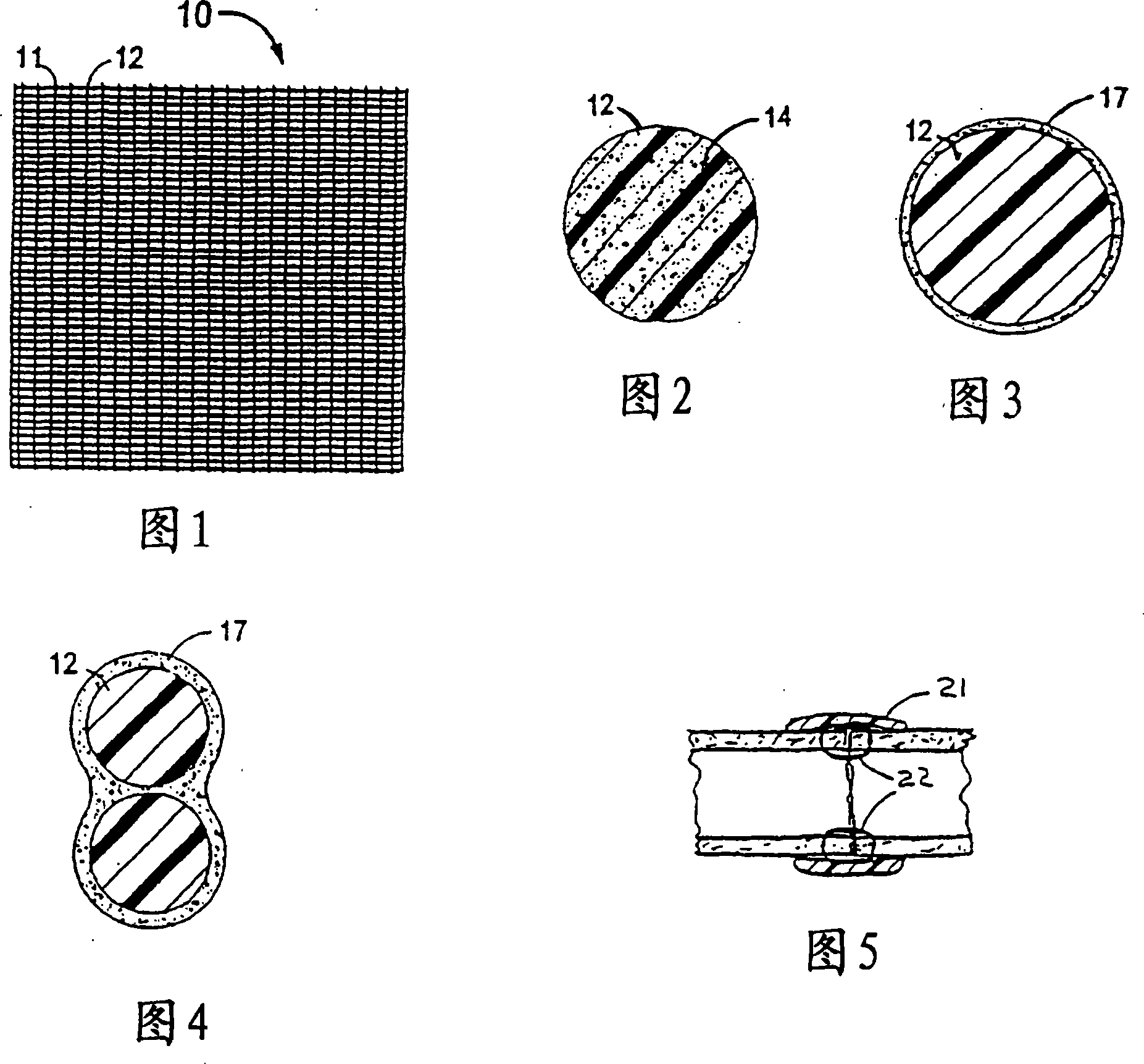
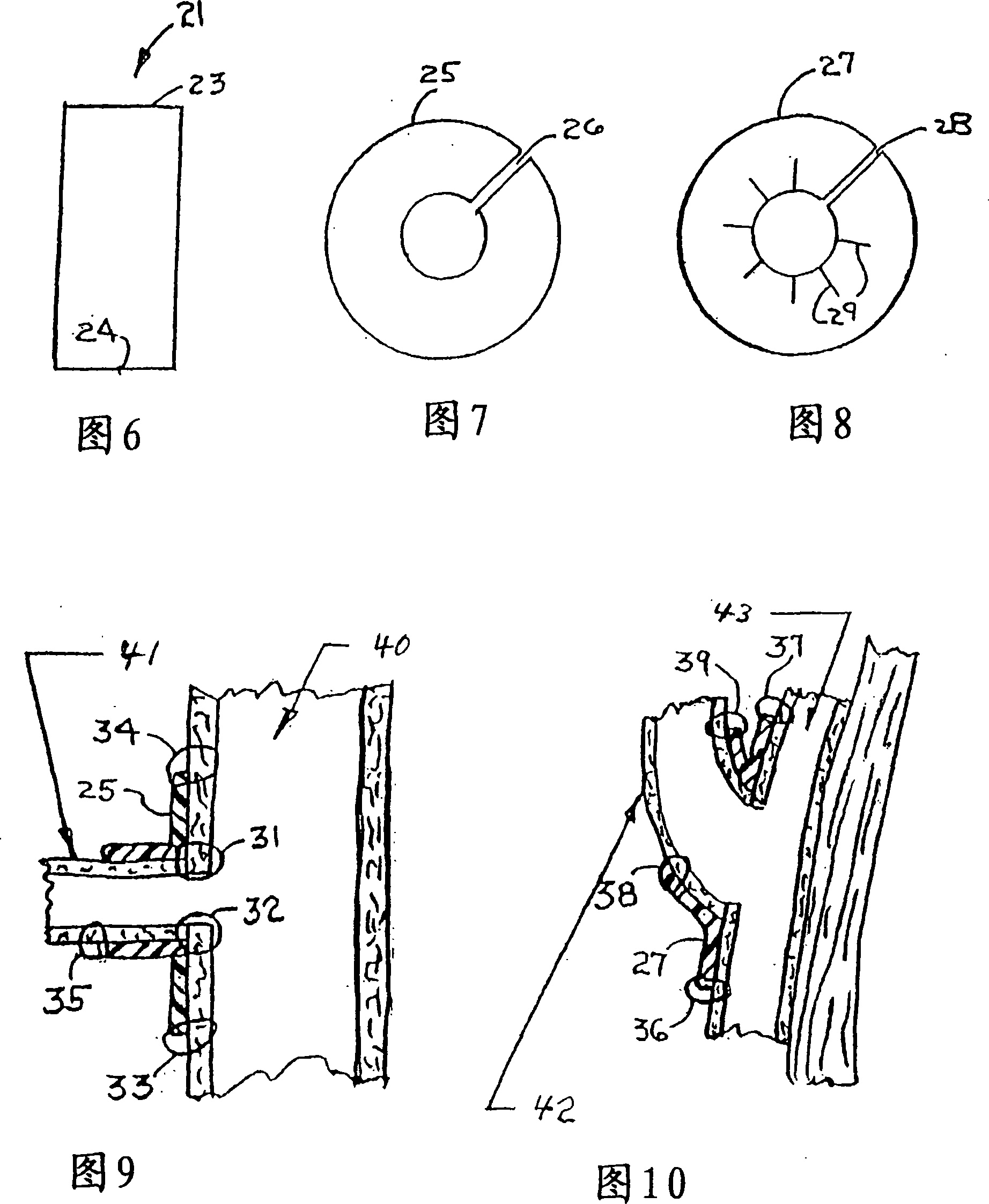
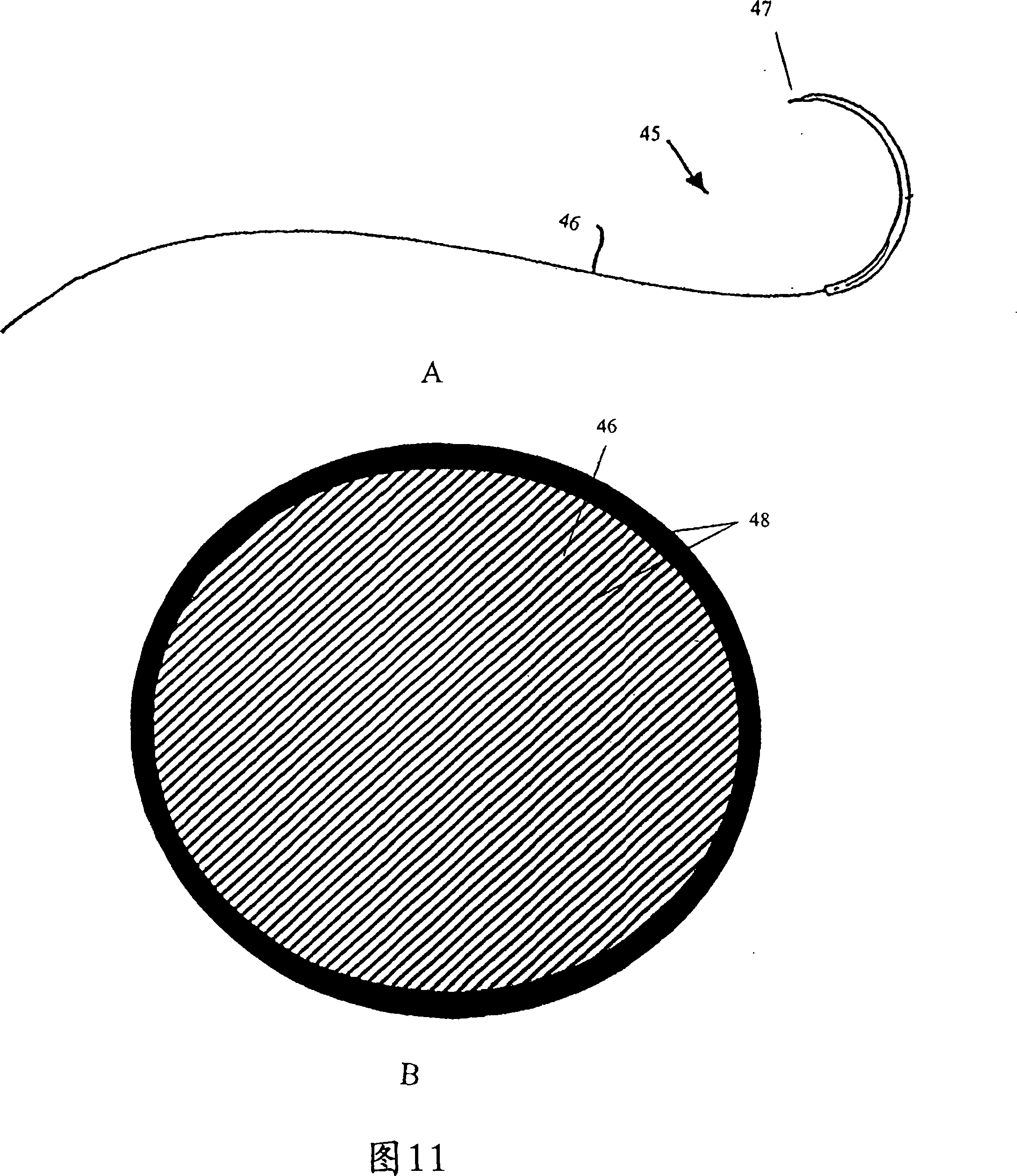
Combination drug therapy for reducing scar tissue formation
A scar and tissue technology, applied in the fields of medical devices for preventing scar tissue and/or adhesion formation, medical devices for anti-proliferative drugs, and devices for anti-platelet drugs, can solve the problems of low medical risk and high therapeutic benefit.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0306] Study on Prevention of Pericardial Adhesion in Rabbits
[0307] This example predicts that one embodiment of a hydrogel-based bioadhesive comprising sirolimus and analogs of sirolimus, gemmilofiban, and bivalirudin is effective in preventing postoperative adhesions and scarring. ability in. A standardized pericardial attrition protocol known in the art was performed on 18 New Zealand white female rabbits weighing 3-4 kg. Bennett et al., "Next Generation Hydrogel Films