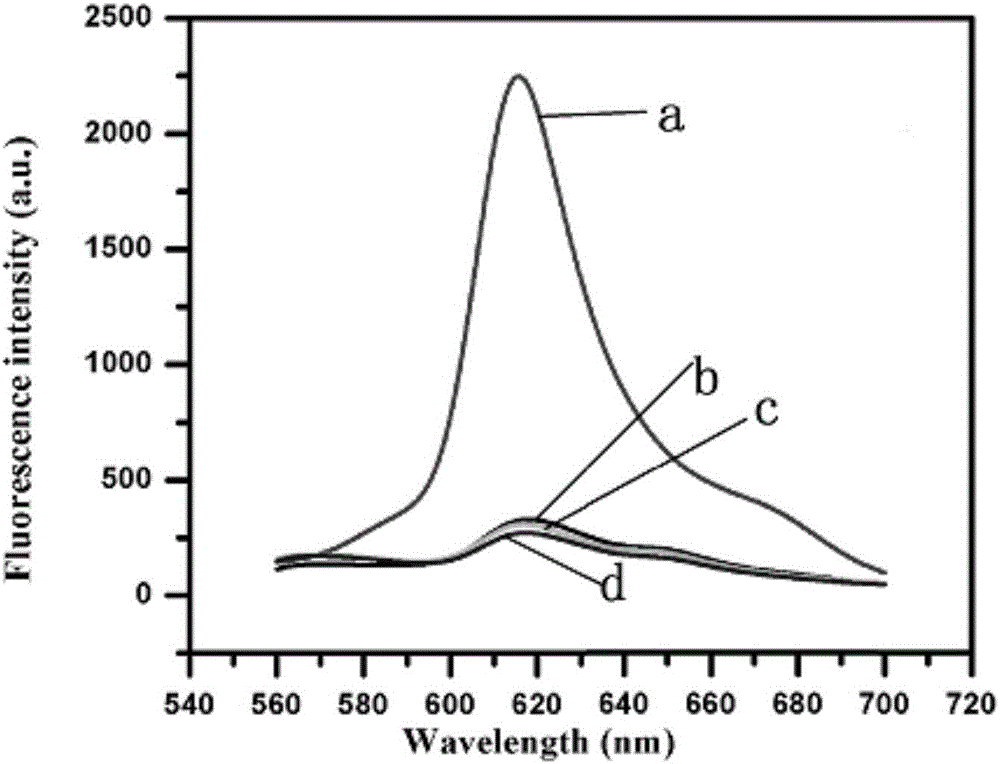
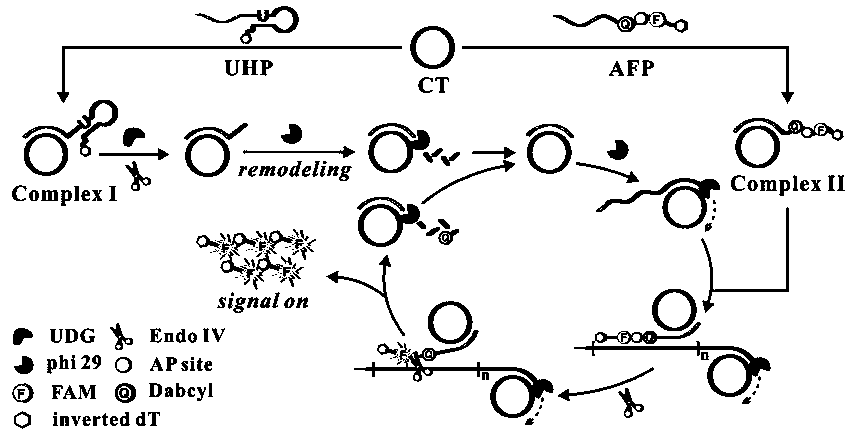
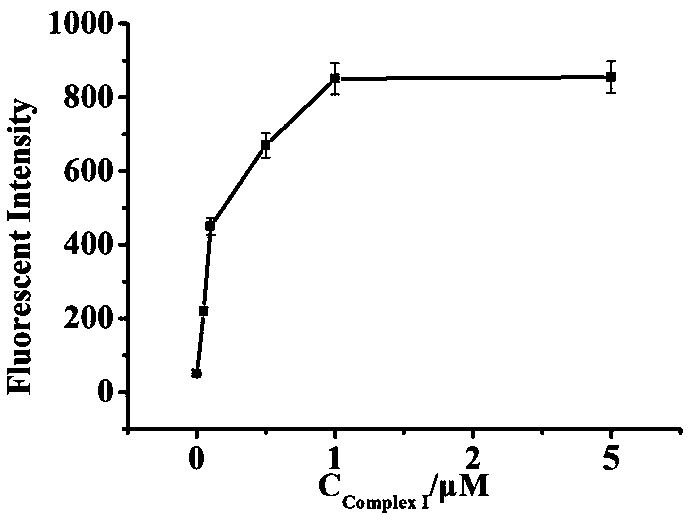
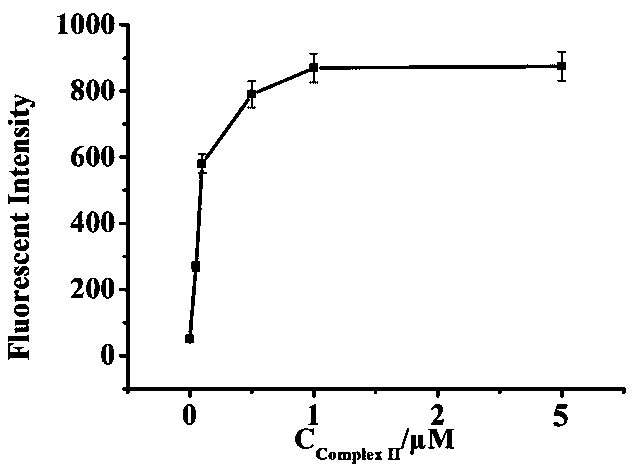
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
78 results about "Uracil-DNA glycosylase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Uracil-DNA glycosylase, also known as UNG or UDG, is an enzyme. The human gene is well researched and orthologs exist ubiquitously among prokaryotes and eukaryotes and even in some DNA viruses. The first uracil DNA-glycosylase was isolated from Escherichia coli.
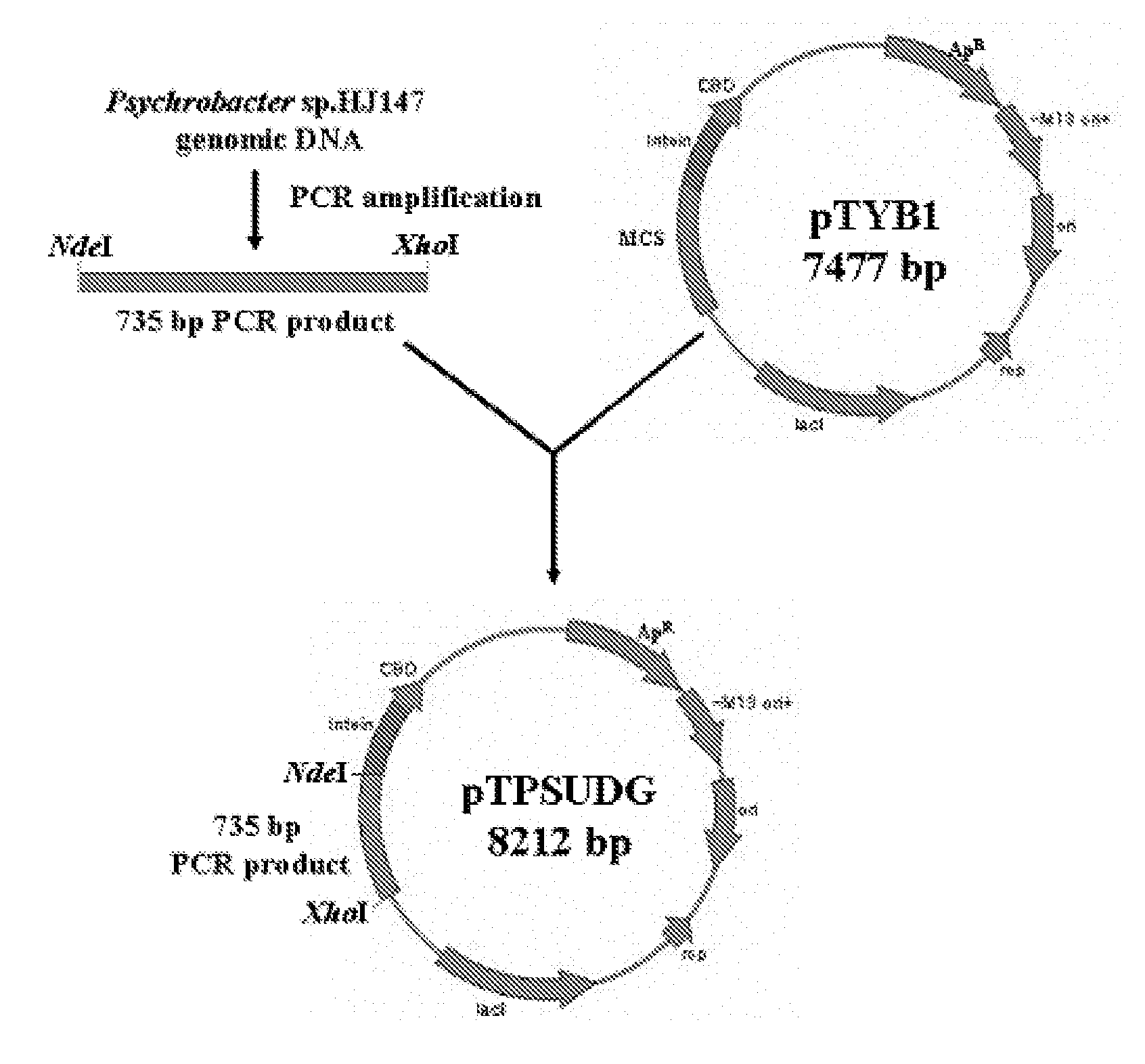
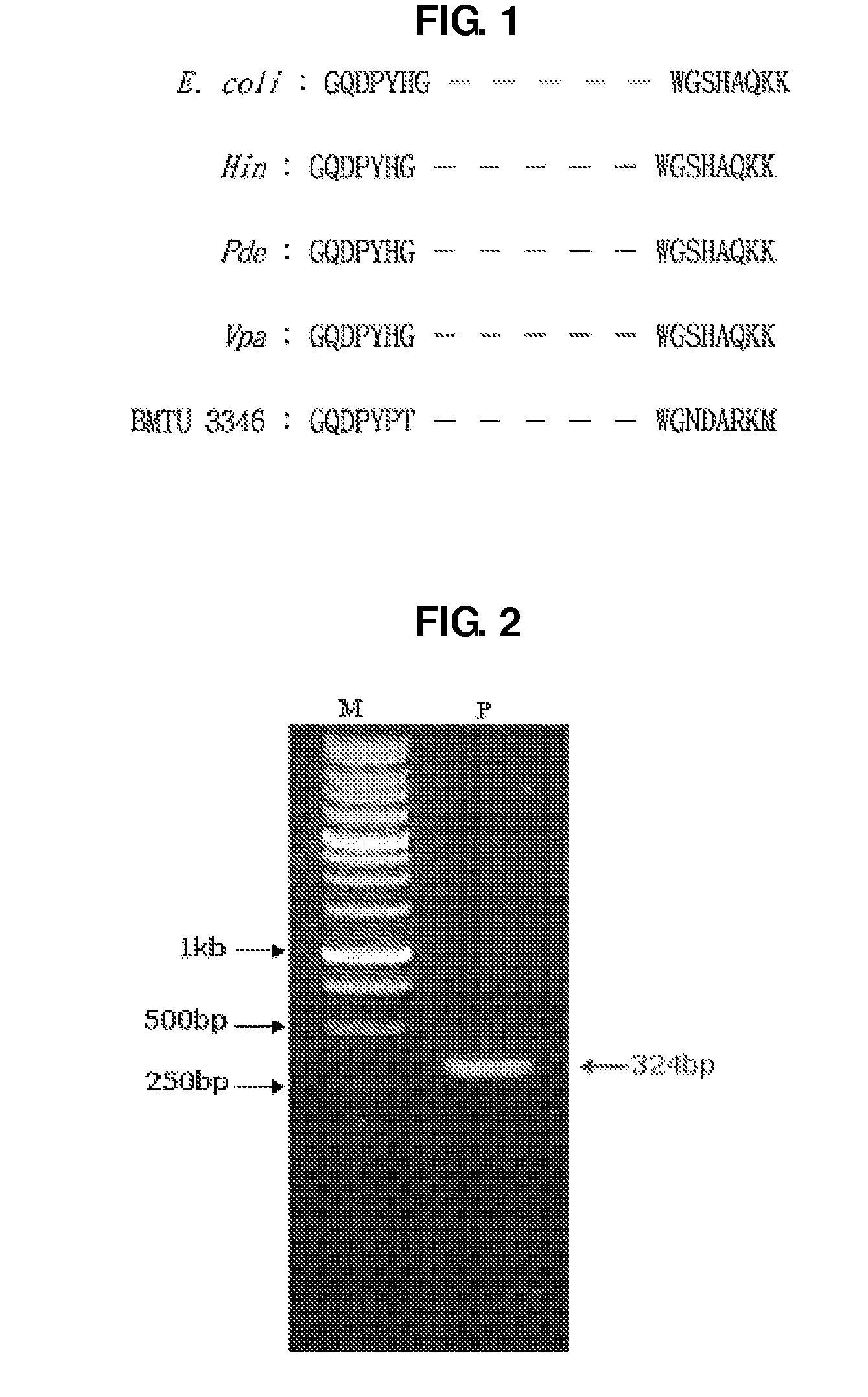
Uracil-DNA glycosylase of psychrobacter sp. HJ147 and use thereof
ActiveUS20080299609A1Eliminating contamination carry-overEliminating cross contaminationBacteriaSugar derivativesEscherichia coliPsychrobacter sp.
