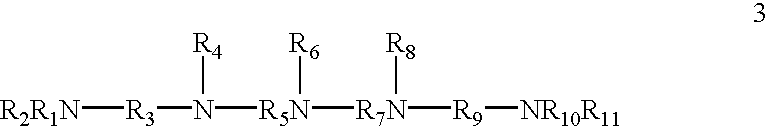
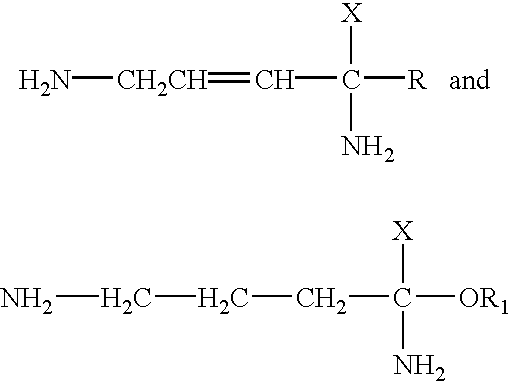
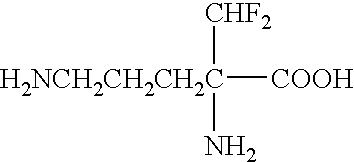
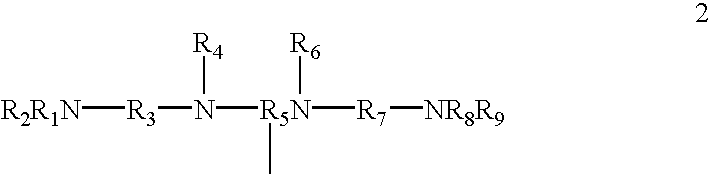Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Antiproliferative Drugs" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sirolimus is a potent immunosuppressive antiproliferative drug that acts to reduce Il-2–induced proliferation of lymphocytes by binding to FK binding protein and forming a complex that binds to the protein kinase mTOR.
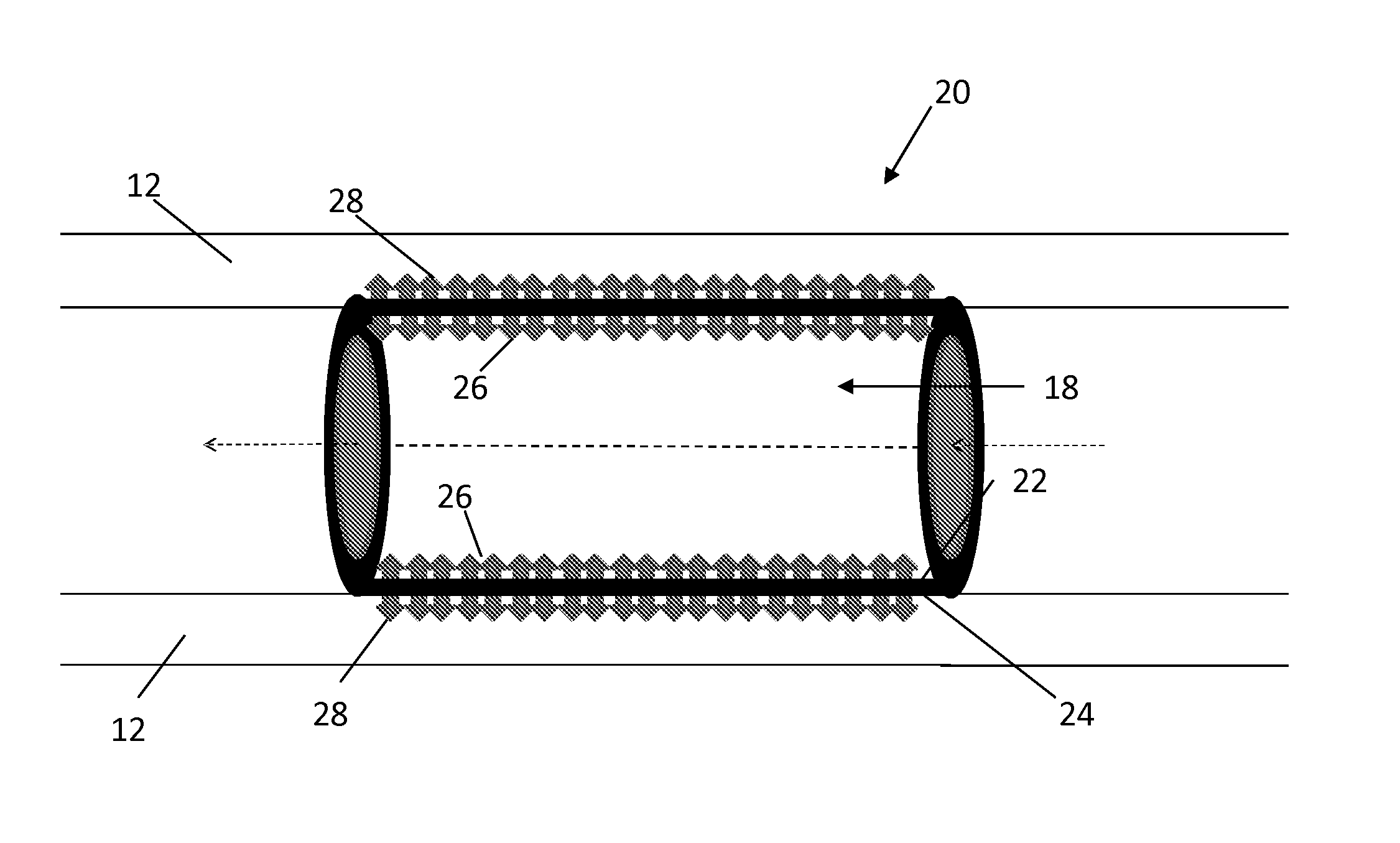
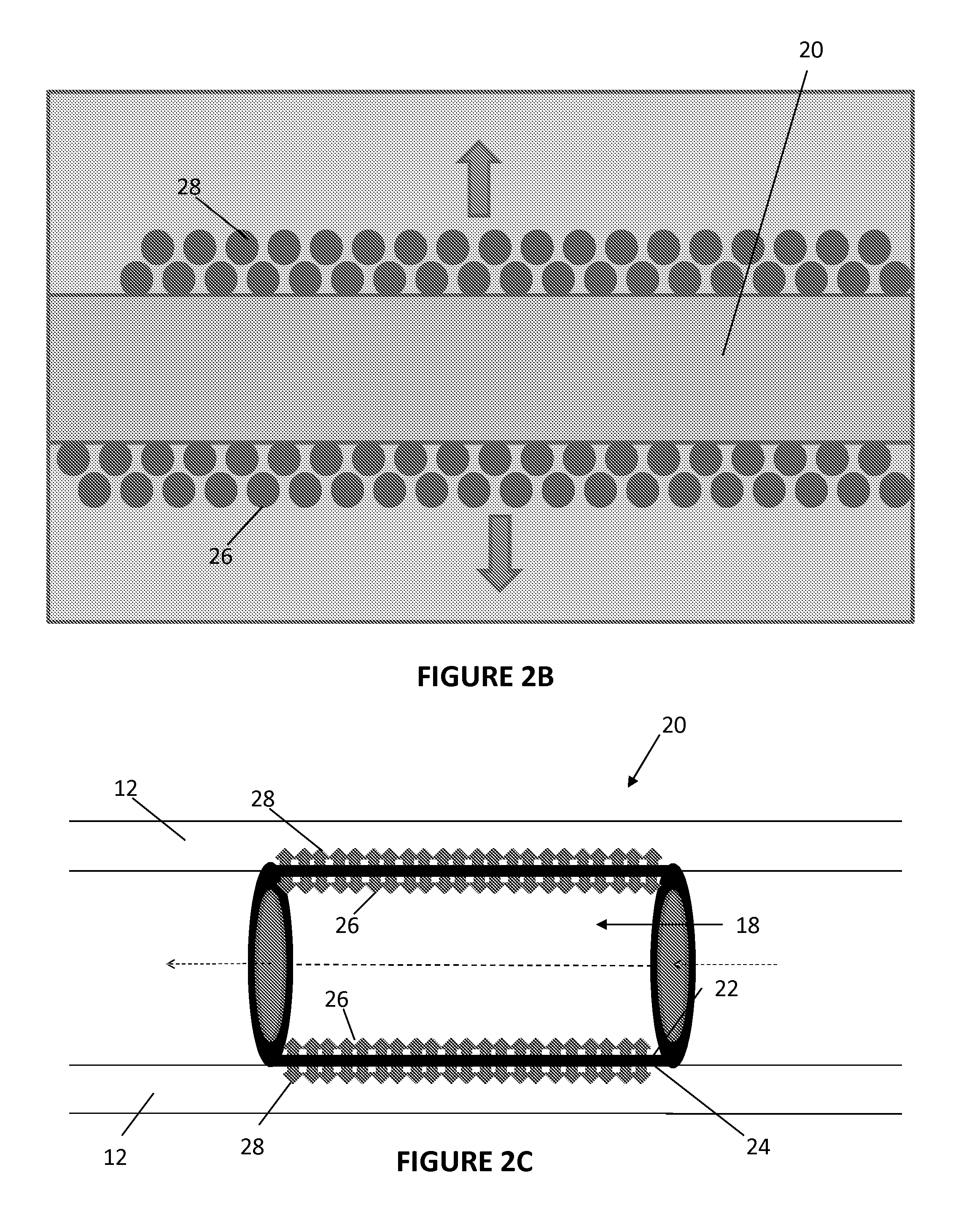
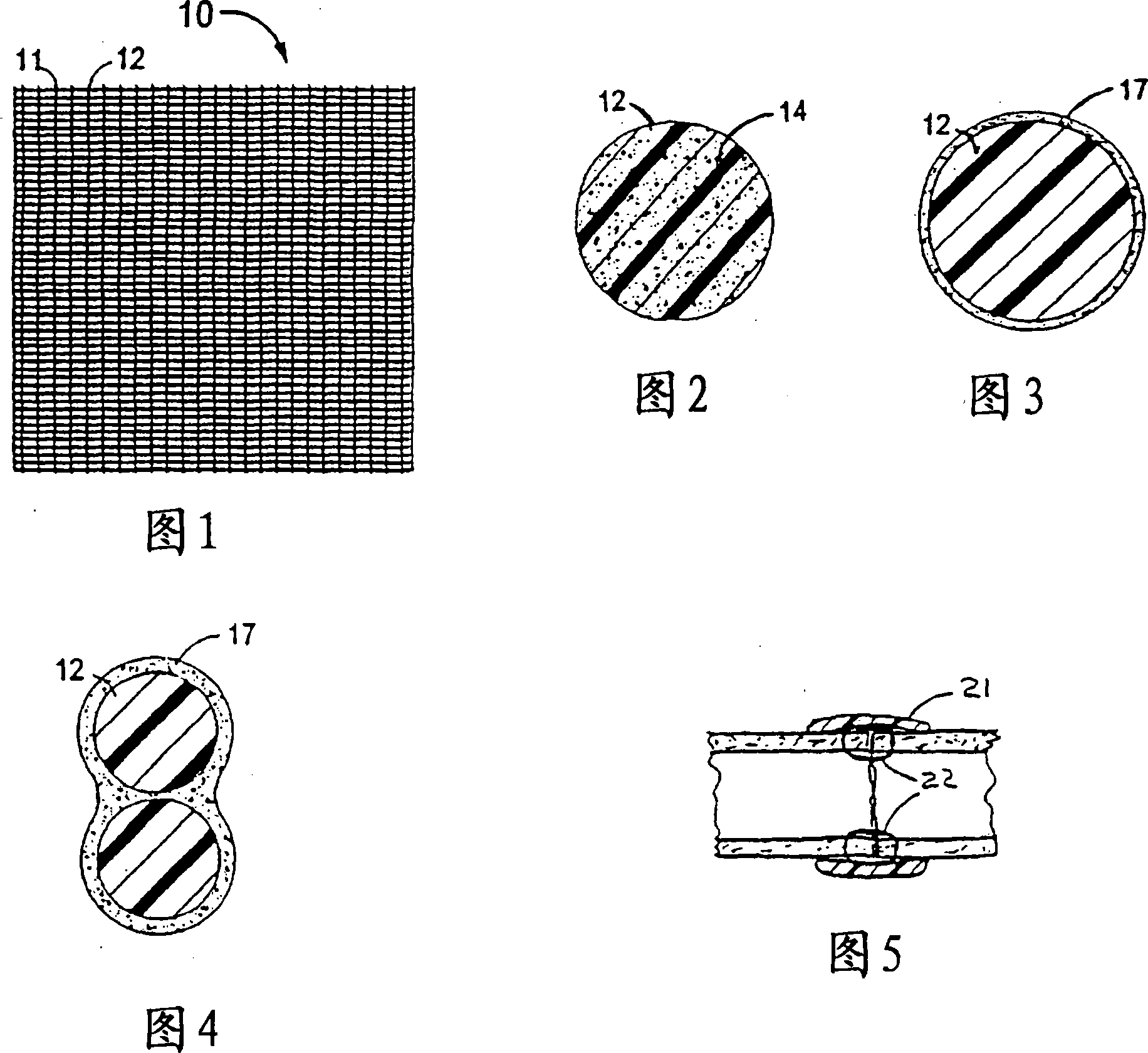
Apparatus and methods for preventing or treating failure of hemodialysis vascular access and other vascular grafts
InactiveUS6726923B2Reduce calcificationInhibiting smooth muscle cell proliferationBiocideOrganic chemistryVascular graftBlood vessel
Owner:VASCULAR THERAPIES INC
Drug-releasing stent with ceramic-containing layer
InactiveUS7713297B2Prevent proliferationPrevent restenosisStentsSurgeryEndoluminal stentAnti platelet
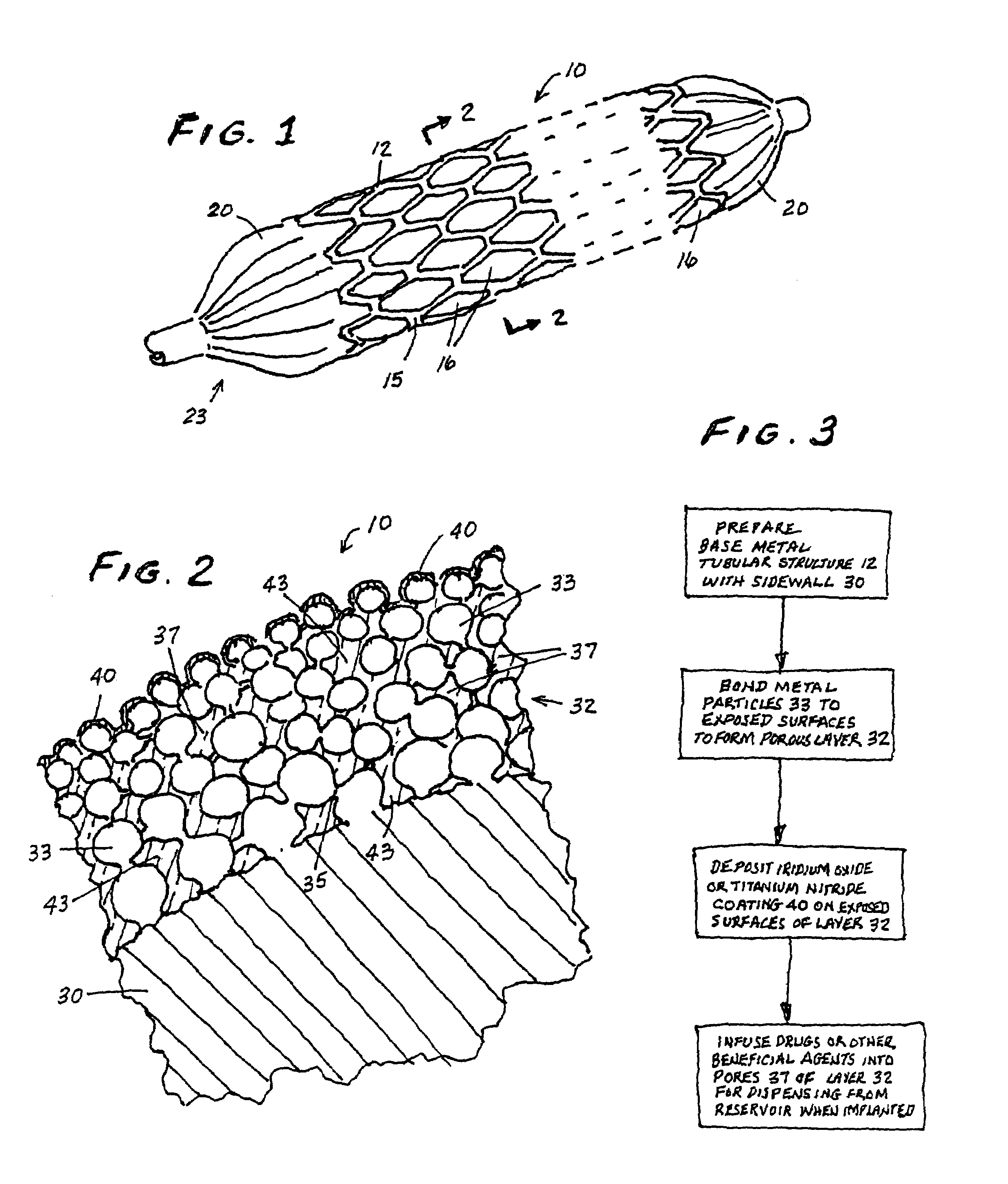
A vascular or endoluminal stent is adapted to be implanted in a vessel, duct or tract of a human body to maintain an open lumen at the site of the implant. The sidewall of the open-ended tubular structure of the stent is a base layer of a metal biologically compatible with blood and tissue of the human body. An intermediate metal particle layer of substantial greater radiopacity overlies the base layer, with particles bonded to the base layer and to each other to leave interstices therebetween as a repository for retaining and dispensing drugs or other agents for time release therefrom after the stent is implanted, to assist the stent in maintaining the lumen open. The particles are composed primarily of a noble metal—an alloy of platinum-iridium. The sidewall has holes extending therethrough, and the particle layer resides along the outward facing and inward facing surfaces, and the edges of the through holes and open ends of the sidewall. The larger particles are bonded to surfaces of the sidewall and progressively smaller particles are bonded to those and to each other up to the outer portion of the particle layer. Exposed surfaces of the particle layer are coated with ceramic-like iridium oxide or titanium nitrate, as a biocompatible material to inhibit irritation of tissue at the inner lining of the vessel when the stent is implanted. One or more anti-thrombotic, anti-platelet, anti-inflammatory and / or anti-proliferative drugs are retained in the interstices, together with a biodegradable carrier for time release therefrom. In an alternative embodiment, the intermediate layer is solid and the biodegradable carrier and drugs or agents therein are applied to the surface of the ceramic-like coating. Gene transfer is alternatively used to control tissue proliferation.
