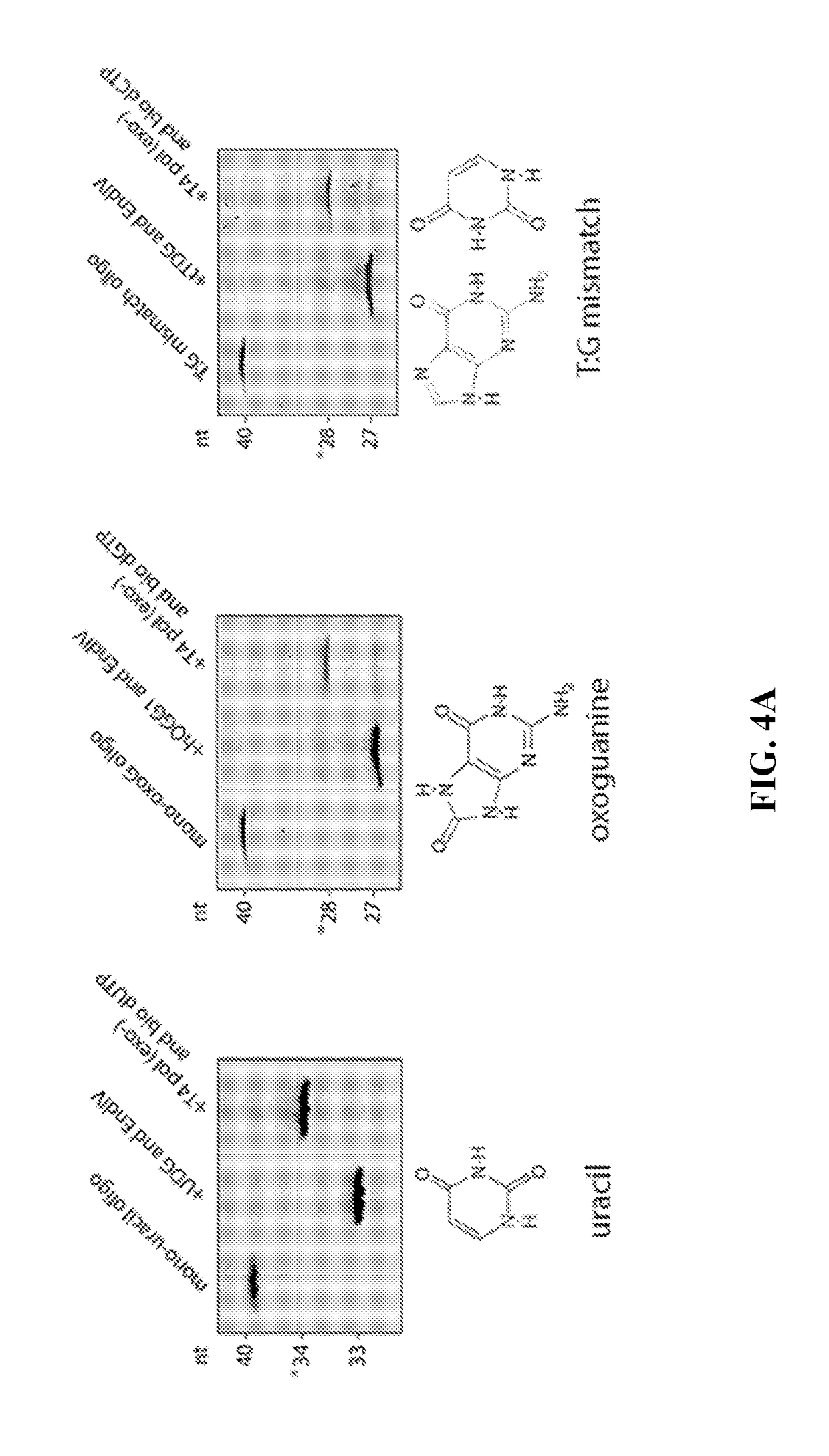
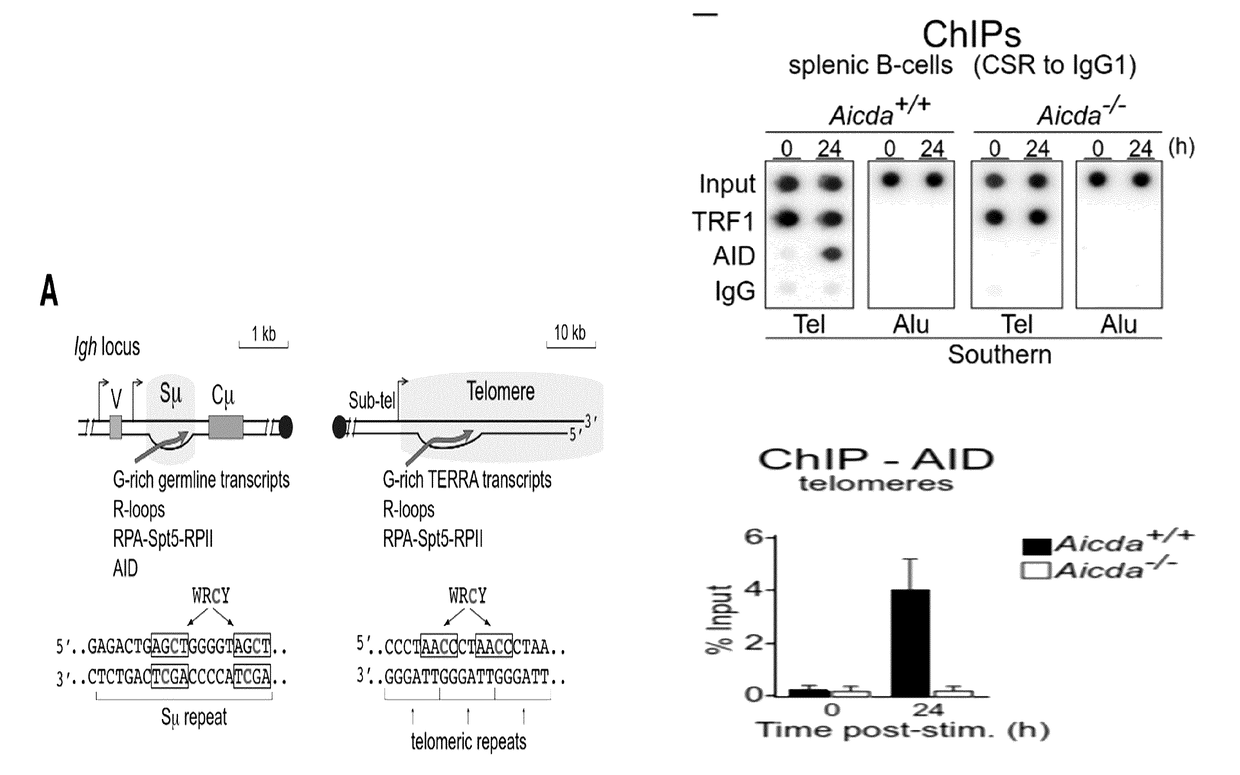
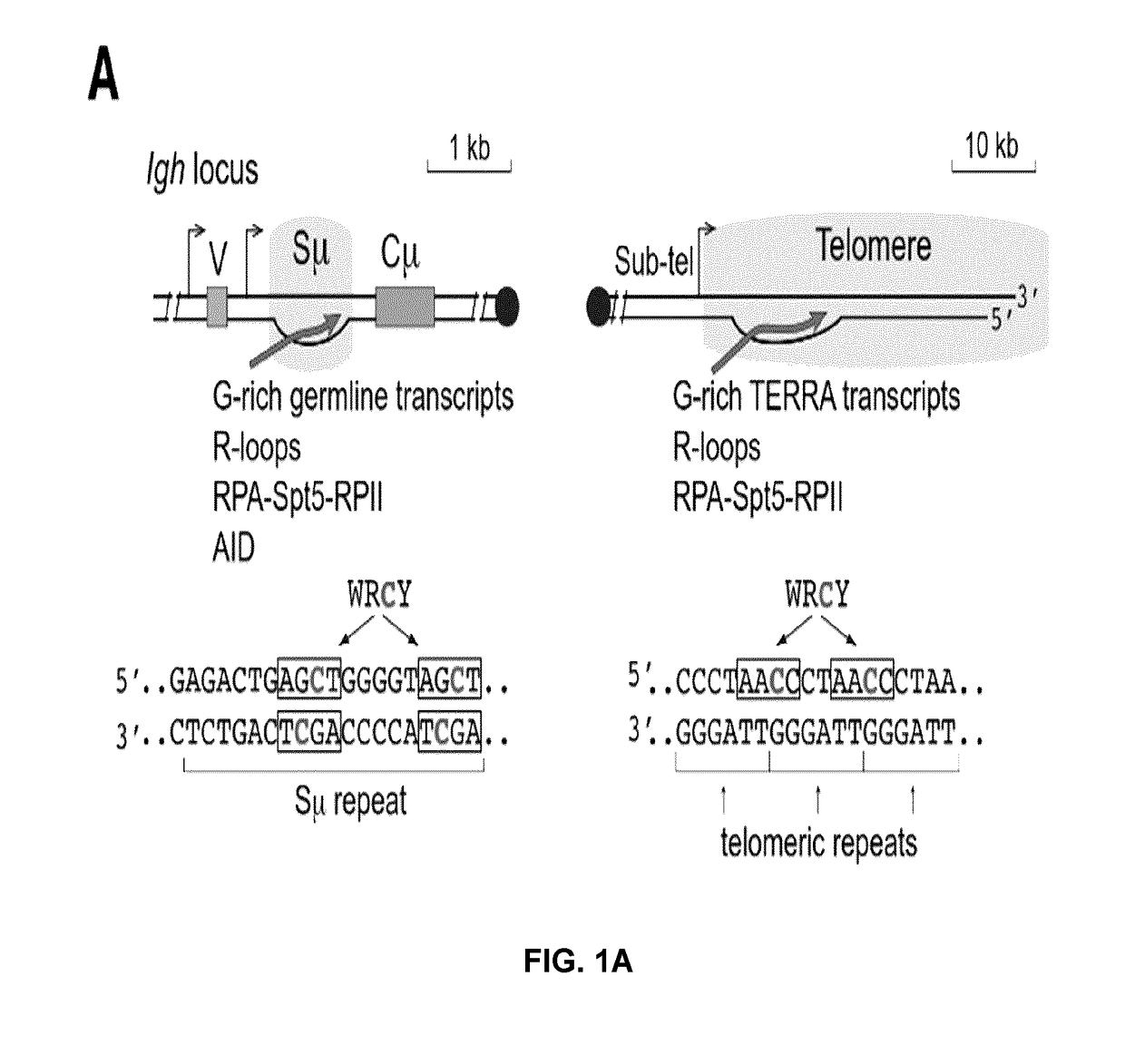
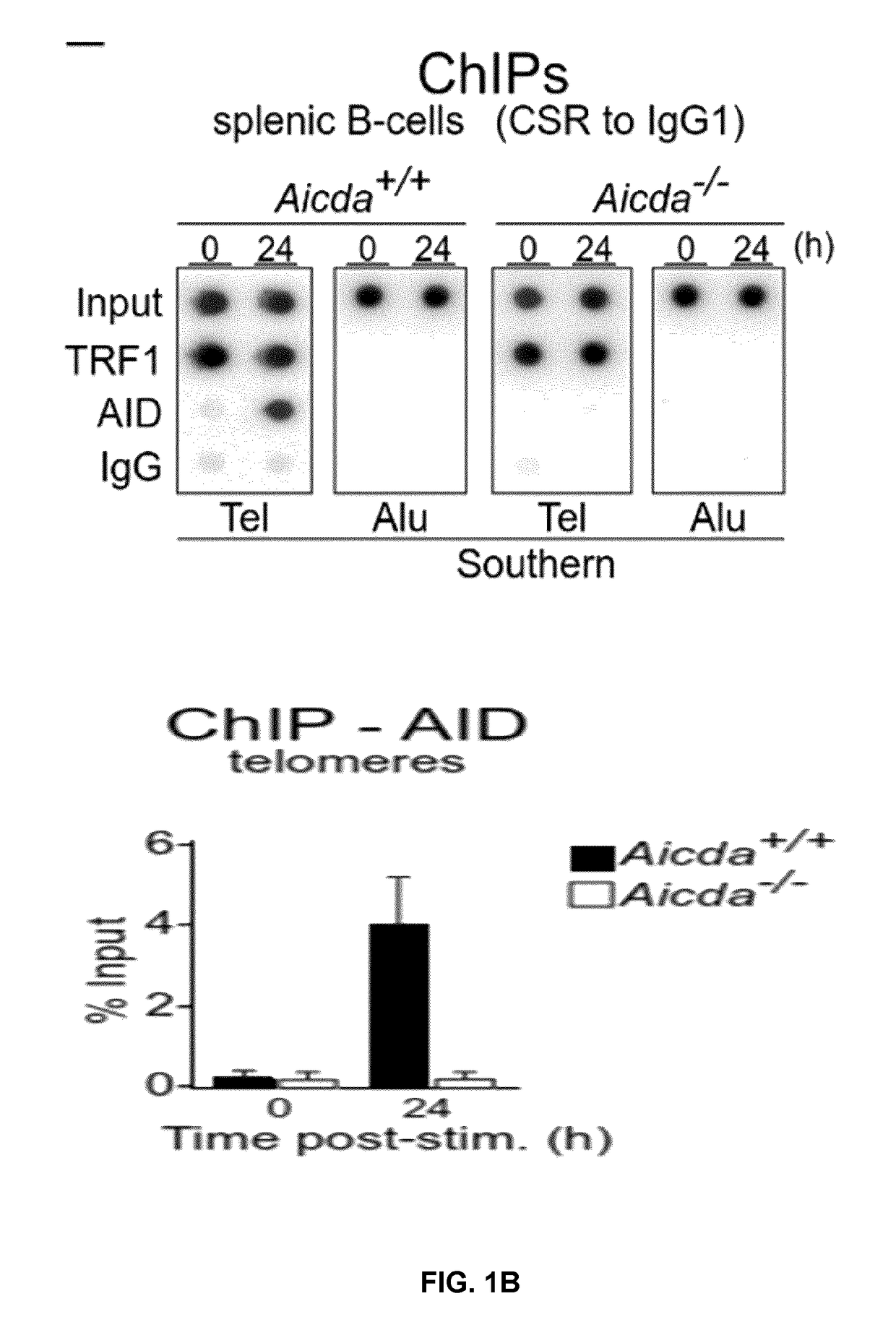
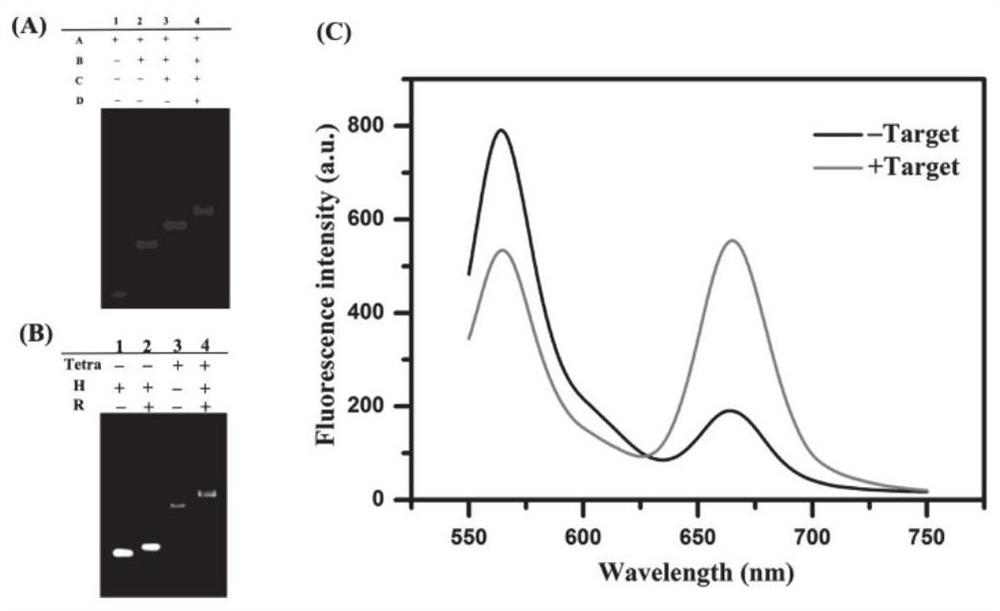
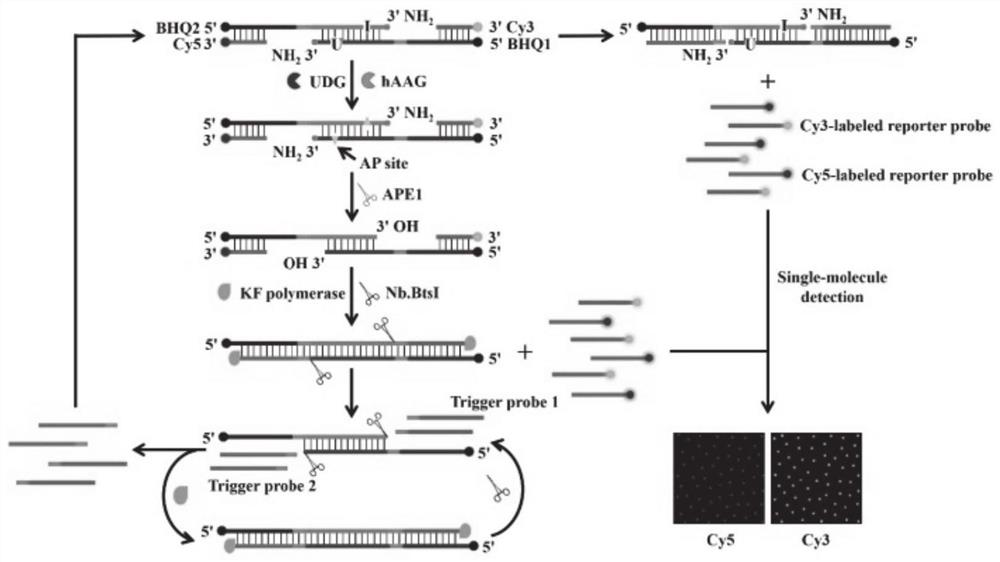
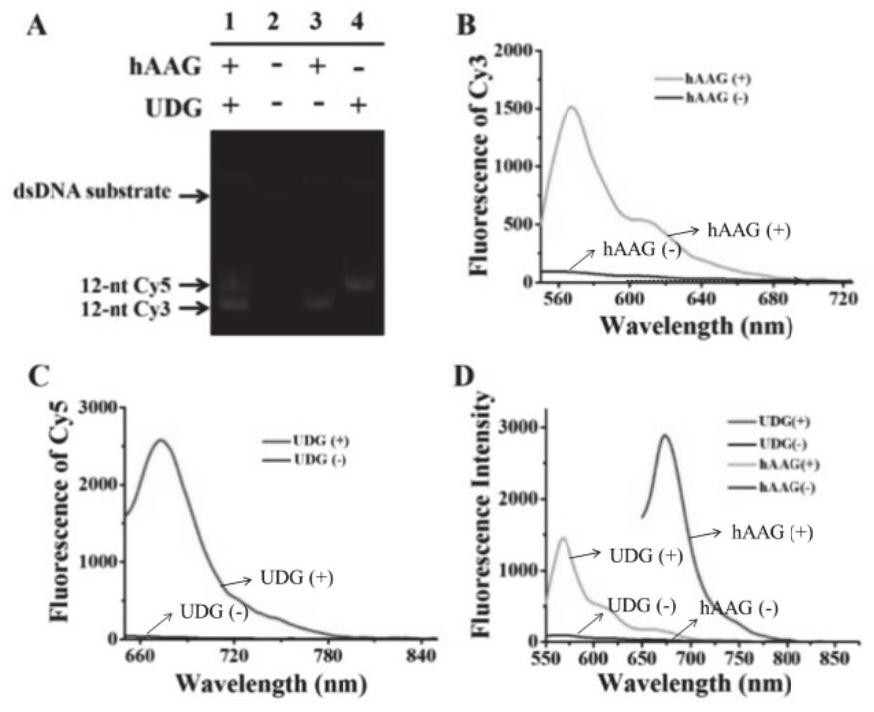
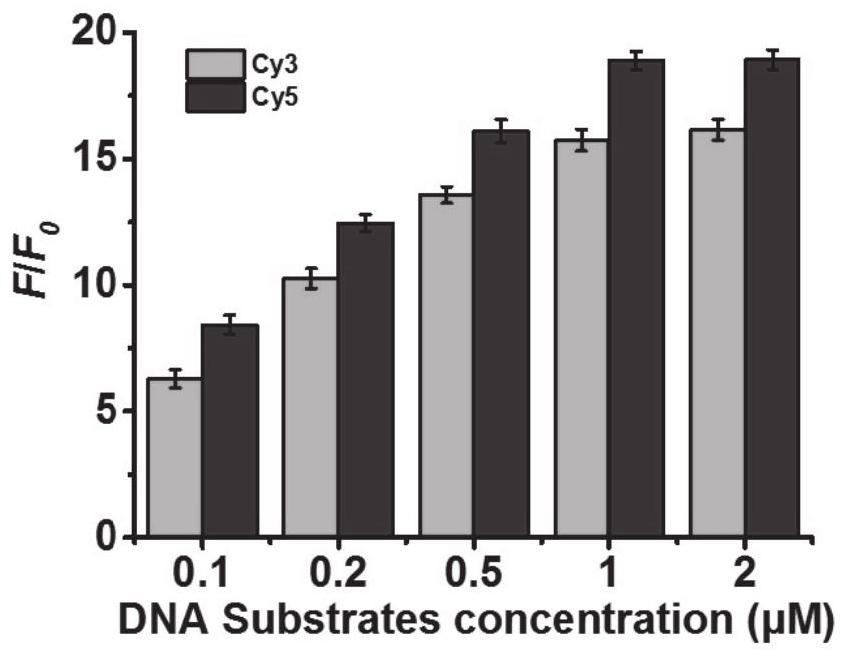
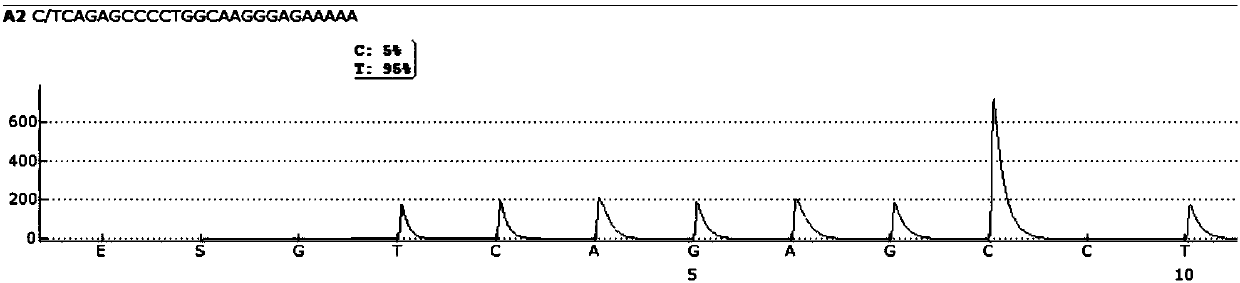
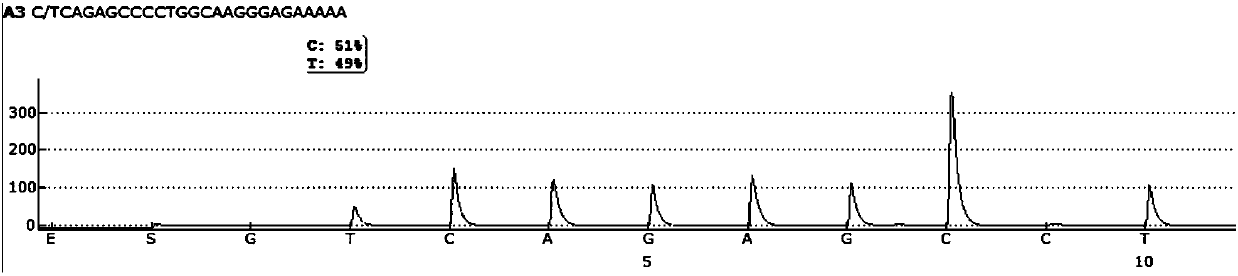
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
44 results about "DNA glycosylase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
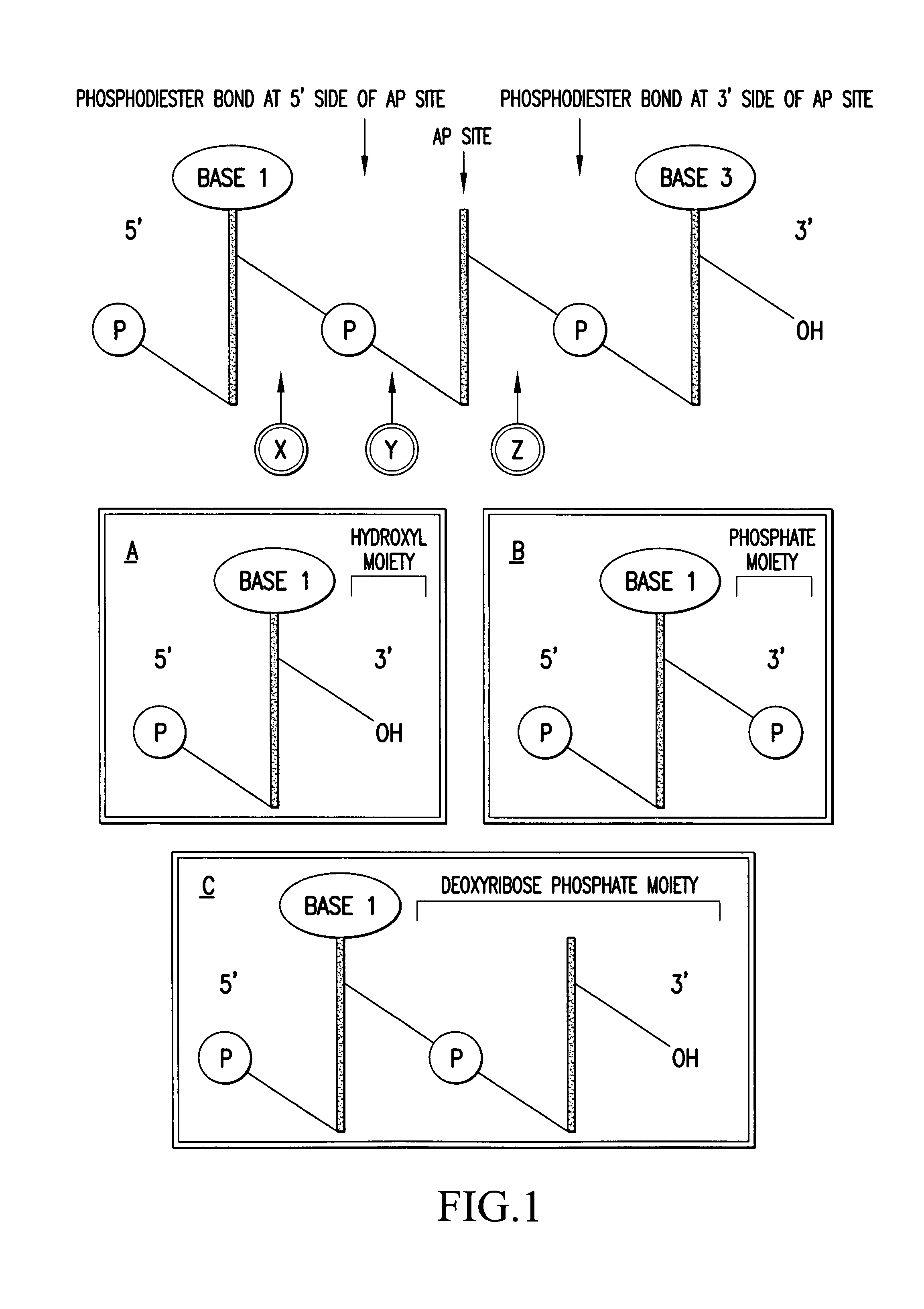