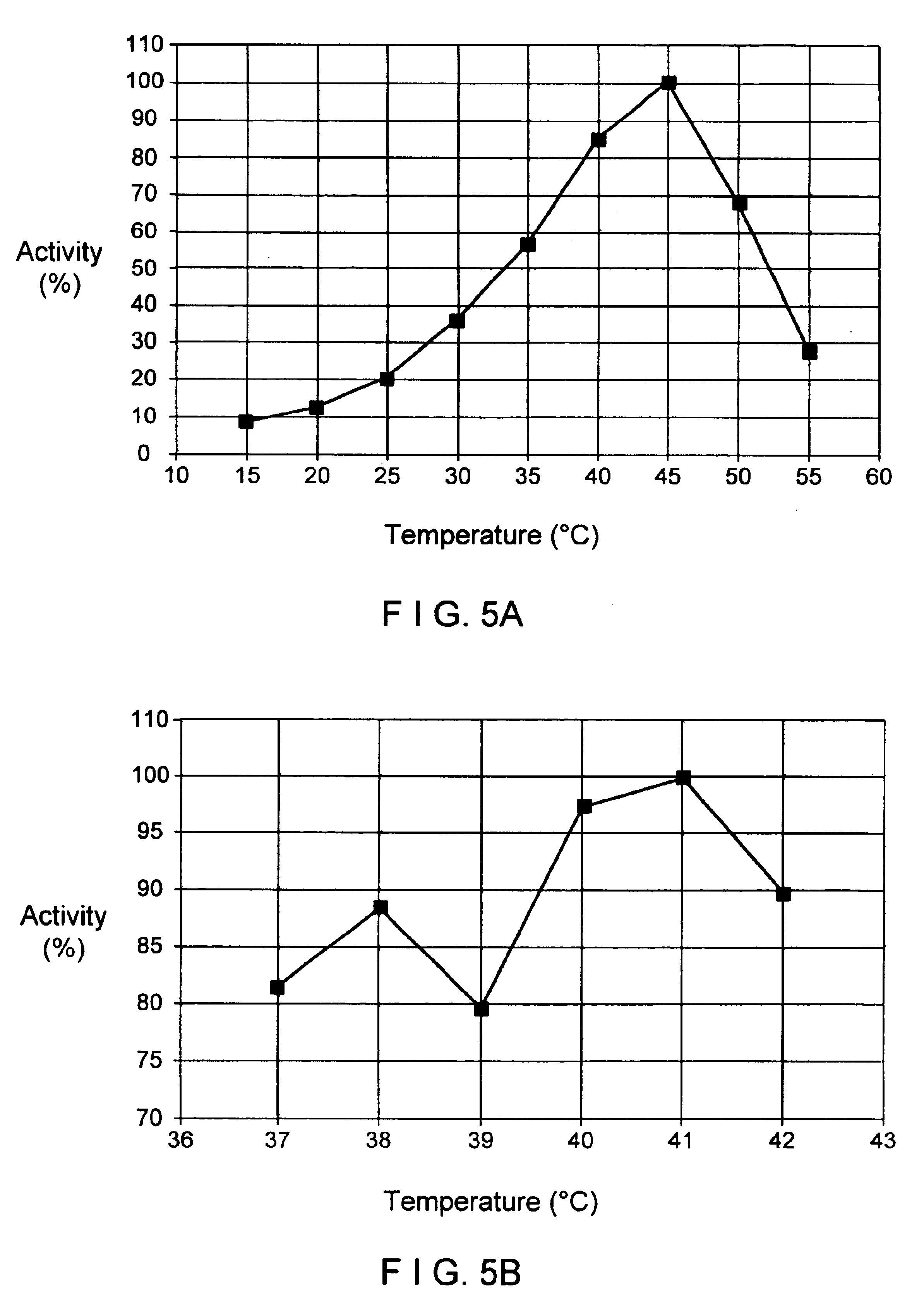
The present invention provides uracil-DNA glycosylase (UDG) gene originating from Psychrobacter sp. HJ147, and amino acid sequences deduced from the gene; expression and purification of Psp HJ147 UDG gene in Escherichia coli; and characterization of UDG obtained therefrom, and the use thereof in a polymerase chain reaction (PCR). The UDG according to the present invention has a specific activity of excising uracil bases in a uracil-containing DNA substrates at a low temperature, and is easily heat-inactivated. It thus can effectively eliminate cross contamination and carry-over contamination of PCR templates often occurring after a PCR process using dUTP. Therefore, it is useful for increasing preciseness (elimination of false positives), purity and amplification efficiency of PCR.