Owner:BOSTON SCI SCIMED INC
Combination of interleukin-6 antagonists and antiproliferative drugs
InactiveUS20090035281A1Good effectCounteracts paracrine actionBiocidePeptide/protein ingredientsDexamethasoneInterleukin 6
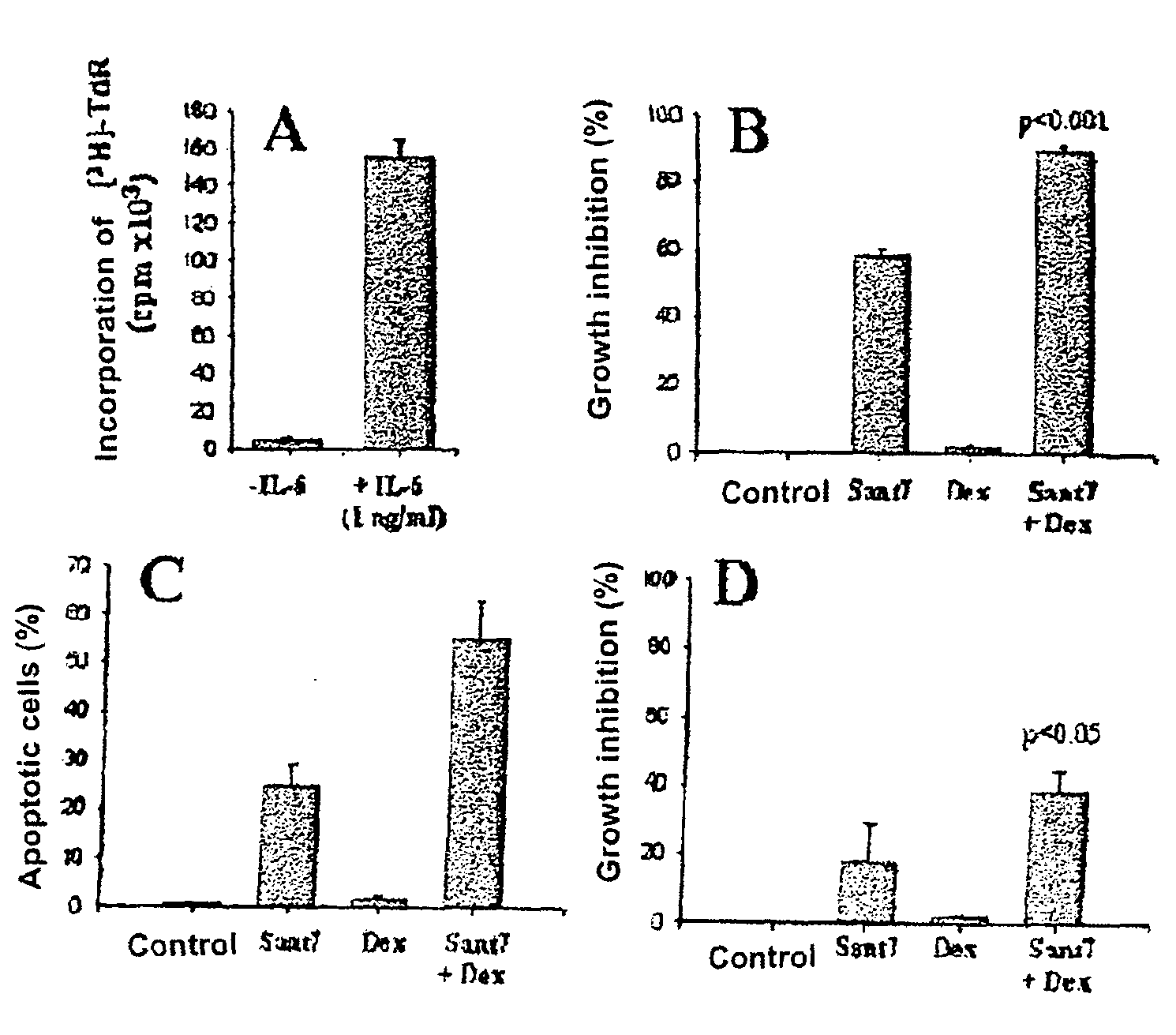
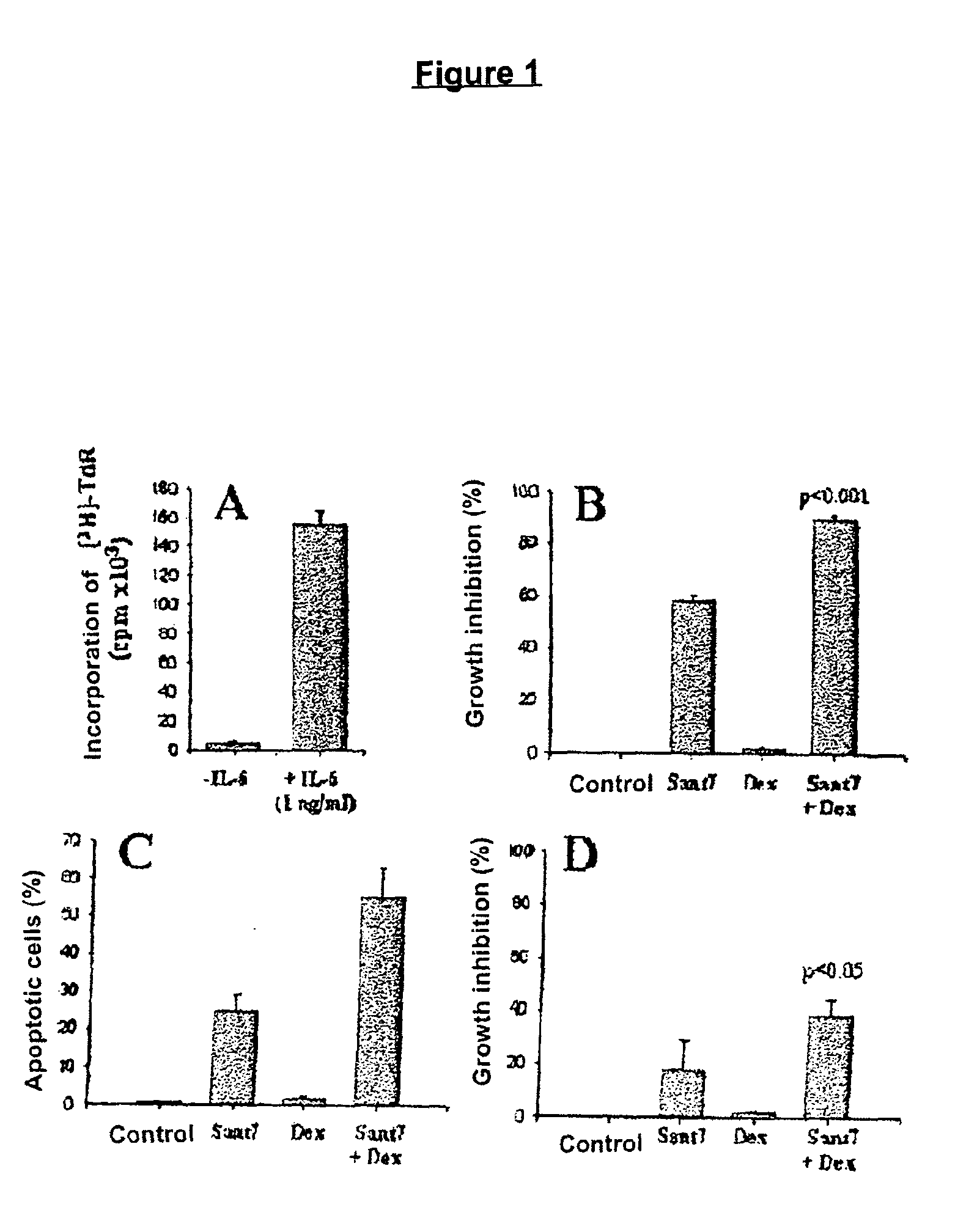
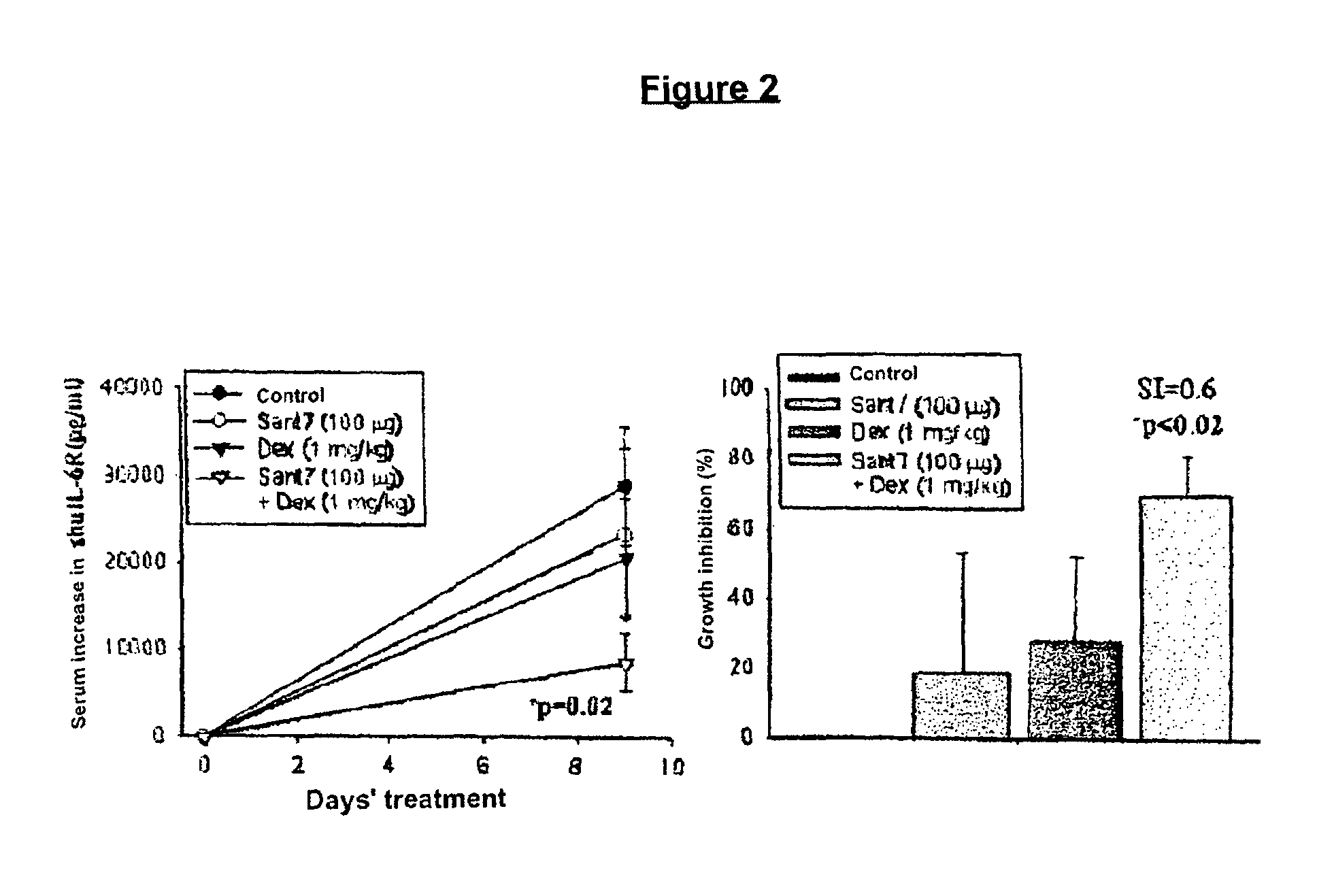
The combination of an interleukin-6 (IL-6) antagonist and an antiproliferative drug is described. In its preferred embodiment, the present invention describes the combination of an IL-6 superantagonist, particularly a superantagonist totally incapable of binding gp130 and an antiproliferative drug belonging to the glucocorticoid class (SANT-7 and dexamethasone). The combination according to the present invention has shown surprising synergism in an animal model of multiple myeloma and the ability to overcome the resistance to the antiproliferative drug developed by myeloid cells. The combination according to the present invention is useful for the preparation of a medicament for the treatment of tumours, particularly IL-6-dependent tumours.
