Treatment with anti-VEGF antibodies
a technology of anti-vegf antibodies and anti-cancer, applied in the field of human diseases and pathological conditions, can solve the problems of numerous side effects, difficult detection and treatment, and current cancer treatment methods that are relatively non-selectiv
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Addition of an Anti-VEGF Antibody to Bolus Irinotecan / Fluorouracil / Leucovorin (IFL) in First Line Metastatic Colorectal Cancer
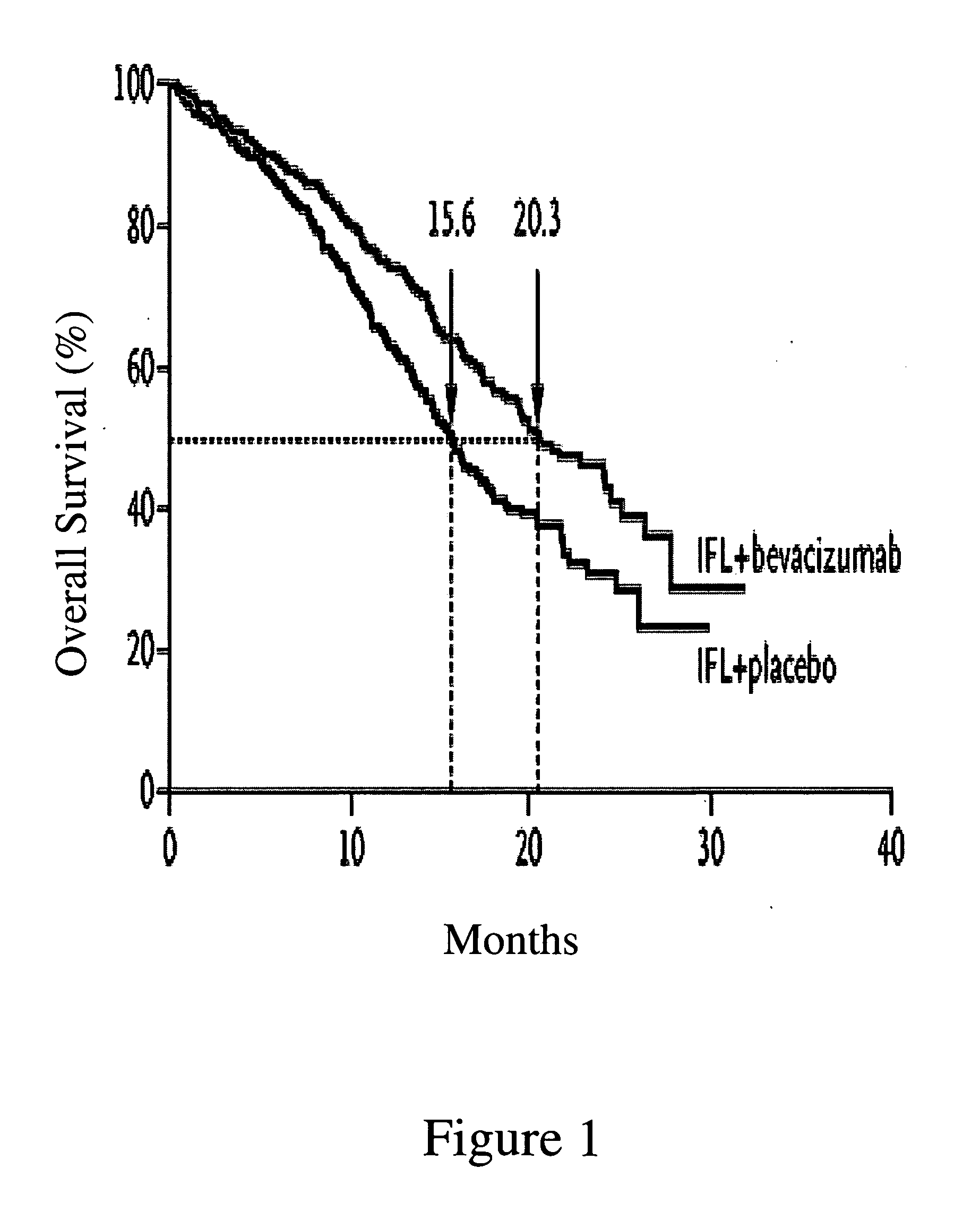
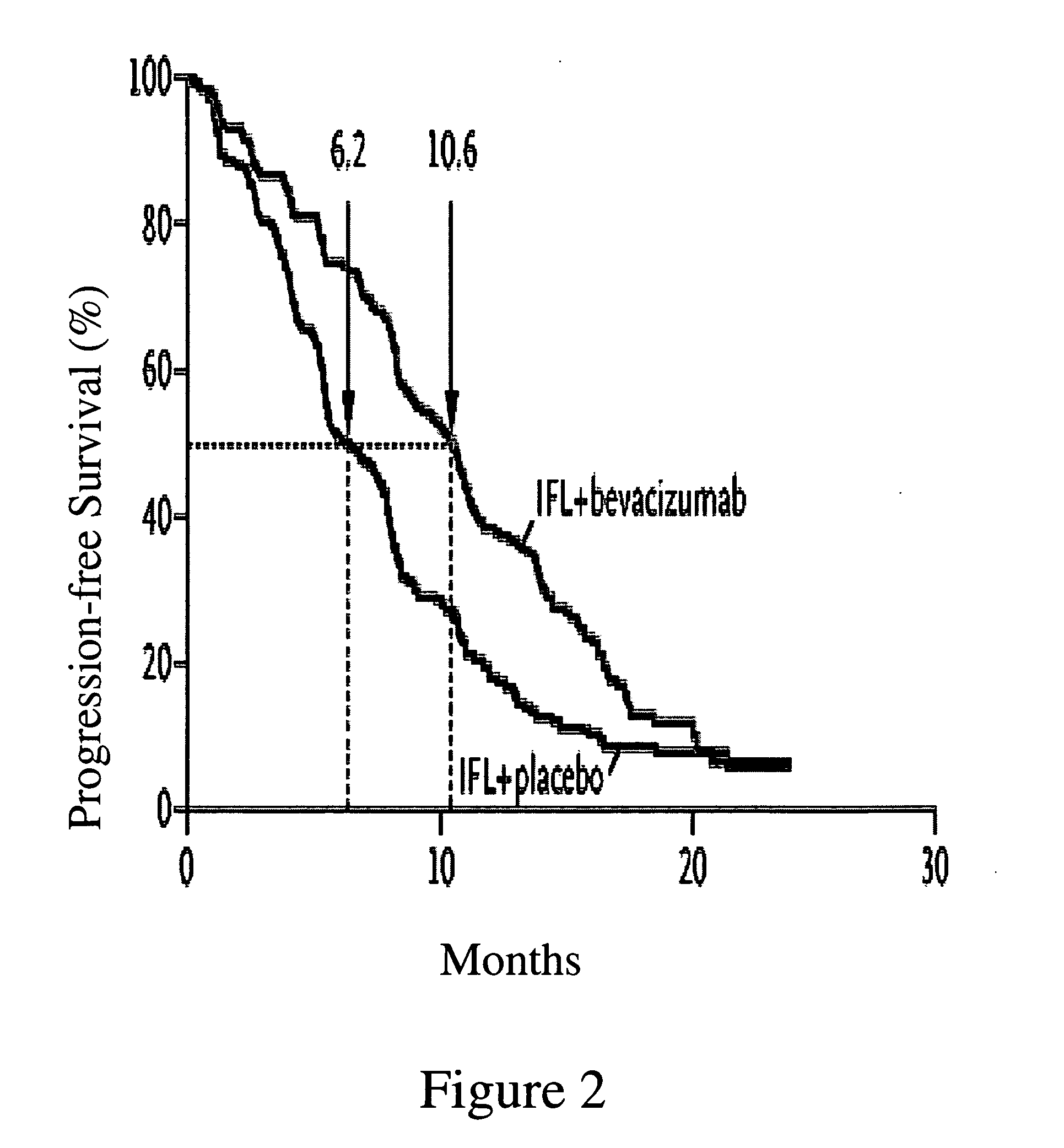
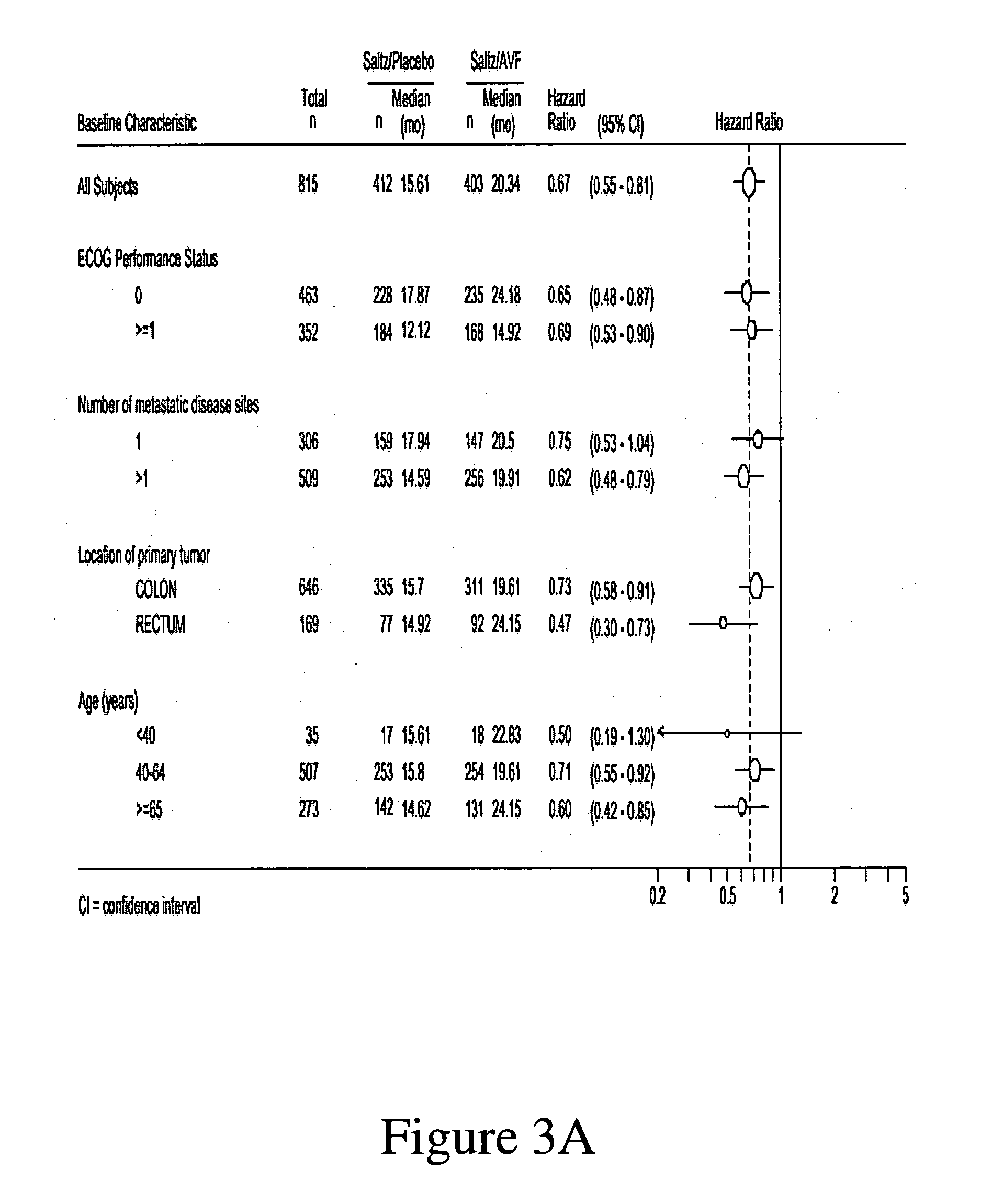
[0193] A multicenter, Phase III, randomized, active-controlled trial was conducted to evaluate the efficacy and safety of bevacizumab when added to standard first-line chemotherapy used to treat metastatic colorectal cancer. The trial enrolled over 900 patients with histologically confirmed, previously untreated, bi-dimensionally measurable metastatic colorectal cancer.
