Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
93 results about "Drug therapy treatment" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Drug therapy is available for detoxification from barbiturates, alcohol, opiates, benzodiazepine, mood stabilizers, anti-depressants, nicotine and other seditives. To treat opiate addiction, some drug treatment centers administer effective and safe medications such as buprenorphine, naltrexone, Subutex and methadone.
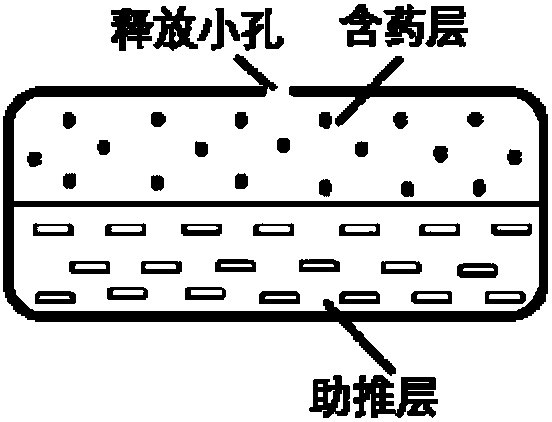
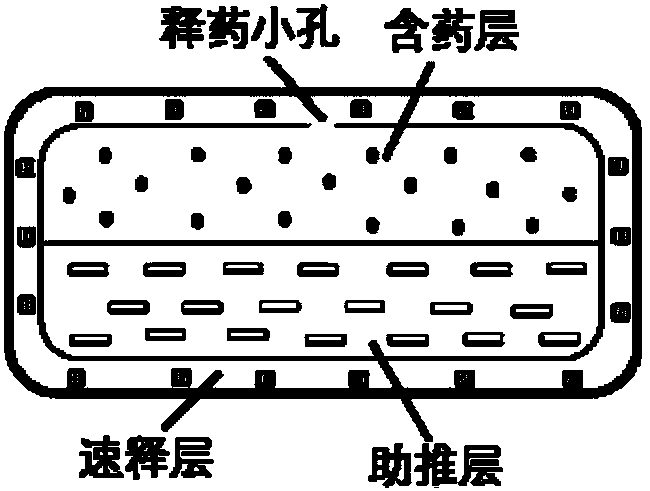
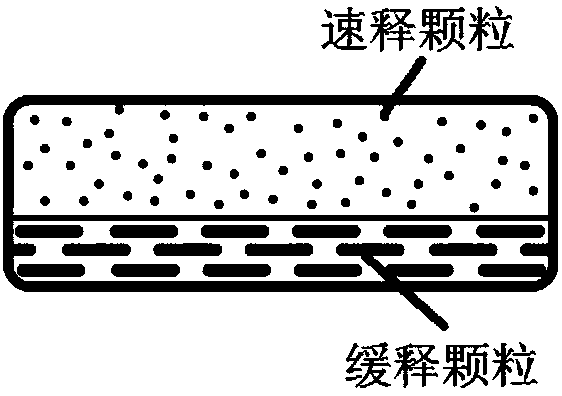
Dosage form containing multiple drugs
A pharmaceutical dosage form comprising a first drug and a second drug, both of which are selected from decongestants, antitussives, expectorants, analgesics and antihistamines. The dosage form provides a plasma concentration within a therapeutic range of the second drug over a period which is coextensive with at least about 70% of a period over which the dosage form provides a plasma concentration within a therapeutic range of the first drug. This Abstract is neither intended to define the invention disclosed in this specification nor intended to limit the scope of the invention in any way.
Owner:SOVEREIGN PHARMA
Dosage form containing a morphine derivative and another drug
InactiveUS20050232987A1Extended maintenance periodBiocidePill deliveryPharmaceutical drugBlood plasma
A pharmaceutical dosage form which comprises a first drug which comprises at least one morphine derivative with antitussive activity and at least one second drug. The dosage form provides a plasma concentration within the therapeutic range of the at least one second drug over a period which is coextensive with at least about 70% of the period over which the dosage form provides a plasma concentration within the therapeutic range of the first drug. This abstract is neither intended to define the invention disclosed in this specification nor intended to limit the scope of the invention in any way.
Owner:SOVEREIGN PHARMA
Drug treatment for restless leg syndrome
A method for the treatment of Restless Leg Syndrome (RLS), which comprises administering an alpha2-agonist and a second agent selected from the group consisting of the dopamine agonists, opioids, benzodiazepines and the combination of L-DOPA plus a decarboxylase inhibitor.
Owner:BRECHT HANS MICHAEL
Anti-cancer activity augmentation compounds and formulations and methods of use thereof
InactiveUS20070219268A1Strong cytotoxicityReduce capacityBiocideHeavy metal active ingredientsMedicineOxidative stress
The field of the present invention comprises pharmaceuticals and pharmaceutical treatments, including, for example, (i) compounds and formulations which cause the augmentation of anti-cancer activity (i.e., by enhancement of the lethal cytotoxic action in stimulatory [inducing oxidative stress] and / or depletive [decreasing anti-oxidative capacity] manner) of chemotherapeutic agents, in a selective manner; (ii) methods of administering said anti-cancer augmentation compounds and formulations; (iii) delivery devices containing said anti-cancer augmentation compounds and formulations; and (iv) methods of using said anti-cancer augmentation compounds, formulations, and devices to treat subjects in need thereof.
Owner:BIONUMERIK PHARMA INC
Nanoparticulate delivery systems for treating multi-drug resistance
InactiveUS20060257493A1Increased endogenous productionPrevent degradationBiocideOrganic active ingredientsDelivery vehicleNanoparticle
An encapsulated delivery system, and, in particular, a nanoparticulate delivery system representing a qualitatively different approach to overcoming multi-drug resistance while simultaneously administering the chosen drug treatment to a patient, e.g., in a site-specific manner, is disclosed. A composition according to the invention includes a therapeutically effective amount of one or more multi-drug resistance reversing agents selected from the group consisting of ceramide and ceramide modulators; and a therapeutically effective amount of a therapeutic agent, wherein the therapeutic agent is different from the one or more multi-drug resistance reversing agents, and the one or more multi-drug resistance reversing agents and the therapeutic agent are encapsulated, preferably co-encapsulated, in a biocompatible, biodegradable delivery vehicle for delivery to a patient in need of treatment, for example, for specific localization at, or higher probability of delivery to, a treatment site in a patient administered the composition. Preferably, the one or more multi-drug resistance reversing agents are ceramide, paclitaxel or tamoxifen.