Owner:UNIV DEGLI STUDI MAGNA GRAECIA DI CATANZARO
Treatment of asthma and chronic obstructive pulmonary disease with Anti-proliferate and Anti-inflammatory drugs
ActiveUS20130261603A1Promote absorptionRespiratorsPowder deliveryDiseaseObstructive Pulmonary Diseases
Embodiments of the present invention provide a method for treatment of respiratory disorders such as asthma, chronic obstructive pulmonary disease, and chronic sinusitis, including cystic fibrosis, interstitial fibrosis, chronic bronchitis, emphysema, bronchopulmonary dysplasia and neoplasia. The method involves administration, preferably oral, nasal or pulmonary administration, of anti-inflammatory and anti-proliferative drugs (rapamycin or paclitaxel and their analogues) and an additive.
Owner:LUTONIX INC
Apparatus and methods for preventing or treating failure of hemodialysis vascular access and other vascular grafts
This invention is a prosthetic device generally placed on the outside surface of the vessel or graft which then elutes antiproliferative drugs or agents from a drug-eluting matrix material. Methods of perivascular antiproliferative drug administration also are disclosed.
Owner:VASCULAR THERAPIES INC
Treatment of neurodegenerative disorders through the modulation of the polyamine pathway
InactiveUS20030158262A1Extend your lifeSlow and arrest progressCompound screeningBiocidePharmacometricsNeuro-degenerative disease
The present invention provides methods and compositions for modulating polyamine pathway activity as a means for ameliorating neurodegenarative disorders. In particular, for ameliorating the symptoms or onset of amyotrophic lateral sclerosis (ALS) by modulating the gene and protein products involved the polyamine pathway, such as by inhibiting the enzyme, ornithine decarboxylase (ODC), involved in the synthesis of the polyamine, putrescine. Compositions and methods are disclosed for inhibiting the polyamine pathway producing lower polyamine levels resulting in a beneficial effect on ALS. This can be accomplished by using modulating agents such as analogs, or polyamine analogs, and antiproliferative drugs. Screening assays for pharmacological agents that are capable of decreasing polyamine levels and / or reducing cell proliferation are also disclosed.
Owner:ALS THERAPY DEV FOUND INC
Use of polyamine analogs for amyotrophic lateral sclerosis
InactiveUS20030130357A1Improve solubilityImprovement in administrationBiocideCompound screeningAmyotrophic lateral sclerosisPathway activity
The present invention provides methods and compositions for modulating polyamine pathway activity as a means for ameliorating neurodegenarative disorders. In particular, for ameliorating the symptoms or onset of amyotrophic lateral sclerosis (ALS) by modulating the gene and protein products involved the polyamine pathway, such as by inhibiting the enzyme, ornithine decarboxylase (ODC), involved in the synthesis of the polyamine, putrescine. Compositions and methods are disclosed for inhibiting the polyamine pathway producing lower polyamine levels resulting in a beneficial effect on ALS. This can be accomplished by using modulating agents such as analogs, or polyamine analogs, and antiproliferative drugs. In particular, the polyamine analog N(1),N(11)-diethylnorspermine (DENSPM) is disclosed as a useful modulating agent in the treatment of ALS. Screening assays for pharmacological agents that are capable of decreasing polyamine levels and / or reducing cell proliferation are also disclosed.
Owner:ALS THERAPY DEV FOUND INC
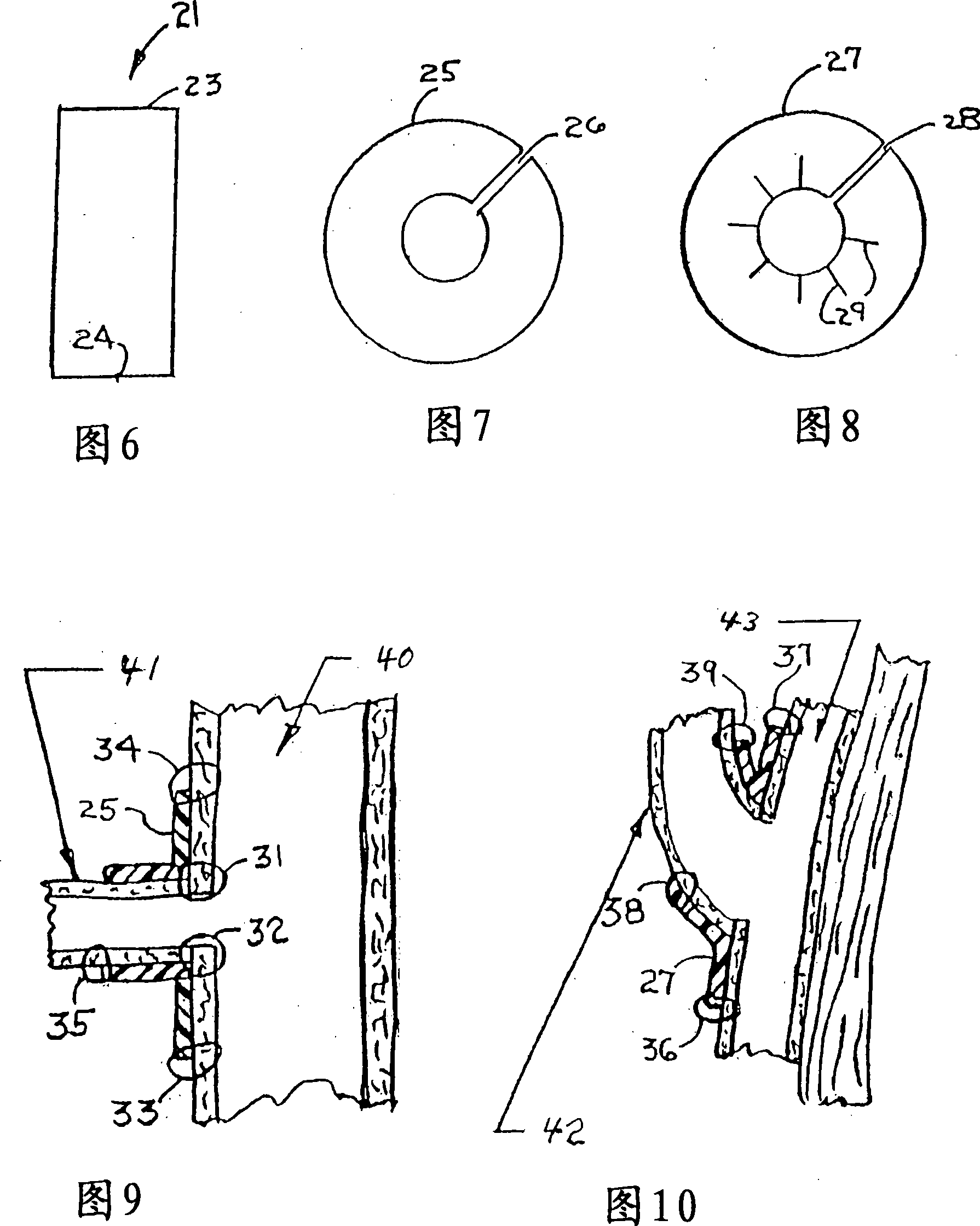
Method of producing personalized biomimetic drug-eluting coronary stents by 3d-printing
ActiveUS20160256610A1Avoid influenceReduce complicationsStentsAdditive manufacturing apparatusThrombusPercent Diameter Stenosis
A method for using 3D printing technology produces personalized biomimetic drug-eluting coronary stent and the product thereof. The process of manufacturing stent, based on coronary angiography imaging data, measures the diameter of diseased coronary and conducts 3D reconstruction. A personalized stent for each patient according to diameter, length, and morphological characteristics of target vessel that suited to the lesion is produced. The coronary stent is formed from biodegradable poly-L-lactic acid (PLLA) or other materials. The stent is modeled by 3D printing and then coated with polymers carrying antiproliferative drug to reduce restenosis (the polymers is a mixture of antiproliferative drug and PDLLA at a ratio of 1:1). The biomimetic drug-eluting coronary stent produced by 3D printing technology is personalized stent for each patient according to different characteristics of diseased coronary, reduces the incidence of vascular injury, thrombosis, dissection and other complications caused by stent and vessel diameter mismatch.