Owner:RES & BUSINESS FOUND SUNGKYUNKWAN UNIV
Application of CRISPR/nCas9 mediated site-directed base substitution in plant
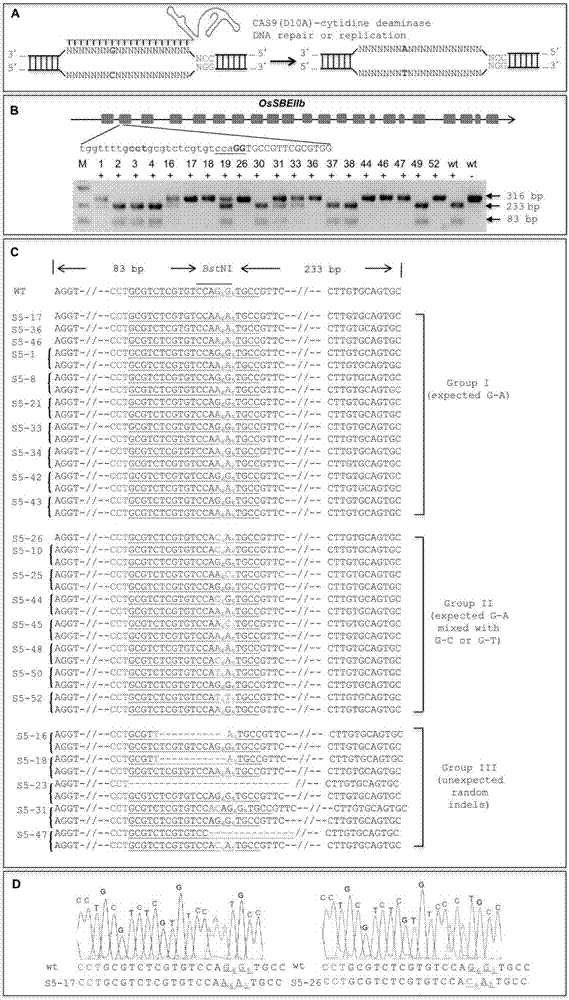
ActiveCN107043779ARapid improvement of agronomic traitsImproved agronomic traitsVectorsVector-based foreign material introductionUracil-DNA glycosylaseSubstitution method
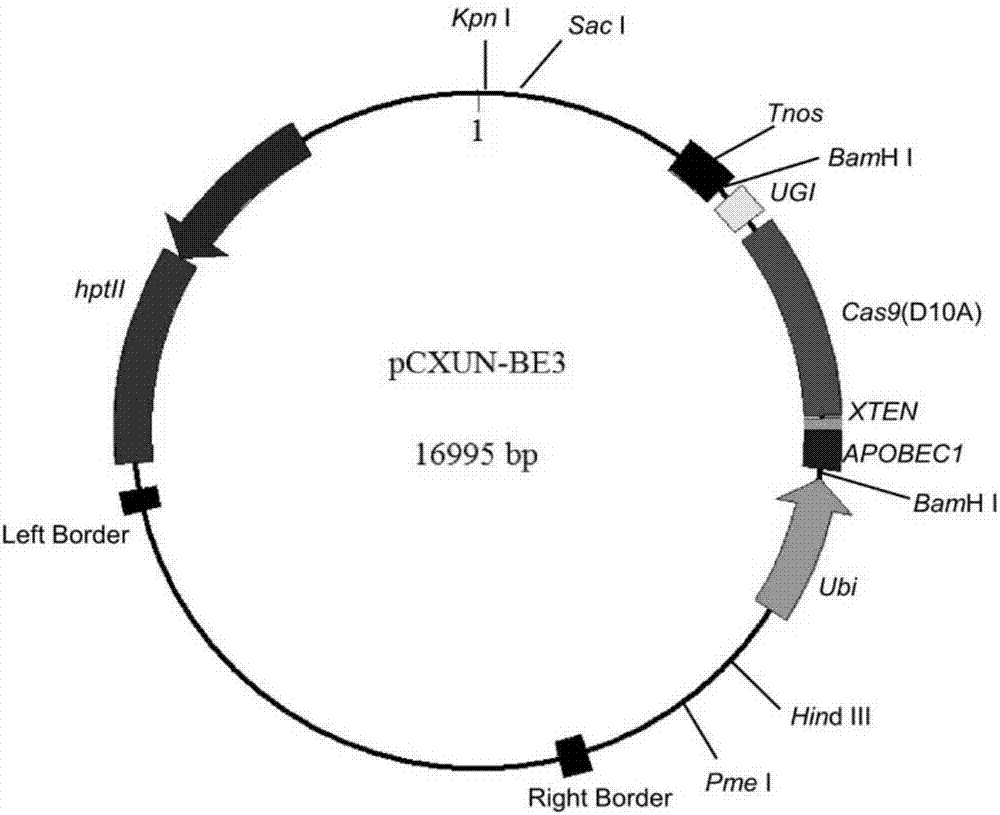
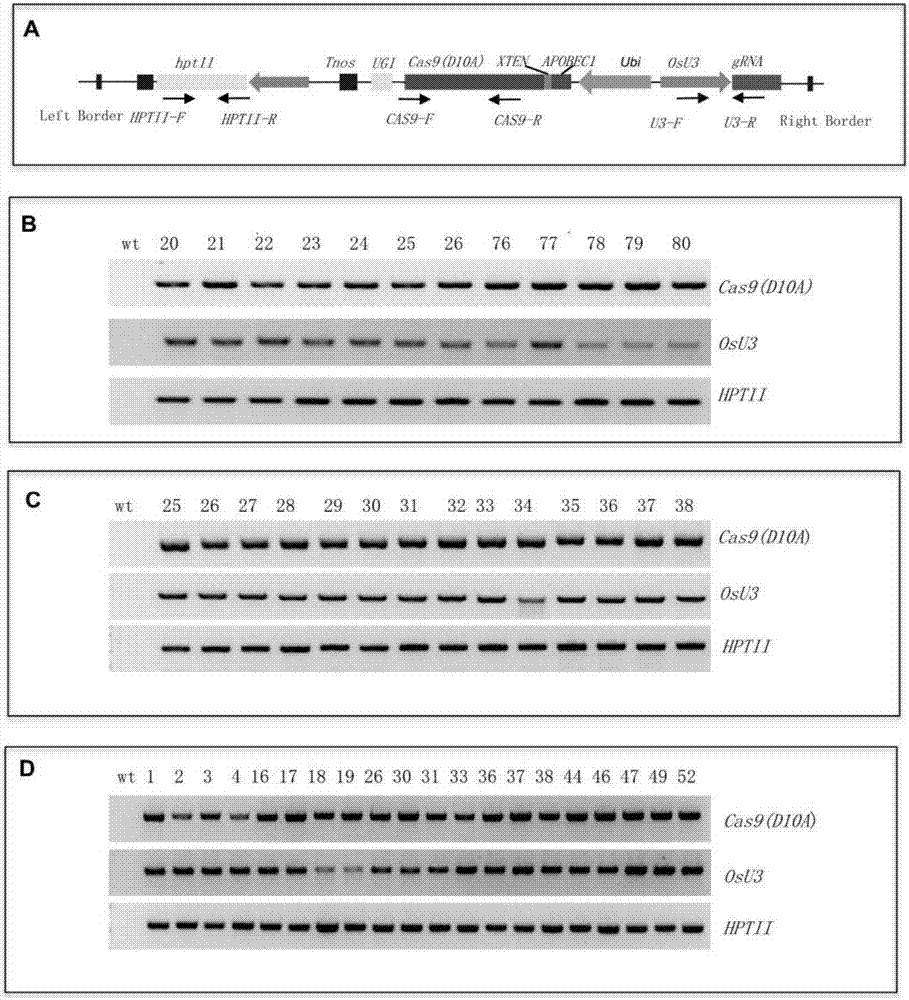
The invention discloses application of CRISPR / nCas9 mediated site-directed base substitution in a plant. The invention provides a plant genome site-directed edition system. The system comprises a BE3 plant expression carrier (expressing a fusion protein composed of nCas9(D10A), deaminase and a uracil DNA glycosylase inhibitory protein), and rice OsPDS and OsSBEIIb are taken as target genes for verifying the system. Results show that an expected site-directed mutant plant is respectively obtained in the three selected target spots, accurate site mutation of a base is realized in rice, and the highest efficiency reaches about 20%, so that a feasible and effective base substitution method is provided for crop breeding, the method has strong application potential in the aspect of agricultural breeding, and a foundation is provided for rapidly improving important agronomic traits of crops.
Owner:INST OF CROP SCI CHINESE ACAD OF AGRI SCI
Cod uracil-DNA glycosylase, gene coding therefore, recombinant DNA containing said gene or operative parts thereof, a method for preparing said protein and the use of said protein or said operative parts thereof in monitoring or controlling PCR
InactiveUS20020155573A1Efficient productionBacteriaSugar derivativesBiotechnologyUracil-DNA glycosylase
It is disclosed a novel enzyme present in cod liver, a DNA sequence encoding the enzyme or operative parts or biologically functional parts thereof, a novel recombinant DNA comprising the gene or tho operative or biologically functional parts thereof, a method of preparing the enzyme from cod liver and from bacteria carrying the gene, the bacteria carrying the gene per se, and the use of the protein in monitoring and / or controlling PCR or related reaction systems.
Owner:BIOTEC PHARMACON
Fluorescence quantitative PCR detection kit of hepatitis B virus and application thereof
ActiveCN101701267AStrong specificityHigh purityMicrobiological testing/measurementMicroorganism based processesPositive controlFluorescence
The invention discloses a fluorescence quantitative PCR detection kit of hepatitis B virus and an application thereof. The kit is composed of the following independent components: DNA extraction solution I, DNA extraction solution II, DNA extraction solution III, DNA extraction solution IV, positive control interior label, PCR reaction liquid, probe HBV-SP, enzyme mixed liquor containing heat resistant DNA polyase and uracil DNA glycosylase, quantitative hepatitis B virus reference material, hepatitis B virus positive control serum and hepatitis B virus negative control serum, wherein DNA extraction solution I contains 0.2-1.0% of lauryl sodium sulphate (mass / volume), 1.0-4.0% of Triton (volume / volume) and 0.2-1.0mol / L of guanidinium isothiocyanate; DNA extraction solution II contains 100-300mmol / L of 4-HEPES, 100-300mmol / L of sodium chloride with pH of 6.5+ / -0.2 and 100-400 mu g / ml of magnetic beads; DNA extraction solution III contains 0.1-1.0% of Triton (volume / volume) and 100-300mmol / L of sodium chloride; DNA extraction solution IV contains mineral oil. The fluorescence quantitative PCR detection kit of hepatitis B virus of the invention can be used for detecting the HBV-DNA concentration in samples of serum, blood plasma or latex and the like.
Owner:SANSURE BIOTECH
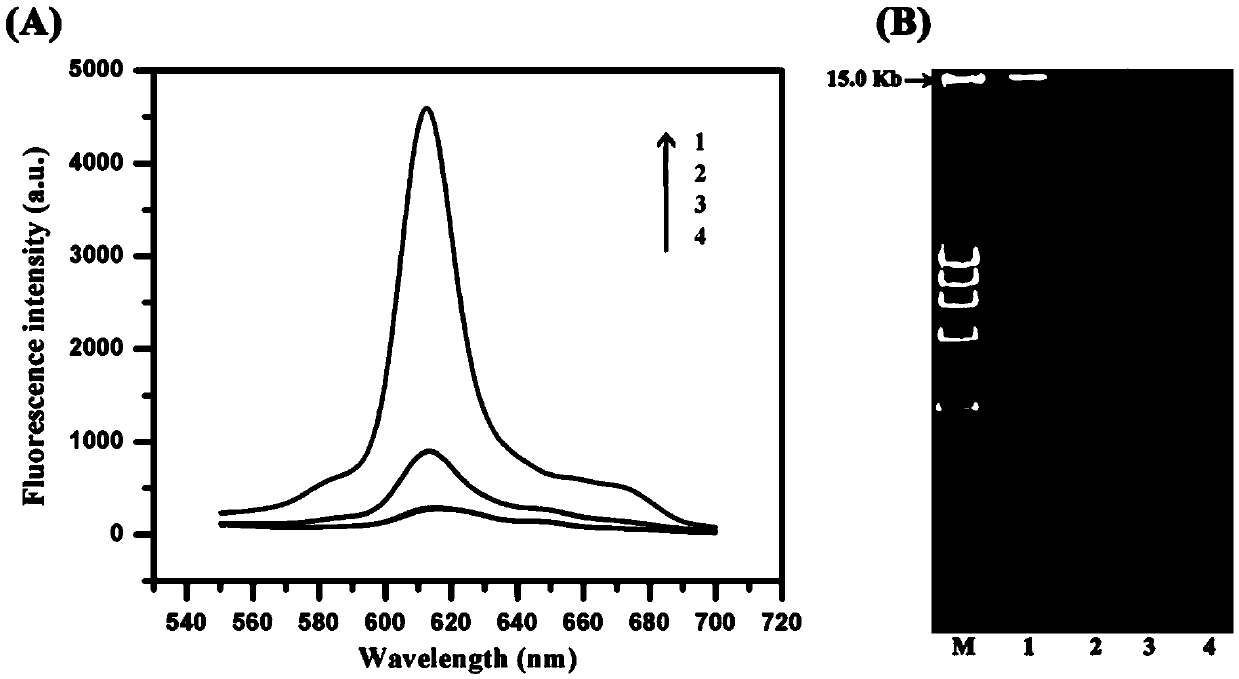
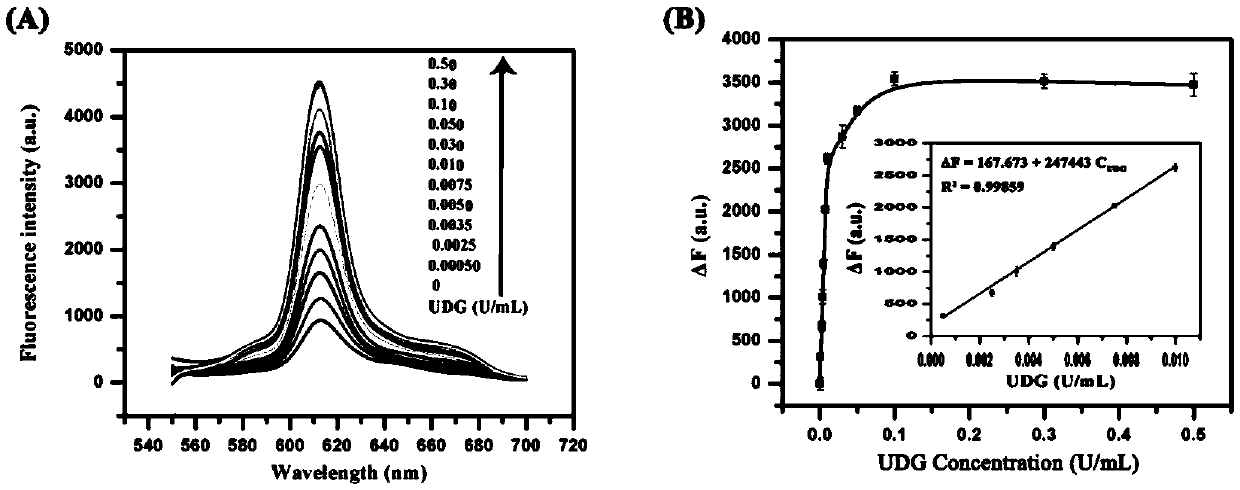