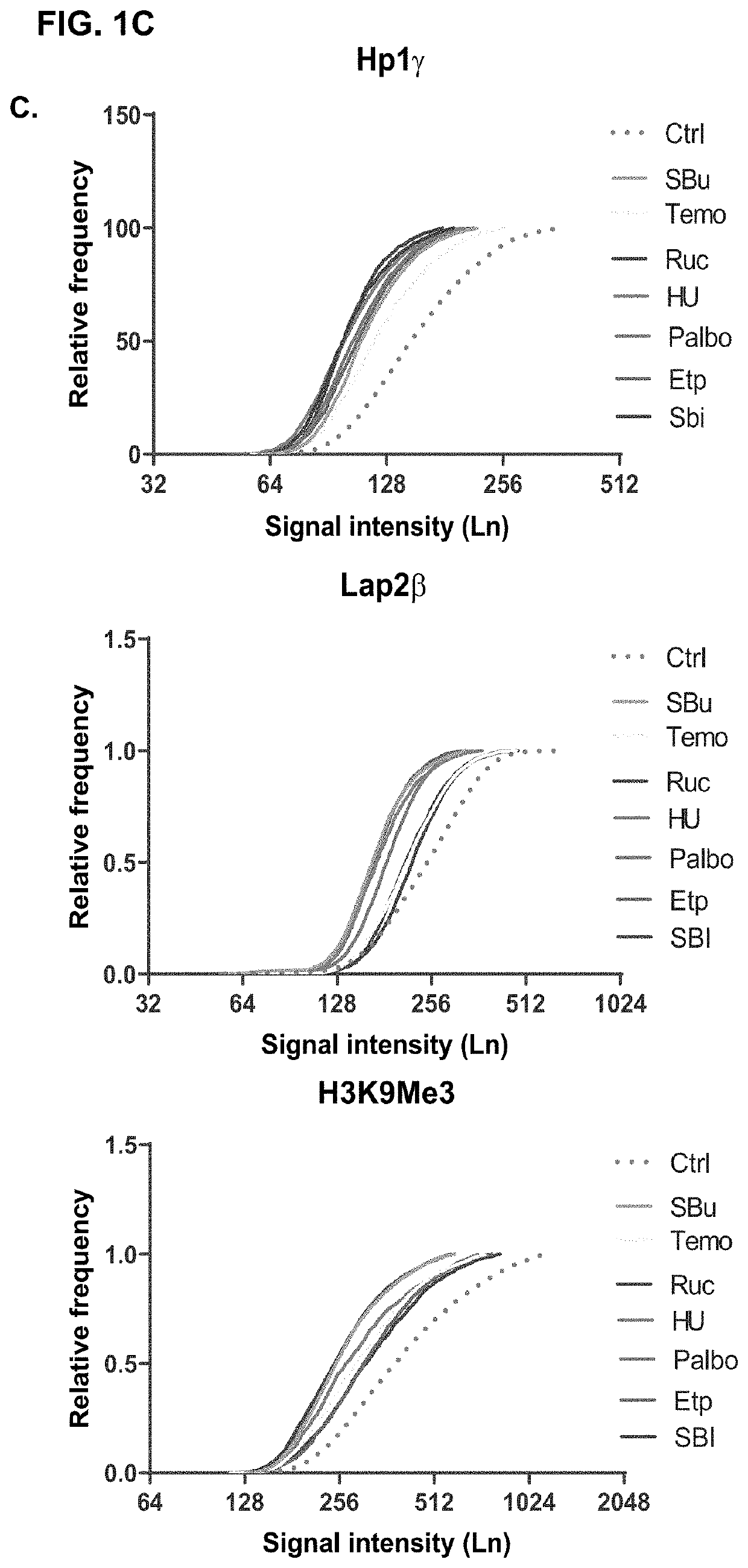
DNA glycosylases are a family of enzymes involved in base excision repair, classified under EC number EC 3.2.2. Base excision repair is the mechanism by which damaged bases in DNA are removed and replaced. DNA glycosylases catalyze the first step of this process. They remove the damaged nitrogenous base while leaving the sugar-phosphate backbone intact, creating an apurinic/apyrimidinic site, commonly referred to as an AP site. This is accomplished by flipping the damaged base out of the double helix followed by cleavage of the N-glycosidic bond.