Owner:NORTHEASTERN UNIV
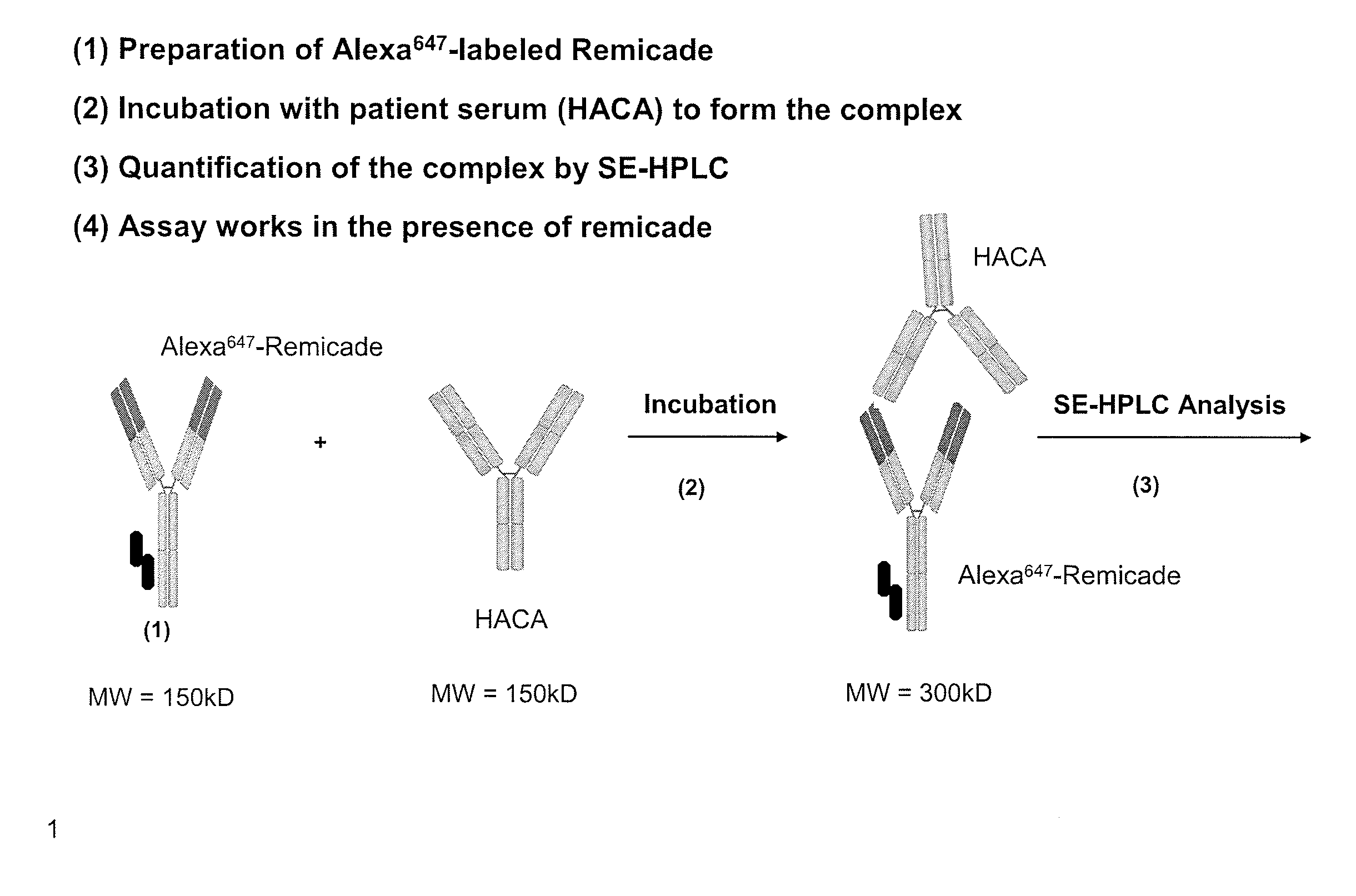
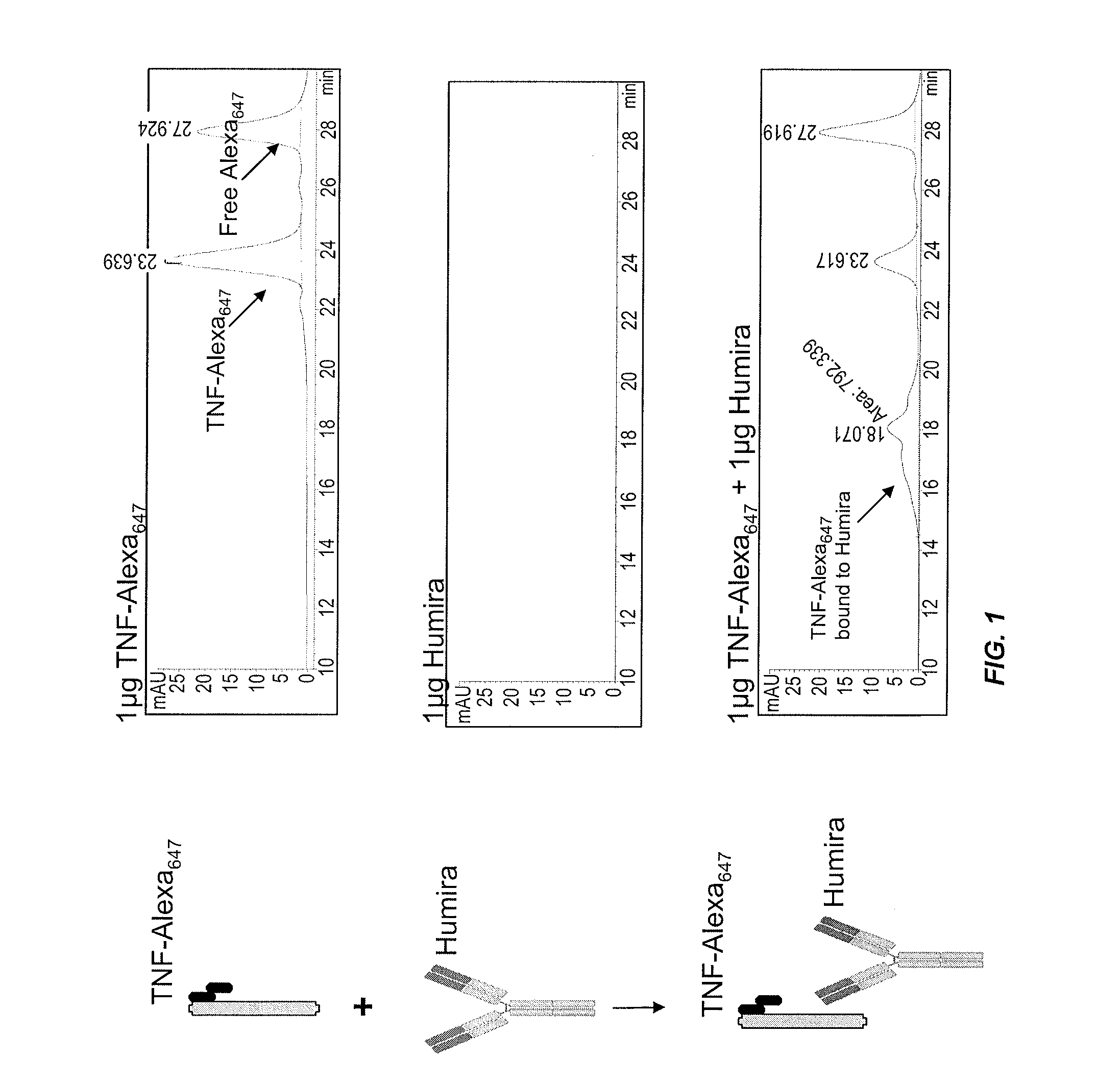
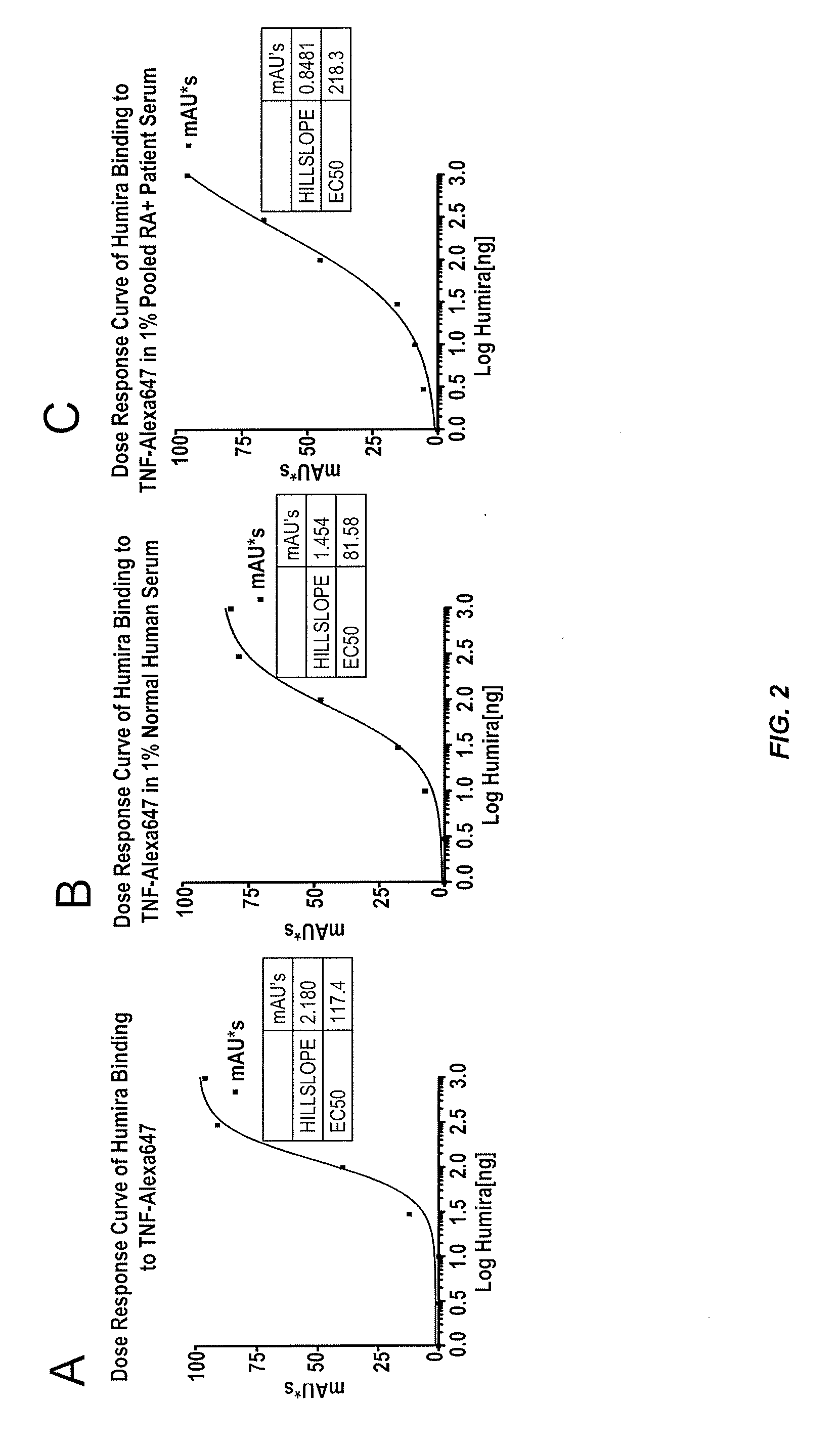
Assays for detecting autoantibodies to Anti-tnfalpha drugs
InactiveUS20140045276A1Low toxicityConvenient treatmentDisease diagnosisBiological testingDiseaseMedicine
The present invention provides assays for detecting and measuring the presence or level of autoantibodies to anti-TNFα drug therapeutics in a sample. The present invention is useful for optimizing therapy and monitoring patients receiving anti-TNFα drug therapeutics to detect the presence or level of autoantibodies against the drug. The present invention also provides methods for selecting therapy, optimizing therapy, and / or reducing toxicity in subjects receiving anti-TNFα drugs for the treatment of TNFα-mediated disease or disorders.
Owner:NESTEC SA +1
Gpc3-targeting drug which is administered to patient responsive to gpc3-targeting drug therapy
ActiveUS20150285806A1Good effectFavorable treatment of cancerDigestive systemImmunoglobulins against cell receptors/antigens/surface-determinantsContinuationDrug regimen
The present invention discloses a method for determining the efficacy of GPC3-targeting drug therapy for cancer in a patient before the start of GPC3-targeting drug therapy or a patient or determining the continuation of GPC3-targeting drug therapy for a patient, including monitoring a concentration of free GPC3 in a biological sample isolated from the patient before the start of GPC3-targeting drug therapy and / or the patient treated with the GPC3-targeting drug therapy, wherein when the concentration of free GPC3 is a predetermined value, the efficacy of the GPC3-targeting drug therapy is determined or the continuation of the GPC3-targeting drug therapy is determined. The present invention also discloses a GPC3-targeting drug or a preparation which is to be further administered to a patient for which the efficacy of the GPC3-targeting drug therapy has been determined or the continuation of the GPC3-targeting drug therapy has been determined.
Owner:CHUGAI PHARMA CO LTD +1
Drug-loaded silicone grease ultrasonic contrast agent and its preparation method and use
InactiveCN104826140AThe synthesis process is simpleStable in vivo and in vitroEnergy modified materialsEchographic/ultrasound-imaging preparationsUltrasound contrast mediaLiquid fluorine
The invention provides a preparation method of a drug-loaded silicone grease ultrasonic contrast agent. The preparation method comprises fully dissolving silicone grease, polyethylene glycol phosphate derivatives and a fat-soluble anticancer drug in an organic solvent, adding water into the solution, carrying out whirling, removing the organic solvent, supplying water with a volume 3 times that of the solution into the solution, carrying out ultrasonic disintegration by an ultrasonic disintegrator, simultaneously and fast adding liquid fluorocarbon into the solution to obtain a pre-emulsion, and carrying out dialysis on the pre-emulsion in PBS for 1h to obtain a product. The preparation method has simple processes. The product has the average particle size of about 100nm and is conducive to aggregation at a tumor position by internal EPR effects. Siloxane silicone grease network distribution substantially improves structure stability and prolongs internal blood circulation time. Through HIFU, the drug-loaded silicone grease ultrasonic contrast agent release drugs at a local part of tumor tissue, drug treatment targeting is improved, HIFU treatment effects are substantially improved and HIFU-chemotherapy combined treatment is realized.