Method for detecting activity of uracil-DNA glycosylase (UDG) based on fluorescence amplification strategy of label-free non-enzyme DNA machines
InactiveCN104630363AImprove hybridization efficiencyEnhanced inhibitory effectMicrobiological testing/measurementFluorescenceNucleotide
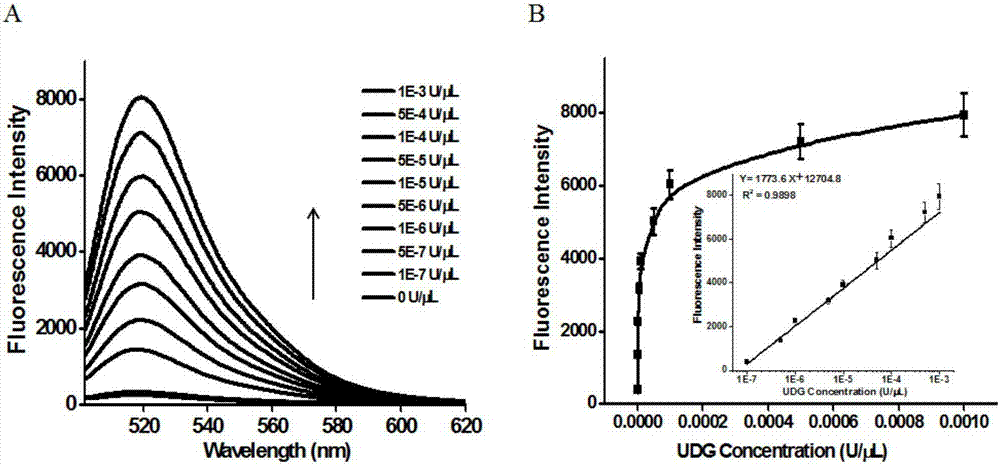
The invention discloses a method for detecting the activity of uracil-DNA glycosylase (UDG) based on a fluorescence amplification strategy of label-free non-enzyme DNA machines, and the method comprises the following steps: (1) the preparation of a probe for the recognition and signal transduction of the UDG: firstly, designing an uracil base and initiation sequence containing double-stranded DNA probe P1-P2, wherein the P1 chain is an inhibition chain and the nucleotide sequence thereof is show in SEQ ID NO.1 in a sequence table; the P2 chain is a uracil-DNA sequence and initiation sequence containing chimeric conjugated chain and the nucleotide sequence thereof is show in SEQ ID NO.2 in the sequence table; and the P1 chain and the P2 chain are partially complemented so as to form the double-stranded DNA probe P1-P2; (2) the construction of a label-free non-enzyme DNA machine: according to an initiation sequence of the P2 chain, designing hairpin probes H1 and H2 which are partially complemented and used for constructing the label-free non-enzyme DNA machine, and grafting a G-quadruplet sequence to the tail end of the hairpin probe H2; and (3) the activity detection of UDG. The method disclosed by the invention successfully realizes background diminishing and signal amplification, and the LOD (limit of detection) is 0.00044 U / mL.
Owner:SHANDONG UNIV
Method for detecting thymine DNA glycosylase activity based on cyclophorase-remediation mediated double-signal amplification strategy
ActiveCN106995840AHigh cycle cutting efficiencyStrong specificityMicrobiological testing/measurementFluorescenceUracil-DNA glycosylase
The invention discloses a method for detecting thymine DNA glycosylase activity based on a cyclophorase-remediation mediated double-signal amplification strategy. A fluorescent method for detecting the thymine DNA glycosylase activity in real time can be achieved through circular rolling circle index amplification triggered by thymine DNA glycosylase and assisted by enzyme remediation and circular cutting catalyzed and mediated by endonuclease IV. As efficiency of circular rolling circle amplification assisted by uracil excision and efficiency of circular cutting based on endonuclease IV are both high and index amplification mediated by uracil can greatly inhibit non-specific amplification, the method has very high detection flexibility; detection shows that lower detection limit of the method to thymine DNA glycosylase is 5.6*10<-7>U / mu L; meanwhile, the method disclosed by the invention utilizes self repair characteristics of the thymine DNA glycosylase and uracil DNA glycosylase to enable the whole repairing reaction to be performed strictly according to a natural repairing mechanism, so that the method disclosed by the scheme of the invention has very high specificity.
Owner:SHANDONG NORMAL UNIV
Methods for fragmentation and analysis of nucleic acid
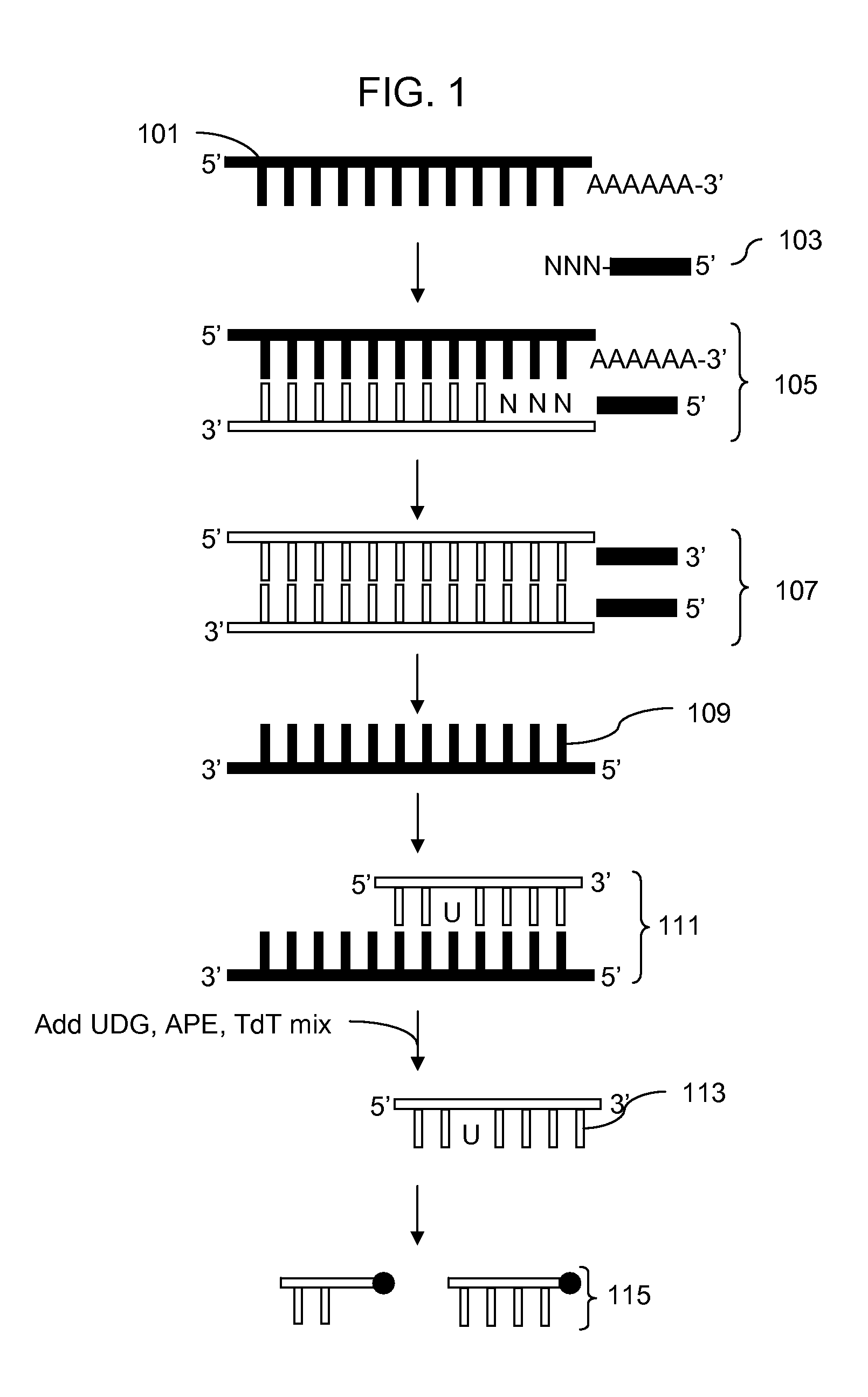
Methods for fragmenting and labeling DNA in a single reaction volume and incubation step using a uracil DNA glycosylase, an apurinic / apyrimidinic endonuclease, and a terminal transferase are disclosed. In a preferred embodiment the UDG, AP and TdT activities are first mixed together to form an enzyme mixture and then the enzyme mixture is mixed with the uracil containing DNA. The fragmentation and labeling reactions thus take place simultaneously as part of the same reaction. The methods may be used in a variety of applications where fragmenting and end-labeling single or double stranded DNA is desired.