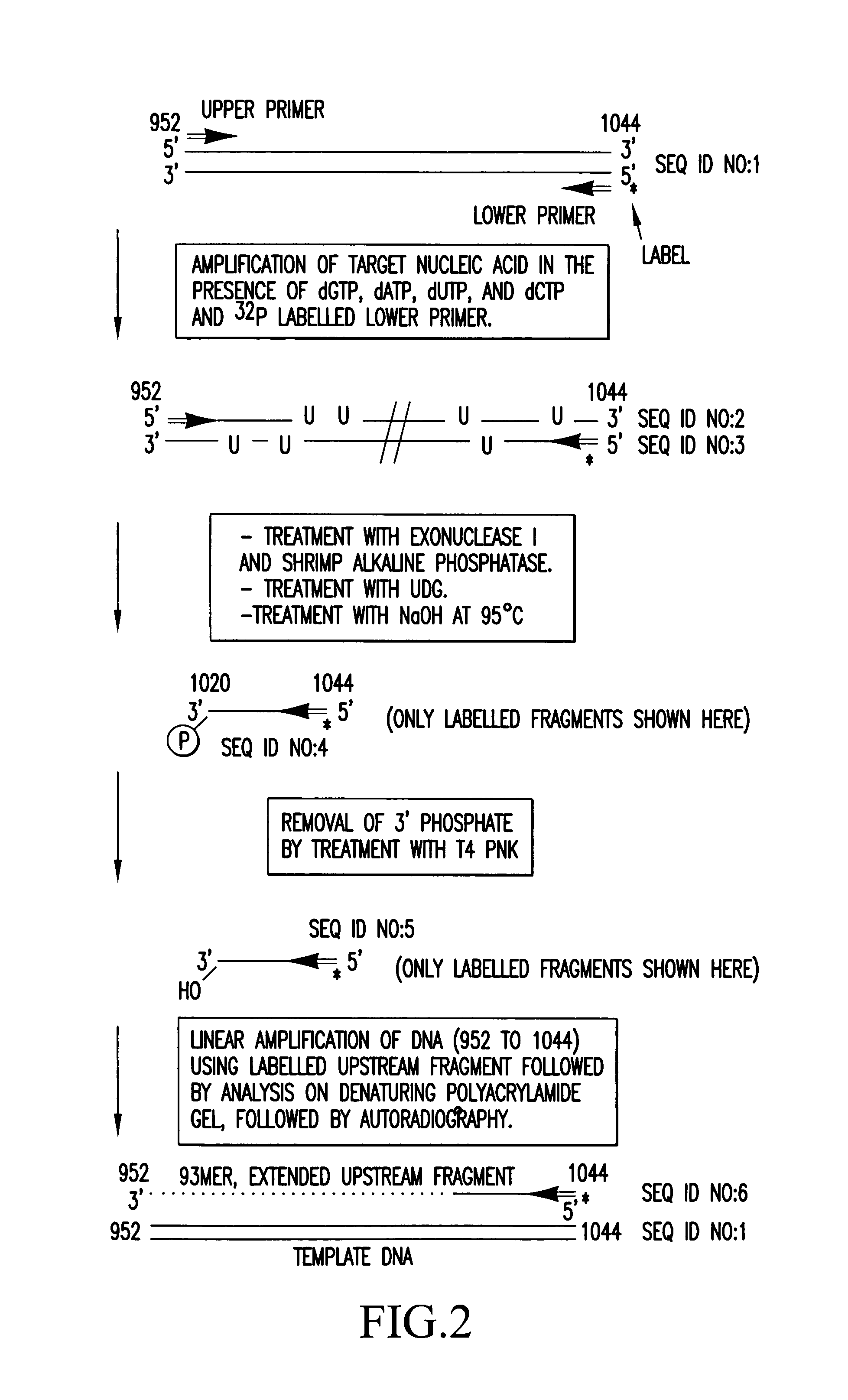
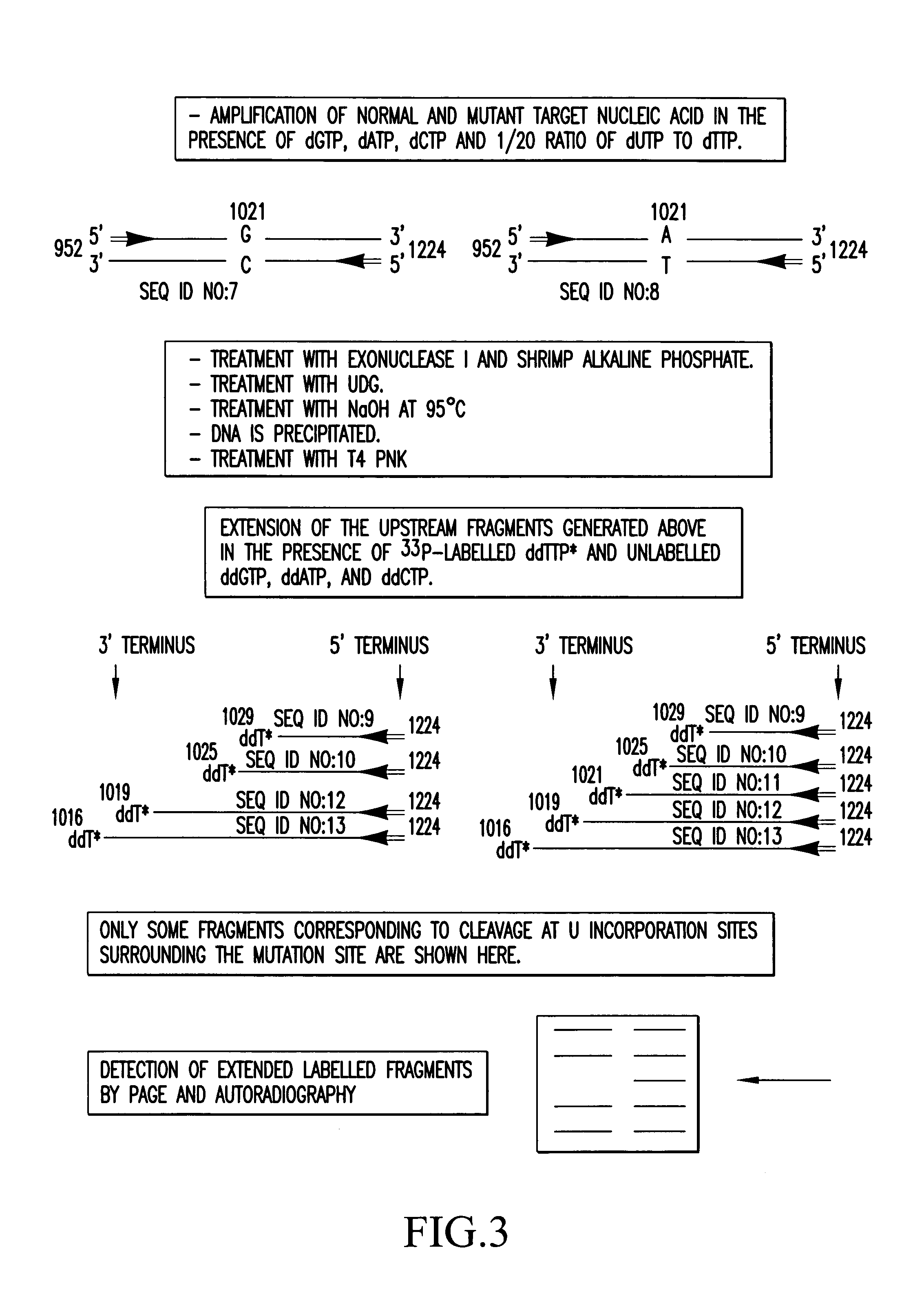
Method for the characterization of nucleic acid molecules involving generation of extendible upstream DNA fragments resulting from the cleavage of nucleic acid at an abasic site
InactiveUS7175982B1Quick filterEasy to detectMicrobiological testing/measurementRecombinant DNA-technologyDNA fragmentationA-DNA
The present invention is drawn to a method for characterising nucleic acid molecules, which comprises the steps of: i) introducing a modified base which is a substrate for a DNA glycosylase into a DNA molecule; ii) excising the modified base with the DNA glycosylase to generate an abasic site; iii) cleaving the DNA at the abasic site to generate and release an extendible upstream DNA fragment having a 3′ hydroxyl terminus; and iv) incubating the released extendible upstream DNA fragment in the presence of an enzyme allowing for extension thereof and an additional template nucleic acid and analysing resultant fragment(s).