Owner:SUN YAT SEN UNIV
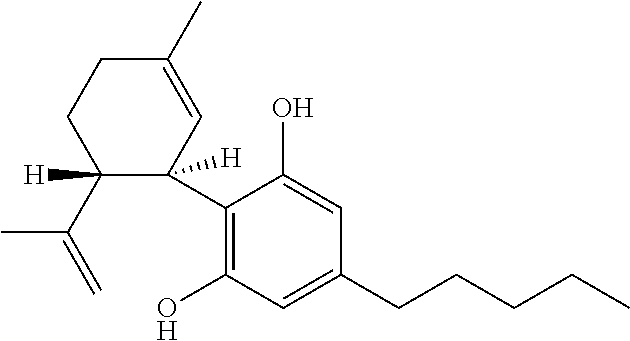
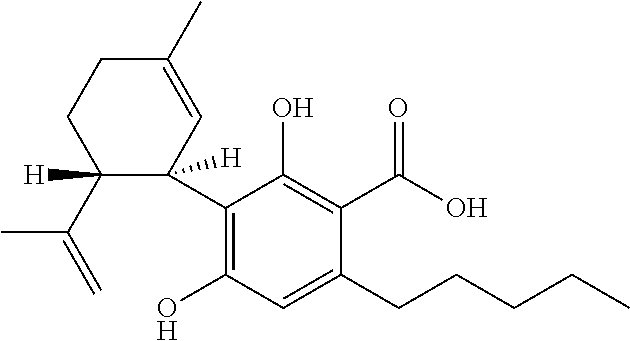
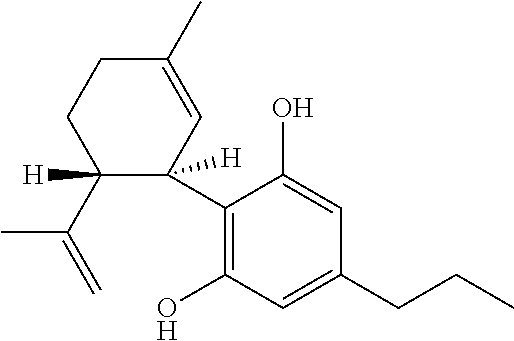
Use of cannabinoids in the treatment of seizures as associated with lennox-gastaut syndrome
InactiveUS20210169824A1Nervous disorderHydroxy compound active ingredientsAntiepileptic drugSeizure frequency
The present invention relates to the use of cannabidiol (CBD) in the treatment of patients with Lennox-Gastaut syndrome (LGS) who are deemed to be treatment failures on their existing medication. In particular the use of CBD was found to provide a statistically significant reduction in both drop seizures and total seizure frequency in patients who have tried and failed anti-epileptic drugs (AEDs) or those who were currently taking AEDs but have uncontrolled seizures. Preferably the AEDs which have been shown to be treatment failures are one or more of rufinamide, lamotrigine, topiramate and / or felbamate. Preferably the CBD used is in the form of a highly purified extract of cannabis such that the CBD is present at greater than 98% of the total extract (w / w) and the other components of the extract are characterised. In particular the cannabinoid tetrahydrocannabinol (THC) has been substantially removed, to a level of not more than 0.15% (w / w) and the propyl analogue of CBD, cannabidivarin, (CBDV) is present in amounts of up to 1%. Alternatively, the CBD may be a synthetically produced CBD.
Owner:GW RES LTD
Traditional Chinese medicine for curing hepatic depression syndrome type melancholia
InactiveCN104258310ACompatibility scienceSimple methodNervous disorderDispersion deliverySide effectPharmaceutical drug
The invention relates to a traditional Chinese medicine for curing hepatic depression syndrome type melancholia. The traditional Chinese medicine for curing hepatic depression syndrome type melancholia can effectively solve the problems of long drug treatment cycle, low curing rate, poor effect and side effects in conventional drug treatment. The traditional Chinese medicine for curing hepatic depression syndrome type melancholia comprises the following crude drugs by weight: 6-10g albizia flower, 6-10g rose, 6-10g Armeniaca mume, 10-12g radix bupleuri, 15-20g radix paeoniae alba, 15-25g radix curcumae, 10-12g rhizoma cyperi, 10-15g Poria cocos Schw. and 12-16g ligusticum chuanxiong, the crude drugs are mixed and then added with water and immersed, soaked for 30-40 min and boiled for twice, during the first time of boiling, strong fire is adopted firstly, then slow fire is adopted for boiling for 30-40 minutes, then filtering is performed, and a first-time decoction is obtained; water is added into dregs of the decoction to immerse the dregs, strong fire is used for boiling, then slowing fire is used for boiling for 20-30 min, filtering is performed, a second-time decoction is obtained, and then the first-time decoction and the second time-decoction are combined together, so that the traditional Chinese medicine can be obtained. The traditional Chinese medicine provided by the invention is scientific in compatibility, the method is simple, the curing cycle is short, the cure rate is high, the effects are good, no side effect can be caused, and traditional Chinese medicine for curing hepatic depression syndrome type melancholia represents a great creation in the traditional Chinese medicines.
Owner:HENAN UNIV OF CHINESE MEDICINE
Pharmaceutical composition for treating hypertension and application thereof
InactiveCN103920025AGood treatment effectNo side effectsHeavy metal active ingredientsMetabolism disorderBelamcanda chinensisEUCOMMIA ULMOIDES BARK
The invention discloses a pharmaceutical composition for treating hypertension. The pharmaceutical composition consists of the following medicinal materials by weight: semen plantaginis, rhizoma alismatis, ginkgo leaf, chrysanthemum, blackberry lily, gastrodia elata, semen cassia, rhynohophylla, concha haliotidis, nacre mother of pearl, leech, seaweed, herba siegesbeckiae, root of kudzu vine, hawthorn, parasitic loranthus, feather cockscomb seed, apocynum venetum, fructus viticis, eucommia ulmoides, root bark of white mulberry, dried rehmannia root, achyranthes root, earthworm, oyster, pulp of dogwood fruit, kelp, lucid ganoderma, magnet, selfheal, corydalis amabilis and cinnamon. The pharmaceutical composition disclosed by the invention is obvious in drug treatment effect, free of side effect, does not produce drug dependence, and is low in treatment cost. And moreover, the pharmaceutical composition has certain treatment effect on hyperglycemia, high cholesterol and hyperlipidemia ropiness while being used for treating the hypertension.
Owner:陈永奇
Method for preparing medicine targeted controlled-release nano eyedrop
InactiveCN101209241AProlong the action timeSustained constant releasePowder deliverySenses disorderHigh concentrationSide effect
The invention discloses a preparation method for drug targeting controlled release nano-particle eye drops, which pertains to the preparation methods of eye preparations. The invention firstly dissolves certain portions by weight of drug and biodegradable polymer in organic solvent so as to obtain solution A; additionally, certain portions by weight of cholesterol modified chitosan is dissolved in water, and solution B is obtained by stirring and rotating; the two are mixed and stirred till the organic solvent is fully volatilized; the drug-carrying polymer nanoparticles are obtained by centrifugal separation, water washing and drying; and then the eye drops are prepared by the general preparation method of the industry of eye drops. The eye drops which are prepared by the method of the invention can ensure the effective drug treatment concentration without the need of frequent and high-concentration drug delivery, can significantly prolong the eye surface acting time of the drug and release the drug continuously and constantly, the eye surface retention time of the drug-carrying particles of the eye drops can be up to six hours, the efficacy is improved and the side effects of the drug are reduced simultaneously.
Owner:天津医科大学眼科中心
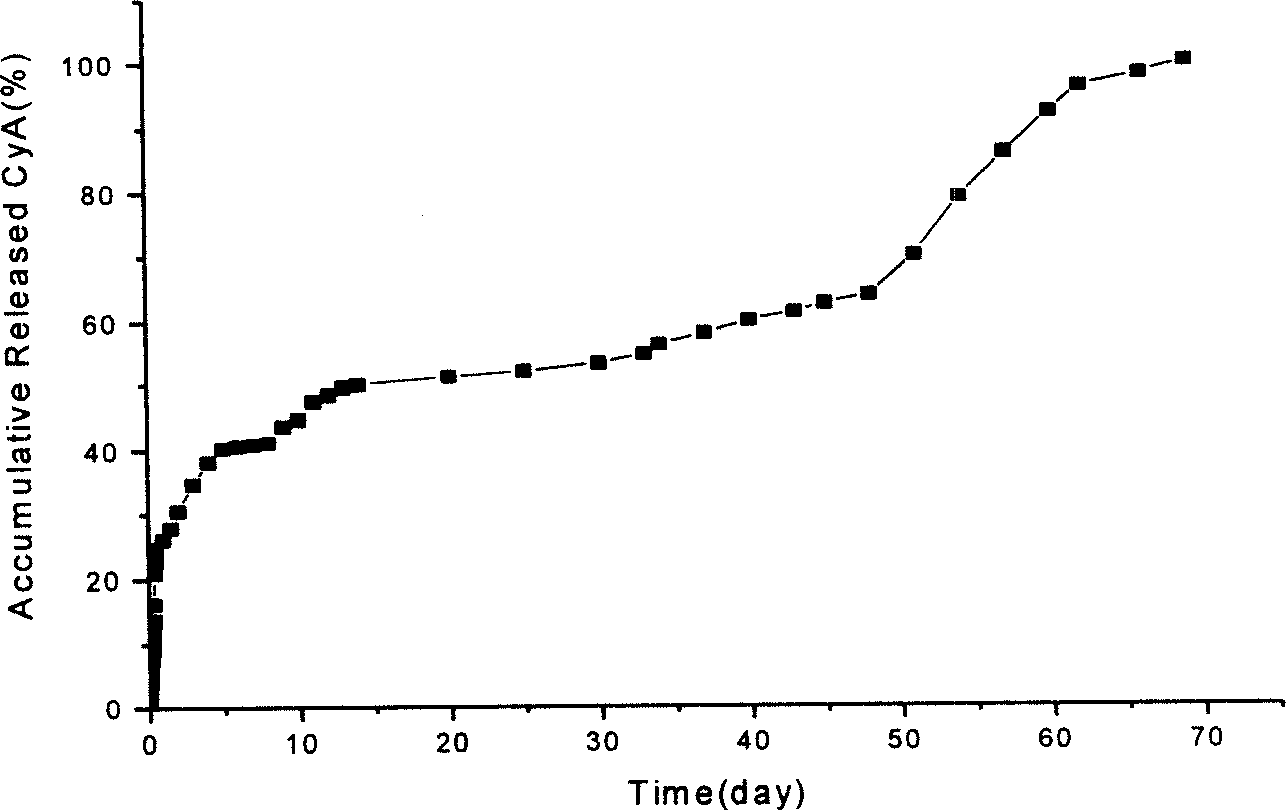
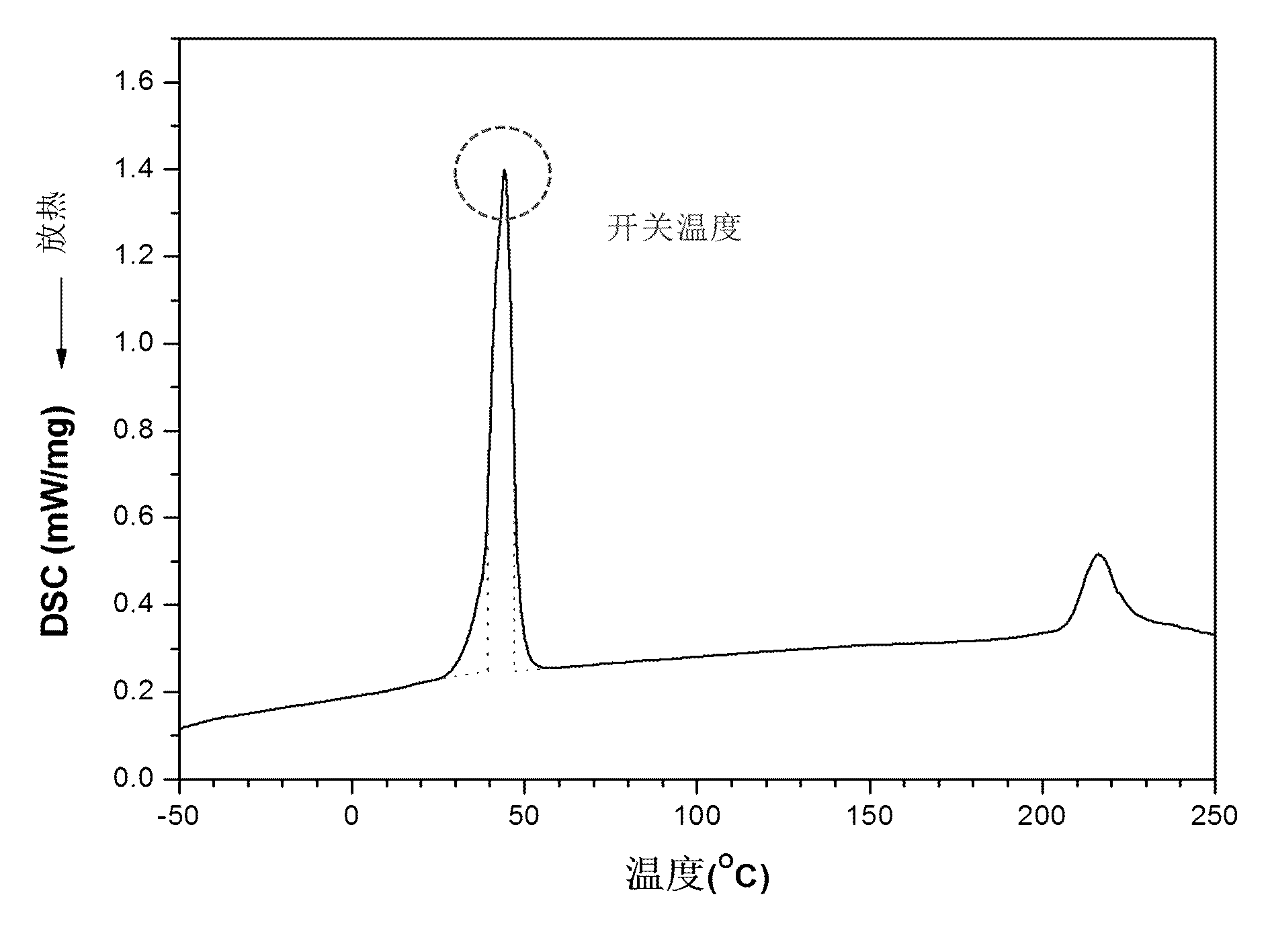
Capsule polyurethane drug controlled release body with temperature control switch, and preparation method thereof
InactiveCN103223173AAchieve releaseAchieve closurePharmaceutical non-active ingredientsPharmaceutical active ingredientsControl releaseMedicine
The invention discloses a capsule polyurethane drug controlled release body with a temperature control switch. The capsule polyurethane drug controlled release body is prepared by loading the drug by a temperature-sensitive polyurethane capsule pipe. In the application of the capsule polyurethane drug controlled release body, a drug release characteristic with temperature response is expressed; and an 'open-close' effect is present. The capsule wall polyurethane molecules are in a rigid crystalline state below the switch temperature, so that diffusion and release of the drug in the controlled release body are blocked; when the temperature rises to higher than the switch temperature, the polyurethane molecules are in a molten state, so that the drug in the body migrates to the capsule wall and the capsule wall drug release outward continuously, with the concentration as a mass ladder force. Besides, the special design of the capsule wall can realize ideal drug diffusion capacity. The invention also discloses a preparation method of the capsule polyurethane drug controlled release body. The temperature-sensitive polyurethane capsule pipe of the drug controlled release body and unsymmetrical structure of the capsule wall design makes the drug controlled release body have fast temperature response speed, good repeatability of the drug release switch, freely adjustable drug release amount, capacity of uniform drug release, no physiological toxicity, so that the drug controlled release body has wide application prospects in clinic local drug treatment.