Owner:AFFYMETRIX INC
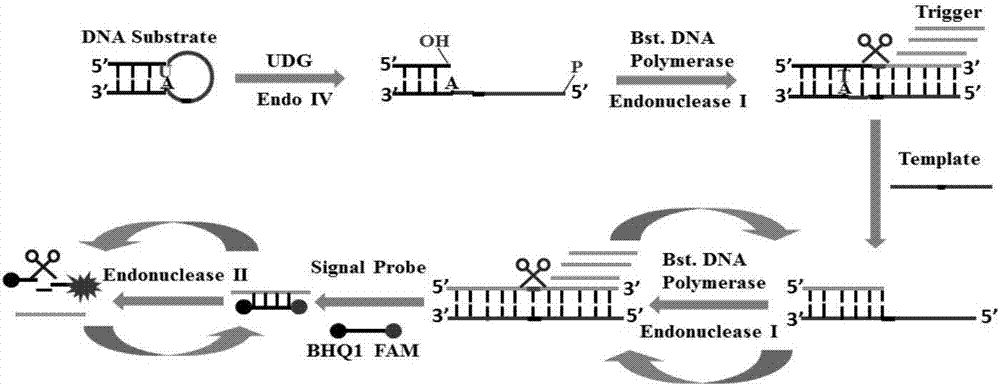
Method of detecting UDG activity by enzyme-mediated two-step serial signal amplification based on excision repair
ActiveCN106929563AHigh sensitivityHigh resolutionMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceDisplacement reactions
The invention discloses a method of detecting the UDG activity by enzyme-mediated two-step serial signal amplification based on excision repair. The method comprises the following steps: (1) adding a UDG active substrate in an excision reaction buffer solution to carry out excision repair reaction, excising a uracil base of the UDG active substrate, and leaving an abasic site; and (2) adding an excision repair reaction product in an amplification reaction buffer solution to carry out enzyme-assisted two-step serial signal amplification reaction, namely inducing strand displacement reaction and index amplification reaction, generating strengthened fluorescence signals in a circulation manner, and determining the UDG activity through weakness of the fluorescence signals. According to the method provided by the invention, ultrahigh sensitive detection to the activity of uracil DNA glycosylase (UDG) is realized by utilizing high amplification efficiency of constant-temperature index amplification reaction and specific circulation digestion of ribonuclease H.
Owner:SHANDONG NORMAL UNIV
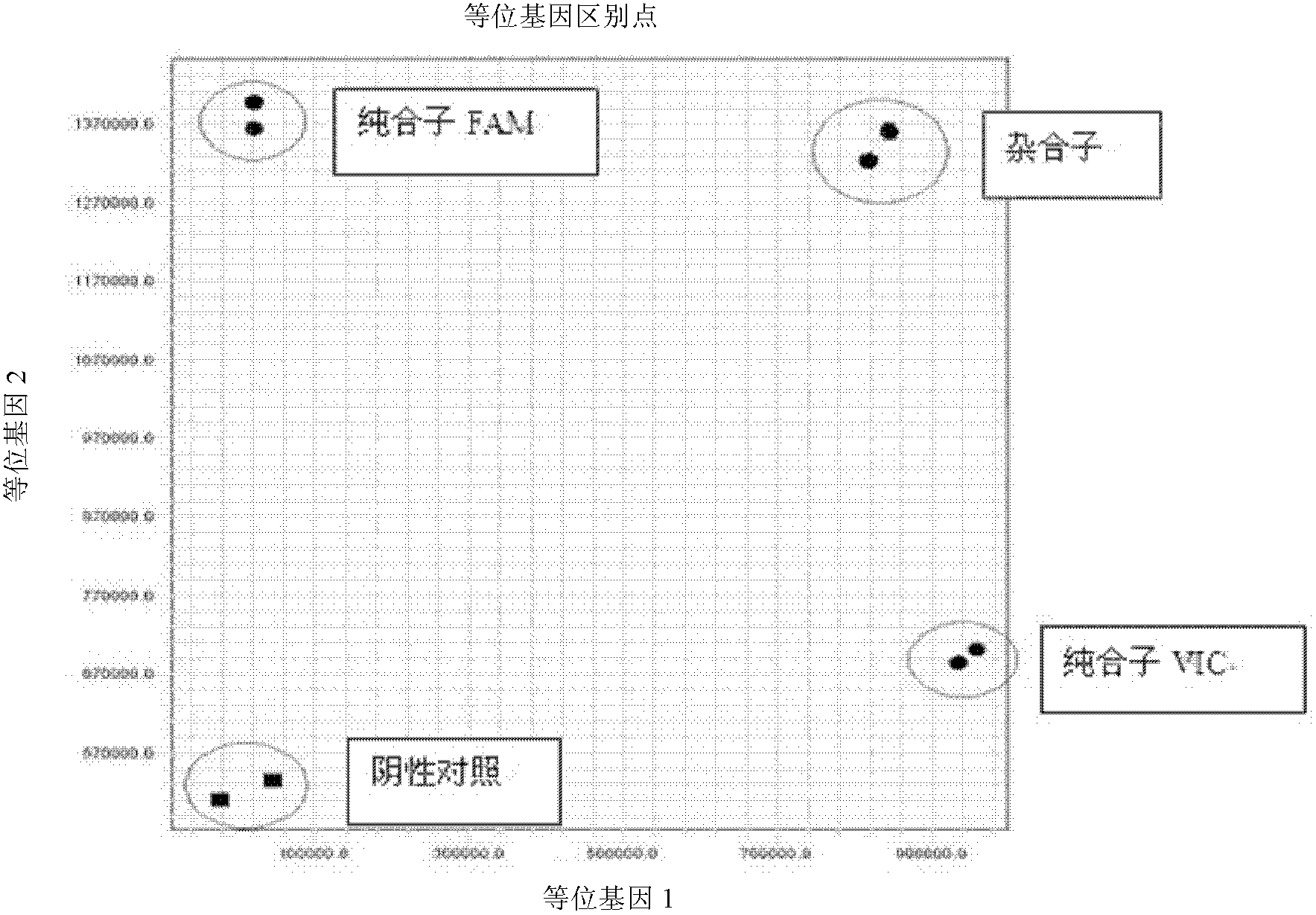
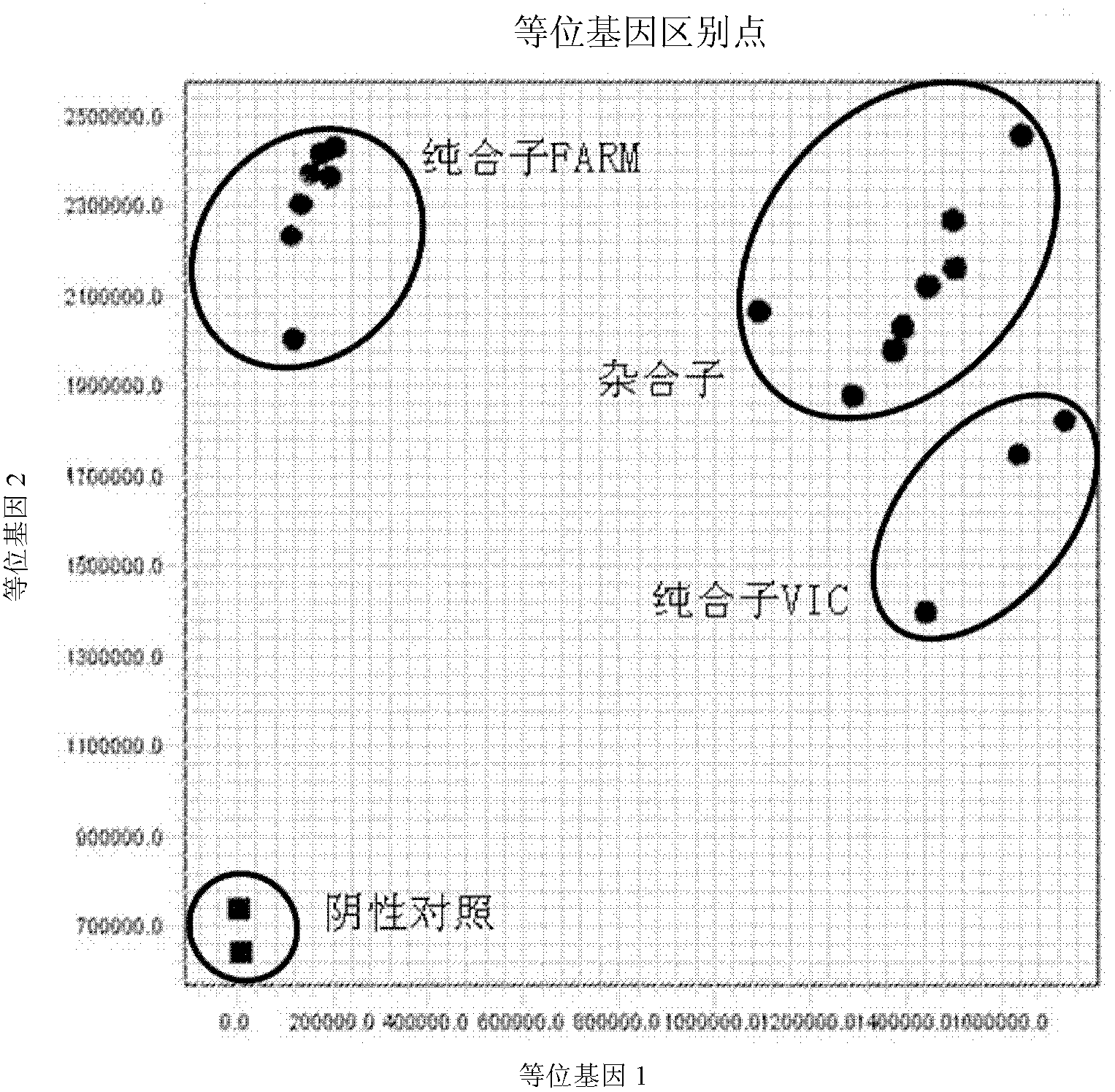
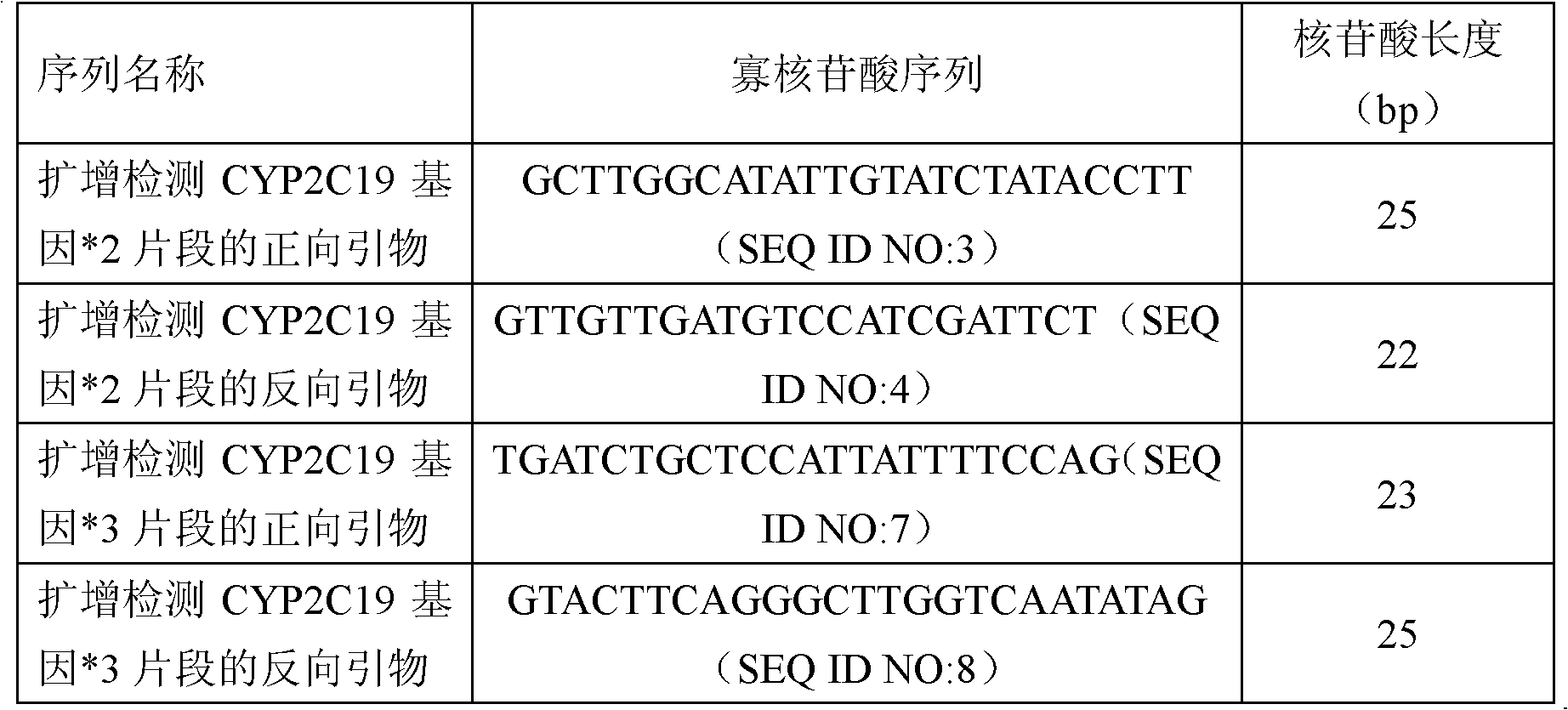
Fluorescent polymerase chain reaction (PCR) kit for detecting CYP2C19 genotypes
InactiveCN102534005AIncreased sensitivityImprove featuresMicrobiological testing/measurementProtein detectionNucleotide
The invention provides a fluorescent polymerase chain reaction (PCR) kit for detecting CYP2C19 genotypes, and belongs to the field of in-vitro nucleic acid testing. The fluorescent PCR kit comprises PCR reaction liquids for detecting CYP2C19*2 and CYP2C19*3 genotypes, Taq DNA polymerase, and uracil-DNA glycosylase, wherein the PCR reaction liquids for detecting the CYP2C19*2 and CYP2C19*3 genotypes respectively comprise PCR amplification primers, minor groove binder (MGB) probes and the like; and nucleotide sequences for detecting the CYP2C19*2 and CYP2C19*3 genotypes are shown as SEQ ID NO:3-4 and SEQ ID NO:5-6 respectively. The kit has high sensitivity and specificity, can monitor the reaction progress in real time, ensures short reaction time, avoids subsequent treatment, can avoid reaction product pollution to the greatest extent, and can replace the traditional protein detection or the common PCR detection to diagnose the CYP2C19 genotypes.