Owner:ZHOU YUJIE +3
Combination drug therapy for reducing scar tissue formation
InactiveUS20080039362A1Reduce spreadPrevents a permanent catheter tip splayBiocidePeptide/protein ingredientsCombination drug therapySurgical site
The present invention describes various devices and methods wherein a cytostatic antiproliferative drug, either alone or in combination with other drugs, is placed between internal body tissues to prevent the formation of scar tissue and / or adhesions during healing of a wound or surgical site. Specific devices to achieve this administration include, but are not limited to, a permanent implant or a biodegradable material having an attached antiproliferative drug such as sirolimus. These antiproliferative drugs may be combined with other drugs including, but not limited to, antiplatelets, antithrombotics or anticoagulants. The present invention also contemplates methods to a reduce scar tissue and / or adhesions or adhesion formation at an anastomosis site. In particular, a cytostatic antiproliferative drug is administered to an arteriovenous shunt anastomoses in patients having end-stage renal disease.
Owner:AFMEDICA INC
Use of difluoromethylornithine (DFMO) for the treatment of amyotrophic lateral sclerosis
InactiveUS20030130350A1Improve solubilityImprovement in administrationCompound screeningBiocideAmyotrophic lateral sclerosisPathway activity
The present invention provides methods and compositions for modulating polyamine pathway activity as a means for ameliorating neurodegenarative disorders. In particular, for ameliorating the symptoms or onset of amyotrophic lateral sclerosis (ALS) by modulating the gene and protein products involved the polyamine pathway, such as by inhibiting the enzyme, ornithine decarboxylase (ODC), involved in the synthesis of the polyamine, putrescine. Compositions and methods are disclosed for inhibiting the polyamine pathway producing lower polyamine levels resulting in a beneficial effect on ALS. This can be accomplished by using modulating agents such as analogs, or polyamine analogs, and antiproliferative drugs. In particular, the ODC inhibitor difuoromethylornithine (DFMO) is disclosed as a useful pharmacological agent in the modulation and treatment of ALS. Screening assays for pharmacological agents that are capable of decreasing polyamine levels and / or reducing cell proliferation are also disclosed.
Owner:ALS THERAPY DEV FOUND INC
Peptide conjugated anti-cancer prodrugs
The present invention relates to prodrug molecules comprising conjugates of an antiproliferative drug, a protease specific cleavable peptide, and, optionally, a targeting peptide, with the prodrugs being substantially inactive prior to degradation of the cleavable sequence by proteolytic enzymes abundant within or in close proximity to the target cancer cell. Also, pharmaceutical compositions of the conjugates and the use of these compositions for treatment of cancer are disclosed.
Owner:BIOSIGHT
Conjugates for cancer therapy and diagnosis
ActiveUS20070072800A1Promote absorptionBiocideTripeptide ingredientsAbnormal tissue growthLymphatic Spread
The present invention relates to conjugates of a drug and an amino acid or an amino acid derivative or analog, pharmaceutical compositions that include the conjugates and methods of use thereof. In particular, the present invention relates to conjugates of anti-proliferative drugs and asparagine and glutamine and analogs thereof as compositions for treatment of cancer, and conjugates of imaging agent carriers and amino acids for the diagnosis of tumors and metastases.
Owner:BIOSIGHT
Drug coated balloon catheters for nonvascular strictures
ActiveUS10888640B2Promote absorptionAvoid dependenceOrganic active ingredientsBalloon catheterBile duct stricturesVascular body
Owner:UROTRONIC
Method of producing personalized biomimetic drug-eluting coronary stents by 3D-printing
ActiveUS9943627B2Good effectSave more lives of patientsStentsAdditive manufacturing apparatusThrombusPercent Diameter Stenosis
A method for using 3D printing technology produces personalized biomimetic drug-eluting coronary stent and the product thereof. The process of manufacturing stent, based on coronary angiography imaging data, measures the diameter of diseased coronary and conducts 3D reconstruction. A personalized stent for each patient according to diameter, length, and morphological characteristics of target vessel that suited to the lesion is produced. The coronary stent is formed from biodegradable poly-L-lactic acid (PLLA) or other materials. The stent is modeled by 3D printing and then coated with polymers carrying antiproliferative drug to reduce restenosis (the polymers is a mixture of antiproliferative drug and PDLLA at a ratio of 1:1). The biomimetic drug-eluting coronary stent produced by 3D printing technology is personalized stent for each patient according to different characteristics of diseased coronary, reduces the incidence of vascular injury, thrombosis, dissection and other complications caused by stent and vessel diameter mismatch.
