20120115802A1Reduce concentrationIncrease exposureBiocideCarbohydrate active ingredientsMetaboliteIsozyme
The invention relates to a method of using ulipristal acetate or a metabolite thereof for providing contraception or for treating a patient's condition, comprising providing a patient with ulipristal acetate or a metabolite thereof, and informing the patient or a medical care worker that ulipristal acetate or a metabolite thereof affects activity of a cytochrome p450 isozyme, and that administration of ulipristal acetate or a metabolite thereof with a substance that affects activity of a cytochrome p450 isozyme can affect plasma concentration, safety, efficacy or any combination thereof of ulipristal acetate or a metabolite thereof, the substance, or both.
Owner:LAB HRA PHARMA SA
Formulation of ticagrelor or pharmaceutically acceptable salt thereof
The invention relates to a formulation of ticagrelor or a pharmaceutically acceptable salt thereof. In particular, the present invention relates to an improved formulation of ticagrelor, or a pharmaceutically acceptable salt thereof, administered once a day. The present invention can achieve a plasma concentration of ticagrelor of greater than about 0.2 [mu] g / mL within 2 hours in a subject; and aplasma concentration of ticagrelor of greater than about 0.2 [mu] g / mL can still be achieved after administration in a subject for 12 hours; and generating a maximum plasma concentration (Cmax) of ticagrelor or a pharmaceutically acceptable salt thereof between about 0.2 [mu] g / mL and about 0.8 [mu] g / mL in the subject. According to the preparation of the ticagrelor or the medicinal salt thereof,the administration frequency can be reduced, so that the administration compliance of a patient can be improved, and the risk of myocardial infarction or stroke caused by acute thrombosis due to missing administration of the ticagrelor by the patient is reduced.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Plasma device for foot sterilization
ActiveCN113332585ATargeted optimizationImprove the bactericidal effectElectrotherapyMedical devicesHigh concentrationMedicine
The invention relates to the technical field of disinfection and sterilization, and discloses a plasma device for foot sterilization, which comprises a box body, a support plate is arranged in the box body, the support plate divides the inner space of the box body into a gas cavity and a sterilization cavity, and a plurality of separators are embedded in the upper surface of the support plate. According to the plasma device for foot sterilization, bacteria and fungi on the foot are concentrated on the foot sole and feet, so that the foot sole is disinfected and sterilized in an injection mode when the foot treads on the plasma device, sterilization and disinfection on the foot are achieved, good pertinence is achieved, the sterilization and disinfection effect is enhanced, ionization disinfection is carried out in the small space in the air bag, and the sterilization effect is improved. And meanwhile, the electric field in the air bag is also beneficial to maintaining the existence of plasmas, so that the plasmas generated by ionization are far larger than dissipated plasmas, high-concentration plasmas exist in the sterilization cavity and are in direct contact with the feet, and the optimal sterilization and disinfection effect is achieved.