Owner:CHANGSHA 3G BIOTECH
Method for detecting UDG (Uracil Dna Glycosylase) activity based on sticky end-mediated strand displacement reaction combined with polymerization incising isothermal amplification technology
ActiveCN106244703AHigh sensitivityReduce negative signalMicrobiological testing/measurementDNA/RNA fragmentationDisplacement reactionsUracil-DNA glycosylase
The invention discloses a method for detecting UDG (Uracil Dna Glycosylase) activity based on sticky end-mediated strand displacement reaction combined with polymerization incising isothermal amplification technology. When UDG exists, two U basic groups in a single-stranded DNA probe are removed, and the single-stranded DNA probe containing AP mispairing is obtained. On the basis of a specific recognition capability of TSDR on a few mispairing, the single-stranded DNA probe containing AP mispairing and a hairpin probe containing a sticky end cannot be subjected to TSDR, so that a primer sequence in the single-stranded DNA probe can still exist freely. Later, the primer sequence is hybridized with a signal report probe, so that subsequent polymerization incising isothermal amplification reaction is triggered, the sensitive response to the removal of a few U basic groups is realized, and the sensitivity in detecting the UDG activity is improved. However, when the UDG does not exist, the single-stranded DNA probe containing AP mispairing and the hairpin probe containing the sticky end can be subjected to TSDR, and the primer sequence is closed, so that the subsequent isothermal amplification reaction cannot be triggered, and a negative signal is reduced. The method can be used for detecting the UDG activity as low as 0.000027U / mL.
Owner:SHANDONG UNIV
Fluorescence biosensor for detecting UDG (Uracil-DNA Glycosylase) and preparation method thereof
ActiveCN109444105ARealize highly sensitive detectionLow detection limitFluorescence/phosphorescenceEnergy transferResonance
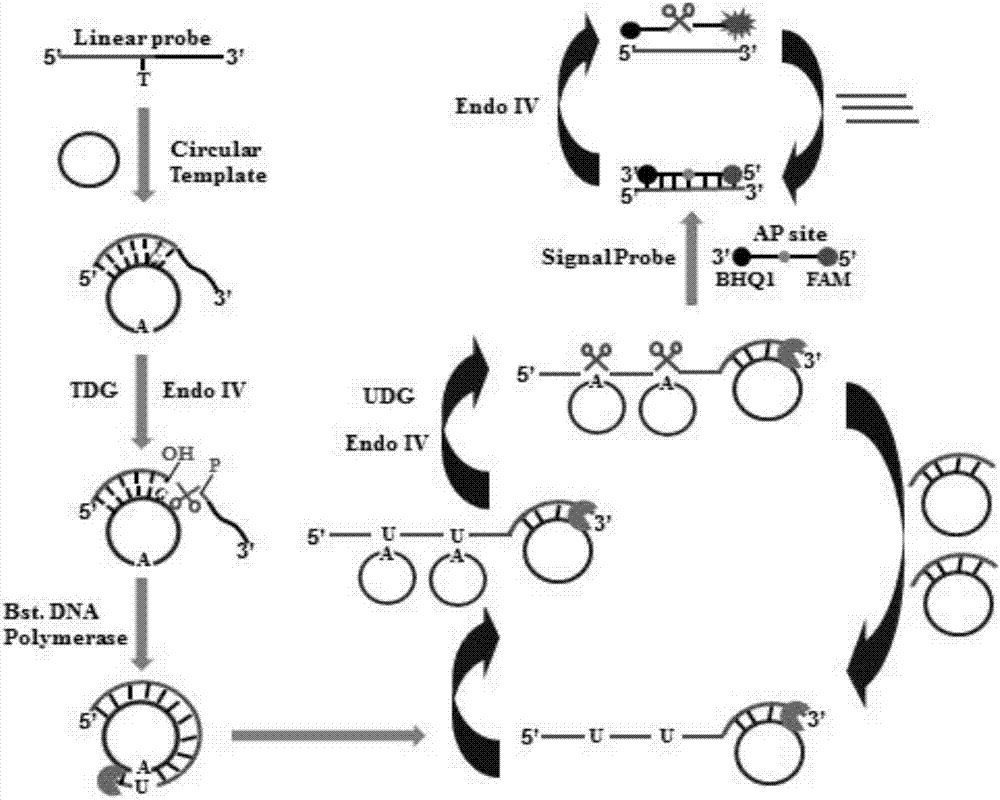
The invention relates to the technical field of a biosensor, and particularly relates to a fluorescence biosensor for detecting UDG (Uracil-DNA Glycosylase) on the basis of a polymerase-assisted feedback rolling circle amplification and endonuclease amplification fluorescence method. The invention aims to solve problems of both low specificity and low sensitivity of a method for detecting UDG in the prior art. The biosensor for detecting the UDG on the basis of a feedback rolling circle amplification technology achieves a rolling circle amplification effect on matching of phi29 polymerase andendonuclease IV, implements fluorescence resonance energy transfer of a fluorophore and a Quenching group and performs a homogeneous reaction on mixed liquid. A preparation method comprises: constructing a circular template and a composite probe; feeding back a rolling circle amplification signal and carrying out fluorescence detection. Specific hydrolysis of the UDG on a basic group U is utilized, and by utilizing such specific reaction, the UDG can be accurately measured and meanwhile, interference can also be avoided; by utilizing endonuclease IV circle amplification, a signal amplificationeffect is achieved.
Owner:UNIV OF JINAN
Method for parallel determination of activity of uracil-DNA glycosylase and endonuclease IV, application thereof and reagent kit
ActiveCN105506078ASimple designLow selectivityMicrobiological testing/measurementFluorescenceUracil-DNA glycosylase
The invention provides a method for parallel determination of the activity of uracil-DNA glycosylase and endonuclease IV, application thereof and a reagent kit. When a target object is UDG, if UDG exists, U basic groups in a hairpin probe are removed, an AP locus is generated, the generated AP locus is cut through tool enzyme Endo IV, and a primer sequence containing free 3' terminal is released and used for initiating subsequent rolling circle amplification reaction (RCA); if UDG does not exist, the 3' terminals are closed in the hairpin probe, the RCA process can not be carried out. When a target object is Endo IV, if Endo IV exists, the RCA process is the same as that in UDG activity detection; if Endo IV does not exist, the 3' terminals are closed in the hairpin probe, and the RCA process can not be conducted. The hairpin probe can be used as a recognition probe of UDG and can also be used for precursor recognition probe of Endo IV, and accordingly design of the probe is simplified. The detection limits of UDG and Endo IV are reduced to 0.00017 U / mL and 0.11 U / mL respectively, and results are better than or the same as those of other label-free fluorescence methods.
Owner:SHANDONG UNIV
Fluorescent biological sensor for detecting uracil-DNA glycosylase (UDG)
InactiveCN108088826AStrong specificityHigh sensitivityFluorescence/phosphorescenceSilver clusterUracil-DNA glycosylase
The invention provides a fluorescent biological sensor for detecting uracil-DNA glycosylase (UDG). The fluorescent biological sensor comprises an annular template, a hairpin probe, a fluorescent probe, dNTP, phi 29 DNA polymerase and endonuclease IV. The biological sensor disclosed by the invention has the advantages of good specificity, high flexibility, moderate reaction condition and quick reaction speed; by means of silver cluster fluorescent detection, the biological sensor has the advantages of convenience in operation, short detection period, easiness in carrying, low technological cost, suitability for an industrial inexpensive requirement; a preparation method has the advantages of simpleness, stable performance and good repeatability.
Owner:UNIV OF JINAN
Prevention and alleviation of steric hindrance during single molecule synthesis
ActiveUS20100035269A1Preventing and alleviating steric hindranceReducing steric hindranceMicrobiological testing/measurementFermentationPolymerase LUracil-DNA glycosylase
The present invention provides compositions and methods for reducing steric hindrance in the product of nucleic acid polymerase reaction. Methods and compositions of the invention encompass application of exonucleases, endonucleases, and uracil-DNA glycosylases to a nucleic acid polymerase reaction such that newly formed nucleic acid strands are modified (e.g., cleaved) while the polymerase reaction continues to proceed.