Owner:ZHOU YUJIE +3
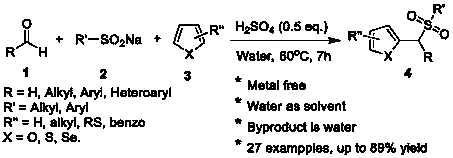
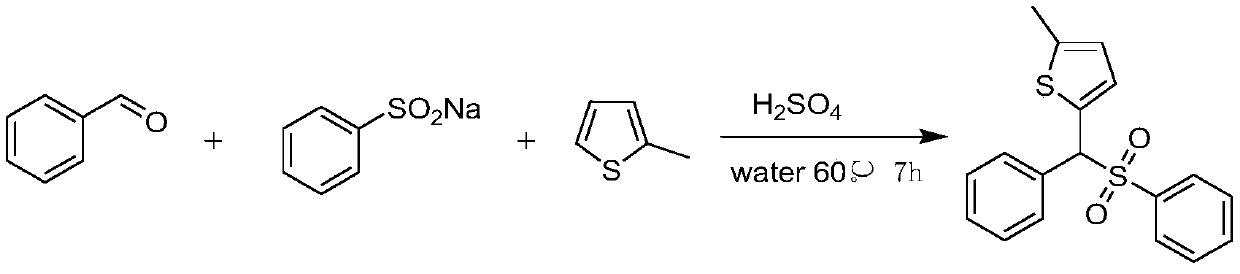
Synthesis method of aryl(chalcogen-heteroaryl)methyl sulfone
Sulfone is an important drug and bioactive compound. Sulfone is widely used as a medicament, such as a medicament gamma-secretase inhibitor for preventing Alzheimer's disease, and is widely applied tobioactive compounds, natural products and agricultural chemicals, such as currently popular herbicide mesotrione. Sulfone is also most often used as an intermediate for organic synthesis. On the other hand, a chalcogen heterocyclic stent has biological activity, such as antitumor drugs and antiproliferative drugs. As is well-known, thiophene, furan and selenium compounds have a variety of biological activities, such as anti-inflammatory agents, anti-HIV PR inhibitors, NQO2 inhibitors, and anticancer agents. The invention develops a simple, convenient and efficient Bronsted acid, i.e., sulfuric acid and catalyzed three components react in water to synthesize aryl(chalcogen-heteroaryl)methyl sulfone, the yield is good to be very high, and the substrate range is wide. The synthesis method isenvironment-friendly and economic, and does not need metal catalysis. The sulfone product can be effectively converted into bactericide analogues and aryl heteroaryl ketones.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
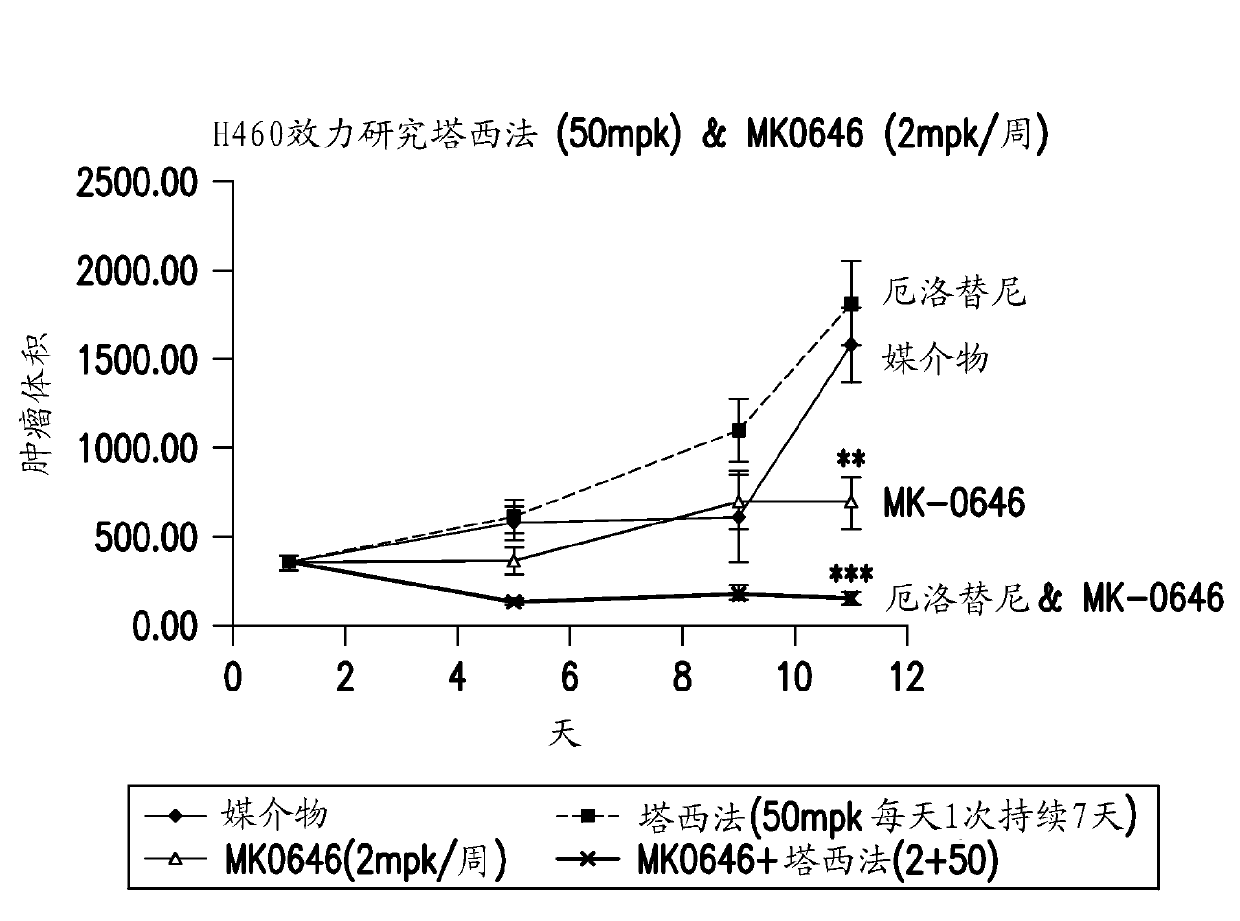
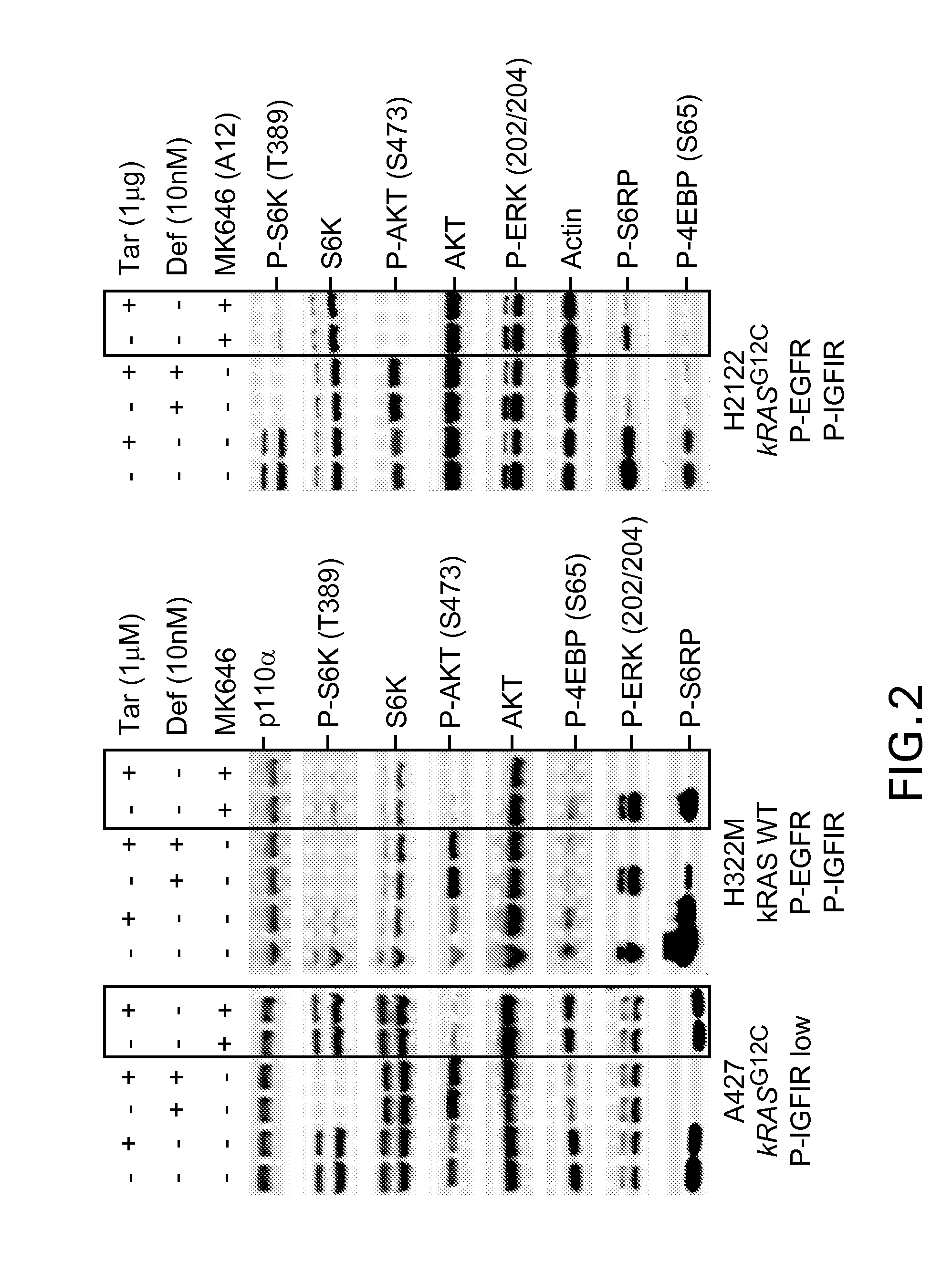
Combination therapy using an anti-egfr agent(s) and igf-1r specific inhibitors
The combination of an IGF-1R antagonist such as a humanized antibody and an anti-proliferative drug is described. In a preferred embodiment, the present invention describes the combination of an IGF-1R antibody and an anti-proliferative drug belonging to the EGFR-inhibitor class, which is preferably erlotinib. The combination according to the present invention is useful for the treatment of tumours, including IGF-1R and / or EGFR mediated or dependent tumours.
Owner:SCHERING AG
Biodegradable lacrimal duct embolus and preparation method thereof
ActiveCN102018995AGood biocompatibilityAvoid secondary surgerySurgeryLactide-caprolactone copolymerLactide
The invention discloses a biodegradable lacrimal duct embolus and a preparation method thereof. The lacrimal duct embolus is prepared by using one or more of a lactide / caprolactone copolymer, a glycolide / caprolactone copolymer, a lactide / dioxane ketone copolymer and a lactide / trimethylene carbonate copolymer as raw materials through the following steps of sequentially carrying out melt extrusion,hot stretching and cold cutting on the raw materials in an inert gas atmosphere. The biodegradable lacrimal duct embolus is used for treating dry eye syndromes; in the process of operation, a part ofthe lacrimal duct embolus is implanted into a lacrimal duct, and the rest of the lacrimal duct embolus can automatically be shrunk into the lacrimal duct, therefore, in the process of operation, a patient feels no pain, the diameter of the lacrimal duct of the patient is not required to be measured before operation, and the lacrimal duct embolus in one product specification can be suitable for the lacrimal ducts in different sizes; in the process of preparing the biodegradable lacrimal duct embolus, the embolus can load anti-inflammatory agents, antibiotics and antiproliferative drugs so as to avoid the generation of complications such as inflammations, wrapping and the like; and in addition, the lacrimal duct embolus is good in biocompatibility and can be completely biodegraded in the lacrimal duct, and the final products of the embolus are non-toxic water and carbon dioxide, thereby avoiding secondary surgery of the patient.
Owner:HE EYE HOSPITAL SHENYANG
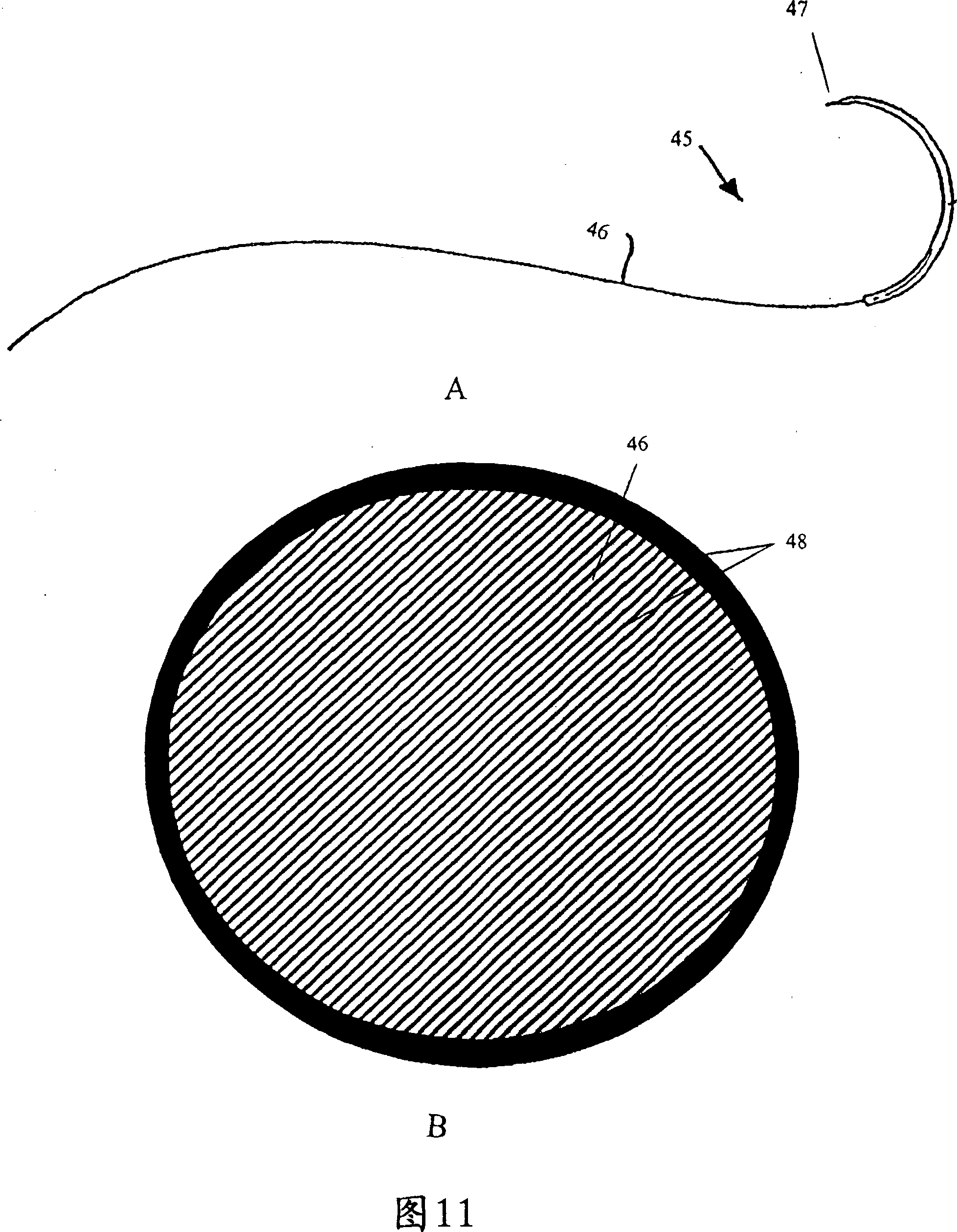
Methods, Systems, and Devices Relating to Directional Eluting Implantable Medical Devices
Implantable medical devices may directionally elute a first therapeutic agent that promotes the growth of endothelial cells and a second therapeutic agent that inhibits the growth of smooth muscle cells. In some embodiments, implantable medical devices may elute a first therapeutic agent such as an anti-proliferative drug from an abluminal side of the implantable medical device and a second therapeutic agent such as an endothelialization agent from a luminal side of the implantable medical device.