Owner:ENTERPRISE IRELAND
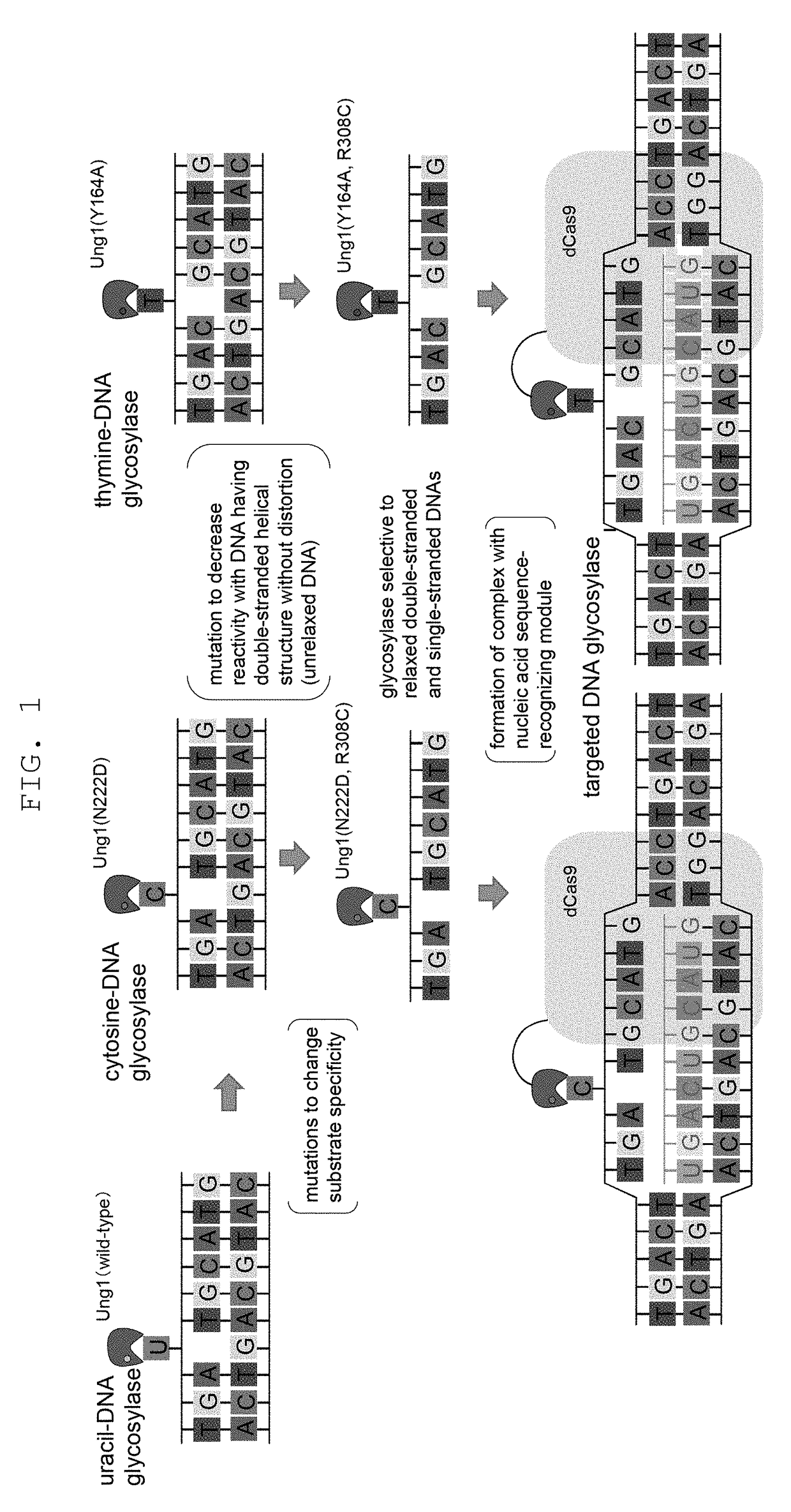
Method for modifying genome sequence to introduce specific mutation to targeted DNA sequence by base-removal reaction, and molecular complex used therein
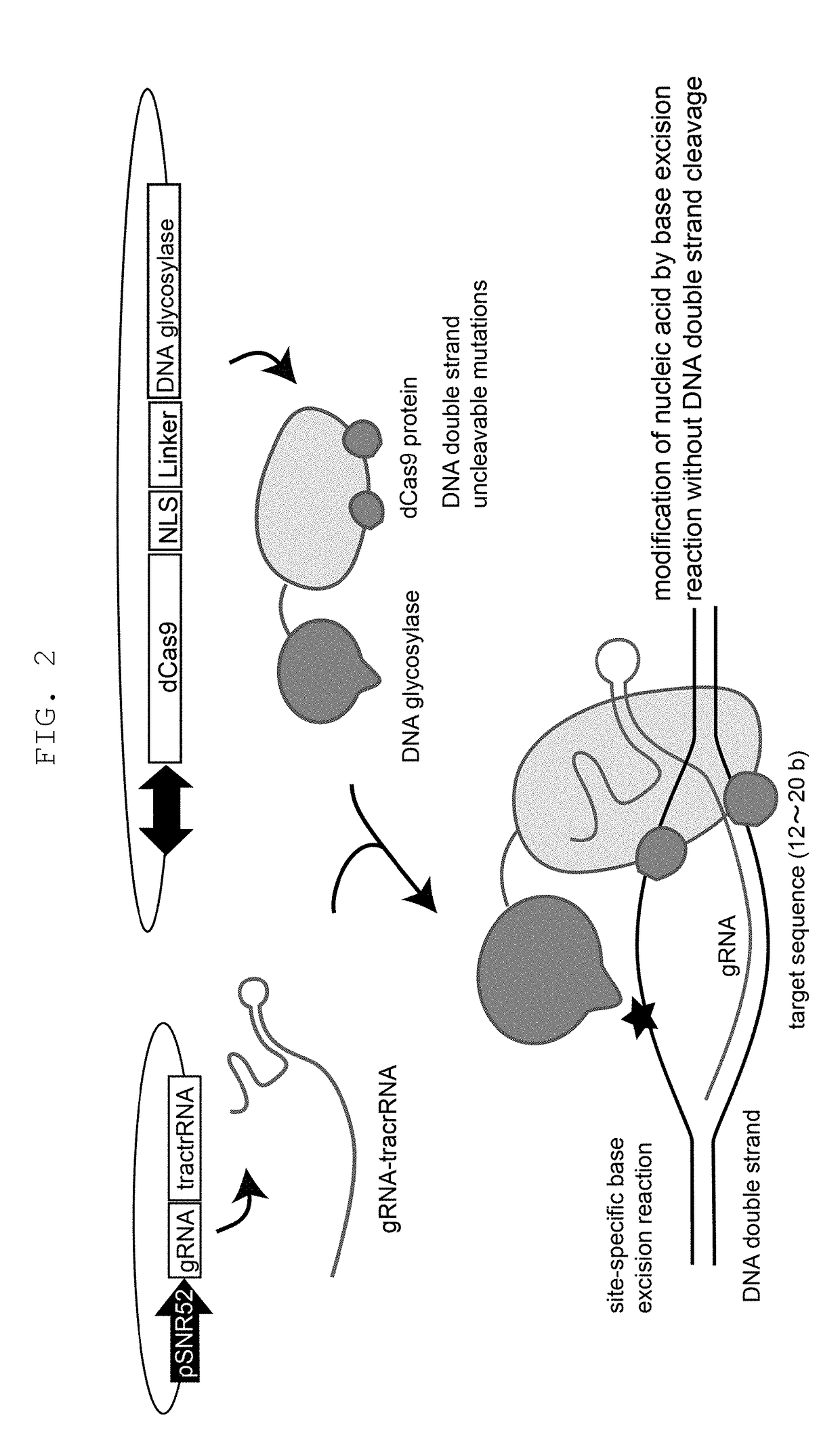
The present invention provides a method of modifying a targeted site of a double stranded DNA, including a step of contacting a complex wherein a nucleic acid sequence-recognizing module that specifically binds to a target nucleotide sequence in a selected double stranded DNA and DNA glycosylase with sufficiently low reactivity with a DNA having an unrelaxed double helix structure (unrelaxed DNA) are bonded, with the double stranded DNA, to convert one or more nucleotides in the targeted site to other one or more nucleotides or delete one or more nucleotides, or insert one or more nucleotides into the targeted site, without cleaving at least one strand of the double stranded DNA in the targeted site.
Owner:KOBE UNIV
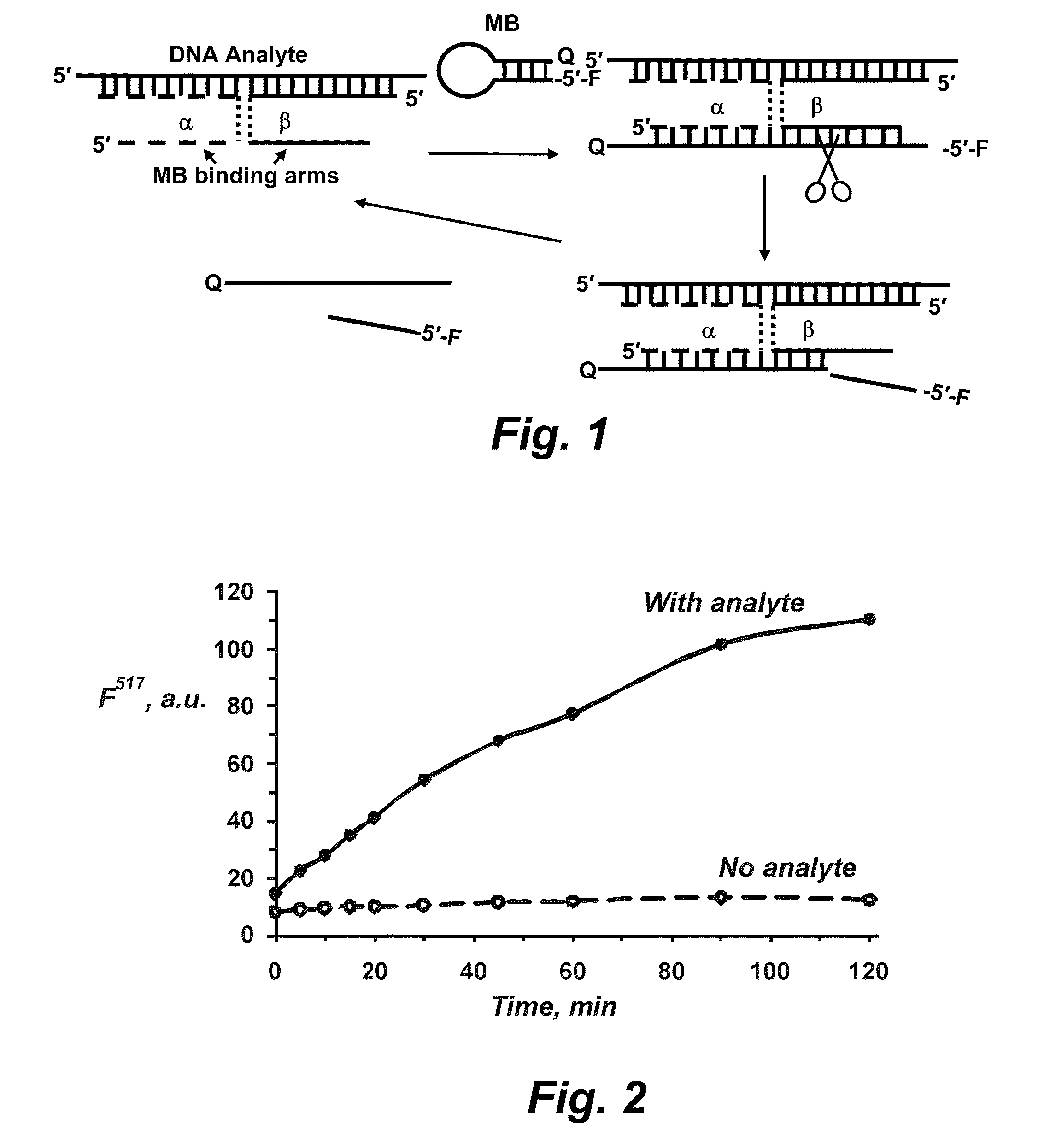
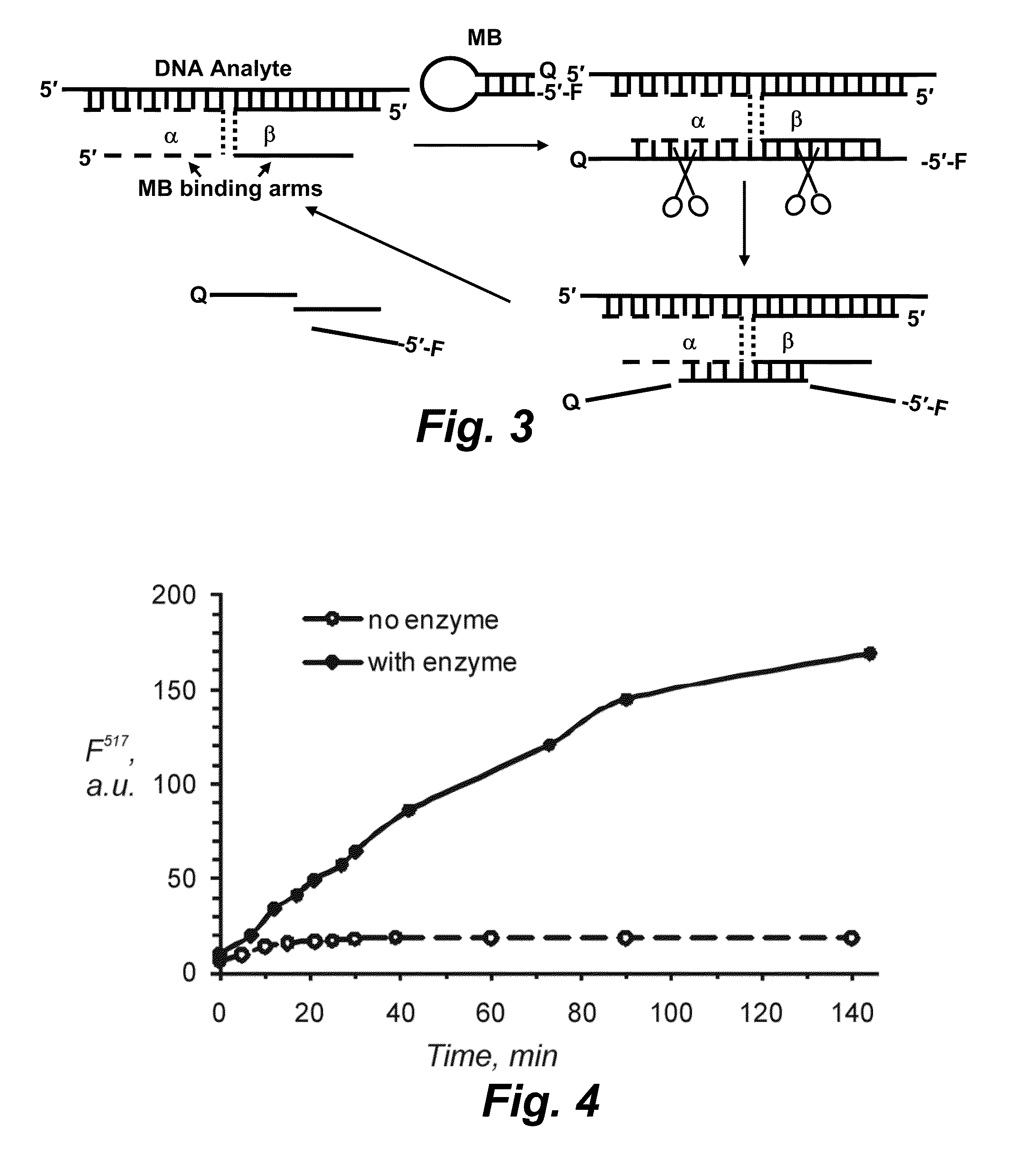
Binary probe system for sensitive detection of target analytes
The present disclosure encompasses systems, and their methods of use, for detecting a target analyte, the systems comprising: (a) a first oligonucleotide probe comprising a reporter oligonucleotide-binding arm complementary to the nucleotide sequence of a first region of a reporter oligonucleotide and an analyte-binding arm selectively binding to a first region of a target analyte, a second oligonucleotide probe comprising a reporter oligonucleotide-binding arm having a nucleotide sequence complementary to the nucleotide sequence of a second region of a reporter oligonucleotide, and an analyte-binding arm having a nucleotide sequence characterized as selectively binding to a second region of a target analyte; and a reporter oligonucleotide comprising a region complementary to the reporter oligonucleotide-binding arm of the first oligonucleotide probe, a second region complementary to the reporter oligonucleotide-binding arm of the second oligonucleotide probe, a fluorophore and a quencher disposed on the reporter oligonucleotide and a cleavable site disposed between the fluorescent label and the quencher. The cleavable site of the reporter oligonucleotide when in the presence of a target analyte can be cleaved by an enzyme such as a restriction endonuclease, an RNase H, a Flap-endonuclease-1 (FEN-1), and a DNA glycosylase.