Owner:SICHUAN UNIV
Drug carrier and system for combined medication
ActiveCN110237373AEasy to useAvoid chemical reactionsMedical devicesInhalatorsChemical reactionMedical product
The invention relates to a medical product, and provides a drug carrier for combined medication. The drug carrier includes a separable base member and a cover member, the separable base member is provided with a plurality of spaced apart cavity bags thereon, the cover member hermetically covers the base member, each cavity bag stores a single-dose pharmaceutical preparation, and two or more single-dose preparations containing different active ingredients are periodically stored in the cavity bags. According to the invention, aiming at different patient groups, in particular to patients who need to be treated with the relieving drugs according to requirements while using the controlling drugs, two or more products are combined into a whole into a delivery device, so that the medication compliance of the patients is improved, and the potential chemical reaction of different drugs in the storage process is avoided.
Owner:RESPIRENT PHARM CO LTD
Construction and application of endoplasmic reticulum targeted nano drug delivery system
ActiveCN112472822APromote accumulationGood treatment effectMaterial nanotechnologyAntipyreticLysosomeReticulum cell
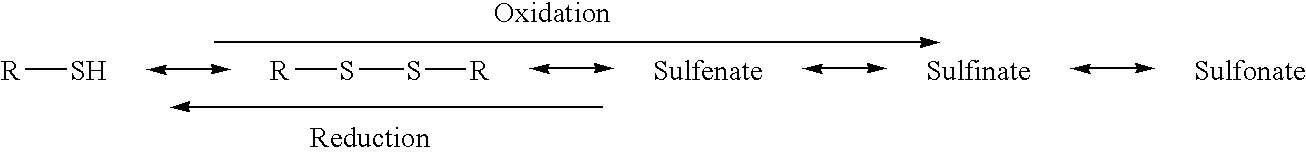
The invention provides construction and application of an endoplasmic reticulum targeted nano drug delivery system. By modifying sulfamide or sulfonylurea compounds with endoplasmic reticulum tendencyinto endoplasmic reticulum targeting nano-compounds constructed in a nano-carrier, the constructed novel endoplasmic reticulum targeting liposome can load water-soluble and fat-soluble drugs and quickly transfer the drugs to the endoplasmic reticulum part, so that accumulation of the drugs at the action part of the liposome is improved, and the bioavailability of the drugs is improved. The medicine treatment effect is enhanced, and the toxic and side effect is weakened; the delivery of nucleic acid drugs needing lysosome escape can be remarkably improved, and a new strategy is provided for exerting the curative effect of the drugs. The nano-carrier disclosed by the invention is not only limited to lipidosome, but also can be solid lipid nanoparticles, nano-emulsion, polymer micelles and the like. The endoplasmic reticulum targeted modification nano-carrier provided by the invention is novel in scheme and simple in preparation method, and has good industrial production and applicationprospects.
Owner:ZHEJIANG UNIV
Methods and devices for providing prolonged drug therapy
InactiveCN1209098CNervous disorderInorganic non-active ingredientsTherapeutic drug effectPharmaceutical drug