Owner:PACIFIC BIOSCIENCES
Uracil-DNA glycosylase of Psychrobacter sp. HJ147 and use thereof
The present invention provides uracil-DNA glycosylase (UDG) gene originating from Psychrobacter sp. HJ147, and amino acid sequences deduced from the gene; expression and purification of Psp HJ147 UDG gene in Escherichia coli; and characterization of UDG obtained therefrom, and the use thereof in a polymerase chain reaction (PCR). The UDG according to the present invention has a specific activity of excising uracil bases in a uracil-containing DNA substrates at a low temperature, and is easily heat-inactivated. It thus can effectively eliminate cross contamination and carry-over contamination of PCR templates often occurring after a PCR process using dUTP. Therefore, it is useful for increasing preciseness (elimination of false positives), purity and amplification efficiency of PCR.
Owner:RES & BUSINESS FOUND SUNGKYUNKWAN UNIV
Primers and kit for detecting fusarium on basis of loop-mediated isothermal amplification technology
InactiveCN102251055ASensitive detectionQuick checkMicrobiological testing/measurementMicroorganism based processesUracil-DNA glycosylaseLoop-mediated isothermal amplification
The invention relates to a loop-mediated isothermal amplification kit and method for detecting fusarium. The kit comprises loop-mediated isothermal amplification reaction liquid, UNG (uracil-DNA-glycosylase), Bst DNA polymerase, developer and fusarium positive DNA, wherein the reaction liquid contains forward / reverse inner and outer primers, of which the sequences are respectively SEQ ID NO: 1-4. The detection method comprises the steps of fungus DNA extraction, loop-mediated isothermal amplification and development detection. The loop-mediated isothermal amplification primers are designed according to the fusarium gene conserved region sequences, and the fusarium in the sample is detected by loop-mediated isothermal amplification technology. The invention has the advantages of high speed, high sensitivity, high specificity, low cost and the like; and the whole process is not related to toxic reagents, thereby ensuring the safety of operating personnel and environment. Meanwhile, the UNG adopted in the invention can thoroughly eliminate false positive caused by nucleic acid pollution in multiple detection processes.
Owner:SHANDONG EYE INST
Cod uracil-DNA glycosylase, gene coding therefore, recombinant DNA containing said gene or operative parts thereof, a method for preparing said protein and the use of said protein or said operative parts thereof in monitoring or controlling PCR
It is disclosed a novel enzyme present in cod liver, a DNA sequence encoding the enzyme or operative parts or biologically functional parts thereof, a novel recombinant DNA comprising the gene or the operative or biologically functional parts thereof, a method of preparing the enzyme from cod liver and from bacteria carrying the gene, the bacteria carrying the gene per se, and the use of the protein in monitoring and / or controlling PCR or related reaction systems.
Owner:BIOTEC PHARMACON
High-sensitivity uracil DNA glycosylase (UDG) detection using DNA three-direction section activated hybridization chain reaction
ActiveCN105132522AImplement the buildLow detection limitMicrobiological testing/measurementNano-deviceDNA Modification
The invention relates to high-sensitivity uracil DNA glycosylase (UDG) detection using DNA three-direction section activated hybridization chain reaction. UDG is used as a model to develop an identification mechanism of hairpin reconstruction, and action of DNA modification enzyme on substrate is converted into the triggering process of the hybridization chain reaction so as to cause the self-assembling process of DNA and build a universal DNA nano device. The device can be used for the high-sensitivity detection and inhibitor screening of the DNA modification enzyme (especially the UDG), and has potential application value.
Owner:SHANDONG UNIV
PCR detection kit for specific detection of fowl adenovirus group I and detection method thereof
InactiveCN106435031AStrong specificitySpecific and accurate detectionMicrobiological testing/measurementMicroorganism based processesSpecific detectionMicrometer
The invention relates to a PCR detection kit for specific detection of fowl adenovirus group I and a detection method thereof. The detection kit comprises a fowl adenovirus group I PCR reaction liquid, 10*PCR buffer for UNG plus, hot start type DNA polymerase, dU plus dNTP mix, uracil DNA glycosylase, positive quality control serum and negative quality control serum, wherein the fowl adenovirus group I PCR reaction liquid comprises an upstream primer with a concentration of 5 micrometer and the sequence is shown as the specifications as well as a downstream primer with a concentration of 5 micrometer and the sequence is shown as the specifications. When detection is conducted through the PCR detection kit, conditions for a PCR amplified reaction are that UNG treatment is conducted at a temperature of 25 DEG C for 10 min, and predegeneration is conducted at a temperature of 95 DEG C for 2 min; degeneration is conducted at a temperature of 98 DEG C for 10 s, annealing is conducted at a temperature of 55 DEG C, extension is conducted at a temperature of 72 DEG C for 1 min, and all that are conducted for 30 work cycling; extension is conducted at a temperature of 72 DEG C for 10 min. The PCR detection kit for the specific detection of the fowl adenovirus group I has the advantages of being high in sensitivity (still positive when the reaction liquid is diluted to 1:105) and good in repeatability. The PCR detection method for the specific detection of the fowl adenovirus group I is easy, convenient and fast to operate, suitable for early diagnosis of fowl adenovirus group I virus, and can meet the demand for disease prevention and control in time.
Owner:INST OF ANIMAL SCI & VETERINARY HUBEI ACADEMY OF AGRI SCI
Basic group editing system and method for specifically repairing HBB gene mutation, kit and application of system and method and kit in human genital system
The invention provides a basic group editing method for specifically repairing HBB gene mutation in a human genital system. The method comprises the steps of delivering a basic group editing system used for specifically repairing HBB gene mutation so that the basic group editing system is close to relevant sequences of HBB mutant genes, and obtaining repaired HBB genes, wherein the basic group editing system comprises a basic group editing enzyme and gRNA, the basic group editing enzyme is fusion protein, and the fusion protein includes effect protein structural domains, cytidine deaminase structural domains and uracil DNA glycosylase inhibitor structural domains of a CRISPR / Cas system. The gene editing system is guided into a human germ cell, a human fertilized ovum or a human embryo, A>Gpathogenic mutation can be precisely repaired, and thus beta-mediterranean anemia is cured. The basic group editing method has a wide application prospect in the field of genetic therapy.
Owner:SUN YAT SEN UNIV
Uracil-DNA glycosylase activity measurement method
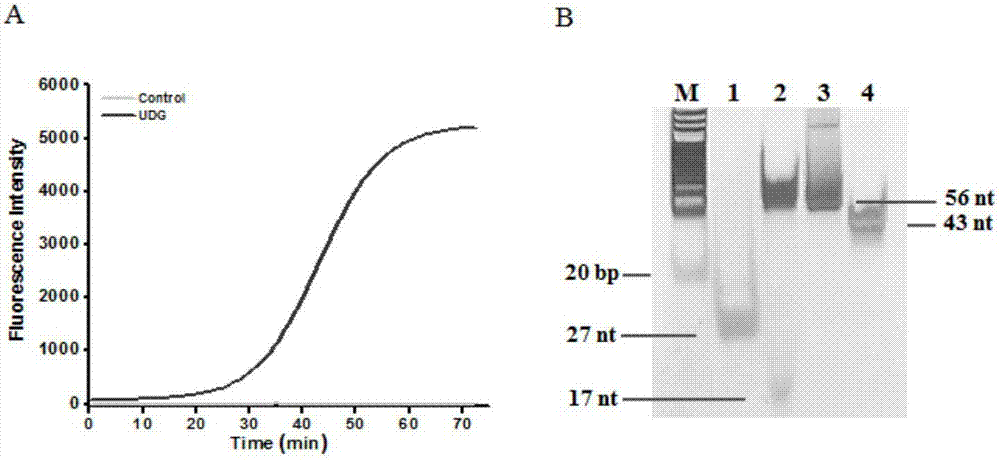
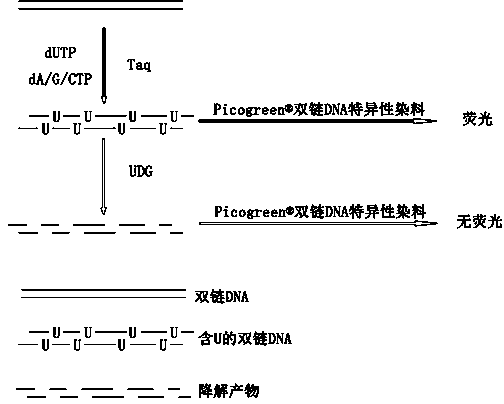
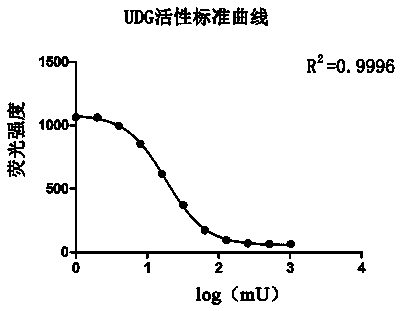
InactiveCN104293927AEasy to operateImprove throughputMicrobiological testing/measurementPolymerase LUracil-DNA glycosylase
The invention discloses a uracil-DNA glycosylase activity measurement method. The method comprises the following steps: firstly, carrying out PCR amplified reaction; with a double-chain DNA as a template, carrying out polymerization reaction by using polymerase in the presence of a dU / A / G / CTP mixture and a primer, and carrying out amplification to generate amplification product UDNA double chains containing dU; secondly, carrying out uracil-DNA glycosylase reaction, and forming a lot of DNA chains in base deletion from the amplification product through the uracil-DNA glycosylase action; and finally, measuring the relative quantity of a single-chain DNA product or UDNA double chains in the reaction system, and deriving the uracil-DNA glycosylase activity degree according to the measurement result, wherein the uracil-DNA glycosylase activity degree is inversely proportional to the quantity of the DUNA double chains in the reaction system, and is directly proportional to the quantity of the single-chain DNA product. The method disclosed by the invention is free of radioactive pollution, simple and rapid in operation, high in sensitivity, and stable, and quantitative determination can be carried out.
