Construction and application of endoplasmic reticulum targeted nano drug delivery system
A nano-drug loading and endoplasmic reticulum technology, which is applied in nano-drugs, endocrine system diseases, nanotechnology, etc., can solve the problems of inability to deliver active molecules in a targeted manner, and cannot realize carrier functions, etc., and achieve good industrial production and application prospects, design Novel schemes to improve the effect of accumulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Synthesis of N-dodecyl-4-methylbenzenesulfonamide modification: Weigh dodecylamine and dissolve it in an appropriate amount of dichloromethane (DCM), add triethylamine as an acid-binding agent, weigh and place p-toluene Sulfonyl chloride was dissolved in an appropriate amount of DCM, and slowly added dropwise to the reaction system under stirring in an ice bath. The molar ratio of p-toluenesulfonyl chloride to dodecylamine was 1.2-1.5:1. The reaction was carried out in an ice bath for 1 h, then transferred to room temperature for 4-10 h. During this period, the reaction progress was detected by thin-layer chromatography (TLC), and the system was clear and transparent. After the reaction, the residual triacetic acid was neutralized with 2M dilute hydrochloric acid, the pH was adjusted to neutral by pH test paper, the organic phase was collected after extraction with saturated saline, and the organic solvent was evaporated under reduced pressure in vacuo to obtain a crude ...
Embodiment 2
[0054] Synthesis of p-dodecylbenzenesulfonamide modification: Weigh p-dodecylbenzenesulfonyl chloride, dissolve it in chloroform and add it to a 25mL three-necked flask, add concentrated ammonia water under ice bath conditions, stir magnetically for 30min, and convert to Reaction at room temperature for another 2-6 h, TLC detection of the reaction process. The molar ratio of p-dodecylbenzenesulfonyl chloride to ammonia water is 1:3-10. After the reaction, use chloroform to extract and collect the organic phase, dry it with anhydrous sodium sulfate, filter, collect the organic phase, and evaporate under reduced pressure to remove the organic solvent , to get crude products. Purify by silica gel chromatographic column afterwards, the eluent system is: heptane:ethyl acetate=4:1 (volume ratio), collect target product, evaporate under reduced pressure to remove organic solvent, obtain the target product of purification, it is pale yellow viscous thick liquid, weighed, and calculat...
Embodiment 3
[0060] Synthesis of dodecanesulfonamide modification: first measure ammonia water and add it to a three-necked flask, then add an appropriate amount of methanol, weigh dodecanesulfonyl chloride and dissolve it in an appropriate amount of methanol, slowly add it dropwise under ice-bath stirring conditions, React for 30 minutes, then turn to room temperature and react overnight. After the reaction, use saturated brine and ethyl acetate to extract, collect the organic phase, dry over anhydrous sodium sulfate and filter, then collect the organic phase, and evaporate the organic solvent under reduced pressure to obtain a white crystalline product. Weigh and calculate yield. Store at room temperature or 4°C.