as Tissue Sealants and Adhesion Barriers" - J Card Surg 18:1-6 (2003); and Wiseman et al., "Fibrinolytic Drugs to Prevent Adhesions of the Rabbit Pericardium" - J Surg Res 53:362-368 (1992).
[0308] Rabbits were sedated, placed supine, intubated and maintained under inhalation anesthesia. A median sternotomy was performed to expose the heart. The pericardium was opened and standardized superficial abrasion with dry gauze on the anterior (anterior) surface of the heart produced a "central streak" (CS...
Embodiment 2
[0312] General PEA Polymer Materials and Methods
[0313] This example represents the base material used in the following examples concerning PEA efficacy, biocompatibility.
[0314] polymer
[0315] Poly(ester amides) (PEA) are manufactured by MediVas, Inc. Poly(D,L-lactide-co-glycolide) (PLGA) was purchased from Boehringer-Ingelheim. Poly(n-butyl methacrylate) (PBMA) was purchased from Polysciences.
[0316] synthesis
[0317] PEA is prepared by synthesizing monomers of two alpha amino acids, L-leucine and L-lysine, from diols (x) and diacids (y) in the presence of hexanediol and sebacic acid. See accompanying drawing 15. The carboxyl group of the side L-lysine of the polymer chain (BnO) serves as the binding site for coupling drugs or biologics to the polymer backbone. For this study, nitrous oxide-based 4-aminoTEMPO was conjugated to PEA. See accompanying drawing 16.
[0318] cell culture
[0319] Human peripheral blood mononuclear cells were isolated by density...
Embodiment 3
[0321] macrophage development
[0322] Monocyte-to-macrophage phenotype progression and contact-induced fusion to form multinucleated cells proceeded at similar rates during the three-week culture. PEA surfaces support human monocyte adhesion and differentiation, but qualitatively, as judged by morphology and differentiation / fusion rates, PEA surfaces do not exhibit an induced hyperactivation state. See accompanying drawing 17.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com