Methods and Materials
[0194] Anti-VEGF Antibody Bevacizumab
[0195] The anti-VEGF antibody “Bevacizumab (BV)”, also known as “rhuMAb VEGF” or “Avastin™” is a recombinant humanized anti-VEGF monoclonal antibody generated according to Presta et al. (1997) Cancer Res. 57:4593-4599. It comprises mutated human IgG1 framework regions and antigen-binding complementarity-determining regions from the murine anti-hVEGF monoclonal antibody A.4.6.1 that blocks binding of human VEGF to its receptors. U.S....
example 2
Addition of Bevacizumab to Bolus 5-FU / Leucovorin in First-Line Metastatic Colorectal Cancer
[0229] This randomized, phase II trial compared bevacizumab plus 5-fluorouracil and leucovorin (5-FU / LV) versus placebo plus 5-FU / LV as first-line therapy in patients considered non-optimal candidates for first-line irinotecan.
Patients and Methods
Patient Eligibility
[0230] Patients with histologically confirmed, previously untreated, measurable metastatic colorectal cancer were eligible if, in the judgment of the investigator, they were not optimal candidates for first-line irinotecan-containing therapy and had at least one of the following characteristics: age above 65 years, ECOG PS of 1 or 2, serum albumin equal or less than 3.5 g / dL, or prior radiotherapy to abdomen or pelvis. Patients were excluded if they had undergone major surgical procedures or open biopsy, or had experienced significant traumatic injury, within 28 days prior to study entry; anticipated need for major surgery dur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| molecular mass | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com