Owner:SOUTH DAKOTA BOARD OF REGENTS
Antiproliferative Drug
InactiveUS20080200428A1Strong antiproliferative activityLow toxicityBiocideOrganic active ingredientsHyaluronic acidOrganic chemistry
Object of the present invention are esterified conjugates of hyaluronic acid having anti-proliferative activity.
Owner:APTALIS PHARMA
Devices and methods for reducing scar tissue formation
InactiveUS20070110796A1Inhibition formationReduce cell proliferationSuture equipmentsBiocideCytostatic drugsHemostat
Disclosed is a cytostatic drug attached to a sterile sheet that is designed to be placed between internal body tissues to prevent the formation of post-operative adhesions, which adhesions are really scar tissue formation. This sheet onto or into which the drug is placed may be either a permanent implant or it may be biodegradable. By impregnating an existing product such as the Johnson & Johnson SURGICEL™ absorbable hemostat gauze-like sheet with an anti-proliferative drug such as sirolimus, the biodegradable, drug impregnated mesh would act as a barrier to cell proliferation and hence be a deterrent to the formation of adhesions or scar tissue. Another embodiment of this invention is a cytostatic drug attached to a sheet that is placed at the site of an anastamosis to decrease scar tissue formation from within the vessel at the site of the anastomosis.
Owner:AFMEDICA INC
Antibodies
InactiveUS20120058112A1Good curative effectOrganic active ingredientsBiocideErlotinibHumanized antibody
The combination of an IGF-1R antagonist such as a humanized antibody and an anti-proliferative drug is described. In a preferred embodiment, the present invention describes the combination of an IGF-1R antibody and an anti-proliferative drug belonging to the EGFR-inhibitor class, which is preferably erlotinib. The combination according to the present invention is useful for the treatment of tumours, including IGF-1R and / or EGFR mediated or dependent tumours.
Owner:MERCK SHARP & DOHME CORP
Novel PTCA balloon catheter
InactiveCN112007215AImprove fusion effectGood compatibilityBalloon catheterPharmaceutical delivery mechanismHydrophilic coatingPharmaceutical drug
The invention provides a novel PTCA balloon catheter. The catheter comprises a far-end tube, a near-end tube and a balloon, a near-end tube is arranged at the bottom end of the far-end tube, the balloon sleeves the outside of the near-end tube, the balloon is provided with three layers and comprises a hydrophilic coating, a compatibility layer and an anti-proliferative drug layer, an inner flow layer and an outer flow layer are arranged on the inner wall of the balloon, the inner flow layer is attached to the outer flow layer, the inner flow layer is formed by staggered limiting slim tubes, and the outer flow layer is formed by a fine hair structure. The PTCA balloon catheter adopts the hydrophilic coating, the compatibility layer and the anti-proliferative drug layer to improved the intermiscibility of organisms; and during the flow process, a liquid flows into the inner flow layer from the outer flow layer, the fusion of the liquid is improved by a plurality of groups of the limitingslim tubes, so that the biocompatibility of organisms can be greatly improved; the biological liquid flows to the outer flow layer after being dissolved, and the liquid tends to be stable after beingfused due to the action of a plurality of fine hairs; and in addition, the compatibility of the liquid can be further improved due to the action of the fine hairs.
Owner:浙江桐轩医疗科技有限公司
Combination drug therapy for reducing scar tissue formation
The present invention describes various devices and methods wherein a cytostatic antiproliferative drug, either alone or in combination with other drugs, is placed between internal body tissues to prevent the formation of scar tissue and / or adhesions during healing of a wound or surgical site. Specific devices to achieve this administration include, but are not limited to, a permanent implant or a biodegradable material having an attached antiproliferative drug such as sirolimus. These antiproliferative drugs may be combined with other drugs including, but not limited to, antiplatelets, antithrombotics or anticoagulants. The present invention also contemplates methods to a reduce scar tissue and / or adhesions or adhesion formation at an anastomosis site. In particular, a cytostatic antiproliferative drug is administered to an arteriovenous shunt anastomoses in patients having end-stage renal disease.
Owner:AFMEDICA INC
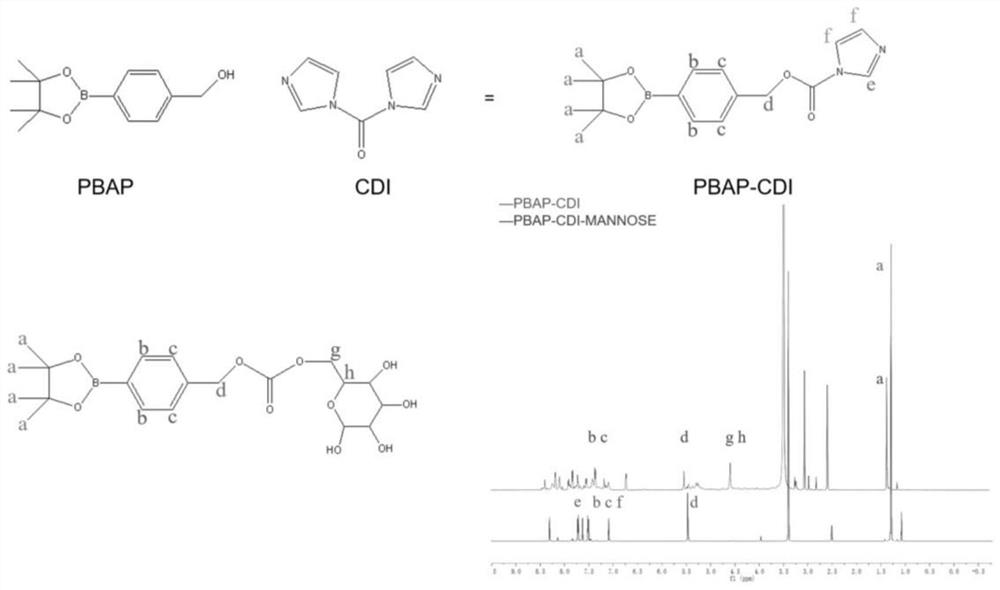
Nano-drug for inhibiting injured vascular intimal hyperplasia and application of nano-drug
PendingCN114057818AAchieve solubilizationStrong targetingOrganic active ingredientsSugar derivativesBlood vessel injuryBiocompatibility
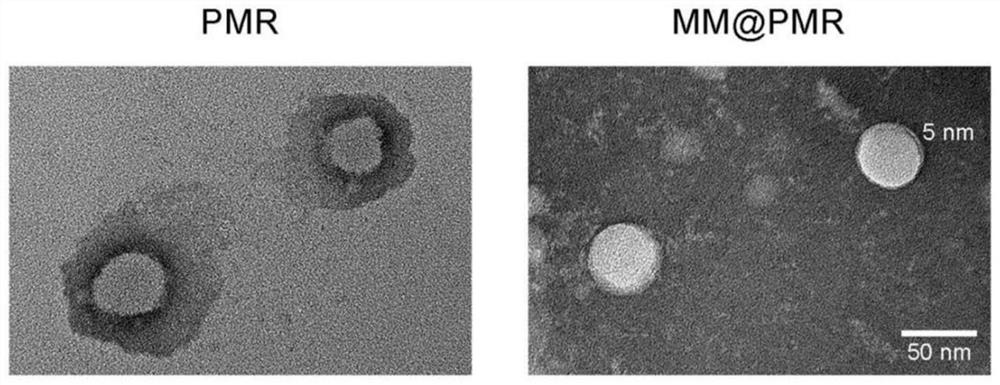
The invention relates to the field of biological medicine, and particularly discloses a nano-drug for inhibiting injured endometrial hyperplasia and application thereof. According to the invention, an anti-proliferation drug RAPA is used as a model drug, phenylboronic acid and mannose are used for synthesizing an amphiphilic carrier PBAP-CDI-Mannose (PCM) with ROS responsiveness, the amphiphilic carrier is used for entrapping the RAPA to form a nanoparticle PMR, and a macrophage cell membrane is coated on the nanoparticle PMR to prepare the multifunctional bionic nanoparticle MM-PMR. The bionic nano particle MM (at) PMR prepared by the invention is uniform in particle size, good in dispersity and relatively good in biocompatibility, has an immune escape function, can be used for targeted pathological site drug delivery, and can be used for inhibiting injured vascular intimal hyperplasia when being applied to preparation of drugs for treating vascular injury.
