Method for qualitatively detecting HLA-B*1502 gene with PCR-SSP method and clinical kit
A technology of B1502-SSP-F2 and B1502-SSP-R5, which is applied in the field of qualitative detection of HLA-B*1502 gene and clinical detection kits, can solve the problems of damage, pollution, and false positives, and achieve the control of false positives , increase the effect of pollution system and low instrument requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0036] 1. Extract the genomic DNA of the subject's whole blood, saliva or other tissues according to the manufacturer's instructions.
[0037] 2. Main reagents: HLA-B*1502 clinical kit reagents. The main components include 2×PCR Mix (Roche), dUTP, UDG enzyme (Thermo Scientific).
[0038] 3. Reaction system and PCR program.
[0039] Configure 12 μl PCR reaction system: including 11 μl kit reagents, 1 μl (1-10ng) of subject sample DNA. PCR reaction program: first incubate at 37°C for 3min, then hot start at 95°C for 10min, 1 cycle; then denature at 95°C for 20sec, extend at 68°C for 50sec, a total of 40 cycles; finally, 72°C for 5min.
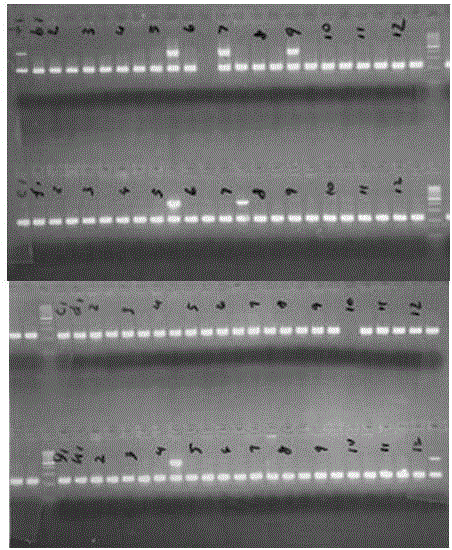
[0040] 4. Separation of PCR fragments: 2% agarose gel electrophoresis detection, electric field strength not higher than 5V / cm, time 20-30min, gel imaging system imaging. If there is an HLA-B*1502-specific band with a length of 430 bp bases and an internal reference control band with a length of 219 bp bases, the tested sample is positive for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com