Methods and devices for maintaining a desired therapeutic drug effect over a prolonged therapy period are provided. In particular, oral dosage forms that release drug within the gastrointestinal tract at an ascending release rate over an extended period of time are provided. The dosage forms may additionally comprise an immediate-release dose of drug. <IMAGE>
Owner:ALZA CORP
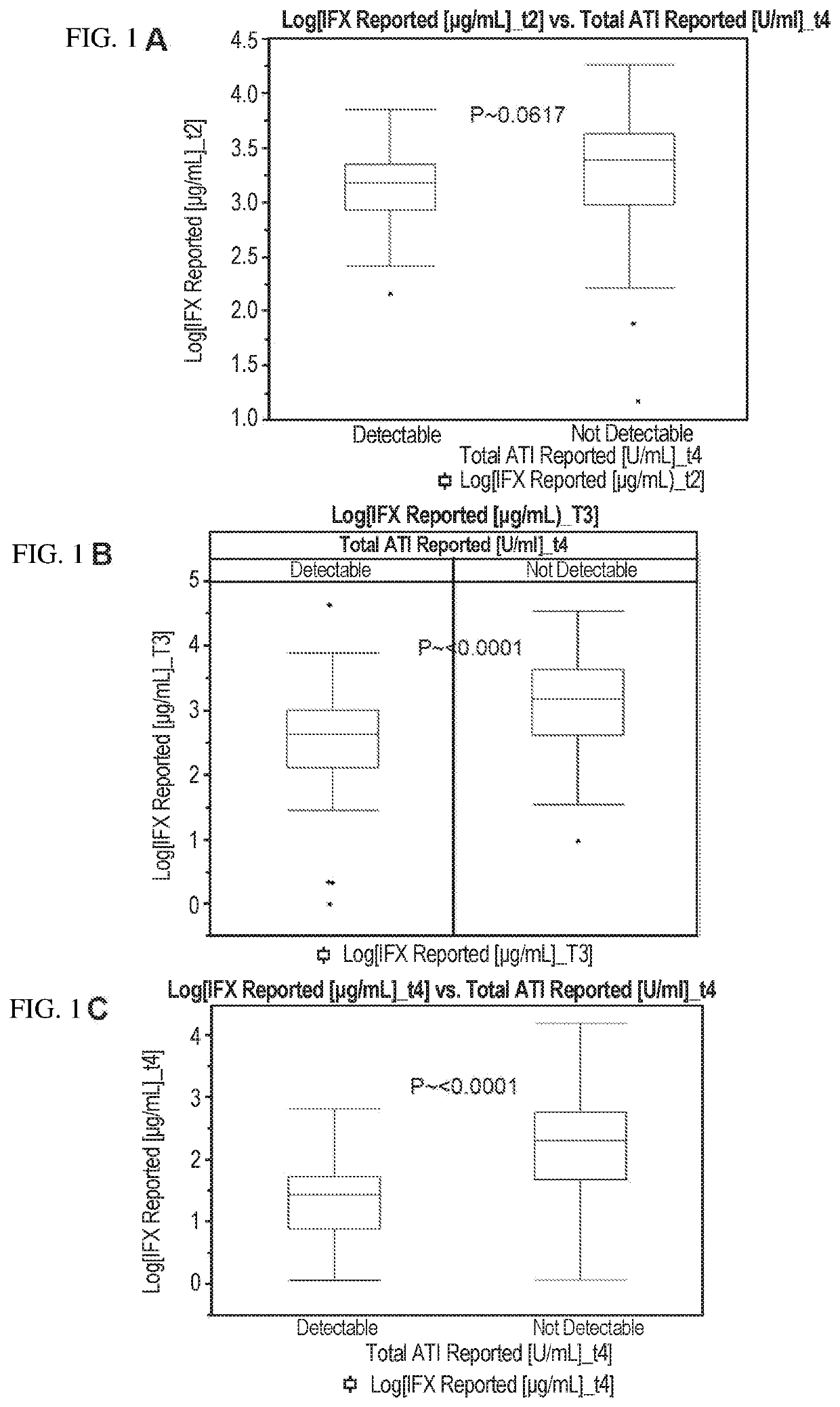
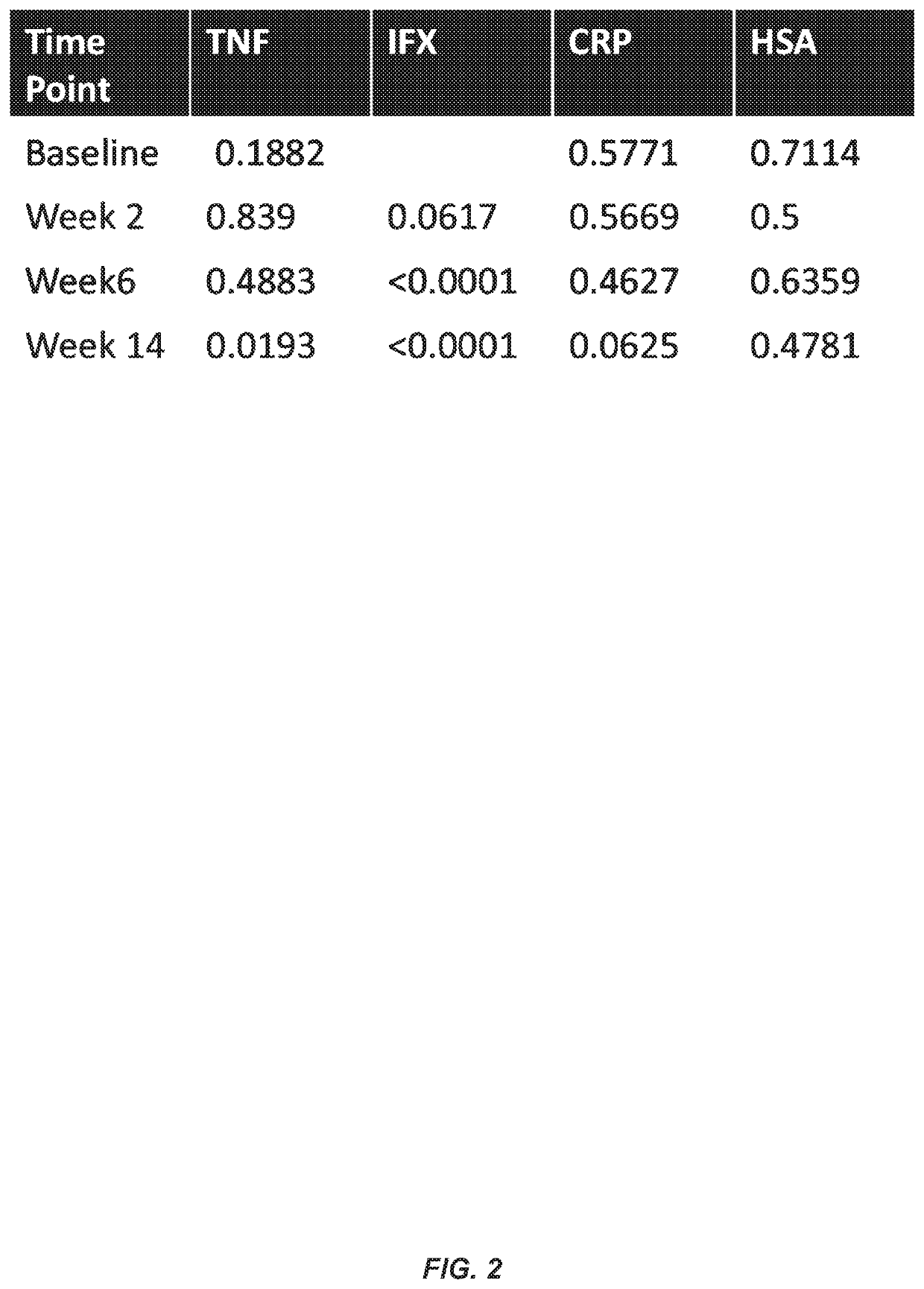
Methods for prediction of anti-TNFα drug levels and autoantibody formation
In some aspects, the present invention provides methods for predicting whether a subject will develop autoantibodies to an anti-TNFα drug during the course of anti-TNFα drug therapy. In other aspects, the present invention provides methods for predicting the level of an anti-TNFα drug in a subject during the course of anti-TNFα drug therapy. Systems for predicting anti-TNFα drug levels and the likelihood of autoantibody formation during the course of anti-TNFα drug therapy are also provided herein. The present invention further provides methods for predicting a clinical outcome (e.g., endoscopic response) of a subject on anti-TNFα drug therapy.
Owner:PROMETHEUS LAB
Stimuli-responsive size-adjustable nano hydrogel drug delivery system and preparation method thereof
InactiveCN113244165AReasonable structural designLow costOrganic active ingredientsAmino compound purification/separationEngineeringCombined treatment
The invention provides a stimuli-responsive size-adjustable nano hydrogel drug delivery system and a preparation method thereof. The drug delivery system aims to meet the requirements that single drug treatment is easy to generate drug resistance, drugs are difficult to enter tumor and the like. The preparation method comprises the following steps of synthesizing nano-hydrogel from monomer acrylic acid and monomer N-isopropyl acrylamide through a reflux precipitation method, promoting connection of aniline tetramer to the nano-hydrogel through EDC / NHS, and loading an anti-cancer drug through electrostatic adsorption to form the nano-hydrogel drug delivery system. The temperature-sensitive material N-isopropyl acrylamide in the drug delivery system enables the nano-hydrogel to have a temperature-controlled size-adjustable property, and the excellent photo-thermal and photo-acoustic imaging property of aniline tetramer is combined, so that the stimuli-responsive size-adjustable nano-hydrogel drug delivery system can realize image-guided photo-thermal / chemotherapy / deep treatment combined treatment.
Owner:NANJING UNIV OF POSTS & TELECOMM
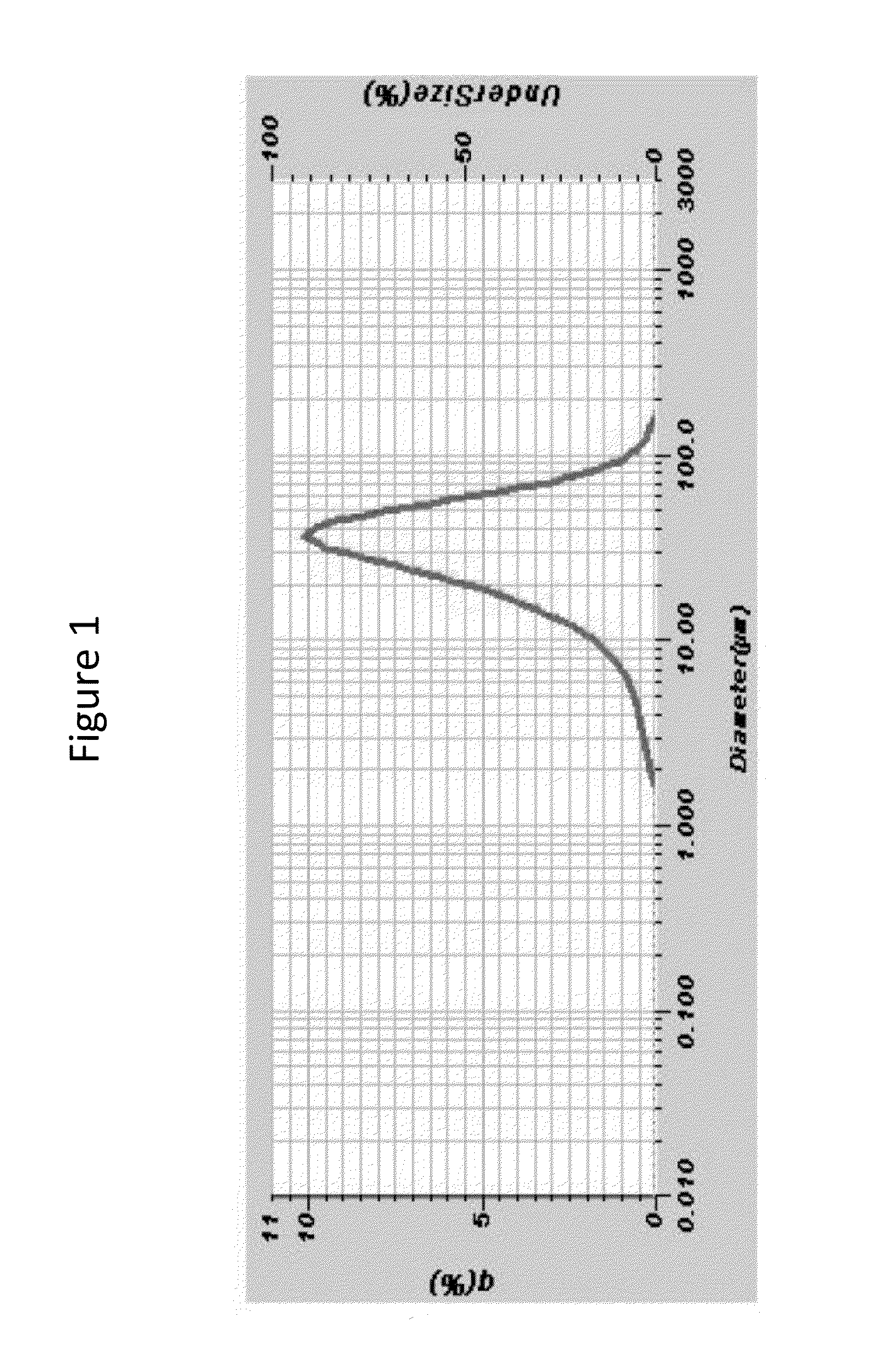
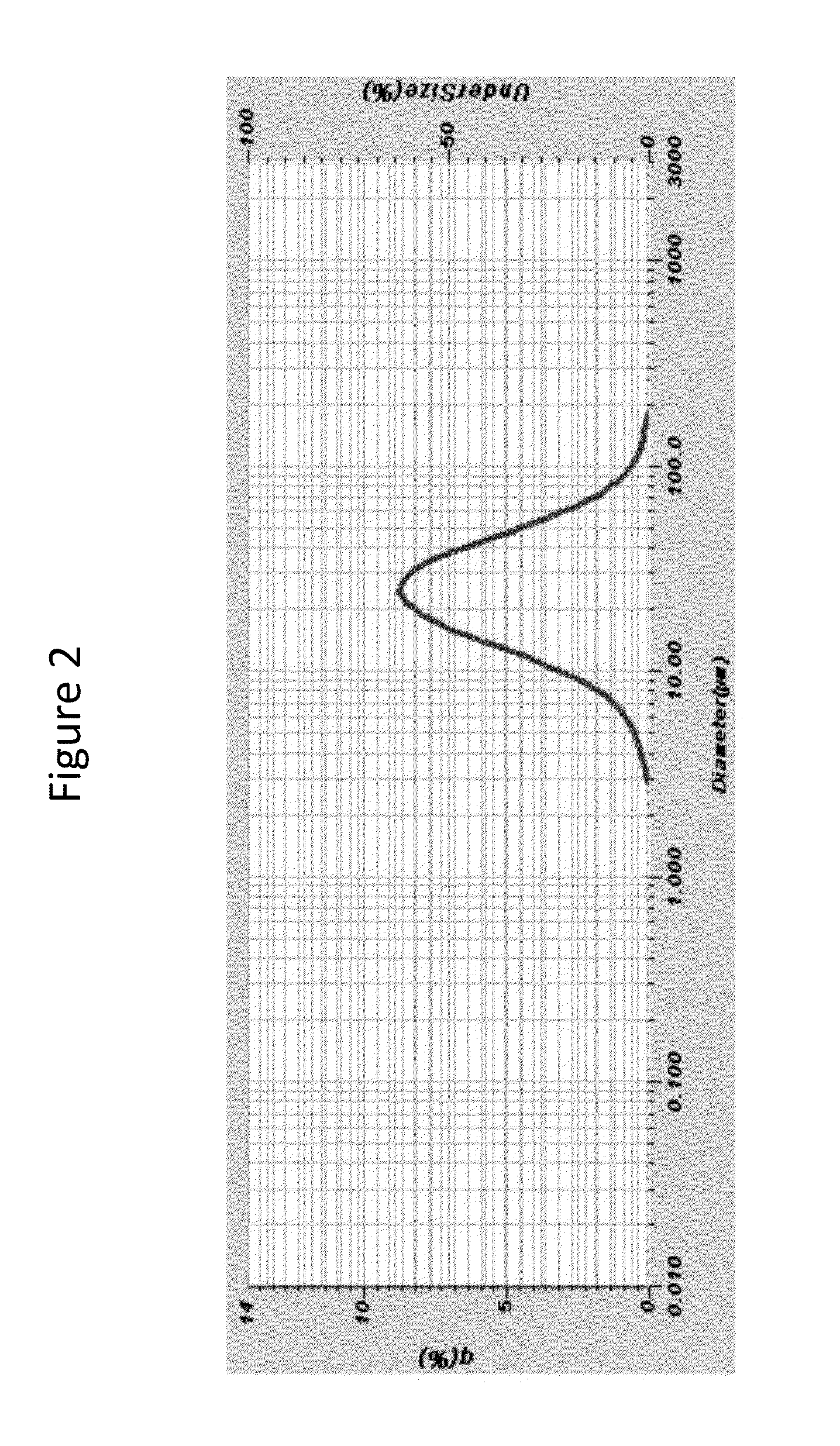