Owner:UNIV OF CENT FLORIDA RES FOUND INC
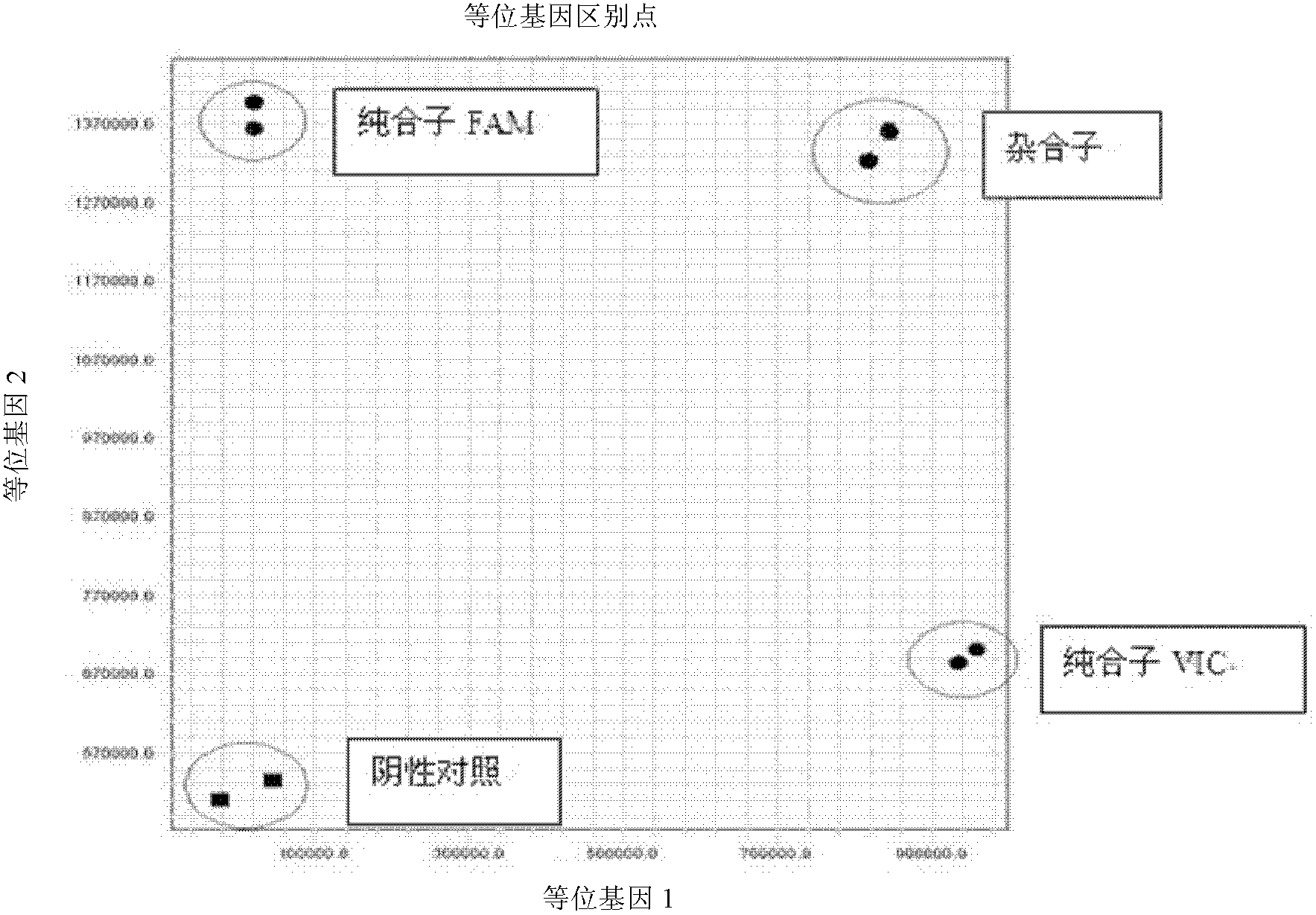
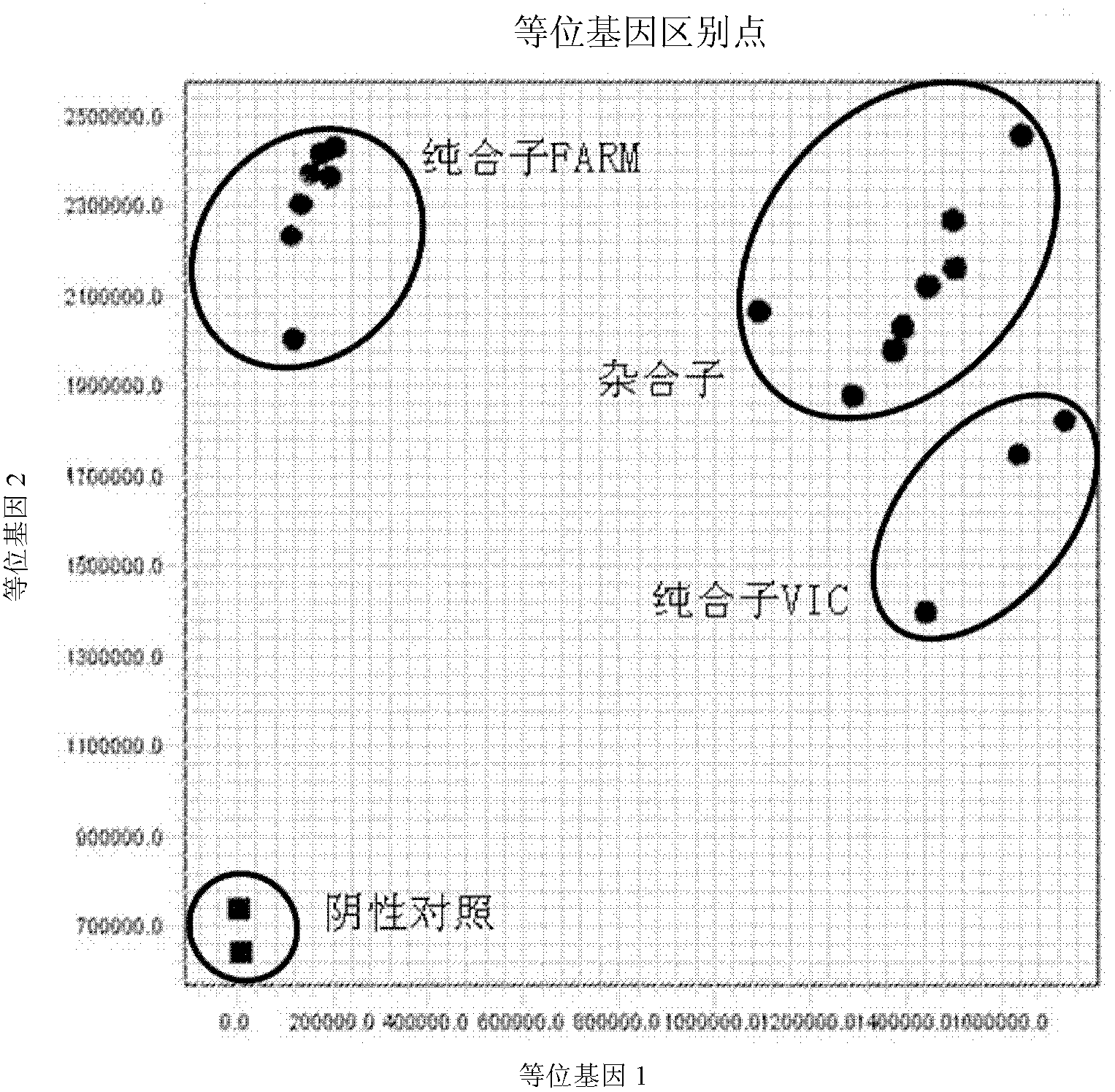
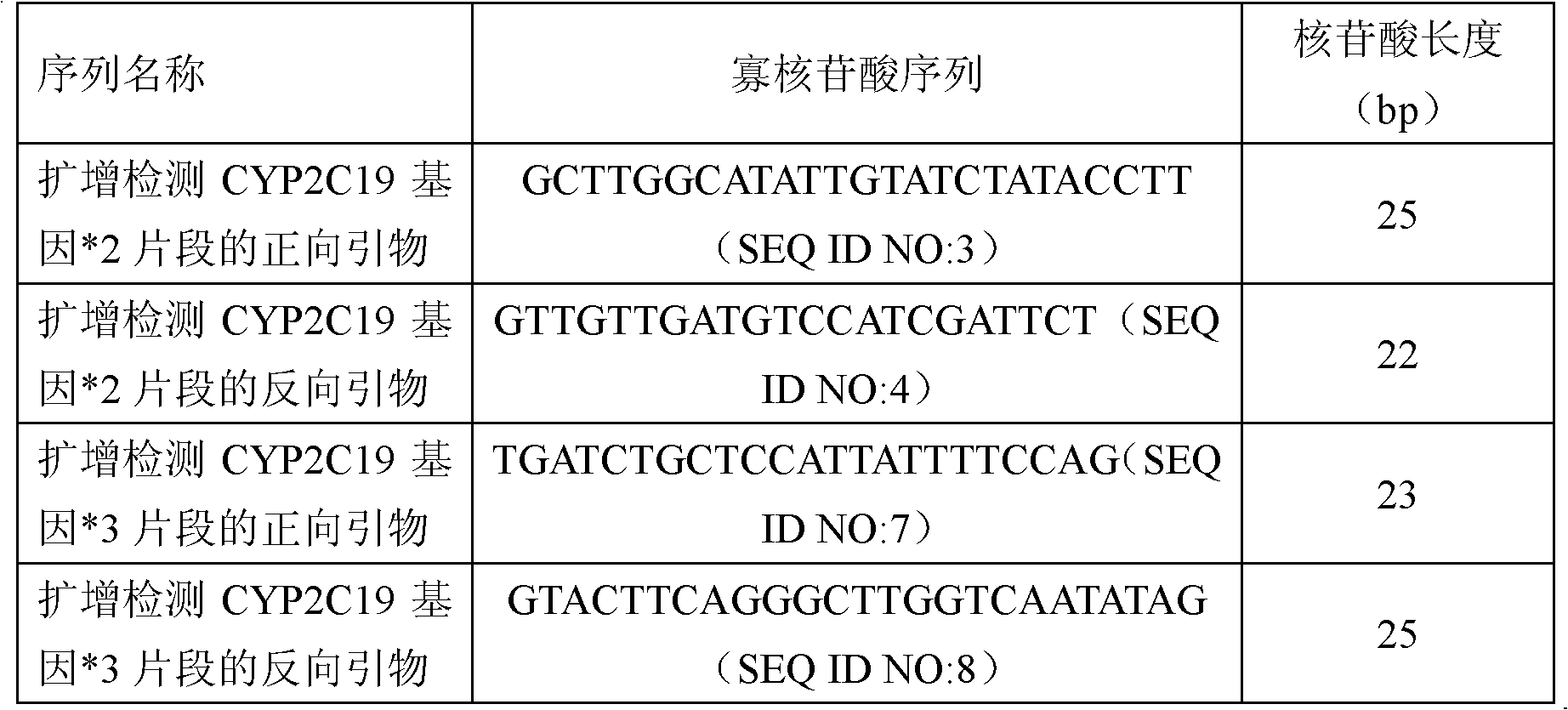
Fluorescent polymerase chain reaction (PCR) kit for detecting CYP2C19 genotypes
InactiveCN102534005AIncreased sensitivityImprove featuresMicrobiological testing/measurementProtein detectionNucleotide
The invention provides a fluorescent polymerase chain reaction (PCR) kit for detecting CYP2C19 genotypes, and belongs to the field of in-vitro nucleic acid testing. The fluorescent PCR kit comprises PCR reaction liquids for detecting CYP2C19*2 and CYP2C19*3 genotypes, Taq DNA polymerase, and uracil-DNA glycosylase, wherein the PCR reaction liquids for detecting the CYP2C19*2 and CYP2C19*3 genotypes respectively comprise PCR amplification primers, minor groove binder (MGB) probes and the like; and nucleotide sequences for detecting the CYP2C19*2 and CYP2C19*3 genotypes are shown as SEQ ID NO:3-4 and SEQ ID NO:5-6 respectively. The kit has high sensitivity and specificity, can monitor the reaction progress in real time, ensures short reaction time, avoids subsequent treatment, can avoid reaction product pollution to the greatest extent, and can replace the traditional protein detection or the common PCR detection to diagnose the CYP2C19 genotypes.
Owner:CHANGSHA 3G BIOTECH
Sequencing primer for qualitative detection of cytochrome oxidase CYP2C19 genetic typing and kit of sequencing primer
InactiveCN102912013AQualitatively accurateIncreased sensitivityMicrobiological testing/measurementDNA/RNA fragmentationPositive controlTaq polymerase
The invention provides a sequencing primer for qualitative detection of cytochrome oxidase CYP2C19 genetic typing and a kit of the sequencing primer and belongs to the field of external nucleic acid detection. The kit comprises uracil DNA (Deoxyribonucleic Acid) glycosylase, Taq polymerase, PCR (Polymerase Chain Reaction) reaction liquor, PCR amplification primers, pyrophosphoric acid sequencing primers and positive control products. The kit is high in sensitivity and good in specificity, PCR products can be simply treated for a pyrophosphoric acid sequenator for sequencing, operation is simple, reaction time is short, the sensitivity of the kit is higher than that of a golden standard-capillary electrophoresis sequencing, and the kit is suitable for use for mutation analysis.
Owner:CHANGSHA 3G BIOTECH
Primer pair and kit for detecting ALDH2 (Aldehyde Dehydrogenase 2) genotype with pyrosequencing method
InactiveCN105177159AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationGenotypeBiotin
The invention relates to a sequencing primer pair for detecting an ALDH2 (Aldehyde Dehydrogenase 2) genotype with a pyrosequencing method and a kit thereof, and belongs to the technical field of in-vitro nucleic acid detection. The primer pair comprises a positive amplification primer, a reverse amplification primer and a sequencing primer, wherein a 5' end of the positive amplifier primer is subjected to biotin labeling. The kit comprises the positive amplification primer, PCR (Polymerase Chain Reaction) reaction liquid containing the reverse amplification primer, the sequencing primer, uracil DNA (Deoxyribose Nucleic Acid) glycosylase and Taq polymerase. The kit provided by the invention has the advantages of accurate detection result, high specificity, short detection period, easiness in operation, and capability of effectively meeting clinical examination requirements. Moreover, the kit further has the advantages that a reaction process can be monitored in real time, the reaction time is short, a PCR product can be subjected to pyrosequencing by a pyrosequencing instrument and high-flux sample detection through simple treatment, and the sensitivity is higher compared with a golden standard method, namely, a capillary electrophoresis sequencing method.
Owner:CHANGSHA 3G BIOTECH
Detection method of prawn IHHNV (infectious hypodermal and hematopoietic necrosis virus) and used nucleic acid isothermal amplification detection kit
InactiveCN101792817AEfficient detectionEasy to handleMicrobiological testing/measurementSodium acetatePositive control
The invention discloses a prawn IHHNV (infectious hypodermal and hematopoietic necrosis virus) nucleic acid isothermal amplification detection kit which comprises a grinding fluid tube containing a grinding fluid, a nucleic acid extracting solution tube A containing a sodium acetate solution, a nucleic acid extracting solution tube B containing absolute ethyl alcohol, a nucleic acid extracting solution tube C containing an alcohol solution with a mass concentration of 70 percent, a TE (tellurium) buffer solution tube containing a TE buffer solution, a UNG (uracil-DNA glycosidase) enzyme tube containing uracil DNA (deoxyribonucleic acid) glycosylase, an LAMP (Loop-mediated isothermal amplification) reaction liquid tube containing an LAMP reaction liquid, a BstDNA polymerase tube containing Bst DNA polymerase, a color-developing agent tube containing nucleic acid dye SYBR Green I, a positive control nucleic acid tube containing prawn IHHNV positive DNA and a negative control tube containing sterilizing double distilled water. The invention also provides a method for detecting the prawn IHHNV by utilizing the detection kit. The method has the characteristics of low cost, high detection sensitivity and easy site operation.