Owner:CHONGQING UNIV
Combination drug therapy for reducing scar tissue formation
InactiveCN101164617ASuture equipmentsOrganic active ingredientsAntithrombotic AgentCombination drug therapy
The present invention describes various devices and methods wherein a cytostatic antiproliferative drug, either alone or in combination with other drugs, is placed between internal body tissues to prevent the formation of scar tissue and / or adhesions during healing of a wound or surgical site. Specific devices to achieve this administration include, but are not limited to, a permanent implant or a biodegradable material having an attached antiproliferative drug such as sirolimus. These antiproliferative drugs may be combined with other drugs including, but not limited to, antiplatelets, antithrombotics or anticoagulants. The present invention also contemplates methods to a reduce scar tissue and / or adhesions or adhesion formation at an anastomosis site. In particular, a cytostatic antiproliferative drug is administered to an arteriovenous shunt anastomoses in patients having end-stage renal disease.
Owner:AFMEDICA INC
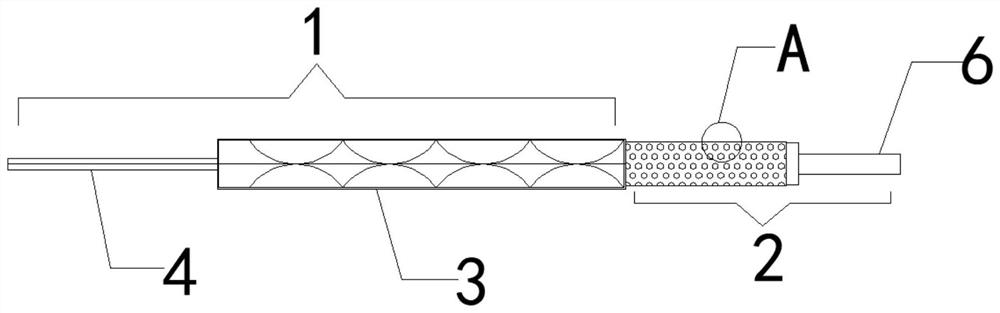
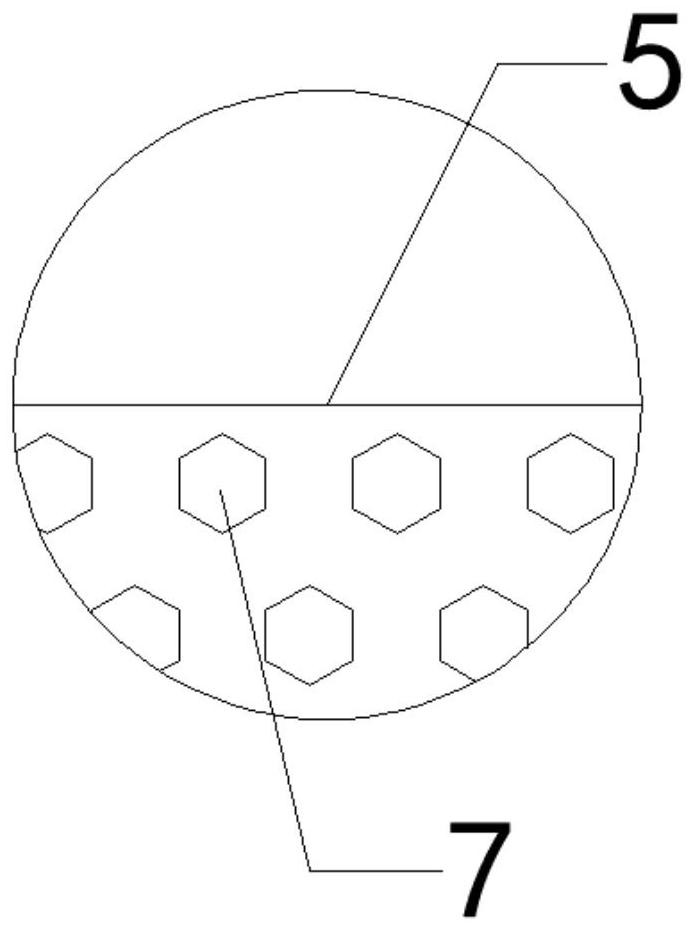
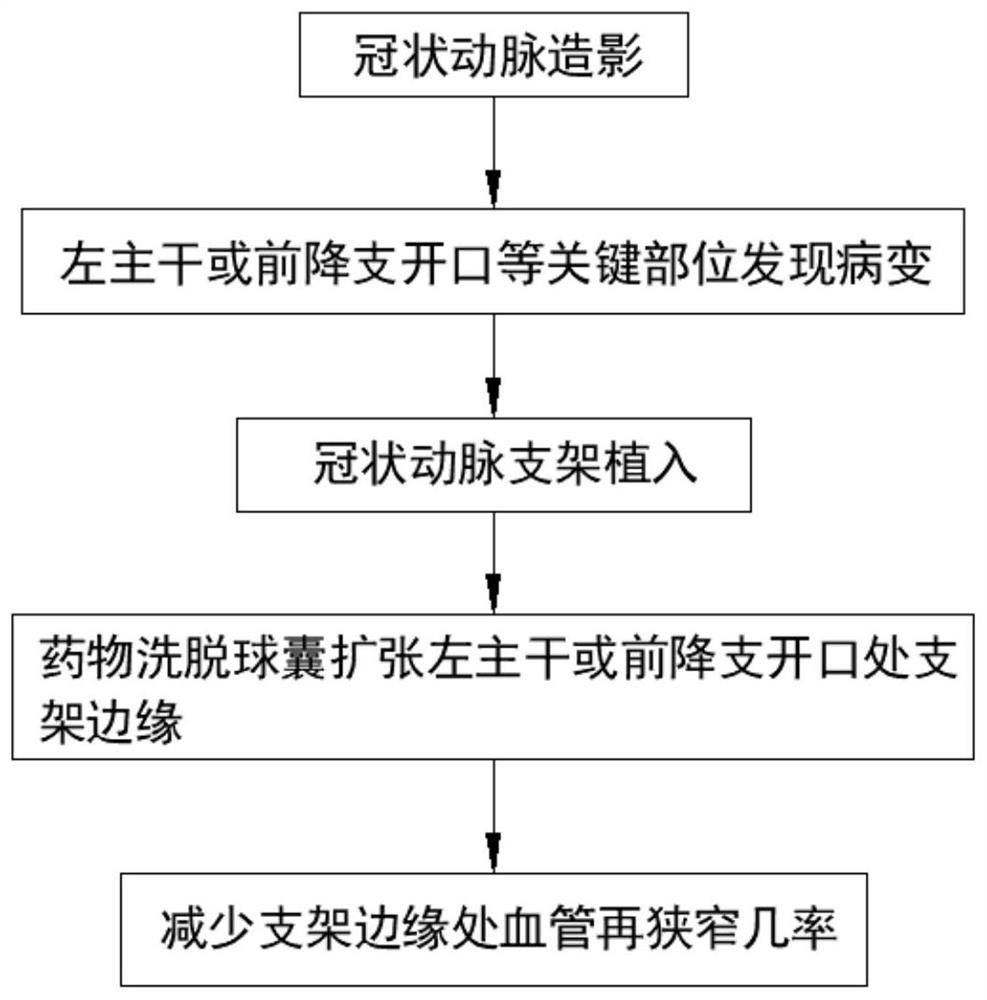
Coronary artery edge protective stent
PendingCN111956373AReduce the chance of interventionInhibit hyperplasiaStentsBalloon catheterCoronary arteriesCoronary artery disease
The invention discloses a coronary artery edge protective stent. The coronary artery edge protective stent comprises a drug-eluting stent and a drug-eluting balloon, wherein the drug-eluting stent comprises a stent body and a delivery system, the drug-eluting balloon comprises a balloon body, the balloon body is cylindrical, a catheter is arranged at one end of the balloon body, a plurality of filling grooves are formed in the outer wall of the balloon body. On the basis of treatment of coronary artery disease by original coronary artery drug-eluting stent implantation, the drug-eluting balloon is used to deliver an anti-proliferative drug to a key part of coronary artery drug-eluting stent implantation, the occurrence of the edge effect of the drug-eluting stent is reduced, the risk of edge restenosis of the stent for a patient is reduced, and the probability of percutaneous coronary intervention is decreased again.
Owner:中山大学附属第八医院
Treatment of ischemia and reperfusion using leptin antagonist
ActiveUS11123461B2Good effectPeptide/protein ingredientsAerosol deliveryStent deploymentPharmacology
A method and device for localized treatment of tissues or organs that were exposed to ischemia and reperfusion (IR) injury, in order to reduce their structural damage and loss of function. The method further includes intra-arterial treatment of transplanted tissues or organs, which are exposed to similar damage of IR and are at risk of impaired function. Leptin antagonist is administered as a bolus injection directly into a re-opened artery, which supplies blood to the tissue or organ involved, immediately after reperfusion. In some cases, after administering a bolus injection of leptin antagonist, the effect of leptin antagonist in the involved organ can be prolonged by deploying a double function drug eluting stent, which elutes leptin antagonist into the lumen, e.g., by sustained release, while eluting antiproliferative drug into the vessel wall to prevent local stenosis, which may appear due to stent deployment.
Owner:REMODELESS CV LTD
Drug eluting stent
Devices and methods for treating ischemia and reperfusion injury (IRI) are configured for sustained-release of anti-proliferative drug into the wall of a blood vessel (to prevent in-stent stenosis), and for sustained-release of leptin antagonist into the lumen to be carried by the blood and be uptaken by tissue cells that were subjected to IRI.
Owner:REMODELESS CV LTD
Treatment of ischemia and reperfusion using leptin antagonist
ActiveUS11033662B2Good effectPeptide/protein ingredientsAerosol deliveryStent deploymentPharmacology
Owner:REMODELESS CV LTD
Antiproliferative drug
InactiveCN101227906AEnhanced anti-proliferative activityLow toxicityAntipyreticAnalgesicsFatty acidAmino acid peptide
Object of the present invention are esterified conjugates of hyaluronic acid having anti-proliferative activity.
Owner:EURAND PHAMACEUTICALS LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com