Owner:SHENZHEN PLAAIR TECH CO LTD
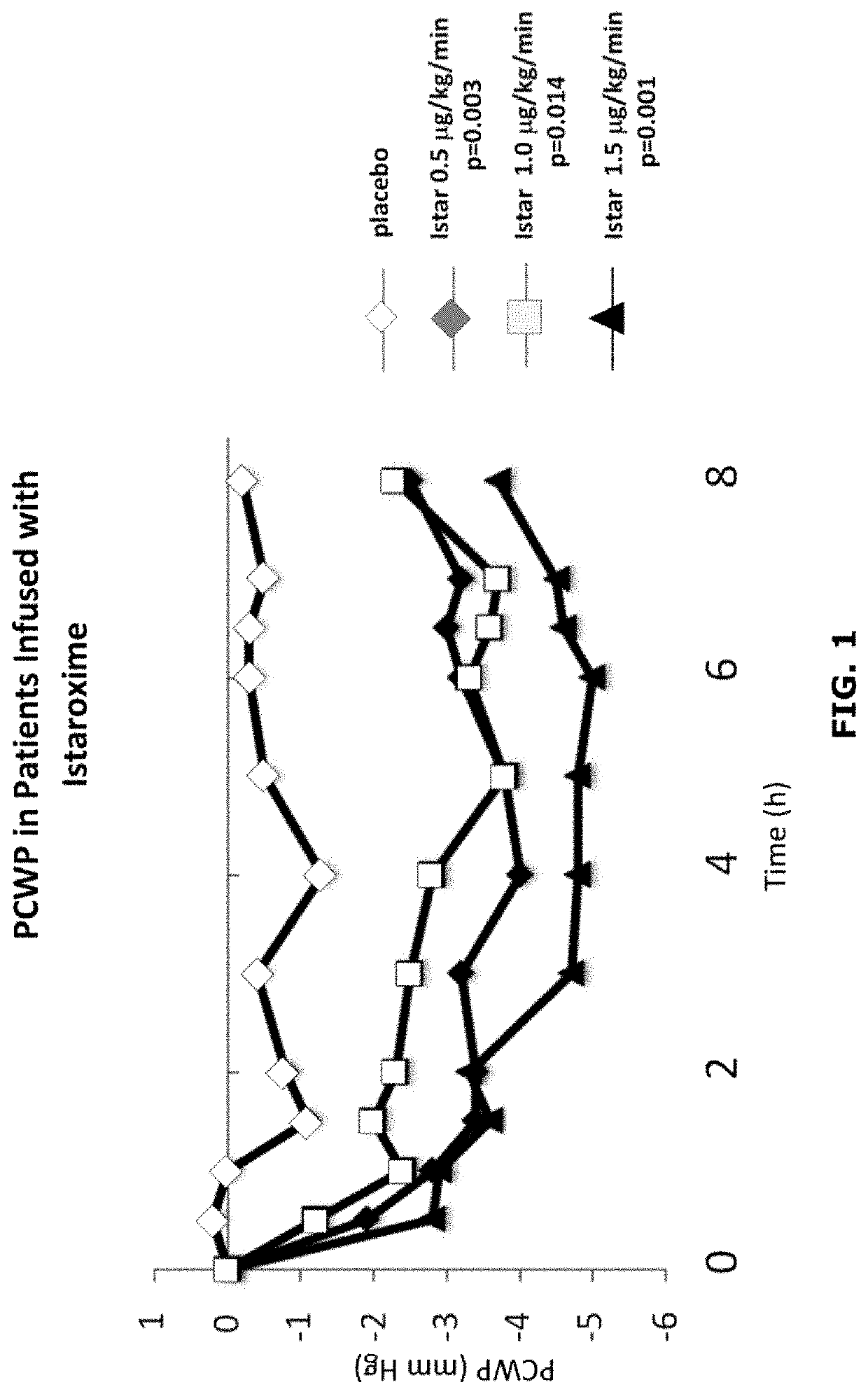
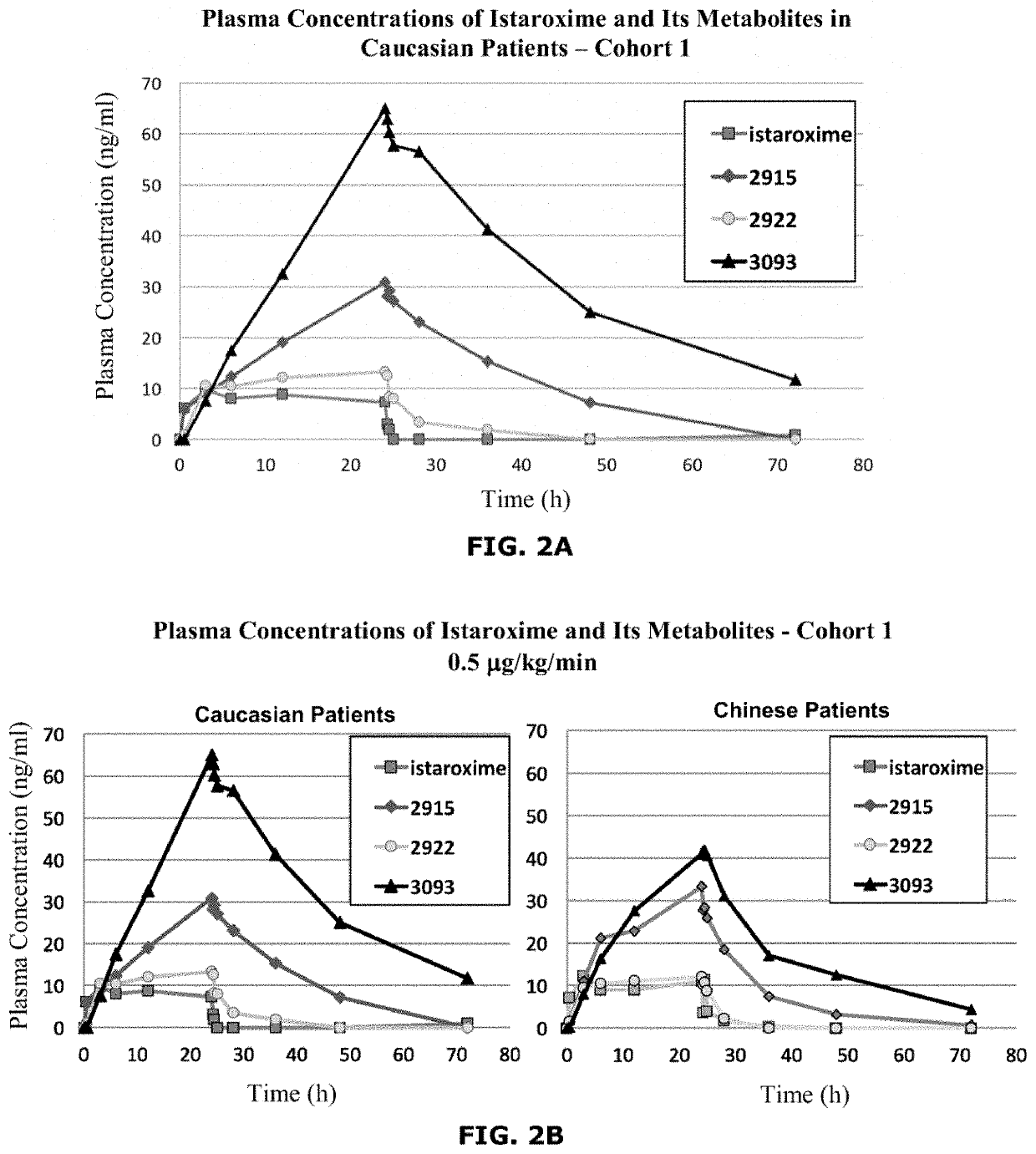
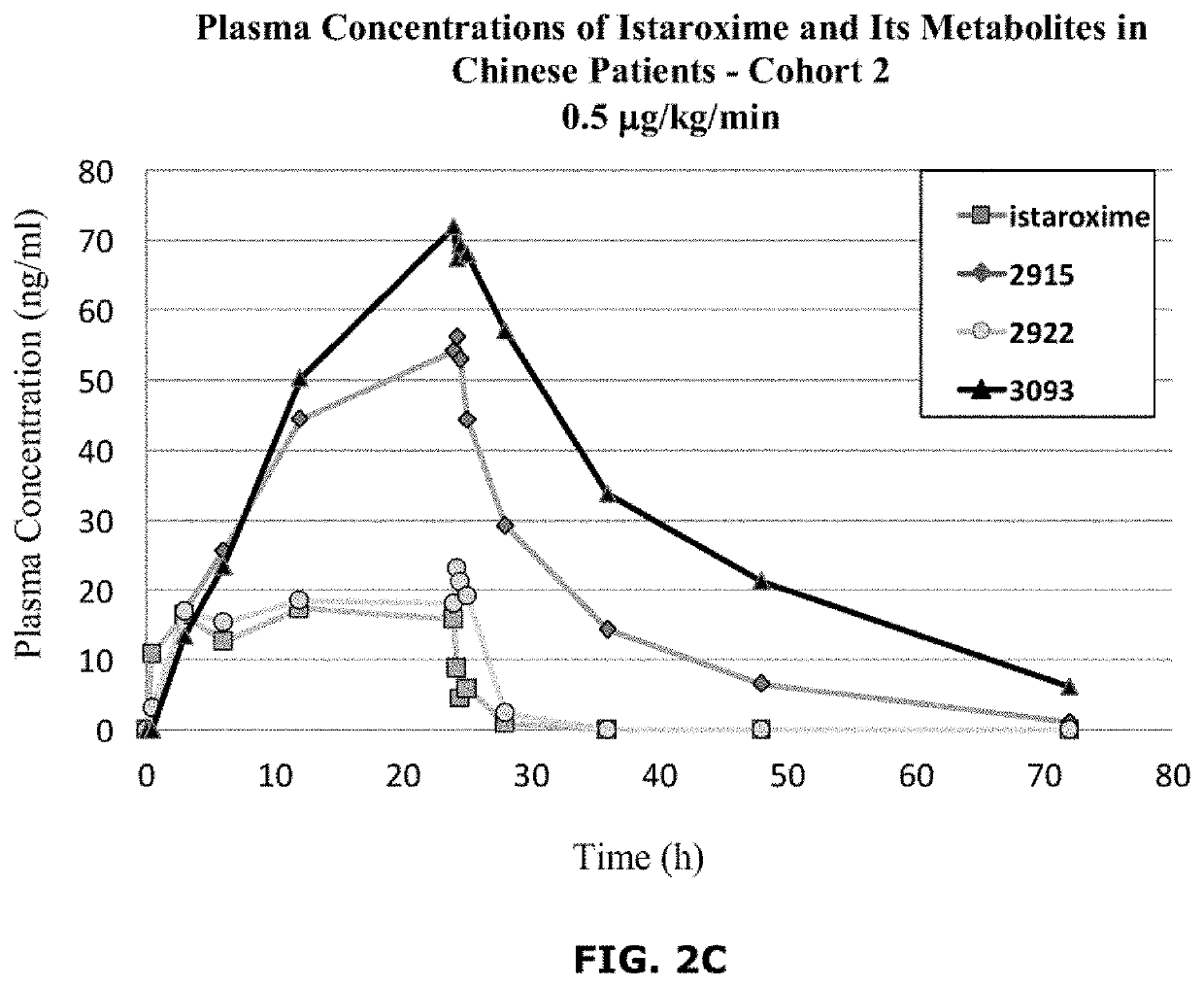
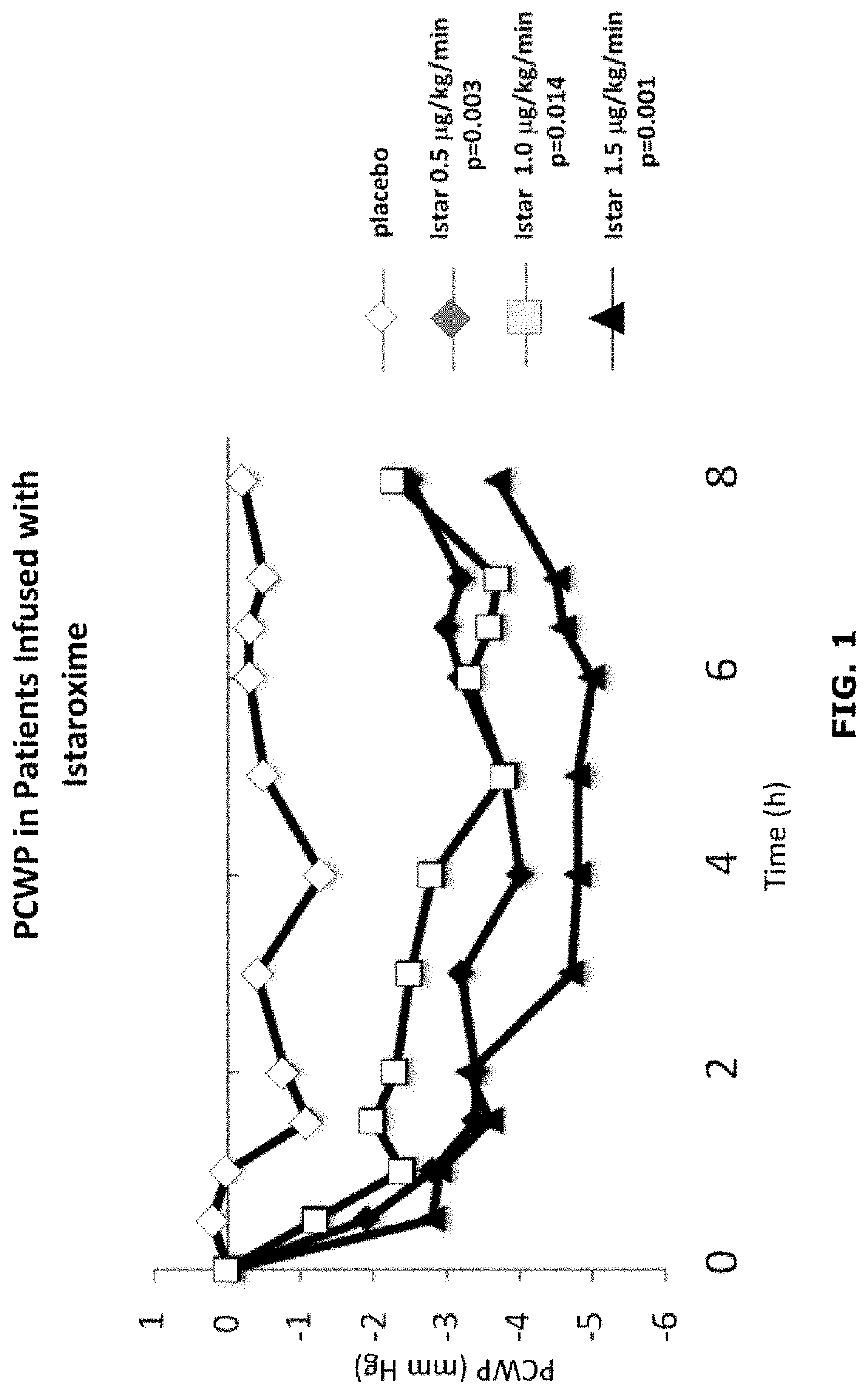
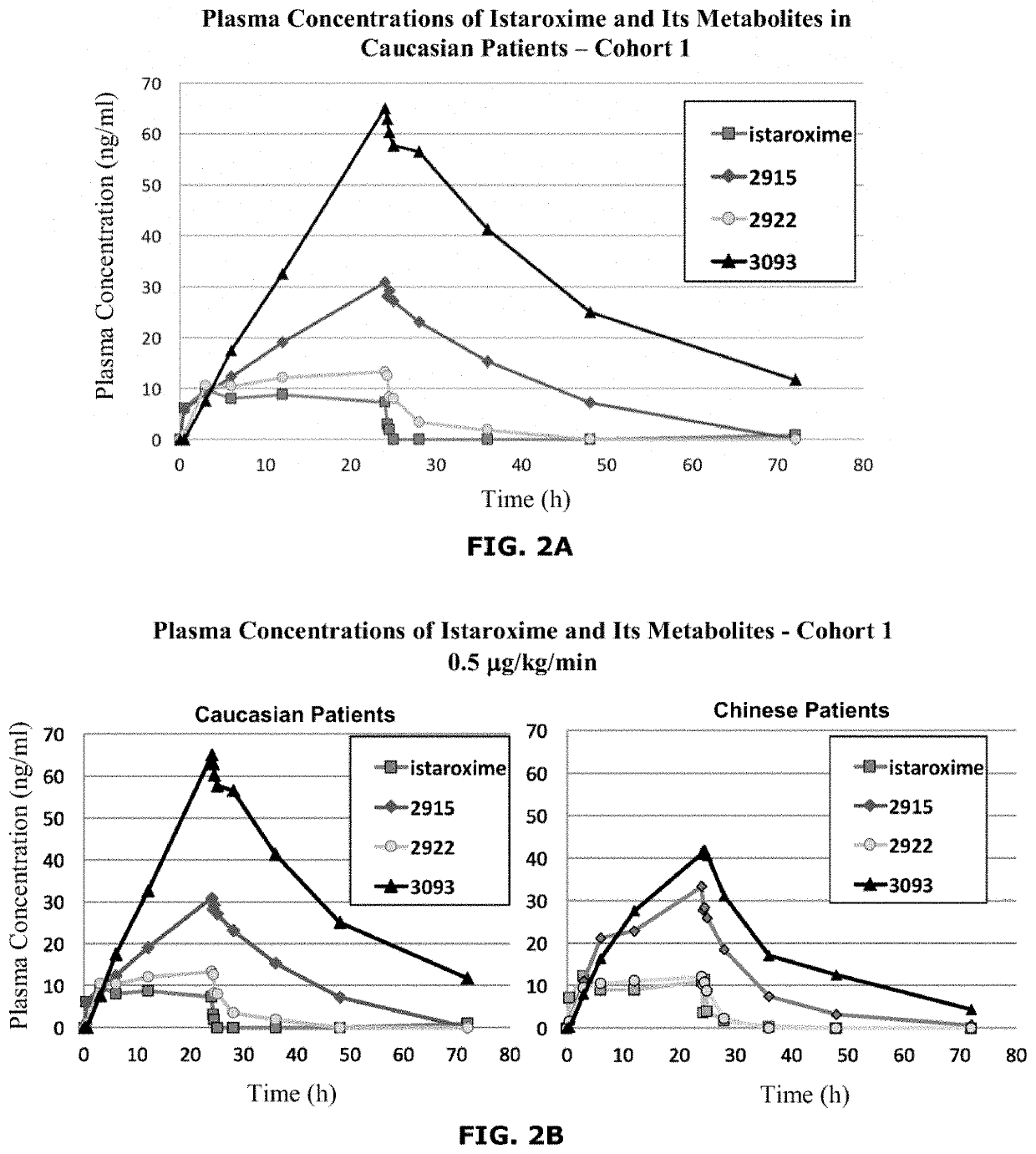
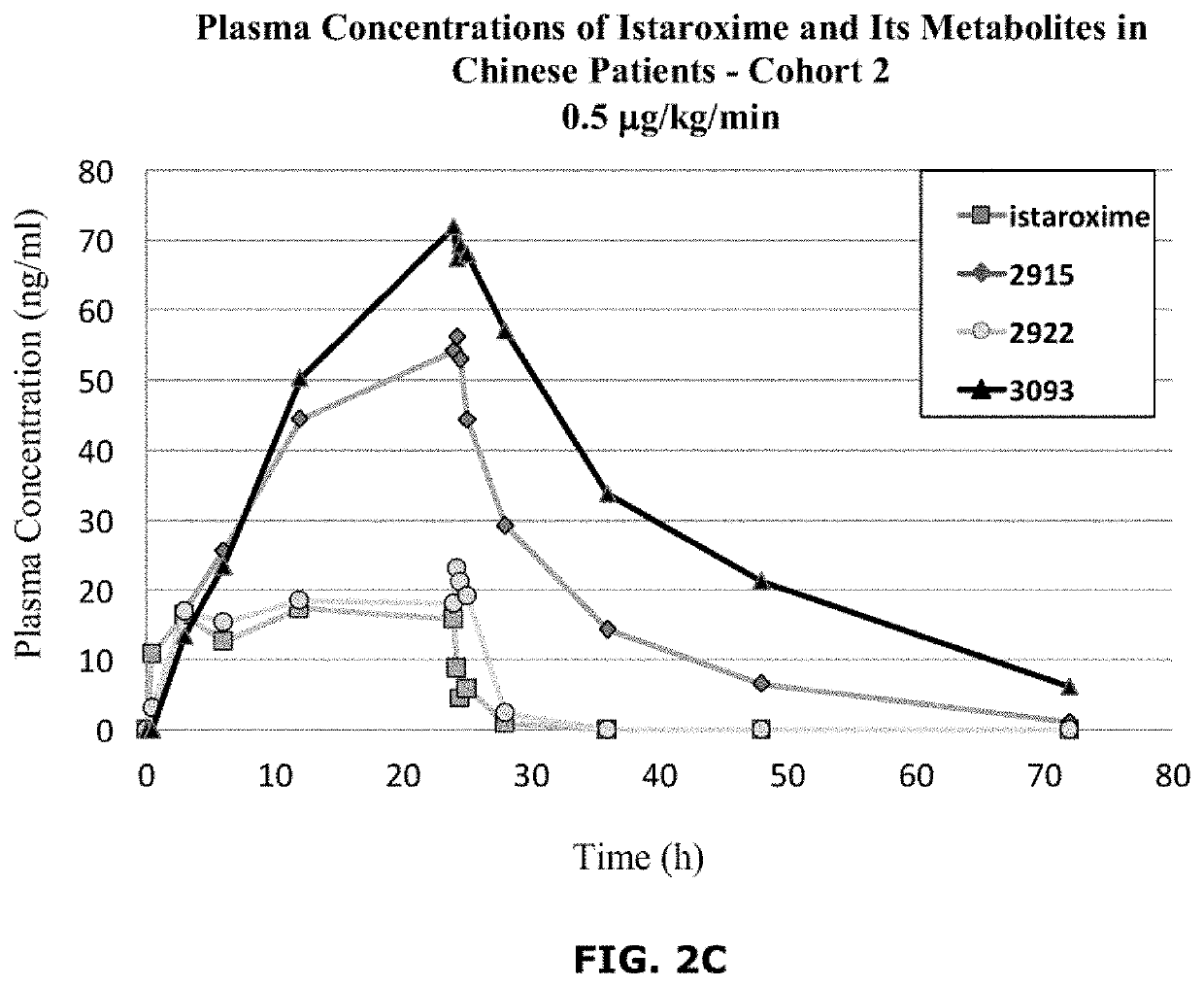
Istaroxime-containing intravenous formulation for the treatment of acute heart failure (AHF)
Compositions for intravenous infusion of istaroxime, or a metabolite of istaroxime, in human patients suffering from