Owner:ZHEJIANG UNIV
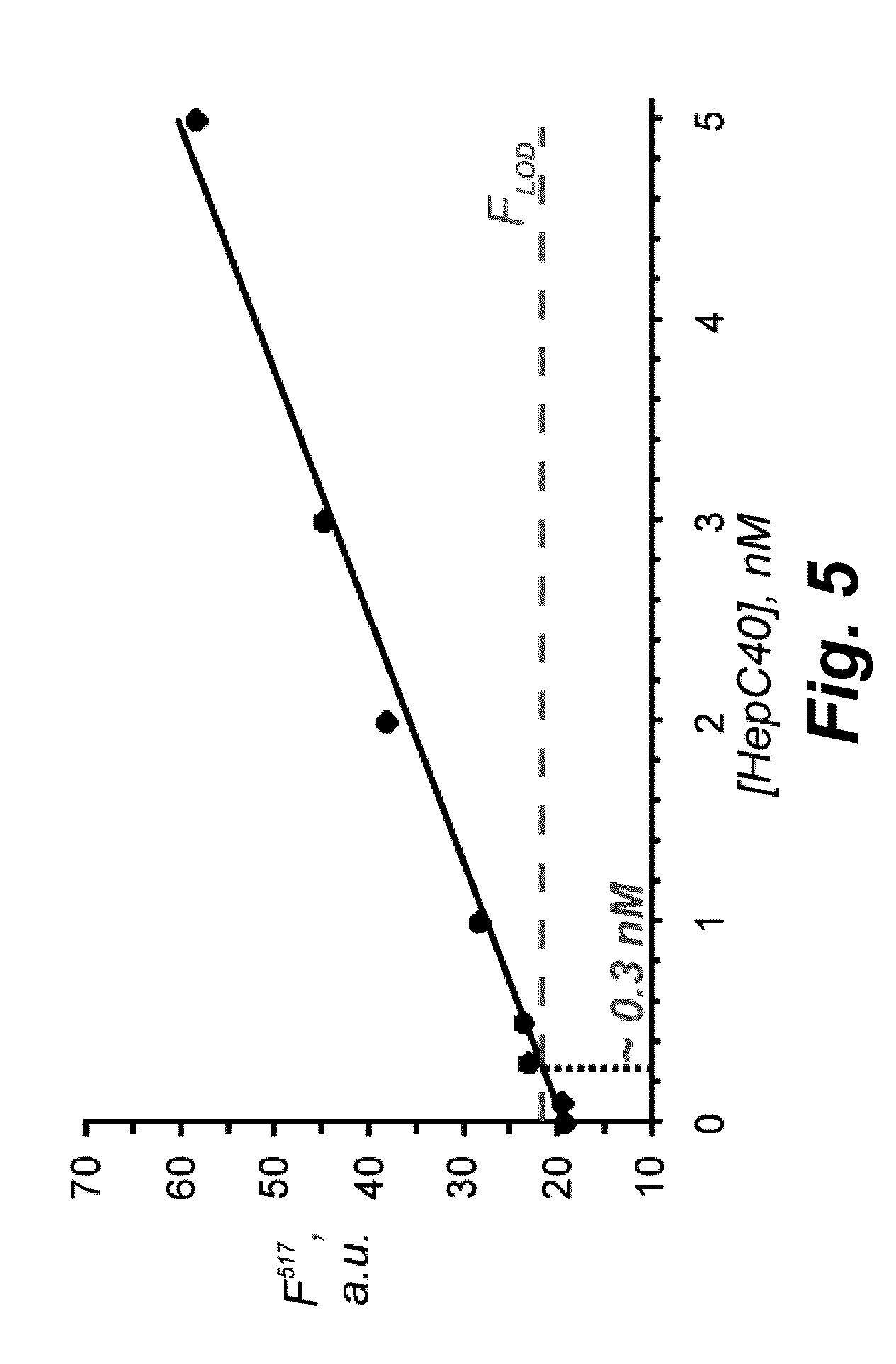
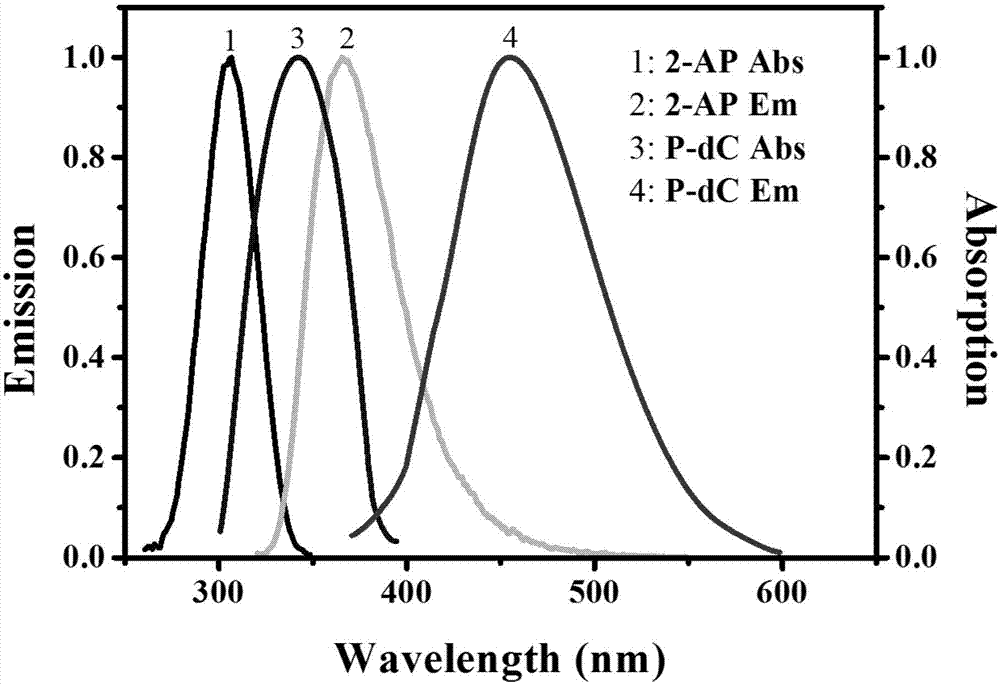
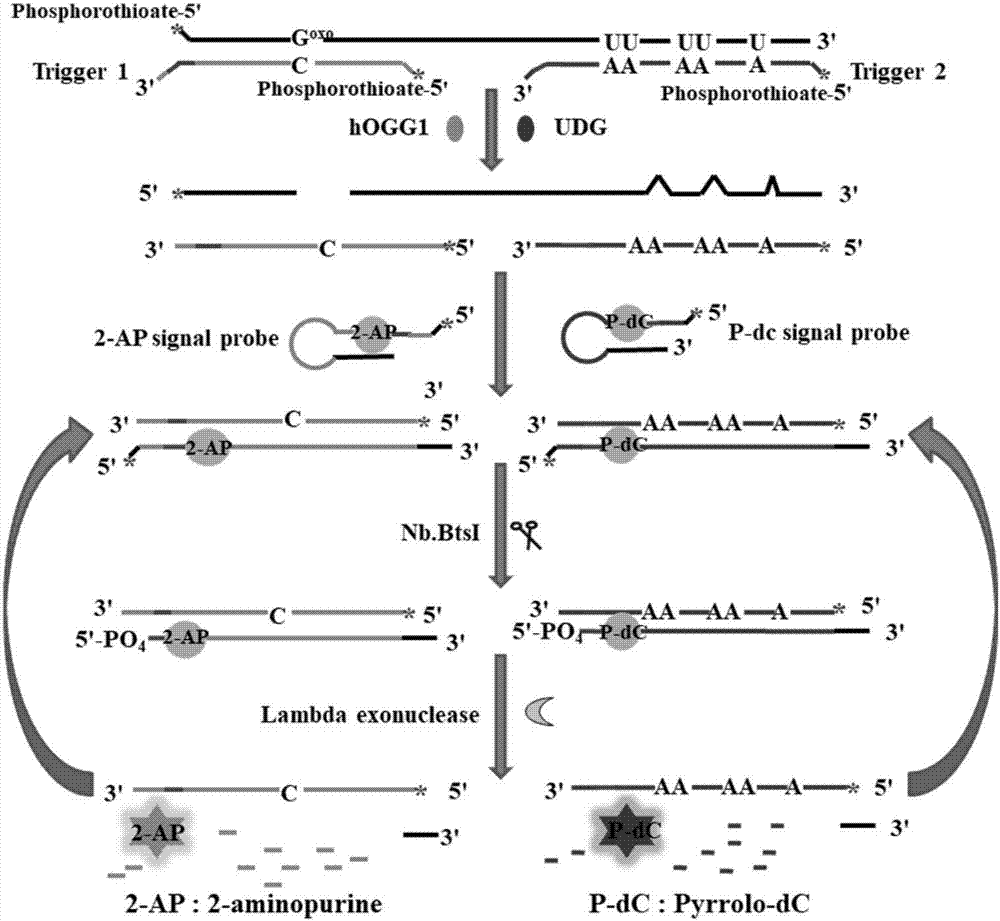
Method for ultra-sensitively simultaneously detecting multiple DNA glycosylases by using intrinsic fluorescent nucleotide
ActiveCN107083437AAchieving Simultaneous DetectionEasy to detectMicrobiological testing/measurementDNA/RNA fragmentationA-DNAFluorophore
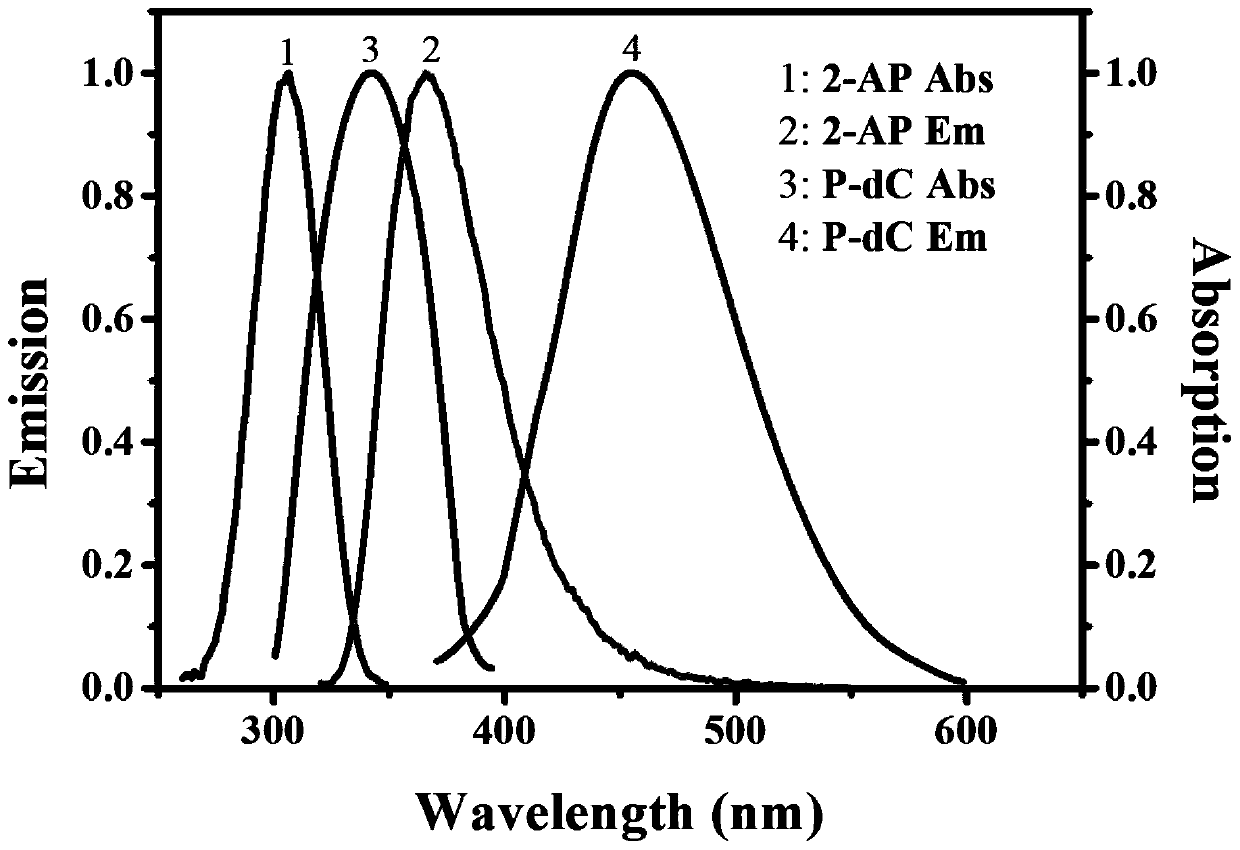
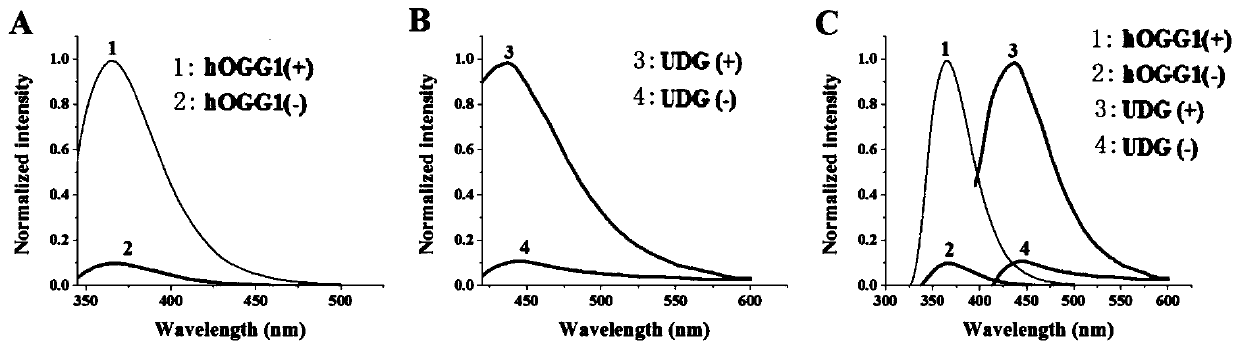
The invention discloses a method for ultra-sensitively simultaneously detecting multiple DNA glycosylases by using intrinsic fluorescent nucleotide. The method is a fast and sensitive fluorescence method for simultaneously detecting multiple DNA glycosylases by adopting 2-aminopurine and pyrrole-deoxycytidine as fluorophores and a DNA molecule as an intrinsic quenching agent and combining excision enzyme-assisted circulating signal amplification; and extra fluorophore and quenching group are not needed for marking, so that the target of simple, intuitive and high-sensitivity detection of an actual sample is achieved, and above all, simultaneous detection of multiple DNA glycosylases is achieved.