N-((1R,2S,5R)-5-(tert-butylamino)-2-((S)-3-(7-tert-butylpyrazolo[1,5-A][1,3,5]triazin-4-ylamino)-2-oxopyrrolidin-1-yl)cyclohexyl)acetamide, a dual modulator of chemokine receptor activity, crystalline forms and processes
ActiveCN102648201AAppropriate pharmacological characteristicsNervous disorderAntipyreticImmunologic disordersMetabolite
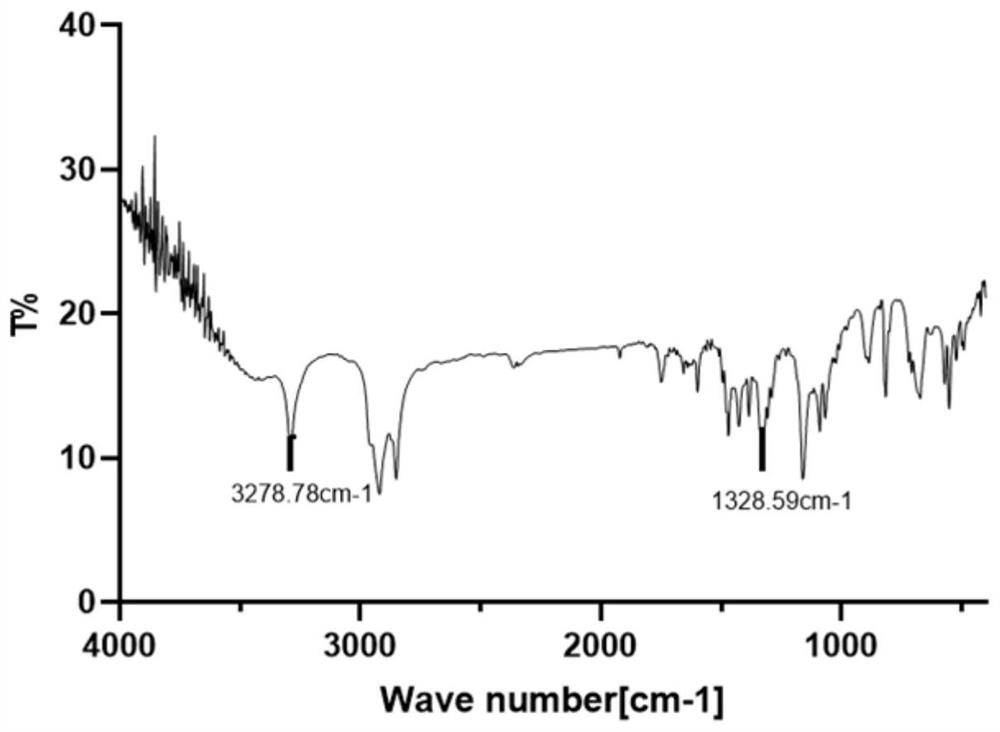
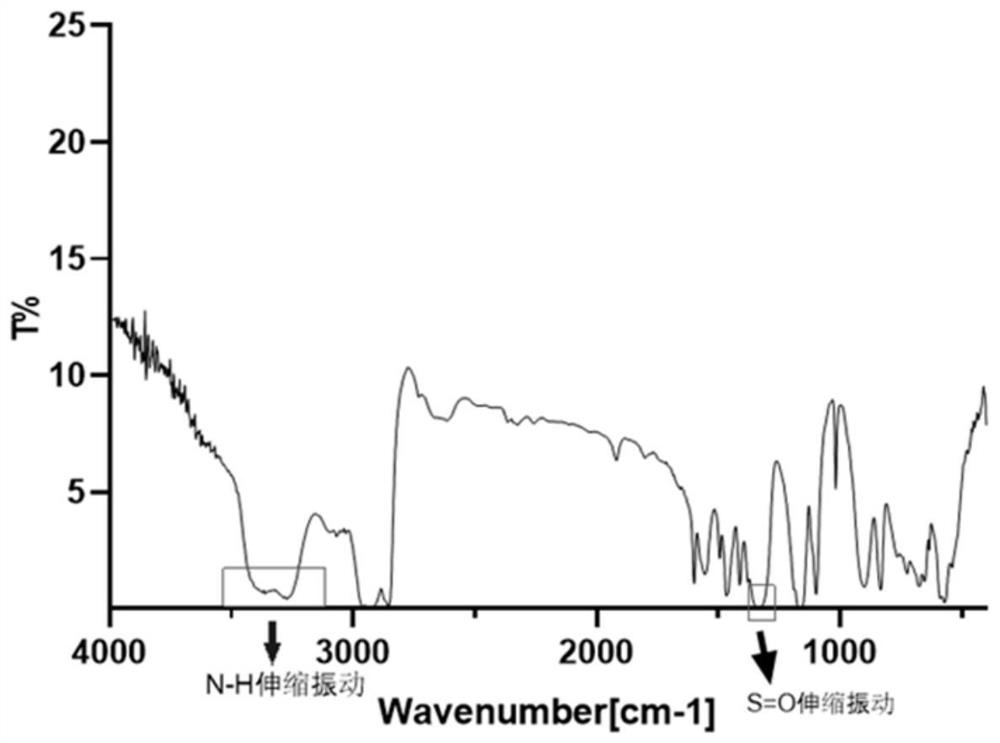
The present invention provides a novel antagonist: N-((1R,2S,5R)-5-(tert-butylamino)-2-((S)-3-(7-tert-butylpyrazolo[1,5-a][1,3,5]triazin-4-ylamino)-2-oxopyrrolidin-1-yl)cyclohexyl)acetamide: or a pharmaceutically acceptable salt, solvate or prodrug, thereof, having unexpected dual CCR-2 and CCR-5 receptor activity. Crystalline forms, metabolites, pharmaceutical compositions containing the same and methods of using the same as agents for the treatment of inflammatory diseases, allergic, autoimmune, metabolic, cancer and / or cardiovascular diseases are also disclosed. The present disclosure also provides processes for preparing compounds of Formula (I) as provided herein, including N-((1R,2S,5R)-5-(tert-butylamino)-2-((S)-3-(7-tert-butylpyrazolo[1,5-a][1,3,5]triazin-4-ylamino)-2-oxopyrrolidin-1-yl)cyclohexyl)acetamide. Compounds that are useful intermediates of the process are also provided herein.
Owner:BRISTOL MYERS SQUIBB CO
Niraparib oral controlled-release and sustained-release pharmaceutical composition and uses thereof
InactiveCN108201537AIncrease doseImprove efficacyOrganic active ingredientsPill deliveryBlood concentrationEnzyme inhibition
The invention discloses a niraparib oral controlled-release and sustained-release pharmaceutical composition and uses thereof. According to the presebt invention, by regulating the niraparib release behavior, the in vivo absorption rate and the absorption time of niraparib can be controlled, and the blood concentration and the blood concentration fluctuation range of niraparib in vivo can be regulated, such that the in vivo PARP enzyme inhibition activity of niraparib can be efficiently and permanently provided so as to provide the antitumor treatment effect in the high-efficiency and low-toxicity manner; a purpose of the present invention is to control the in vivo blood concentration and the efficacy providing of niraparib through the controlled-release and sustained-release preparation so as to improve the treatment effect of the drug and reduce the toxic-side effect of the drug; and the efficient and long-acting PARP enzyme activity inhibition pharmaceutical composition is providedfor patients, wherein the antitumor effect is improved, the acting time is long, the compliance is good, and the toxic-side effect is low.