Owner:VAZYME BIOTECH NANJING
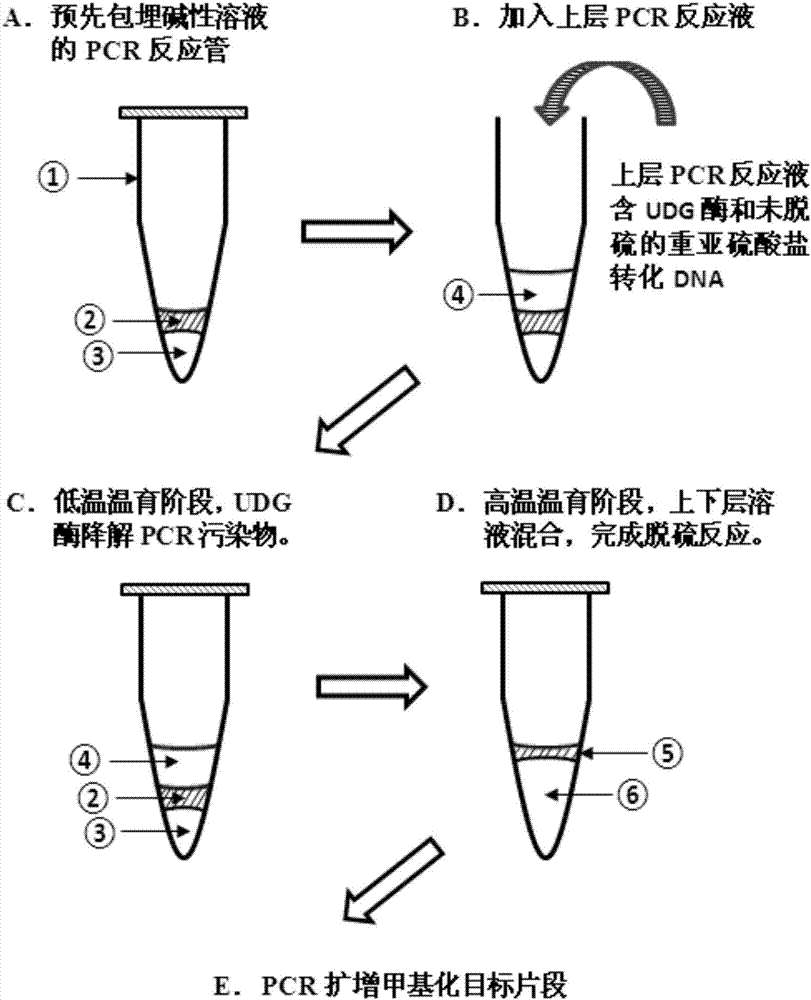
Methylated amplification method for eliminating PCR product pollution and application of methylated amplification method
ActiveCN107488721AEliminate pollutionMicrobiological testing/measurementMicrobiologyUracil-DNA glycosylase
The invention relates to a methylated amplification method for eliminating PCR product pollution and application of the methylated amplification method. Sub-system and sub-step heat treatment is carried out before a PCR reaction, so that uracil-DNA-glycosylase is effectively applied, and the pollution problem of methylated amplification PCR products is effectively solved.
Owner:GENE TECH SHANGHAI COMPANY
General detection method for pathogenic vibrios and nucleic acid isothermal amplification detection kit used in same
InactiveCN102154469AEfficient detectionEasy to handleMicrobiological testing/measurementSodium acetatePositive control
The invention discloses a general nucleic acid isothermal amplification detection kit for pathogenic vibrios of mariculture animals. The detection kit comprises a grinding liquid tube into which grinding liquid is filled, a nucleic acid extracting solution tube A into which solution of sodium acetate is filled, a nucleic acid extracting solution tube B into which absolute ethanol is filled, a nucleic acid extracting solution tube C into which 70 mass percent ethanol solution is filled, a tris-hydrogen chloride ethylene diamine tetraacetic acid (TE) buffer solution tube into which TE buffer solution is filled, a uracil-DNA-glycosylase (UNG) tube into which uracil-DNA-glycosylase is filled, a loop-mediated isothermal amplification (LAMP) reaction liquid tube into which LAMP reaction liquid is filled, a bacillus stearothermophilus deoxyribonucleic acid (Bst DNA) polymerase tube into which Bst DNA polymerase is filled, a color-developing agent tube into which a nucleic acid dye SYBR Green I is filled, a positive control nucleic acid tube into which positive DNA of the vibrios is filled and a negative control tube into which sterilized double distilled water is filled. The invention also discloses a method for detecting the pathogenic vibrios of the mariculture animals by utilizing the detection kit.
Owner:ZHEJIANG UNIV
Primer pair for detecting CYP2C9 genetic typing through pyrosequencing method and kit
InactiveCN105368826AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationNucleic acid detectionBiotin
The invention relates to a primer pair for detecting CYP2C9 genetic typing through a pyrosequencing method and a kit, and belongs to the technical field of in vitro nucleic acid detection. The primer pair comprises a CYP2C9*2 forward amplification primer, a CYP2C9*2 reverse amplification primer, a CYP2C9*2 sequencing primer, a CYP2C9*3 forward amplification primer, a CYP2C9*3 reverse amplification primer and a CYP2C9*3 sequencing primer. Biotin labeling is conducted at the 5' end of the CYP2C9*2 forward amplification primer and the 5' end of the CYP2C9*3 reverse amplification primer. The kit comprises the amplification primers, a PCR reaction solution 1, a PCR reaction solution 2, the sequencing primers, uracil DNA glycosylase and Taq polymerase. The kit has the advantages of being accurate in detection result, high in specificity, short in detection period, easy to operate and capable of effectively meeting the clinical examination requirement.
Owner:CHANGSHA 3G BIOTECH
Sequencing primer for qualitative detection of KRAS genetic typing and kit thereof
ActiveCN102899408AQualitatively accurateIncreased sensitivityMicrobiological testing/measurementDNA/RNA fragmentationUracil-DNA glycosylaseExon
The invention provides a sequencing primer for detecting mutation of codons 12 and 13 in a second exon and a codon 61 in a third exon of a KRAS gene, and a kit thereof, and belongs to the field of in vitro nucleic acid detection. The kit comprises uracil DNA glycosylase, Taq polymerase, PCR amplification primers and sequencing primers. The kit provided by the invention has high sensitivity and good specificity; the PCR products can be simply treated for sequencing on a phosphate sequenator; and the kit has advantages of simple operation, short reaction time, and higher sensitivity than a gold standard-capillary electrophoresis sequencing, and is more suitable for mutation analysis.
Owner:CHANGSHA 3G BIOTECH
Fluorescent PCR kit for qualitative detection of HLA-B*1502 gene subtypes
ActiveCN102660635AStrong specificityQualitatively accurateMicrobiological testing/measurementFluorescence/phosphorescenceNucleic acid detectionFluorescence
The invention provides a fluorescent PCR kit for qualitative detection of HLA-B*1502 gene subtypes, and belongs to the field of in-vitro nucleic acid detection. The kit comprises uracil DNA glycosylase, Taq polymerase, PCR genotyping primers and a fluorescence probe; the PCR genotyping primer sequence is as shown in SEQ ID NO: 3-4 and / or SEQ ID NO: 6-7. The kit provided in the invention has high sensitivity and good specificity, and can monitor reaction process in real time and the reaction time is short; in addition, closed tube operation is performed, and subsequent treatment is not needed, which can maximally avoid the pollution of a reaction product, thus being capable of replacing traditional cell detection.
Owner:CHANGSHA 3G BIOTECH
Method and kit for detecting DNA polymerase activity
ActiveCN107034280ASimple and fast operationGood reproducibilityMicrobiological testing/measurementForward primerPolymerase L
The invention relates to a method and kit for detecting DNA polymerase activity; the method comprises: mixing DNA polymerase under detection and a sufficient template to be filled in, and using, in the presence of dNTP, the DNA polymerase under detection to induce a first DNA fragment in the template to be filled in so as to form a filling-in fragment complementary with a base of a projecting fragment, thereby acquiring a filled-in template; digesting to remove the base U of the filled-in template through sufficient UDG (uracil DNA glycosylase) to obtain a template suitable for PCR (polymerase chain reaction) amplification, designing a forward primer for the projecting fragment and a reverse primer for 5' end of the first DNA fragment, and inducing first template chain amplification, wherein a template which is not filled in and has the base U removed via a digestive enzyme is unable to pair with the forward primer, and PCR amplification cannot be performed; CT (cycle threshold) value of DNA polymerase under detection can be brought to a standard curve established via a DNA polymerase standard, and activity of the DNA polymerase under detection can be acquired just by calculating.
Owner:FAPON BIOTECH INC +1
Sequencing primer for qualitative detection of TPMT genetic typing and kit thereof
ActiveCN102899407AQualitatively accurateIncreased sensitivityMicrobiological testing/measurementDNA/RNA fragmentationNucleic acid detectionCapillary electrophoresis
The invention provides a sequencing primer for qualitative detection of TPMT genetic typing and a kit thereof, and belongs to the field of in vitro nucleic acid detection. The kit comprises uracil DNA glycosylase, Taq polymerase, a PCR reaction liquid, PCR primers, pyrosequencing primers and a reference substance. The kit provided by the invention has high sensitivity and good specificity; the PCR products can be simply treated for sequencing on a phosphate sequenator; and the kit has advantages of simple operation, short reaction time, and higher sensitivity than a gold standard-capillary electrophoresis sequencing, and is more suitable for SNP analysis.
Owner:CHANGSHA 3G BIOTECH
Replication-competent herpes simplex viruses
InactiveUS6509020B1Safe and effective usePeptide/protein ingredientsGenetic material ingredientsUracil-DNA glycosylaseMutant
A promising approach for the therapeutic treatment of brain tumors utilizes replication-competent, neuroattenuated herpes simplex virus-1 (HSV-1) mutants. This approach requires mutation of HSV-1 to eliminate killing of normal, non-dividing cells of the brain (e.g., neurons). The present invention discloses methods for killing malignant brain tumor cells in vivo entails providing replication competent herpes simplex virus vectors to tumor cells. A replication competent herpes simplex virus vector, with defective expression of the gamma 34.5 gene and the uracil DNA glycosylase (UNG) gene, specifically destroys tumor cells, is hypersensitive to anti-viral agents, and is not neurovirulent.
Owner:UNIVERSITY OF CINCINNATI
Pyrosequencing primer pair and kit for qualitatively detecting CYP2D6 genotyping
InactiveCN106244708AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationCapillary electrophoresisElectrophoresis
The invention relates to a pyrosequencing primer pair for qualitatively detecting CYP2D6 genotyping; the primer pair includes a forward amplification primer and a reverse amplification primer, and a sequencing primer, and 5' ends of the forward amplification primer and reverse amplification primer are subjected to biotin labeling respectively; the invention also relates to a pyrosequencing kit for qualitatively detecting CYP2D6 genotyping; the kit includes amplification primers, PCR (polymerase chain reaction) liquid 1, PCR liquid 2, sequencing primers, uracil DNA glycosylase and Taq polymerase. The pyrosequencing primer pair and kit have the advantages that detection results are accurate, the specificity is high, detection period is short, operation is simple and clinical examination requirements can be effectively met and additionally have the advantages that reaction process can be monitored in real time, reaction time is short, PCR products can be fed to a pyrosequencing instrument for sequencing and high-throughput sample detection just through simple treatment, and the sensitivity is higher than that of a golden standard method, namely capillary electrophoresis sequencing method.
Owner:CHANGSHA 3G BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com