Owner:SHANDONG NORMAL UNIV
Method for qualitatively detecting HLA-B*1502 gene with PCR-SSP method and clinical kit
ActiveCN103114138ALow costLow instrument requirementsMicrobiological testing/measurementCarbamazepineMedication use
The invention belongs to the field of biotechnology, and particularly relates to a method for qualitatively detecting HLA-B*1502 gene with a PCR-SSP method and a clinical detection kit. The method comprises the following steps of: finding 6 specific areas capable of effectively identifying HLA-B*1502 allele type; designing 6 specific primers covering the 6 specific areas, and screening out a high-specificity primer pair applicable to PCR-SSP; and adding dUTP and UDG enzyme (uracil-DNA glycosylase) into a reaction system to solve the problem of PCR cross pollution and further improve the detection reliability. An HLA-B*1502 quick and convenient qualitative detection kit is researched and developed accordingly. The method and the clinical detection kit provided by the invention have the advantages of convenience in operation, short time, strong specificity, high accuracy, low cost and the like, and is suitable for realizing individual safe and reasonable drug use through HLA-B*1502 genotype detection before the Chinese or Asian epileptics take carbamazepine and phenytoin sodium.
Owner:GRACELL BIOTECH SHANGHAI CO LTD
Primer pair and kit for detecting VKORC1 (vitamin K epoxide reductase complex subunit 1) genotyping by pyrosequencing
InactiveCN105154569AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationVKORC1Biotin
The invention relates to a primer pair and kit for detecting VKORC1 (vitamin K epoxide reductase complex subunit 1) genotyping by pyrosequencing, belonging to the technical field of in vitro nucleic acid detection. The primer pair comprises VKORC1 G1639A and VKORC1 C1173T forward amplification primers, VKORC1 G1639A and VKORC1 C1173T reverse amplification primers and VKORC1 G1639A and VKORC1 C1173T sequencing primers, wherein 5' terminals of the VKORC1 G1639A forward amplification primer and the VKORC1 C1173T reverse amplification primer are respectively subjected to biotin labelling. The kit comprises the amplification primers, a PCR (polymerase chain reaction) liquid 1, a PCR liquid 2, the sequencing primers, uracil DNA (deoxyribonucleic acid)glycosylase and Taq polymerase. The kit provided by the invention has the advantages of accurate detection results, high specificity, short detection period, simplicity in operation, capability of effectively meeting the requirements of clinical examination, capability of monitoring the reaction process in real time, short reaction time, sequencing of PCR products on a pyrosequencing instrument after the PCR products are simply treated, high throughput sample detection and higher sensitivity than gold standard methods, namely capillary electrophoresis sequencing methods.
Owner:CHANGSHA 3G BIOTECH
Primer pair and kit for detecting CYP3A4 genotyping by pyrosequencing
InactiveCN105154568AEasy to handleHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationBiotinTaq polymerase
The invention relates to a primer pair and kit for detecting CYP3A4 genotyping by pyrosequencing, belonging to the technical field of in vitro nucleic acid detection. The primer pair comprises a forward amplification primer, a reverse amplification primer and a sequencing primer, wherein a 5' terminal of the forward amplification primer is subjected to biotin labelling. The kit comprises the forward amplification primer, a PCR (polymerase chain reaction) liquid containing the reverse amplification primer, the sequencing primer, uracil DNA (deoxyribonucleic acid)glycosylase and Taq polymerase. The kit provided by the invention has the advantages of accurate detection results, high specificity, short detection period, simplicity in operation, capability of effectively meeting the requirements of clinical examination, capability of monitoring the reaction process in real time, short reaction time, sequencing of PCR products on a pyrosequencing instrument after the PCR products are simply treated, high throughput sample detection and higher sensitivity than gold standard methods, namely capillary electrophoresis sequencing methods.
Owner:CHANGSHA 3G BIOTECH
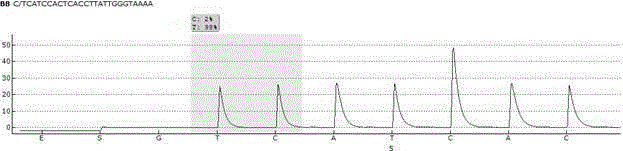
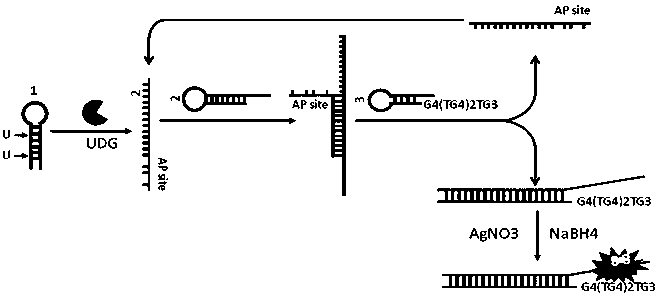
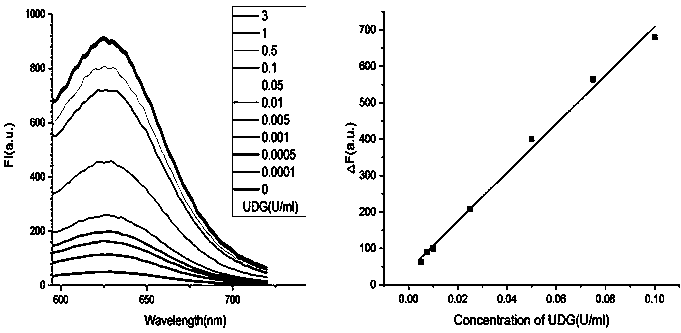
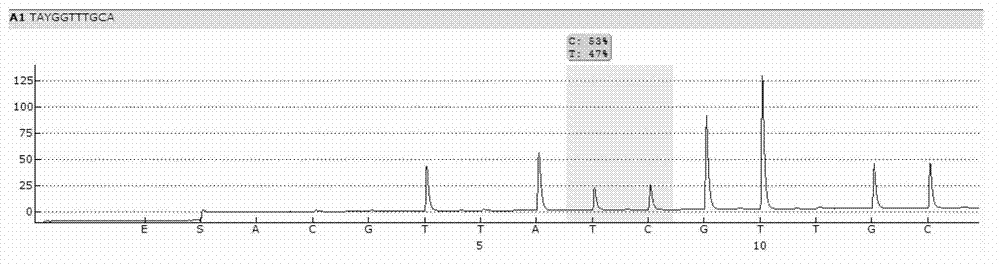
Nonenzymatic biosensor for detecting activity of uracil-DNA glycosylase
ActiveCN110734961AStrong specificityHigh sensitivityMicrobiological testing/measurementBiological material analysisA-DNABio sensor
The invention provides a nonenzymatic biosensor for detecting activity of uracil-DNA glycosylase. The nonenzymatic biosensor comprises a UDG template strand 1, a silver nanocluster DNA template strand2, and a DNA strand 3 rich in cytosine. A fluorescence method is used for detecting the activity of the uracil-DNA glycosylase, the stimulation wave lenagth of fluoroscopic examination is 570nm, thelength of transmitted waves is 630nm, and the detection wave band is 590-720nm. The biosensor provided by the invention is good in specificity, high in sensitivity, mild in reaction condition and highin reaction speed. Because the reaction process is free from addition of external enzymes, and through silver nanocluster fluoroscopic examination, the biosensor is simple to operate, low in reactionrequirements, short in detection cycle, stable in properties, and good in repeatability, and is suitable for detection of UDG in the field of medical health.
Owner:FUZHOU UNIV
Sequencing primer pair for qualitatively detecting human BRAF V600E gene mutation and kit thereof
ActiveCN102925555AQualitatively accurateIncreased sensitivityMicrobiological testing/measurementDNA/RNA fragmentationGenes mutationTaq polymerase
The invention provides a sequencing primer pair for qualitatively detecting human BRAF V600E gene mutation and a kit thereof, belonging to the field of detection for in-vitro nucleic acid. The kit comprises a uracil DNA (deoxyribonucleic acid) glycosylase, a Taq polymerase, PCR (polymerase chain reaction) solution, a PCR amplification primer, a pyrosequencing primer and a positive reference substance. The kit provided by the invention is high in sensitivity, good in specificity, capable of sequencing a PCR product on a pyrosequencer after performing a simple treatment, simple and convenient in operation, short in reaction time, higher in sensitivity compared with golden standard-capillary electrophoresis sequencing, and more suitable for mutation analysis.
Owner:CHANGSHA 3G BIOTECH
Primer pair and kit for detecting folate metabolism-related gene polymorphism in hypertensive patients
InactiveCN107586837AEasy to handleHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationPositive controlBiotin
The invention relates to a primer pair and a kit for detecting folate metabolism-related gene polymorphism in hypertensive patients, and belongs to the technical field of in vitro nucleic acid detection. The primer pair comprises a forward amplification primer, a reverse amplification primer and a sequencing primer, wherein the 5' end of the reverse amplification primer is labeled with biotin. Thekit comprises a PCR (Polymerase Chain Reaction) solution containing a forward amplification primer and a reverse amplification primer, a sequencing primer, uracil DNA (Deoxyribonucleic Acid) glycosylase, Taq polymerase and a positive control product. The kit provided by the invention has the advantages of accurate detection result, high specificity, short detection period, and simple operation, and the clinical inspection requirements can be effectively met; in addition, the kit also has the advantages of real-time monitoring of reaction progress, and short reaction time, the PCR product canbe used for sequencing on a pyrosequencing instrument after being simply treated, the sensitivity is higher than that of a gold standard method, i.e., a capillary electrophoresis sequencing method, and the kit is more applicable to mutation analysis.
Owner:CHANGSHA 3G BIOTECH
Identification of genetic modifications
ActiveUS20190040457A1Minimize side effectsSugar derivativesMicrobiological testing/measurementSide effectNucleotide
Described are methods of detecting modified nucleotide bases in a DNA sample using specific DNA glycosylases to excise target modified bases. DNA molecules are then labeled using a DNA polymerase lacking 3′→5′ exo-nuclease activity and strand displacement activity. The methods can be used to detect epigenetic changes and DNA damage. Provided are methods for diagnosing a disease or condition, determining risk of a disease or condition, identifying appropriate treatment, monitoring effectiveness of treatment, and monitoring side effects of treatment in subjects based on detection of modified bases. Also provided are methods for determining environmental exposure, or an environmental exposure time, of a biological sample containing DNA. Also provided are kits, systems, and devices for performing the described methods.
Owner:WAKE FOREST UNIV HEALTH SCI INC
Modulating uracil-dna glycosylase and uses thereof
InactiveUS20180221438A1Reduces B cell clonal expansionPrevent proliferationOrganic active ingredientsMicrobiological testing/measurementUracil-DNA glycosylaseBiological activation
The present invention concerns a method for the prevention and / or treatment of an activation-induced deaminase (AID)-associated disease in a subject in need thereof, said method comprising administering an effective amount of an uracil-DNA glycosylase (UNG) inhibitor, or a composition comprising the inhibitor, and a pharmaceutically acceptable carrier, to a subject having pathogenic cells expressing AID, uracil-DNA glycosylase (UNG) and mismatch repair pathway (MMR). Also provided are kits comprising an UNG inhibitor, methods of stratifying a subject having an AID-associated disease, uses and compositions for use of the UNG inhibitor.
Owner:UNIV OF MIAMI +1
DTNS-mediated method for detecting activity of 8-OG DNA glycosylase
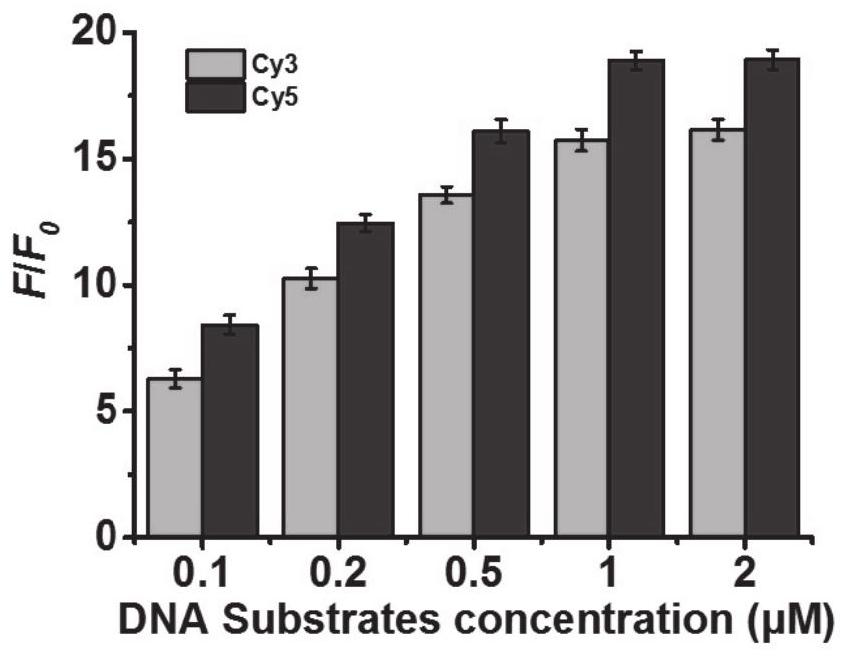
ActiveCN113789363AGood intakeAccurate in situ imagingMicrobiological testing/measurementBiological material analysisExtracellularReceptor
The invention belongs to the technical field of biological detection and molecular biology, and particularly relates to a DTNS-mediated method for detecting the activity of 8-OGDNA glycosylase. Under the action of 8-OG DNA glycosylase, the structure of the DTNS is changed from an open state to a closed state, so that the distance between a Cy3 donor and a Cy5 receptor is close, and efficient FRET is initiated. By utilizing the method, the activity of extracellular 8-OG DNA glycosylase can be sensitively and selectively detected, and false positive signals generated by nuclease degradation can be prevented based on an FRET signal output mode, and accurate imaging in living cells is facilitated.
Owner:LIAOCHENG UNIV
Fluorescence chemical sensor for simultaneously detecting multiple DNA glycosylase and detection method and application thereof
ActiveCN113088557ASimple methodNo troublesome operationMicrobiological testing/measurementFluorophoreRecognition sequence
The invention belongs to the technical field of molecular detection, and particularly relates to a fluorescent chemical sensor for simultaneously detecting multiple DNA glycosylase as well as a detection method and application of the fluorescent chemical sensor. The fluorescent chemical sensor comprises a double-stranded DNA substrate, two or more than two DNA glycosylase recognition sequences and a reporter probe, wherein the double-stranded DNA substrate comprises a recognition sequence for recognizing one or more DNA glycosylase; the 5' tail ends of the two chains of the double-chain DNA substrate are respectively modified with fluorescence quenching groups; the reporter probe is modified by a fluorophore; the base sequence connected with the fluorescence quenching group is hybridized with the reporter probe to form a base pair. According to the invention, the signal amplification technology based on the fluorescence chemical sensor can realize ultra-sensitive detection of various DNA glycosylase.