Owner:SCI RAINBOW BIOPHARMA CO LTD
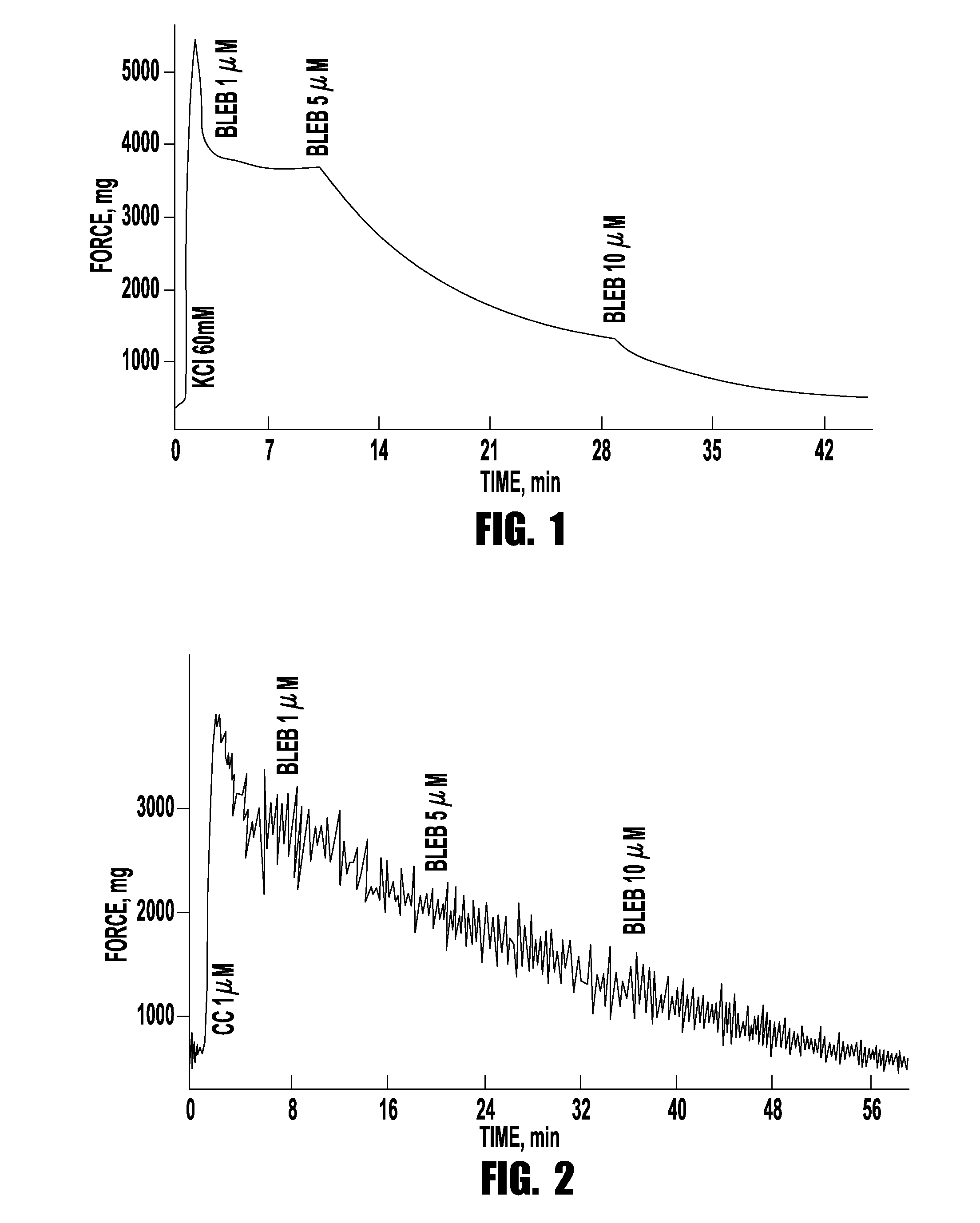
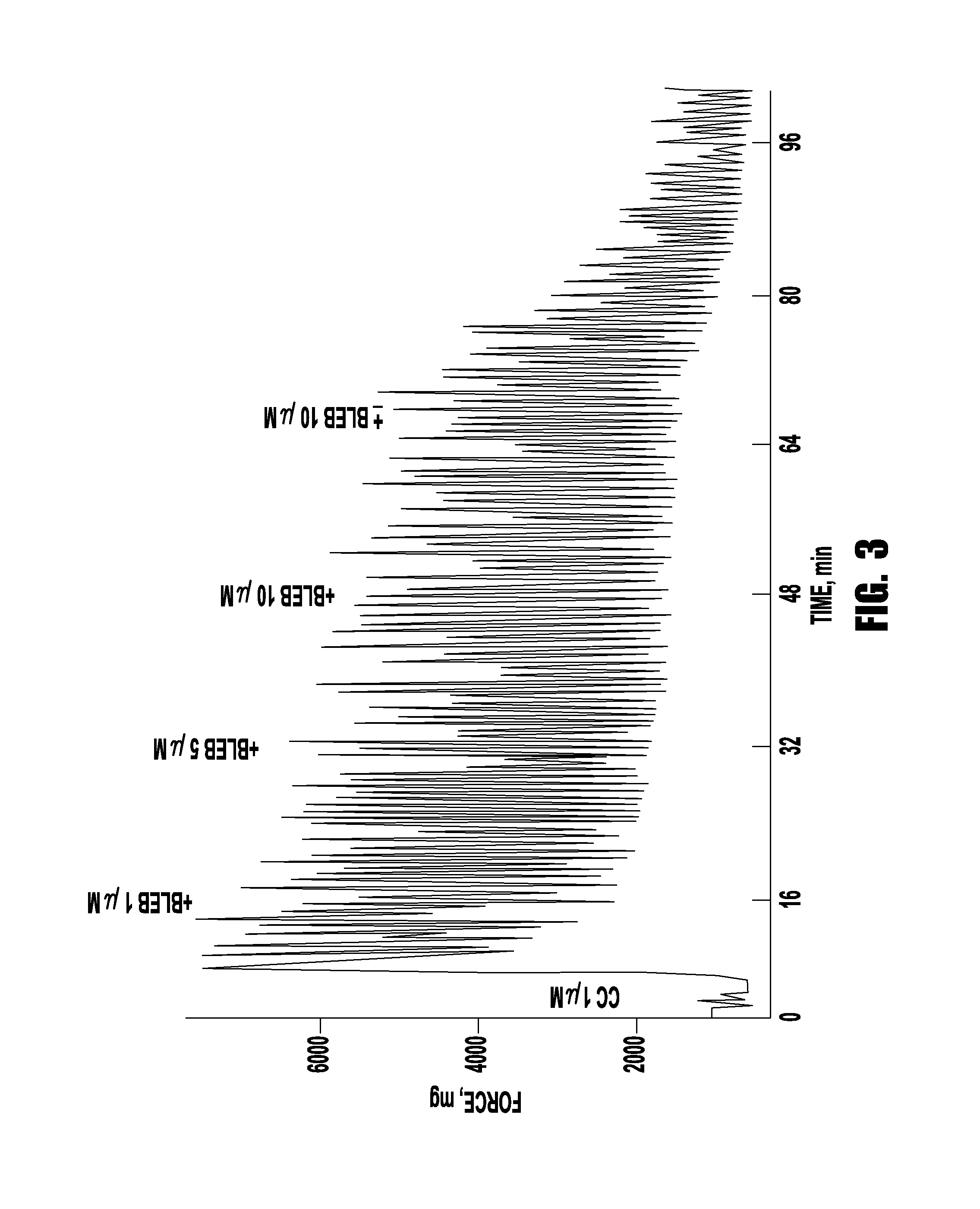
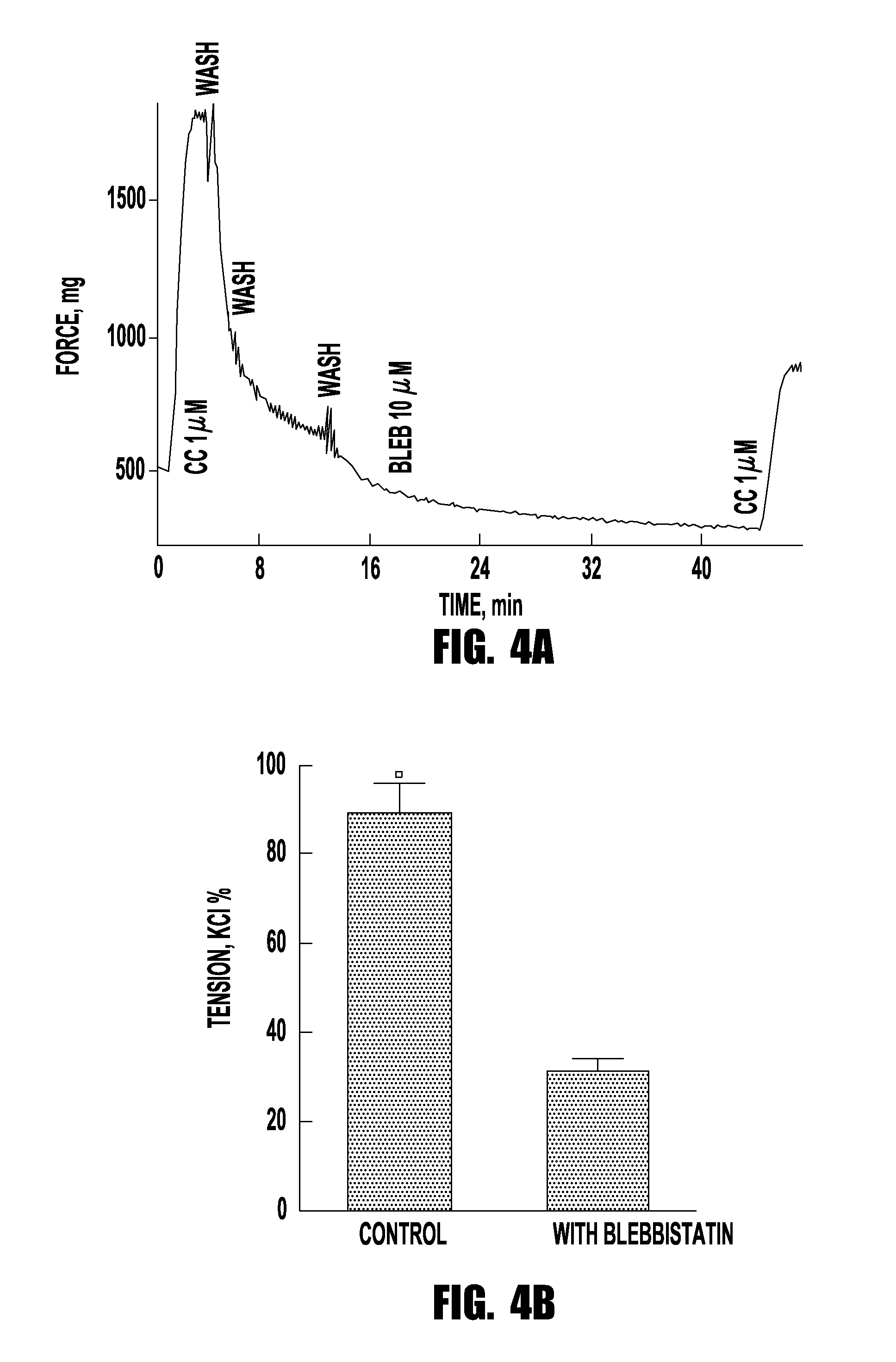
Drug treatment of overactive bladder
Provided are methods of treating an overactive bladder in a patient which include: administering a Myosin II ATPase inhibitor compound; or administering an X group and Y group substituted (3a-hydroxy-1-phenyl-1,2,3,3a-tetrahydro-4H-pyrollo[2, 3b] quinolin-4-one) compound of Formula 1 or administering an X group and Y group substituted (3a-hydroxy-1-phenyl-1,2,3,3a-tetrahydro-4H-pyrollo[2,3b] quinolin-4-one) compound of Formula II; or administering pharmaceutically-acceptable salts, racemic mixtures, enantiomers, or prodrugs of said compounds, useful in their active form as inhibitors of Myosin H ATPase related to over-active bladder. Optionally the compounds are administered intervesicularly into the bladder. Also provided are pharmaceutical compositions comprising said compounds useful in their active form, as methods of treating a patient suffering from an over-active bladder related to inhibition of Myosin II ATPase. These pharmaceutical compositions also may contain one or more other compounds useful in their active form, as methods of treating a patient suffering from an over-active bladder.
Owner:BLEBBICORP LLC
Methods of distributing complement-inhibiting drugs to patients receiving a complement inhibitor
This disclosure relates to methods of authorizing distribution of complement-inhibiting drugs to patients who have a complement-associated disorder in a manner to ensure that the patients are aware of the possible dangers of discontinuing treatment with the drugs. A database is prepared comprising patient information including experiencing adverse clinical events after discontinuing the drug treatment. The information in the database is collected and may be reported. The patients are given a warning as to adverse events that may occur if treatment with the complement inhibiting drugs is discontinued.
Owner:ALEXION PHARMA INC
Compositions for reduction of side effects
ActiveUS20130039960A1Rapid onsetReduce absorptionBiocideOrganic active ingredientsSide effectImmediate release
The present invention provides drug therapy formulations. In some embodiments, the present invention provides combinations of pharmaceutical agents (e.g., stimulant and non-stimulant), pharmaceutical formulations (e.g., nanoparticulate, non-nanoparticulate, etc.), and release profiles (e.g., immediate release, delayed release, sustained release, etc.) to provide therapeutic benefit with reduced side effects.