heart failure are disclosed. Likewise, methods for extended infusion of istaroxime or its metabolites in individuals with heart failure are disclosed. In particular, some methods disclosed herein include the infusion of istaroxime, or a metabolite thereof, for a period of time that is greater than six hours in order to improve cardiac relaxation without triggering arrhythmogenic events in an individual suffering from heart failure. Other methods include administration of istaroxime until certain plasma concentration thresholds of istaroxime metabolites are achieved. Also disclosed are istaroxime metabolites with selective SERCA2a activation.
Owner:DISCOVERY LABORATORIES INC
Istaroxime-containing intravenous formulation for the treatment of acute heart failure (AHF)
Compositions for intravenous infusion of istaroxime, or a metabolite of istaroxime, in human patients suffering from heart failure are disclosed. Likewise, methods for extended infusion of istaroxime or its metabolites in individuals with heart failure are disclosed. In particular, some methods disclosed herein include the infusion of istaroxime, or a metabolite thereof, for a period of time that is greater than six hours in order to improve cardiac relaxation without triggering arrhythmogenic events in an individual suffering from heart failure. Other methods include administration of istaroxime until certain plasma concentration thresholds of istaroxime metabolites are achieved. Also disclosed are istaroxime metabolites with selective SERCA2a activation.
Owner:DISCOVERY LABORATORIES INC
Olaparib oral sustained and controlled release pharmaceutical composition and uses thereof
InactiveUS20200108008A1Accurate in vivo plasma concentrationImprove the level ofOrganic active ingredientsPill deliveryControl releaseEnzyme inhibition
An olaparib oral sustained and controlled release pharmaceutical composition contains an olaparib in an improved dissolution form and a release rate adjusting matrix polymer. The pharmaceutical composition has controllable in-vivo absorption behavior, plasma concentration and PARP enzyme inhibition level, and improved drug load and / or oral absorption and / or bioavailability and / or plasma concentration control and / or enzyme inhibition level control of olaparib, and can be used as the only preparation or in combination with other therapies to treat a cancer.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com