Owner:SHANDONG NORMAL UNIV
Chemical cocktail for inducing senescence in human neurons to promote disease modeling and drug discovery
PendingUS20210311023A1High expressionReduce expressionDrug screeningNervous system cellsCell phenotypeChemical mixtures
Provided herein are methods and compositions for inducing chemical senescence in neurons and methods of using chemically induced senescent neurons for modeling neurodegenerative disease and drug discovery. The methods include contacting human neurons with a culture medium comprising an inhibitor of DNA glycosylase 1, an autophagy inhibitor, and an HIV protease inhibitor to obtain an in vitro population of senescent neurons within about 4 days. When the neurons are obtained from a patient having a neurodegenerative disease, chemically induced senescent neurons obtained by these methods recapitulate cellular and subcellular phenotypes observed in individuals with the neurodegenerative disease.
Owner:WISCONSIN ALUMNI RES FOUND
Method capable of detecting activity of multiple DNA glycosylases
The invention relates to a detection system for detecting DNA glycosylases. The detection system comprises (a) a double-stranded DNA probe, wherein the double-stranded DNA probe comprises two strandsT1 and T2; the two strands T1 and T2 can form a double-stranded DNA structure; the strand T1 comprises at least one base capable of being recognized by to-be-detected DNA glycosylases, a fluorophore and a separate label; and the fluorophore and the separate label are located at two ends of the at least one base capable of being recognized by the DNA glycosylases separately; (b) a component capableof breaking a glucoside-phosphate bond at a nucleic acid abasic site; and (c) a solid-phase carrier with a separate bound label. The detection system provided by the invention is high in flux, smallin required sample volume, large in signal window, simple and convenient to operate and low in cost, and can detect multiple DNA glycosylases.
Owner:EPITAS BIOSCIENCES (SHANGHAI) CO LTD
Primer pair and kit for detecting hepatitis B canceration susceptibility gene polymorphism
InactiveCN107586840AEasy to handleHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationPositive controlBiotin
The invention relates to a primer pair and a reagent kit for detecting hepatitis B canceration susceptibility gene polymorphism, and belongs to the technical field of in vitro nucleic acid detection.The primer pair comprises a forward amplification primer, a reverse amplification primer and a sequencing primer, wherein the 5'-end of the reverse amplification primer is labeled with biotin. The kitcomprises a PCR (Polymerase Chain Reaction) solution containing a forward amplification primer, reverse amplification primer, a sequencing primer, uracil DNA (Deoxyribonucleic Acid) glycosylase, Taqpolymerase and a positive control product. The kit provided by the invention has the advantages of accurate detection result, high specificity, short detection period, and simple operation, and the clinical inspection requirements can be effectively met; in addition, the kit also has the advantages of real-time monitoring of reaction progress, and short reaction time, the PCR product can be used for sequencing on a pyrosequencing instrument after being simply treated, the sensitivity is higher than that of a gold standard method, i.e., a capillary electrophoresis sequencing method, and the kitis more applicable to mutation analysis.
Owner:CHANGSHA 3G BIOTECH
Method for qualitatively detecting HLA-B*1502 gene with PCR-SSP method and clinical kit
ActiveCN103114138BLow costLow instrument requirementsMicrobiological testing/measurementCarbamazepineMedication use
The invention belongs to the field of biotechnology, and particularly relates to a method for qualitatively detecting HLA-B*1502 gene with a PCR-SSP method and a clinical detection kit. The method comprises the following steps of: finding 6 specific areas capable of effectively identifying HLA-B*1502 allele type; designing 6 specific primers covering the 6 specific areas, and screening out a high-specificity primer pair applicable to PCR-SSP; and adding dUTP and UDG enzyme (uracil-DNA glycosylase) into a reaction system to solve the problem of PCR cross pollution and further improve the detection reliability. An HLA-B*1502 quick and convenient qualitative detection kit is researched and developed accordingly. The method and the clinical detection kit provided by the invention have the advantages of convenience in operation, short time, strong specificity, high accuracy, low cost and the like, and is suitable for realizing individual safe and reasonable drug use through HLA-B*1502 genotype detection before the Chinese or Asian epileptics take carbamazepine and phenytoin sodium.
Owner:GRACELL BIOTECH SHANGHAI CO LTD
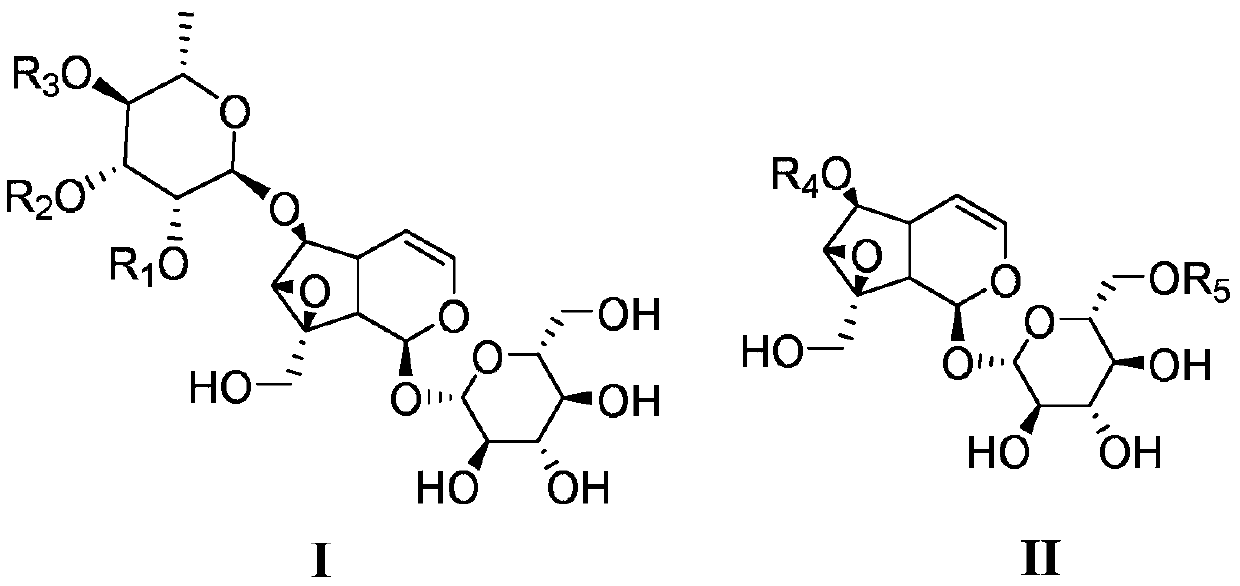
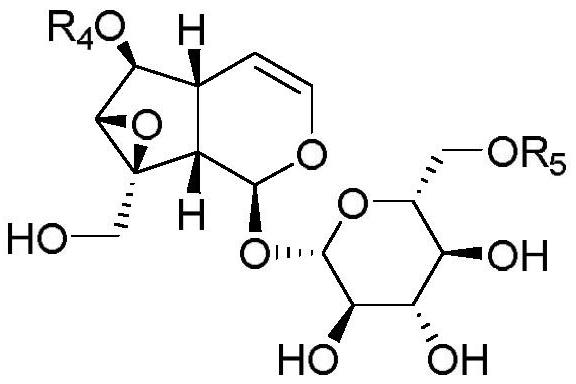
Catalpol derivative and application thereof
ActiveCN109734759AEnhanced inhibitory effectSignificant technological progressSugar derivativesAntipyreticChemical industryStereoisomerism
The invention belongs to the technical field of the medicine and chemical industry, discloses a natural catalpol derivative and a novel modified catalpol derivative derived from catalposide and picroside II, and provides a preparation method of the derivative and application of the derivative in the preparation of anti-inflammatory drugs. The structure of the natural catalpol derivative is shown in a formula (I) or a formula (II), and the structure of the novel modified catalpol derivative derived from the catalposide and the picroside II is shown in a formula (III) or a formula (IV). Experimental results show that compounds of which the structures are shown in formula (I), formula (II), formula (III) and formula (IV) or hydrates of the compounds, pharmaceutically acceptable salt, tautomers, stereisomers and precursor compounds have a significant inhibiting effect on 8-hydroxytridine DNA glycosylase 1 (8-hydroxytridine DNA glycosylase 1, OGG1), and has wide application space in the preparation of the anti-inflammatory drugs and antitumor drugs.
Owner:SHANGHAI UNIV OF T C M
A kind of catalpol derivative and its application
ActiveCN109734759BEnhanced inhibitory effectSignificant technological progressSugar derivativesAntipyreticAntiinflammatory drugPharmaceutical medicine
The invention belongs to the technical field of medicine and chemical industry, and discloses a class of natural catalpol derivatives and a class of new catalpol derivatives derived from catalbin and picroside II modification. The invention also provides the preparation method of the above derivatives and Application in the preparation of anti-inflammatory drugs. The structure of the natural catalpol derivatives involved in the present invention is as shown in formula (I) or formula (II), and the structure of the new catalpol derivatives derived from catalbin and pubetroside II modification is as shown in formula (III) or Shown in formula (IV). Experiments have proved that the compounds of formula (I), formula (II), formula (III) and formula (IV) described in the present invention or their hydrates, pharmaceutically acceptable salts, tautomers, stereoisomers, The precursor compound can significantly inhibit 8-hydroxyguanine DNA glycosylase 1 (8-hydroxytridine DNA glycosylase 1, OGG1), and has a broad application space in the preparation of anti-inflammatory drugs and anti-tumor drugs.
Owner:SHANGHAI UNIV OF T C M
Loop-mediated isothermal amplification primers and kit for detecting acanthamoeba
InactiveCN102230016BSensitive detectionQuick checkMicrobiological testing/measurementDNA/RNA fragmentationUracilKeratitis
The invention discloses loop-mediated isothermal amplification primers and kit for detecting acanthamoeba. The sequences of the primers are represented by SEQ ID No.1, SEQ ID No.2, SEQ ID No.3 and SEQ ID No.4 respectively. The loop-mediated isothermal amplification primers designed according to the sequence of the conserved region of the gene of the acanthamoeba can flexibly, quickly and safely detect acanthamoeba; meanwhile, in the kit disclosed by the invention, false positive caused by nucleic acid pollution in a multiple detection process can be radically eliminated by using uracil-DNA glycosylase (UNG), so the problem of susceptibility to pollution and interference, which limits the wide application of a loop-mediated isothermal amplification technique, is solved. When the kit disclosed by the invention is used, the acanthamoeba causing amebic keratitis can be detected within 3 hours, and instead of expensive instrument and equipment, only a water bath pot or metal bath and a centrifuge are needed in a detection process; the detection result is very easy to determine; and compared with the conventional detection technique, the kit is low in cost, the kit is very safe for operators and the environment for the whole process is free from toxic reagents, and the detection sensitivity of the kit is very high.
Owner:SHANDONG EYE INST
A method for the ultrasensitive simultaneous detection of multiple DNA glycosylases using intrinsically fluorescent nucleotides
ActiveCN107083437BAchieving Simultaneous DetectionStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationNucleotideFluorophore
Owner:SHANDONG NORMAL UNIV
Fluorescent chemical sensor for simultaneous detection of multiple dna glycosylases, detection method and application thereof
ActiveCN113088557BSimple methodNo troublesome operationMicrobiological testing/measurementRecognition sequenceDouble strand
The invention belongs to the technical field of molecular detection, in particular to a fluorescent chemical sensor for simultaneously detecting multiple DNA glycosylases, a detection method and application thereof. The fluorescent chemical sensor includes a double-stranded DNA substrate, two or more DNA glycosylase recognition sequences, and a reporter probe; the double-stranded DNA substrate includes a double-stranded DNA substrate for identifying one or more DNA glycosylation The enzyme recognition sequence, the 5' ends of the two strands of the double-stranded DNA substrate are respectively modified with fluorescent quenching groups, the reporter probe is modified with fluorescent groups, and the base sequence connected to the fluorescent quenching group is the same as that of the reporter probe. The needles hybridize to form base pairs. Signal amplification technology based on fluorescent chemical sensors can realize ultrasensitive detection of various DNA glycosylases.
Owner:SHANDONG NORMAL UNIV
Detection method of prawn IHHNV (infectious hypodermal and hematopoietic necrosis virus) and used nucleic acid isothermal amplification detection kit
InactiveCN101792817BEfficient detectionEasy to handleMicrobiological testing/measurementSodium acetatePositive control
Owner:ZHEJIANG UNIV
8-oxoguanine DNA glycosylase determination method based on background signal inhibition probe, kit and application thereof
ActiveCN113186248AImprove detection limitLow costMicrobiological testing/measurementEnzyme digestionEnzyme assay
The invention belongs to the technical field of biology, and particularly relates to an 8-oxoguanine DNA glycosylase determination method based on a background signal inhibition probe, a kit and an application thereof. According to the present invention, the selectivity of Lambda exonuclease on the substrate structure and the recognition cutting effect of 8-oxo guanine DNA glycosylase on 8-oxo guanine are utilized, and the inhibition effect of the background signal inhibition probe on the lambda exo side activity is combined so as to construct the new 8-oxo guanine DNA glycosylase fluorescence determination method. The method specifically comprises the following steps: mixing a lambda exo buffer solution, a reporter probe sequence containing a fluorophore and a quenching group, and a background signal inhibition probe sequence containing 8-oxo guanine; heating the mixed solution to 85 DEG C, then gradually cooling to 37 DEG C, and adding a sample to be detected; incubating at 37 DEG C; and after incubation is finished, adding lambda exo, carrying out enzyme digestion reaction, and then carrying out fluorescence detection.
Owner:HUAZHONG UNIV OF SCI & TECH
Sequencing primer pair for qualitatively detecting human BRAF V600E gene mutation and kit thereof
ActiveCN102925555BQualitatively accurateIncreased sensitivityMicrobiological testing/measurementDNA/RNA fragmentationTaq polymeraseGene mutation
The invention provides a sequencing primer pair for qualitatively detecting human BRAF V600E gene mutation and a kit thereof, belonging to the field of detection for in-vitro nucleic acid. The kit comprises a uracil DNA (deoxyribonucleic acid) glycosylase, a Taq polymerase, PCR (polymerase chain reaction) solution, a PCR amplification primer, a pyrosequencing primer and a positive reference substance. The kit provided by the invention is high in sensitivity, good in specificity, capable of sequencing a PCR product on a pyrosequencer after performing a simple treatment, simple and convenient in operation, short in reaction time, higher in sensitivity compared with golden standard-capillary electrophoresis sequencing, and more suitable for mutation analysis.
Owner:CHANGSHA 3G BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com