Owner:KEMPHARM INC
Opioid+device combination products with improved safety and efficacy profiles
The present invention describes a novel oral opioid dispensing system comprised of a mobile Opioid Specific App which utilizes a patient and drug specific drug dispensing algorithm. The algorithm incorporates the use of digitally captured and patient self-assessment, self-test, and / or self- report, prescription, and dispenser information / values to: (i) control opioid dispensing to improve medication safety, (ii) personalize oral drug therapy, (iii) improve medication effectiveness while avoiding drug mediated side effects, (iv) decreases misuse, abuse, over dosing, under dosing, dependence, addiction, divergence, accidental ingestion, overdose, and deaths, (v) improve disease management, (vi) ensure prescription compliance, and (vii) promotes prescription persistence on a cost-effective real-time basis.
Owner:埃德蒙 L 瓦伦丁
Use and method for predicting serotonin re-uptake inhibitor medicine effect by polymorphic site
InactiveCN101029337AImprove efficiencyImprove securityNervous disorderMicrobiological testing/measurementSerotoninDepressant
A method for determining HTR1A gene polymorphism site gene and predicting 5-hydroxytrytamine re-ingestion inhibitor medicinal effect and its use are disclosed. It can predict therapeutical effect and acting time by polymorphism site oligonucleotide inspecting biological sample. It's safe, cheap and non-toxic and can be used for thymoleptic and medicine in treatment of sexual disorders.
Owner:BEIJING HUAANFO BIOMEDICAL RES CENT +1
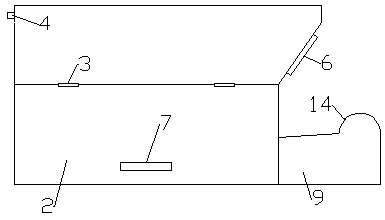
Targeted drug delivery fumigation system and method for gynecology department
ActiveCN109009992APrecise positioningPromote absorptionMedical devicesBathing devicesTreatment effectGynecology department
The invention relates to a targeted drug delivery fumigation system and method for the gynecology department. A water inlet pipe with nozzles is fixedly mounted on the inner top wall of a fumigation box, a heating disinfection lamp is fixedly mounted on one side of the water inlet pipe, a bellows box is fixedly mounted on the portion, at the lower end of the heating and disinfection lamp, of the inner wall of the fumigation box, fans are installed in the bellows box, an opening is formed below an oblique pulling support plate, and a targeted positioning fumigation device is mounted in the portion, below the opening, of the interior of the fumigation box. A positioning targeted drug delivery connector is used for drug delivery so that a female private part can be accurately located; moreover, a universal adjustment rod is used for positioning, fixation and adjustment of the corresponding degree, and the positioning targeted drug delivery connector is provided with an upper drug outlet,a middle drug outlet and a lower drug outlet. According to a human body bionic principle, omnibearing fumigation drug therapy treatment is carried out to a precise position and peripheral positions ofthe female private part, precise targeted drug delivery is achieved, and the treatment effect is remarkable.
Owner:NANYANG CITY CENT HOSPITAL
Disposable thermal therapeutic apparatus and method of thermally controlling the delivery of medication therewith
InactiveUS20130298903A1Quick and reliable and economicalImprove delivery performanceMedical devicesIntravenous devicesThermal energyChemical reaction
A portable, disposable, thermal drug dispensing apparatus and method of thermally controlling the delivery of medication therewith is provided. The apparatus provides a predetermined thermal energy over a predetermined amount of time to allow effective drug delivery regardless of the external environment. The apparatus produces exothermic or endothermic thermal energy, in a balanced and controlled environment via a chemical reaction between reactants contained as an integral part within the apparatus. The reactants are provided and automatically combined in a selective, predetermined manner within the apparatus to provide the desired thermal cycle needed to produce the desired, thermal environment for effective drug treatment.
Owner:WRIGHT DAVID W
Automated techniques for identifying optimal combinations of drugs
PendingUS20200222538A1Improve performanceConvenient treatmentDrug and medicationsData visualisationPharmaceutical drugOmics data
Techniques are provided for administering combination of drug treatments to a patient. Information is analyzed pertaining to individual drug treatments from structurally or functionally defined drugs, drugs with unknown functions, and corresponding effects, wherein the information includes omic data including genes, transcripts, proteins, as well as experimental data from published documents. One or more combinations of drug treatments are identified with combined effects producing a positive result, wherein the positive result is directed to a specific aspect of patient health. The identified combination of drug treatments are administered to a patient.
Owner:IBM CORP
Composition with function of relieving diarrhea and preparing method and application thereof
ActiveCN110448569AUnique two-way regulationInhibition of growth and reproductionAntibacterial agentsOrganic active ingredientsChronic diarrheaCritically ill
The invention provides a composition with the function of relieving diarrhea and a preparing method and application thereof. The composition contains macromolecule glue and a polymer, and is suitablefor tumor-associated diarrhea, antibiotic-and-drug therapeutic diarrhea, intestinal inflammation disease diarrhea, diabetic diarrhea, irritable bowel syndrome diarrhea, persistent diarrhea, chronic diarrhea, viral or bacterial infection diarrhea, and enteral nutrition intolerance diarrhea and alternating diarrhea and constipation of critically-ill patients. The composition can be applied to patients through nasal feeding and oral administration, can be used for both children and adults, has two-way regulation effects on diarrhea and constipation, and can be taken for a long term.
Owner:麦孚营养科技(北京)有限公司 +1
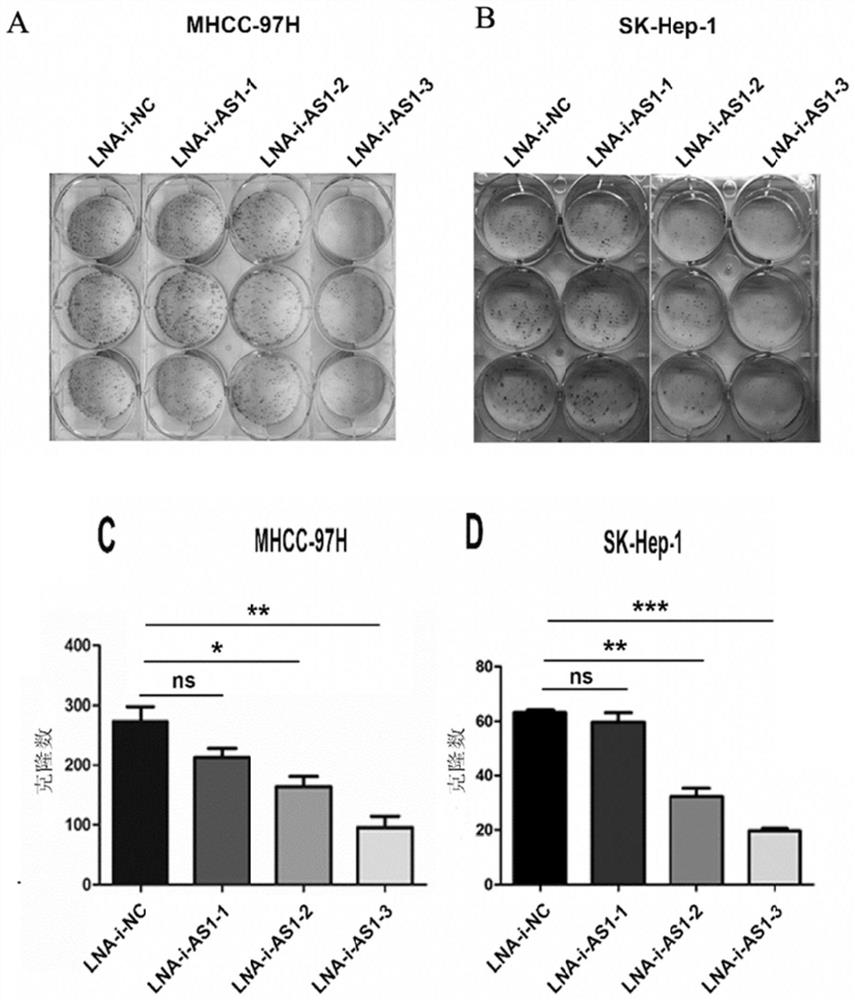
Application of UPK1A-AS1 inhibitor in preparation of antitumor drugs
ActiveCN113476606APrevent proliferationNo significant effect on body weightOrganic active ingredientsAntineoplastic agentsSide effectDepressant
The invention belongs to the technical field of medicines, and discloses application of a UPK1A-AS1 inhibitor in preparation of antitumor drugs. The invention discloses application of the UPK1A-AS1 inhibitor or pharmaceutically acceptable salt thereof in preparation of antitumor drugs for the first time based on the fact that the inventor finds that proliferation of tumor cells and tumor tissues can be inhibited by inhibiting expression and / or activity of UPK1A-AS1. The weight of a drug-administered animal is not remarkably influenced, and meanwhile, during drug administration, no drug treatment side effect is observed; and it is indicated that the UPK1A-AS1 inhibitor or the pharmaceutically acceptable salt thereof can inhibit the proliferation of tumor tissues, has no side effect, and can be used for preparing the antitumor drugs.
Owner:NANFANG HOSPITAL OF SOUTHERN MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



















![N-((1R,2S,5R)-5-(tert-butylamino)-2-((S)-3-(7-tert-butylpyrazolo[1,5-A][1,3,5]triazin-4-ylamino)-2-oxopyrrolidin-1-yl)cyclohexyl)acetamide, a dual modulator of chemokine receptor activity, crystalline forms and processes N-((1R,2S,5R)-5-(tert-butylamino)-2-((S)-3-(7-tert-butylpyrazolo[1,5-A][1,3,5]triazin-4-ylamino)-2-oxopyrrolidin-1-yl)cyclohexyl)acetamide, a dual modulator of chemokine receptor activity, crystalline forms and processes](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/31e8536f-2ac7-4f2e-8df5-b963a43da2a2/HDA00001759433500011.png)
![N-((1R,2S,5R)-5-(tert-butylamino)-2-((S)-3-(7-tert-butylpyrazolo[1,5-A][1,3,5]triazin-4-ylamino)-2-oxopyrrolidin-1-yl)cyclohexyl)acetamide, a dual modulator of chemokine receptor activity, crystalline forms and processes N-((1R,2S,5R)-5-(tert-butylamino)-2-((S)-3-(7-tert-butylpyrazolo[1,5-A][1,3,5]triazin-4-ylamino)-2-oxopyrrolidin-1-yl)cyclohexyl)acetamide, a dual modulator of chemokine receptor activity, crystalline forms and processes](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/31e8536f-2ac7-4f2e-8df5-b963a43da2a2/HDA00001759433500021.png)
![N-((1R,2S,5R)-5-(tert-butylamino)-2-((S)-3-(7-tert-butylpyrazolo[1,5-A][1,3,5]triazin-4-ylamino)-2-oxopyrrolidin-1-yl)cyclohexyl)acetamide, a dual modulator of chemokine receptor activity, crystalline forms and processes N-((1R,2S,5R)-5-(tert-butylamino)-2-((S)-3-(7-tert-butylpyrazolo[1,5-A][1,3,5]triazin-4-ylamino)-2-oxopyrrolidin-1-yl)cyclohexyl)acetamide, a dual modulator of chemokine receptor activity, crystalline forms and processes](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/31e8536f-2ac7-4f2e-8df5-b963a43da2a2/HDA00001759433500031.png)