[0061] Dodecylsulfonamide synthesis reaction equation:
[0062]
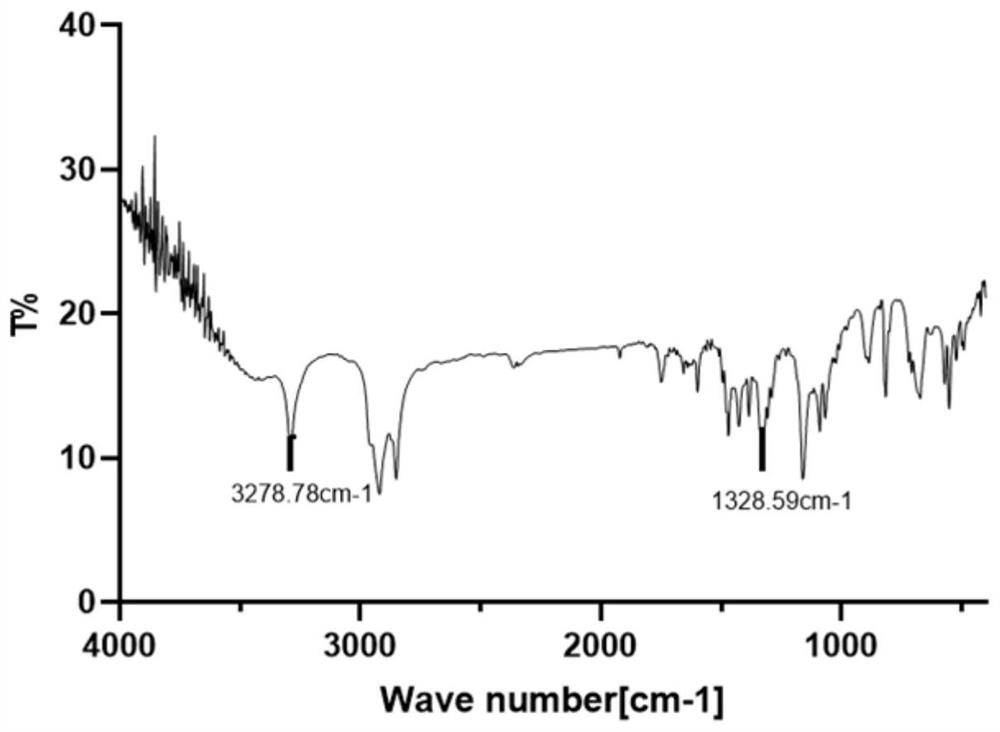
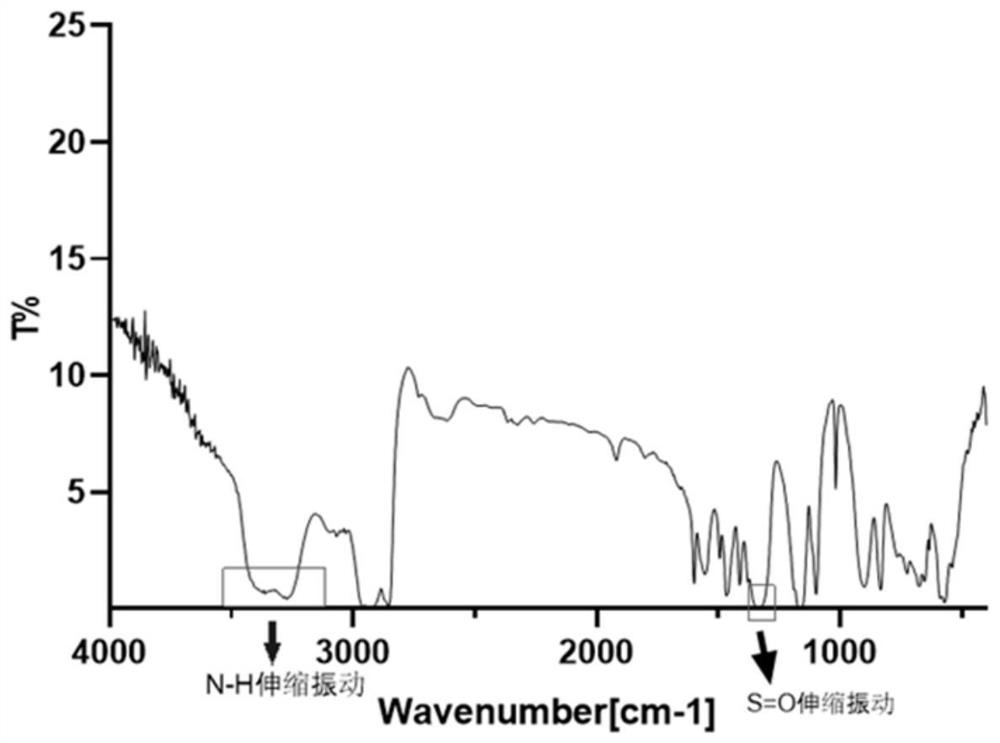
[0063] Confirmation of dodecanesulfonamide structure: (1) Dodecanesulfonamide was carried out by nuclear magnetic resonance 1 H NMR structure confirmation: 1 H NMR (500MHz, CDCl 3 )δ4.49 (s, 2H), 3.14-3....
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com