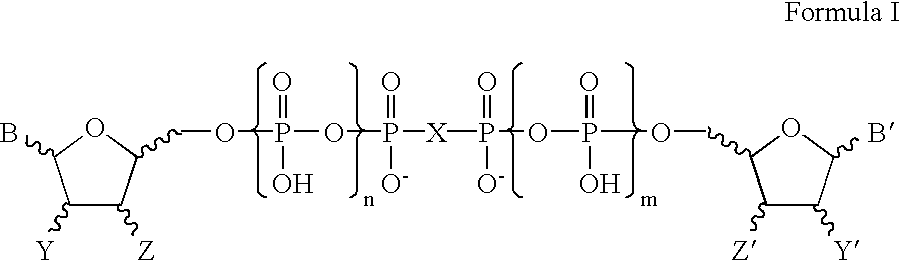
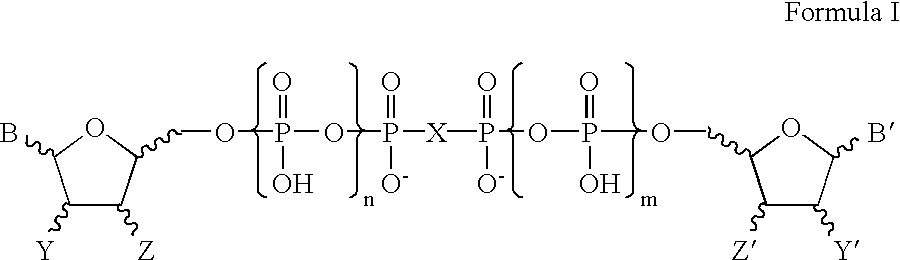
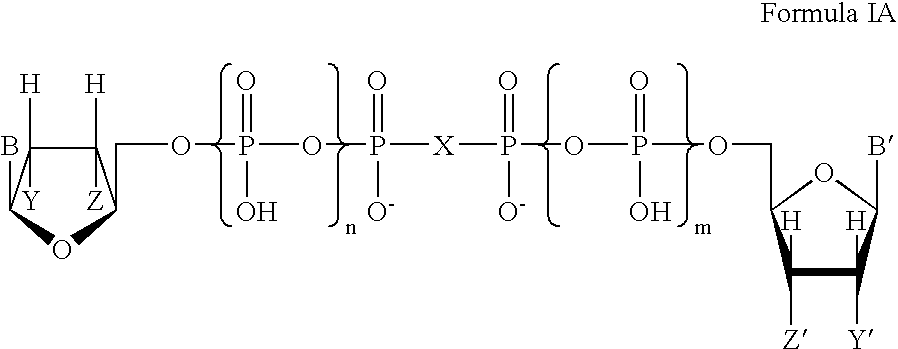Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
132 results about "Lacrimal duct" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
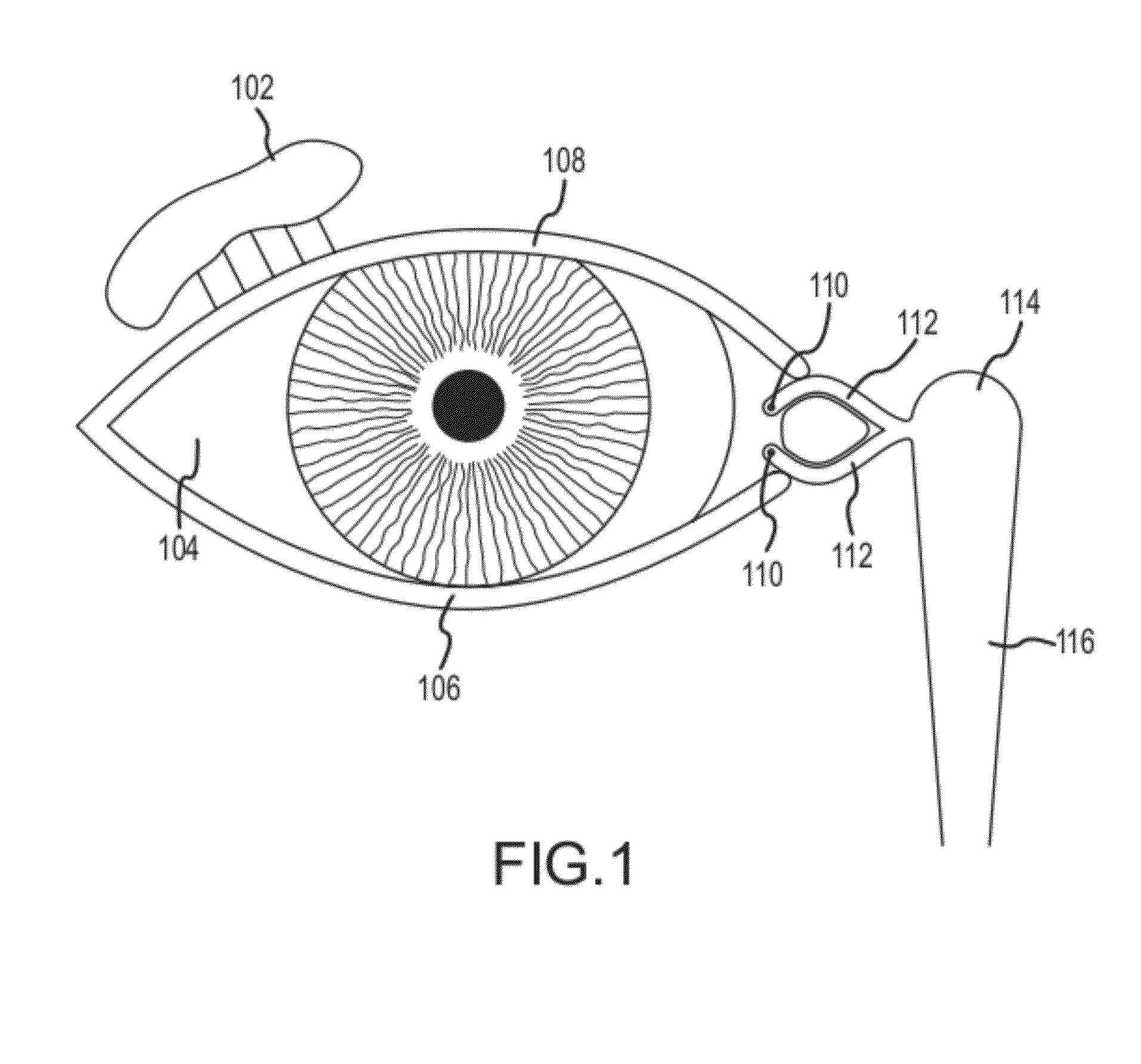
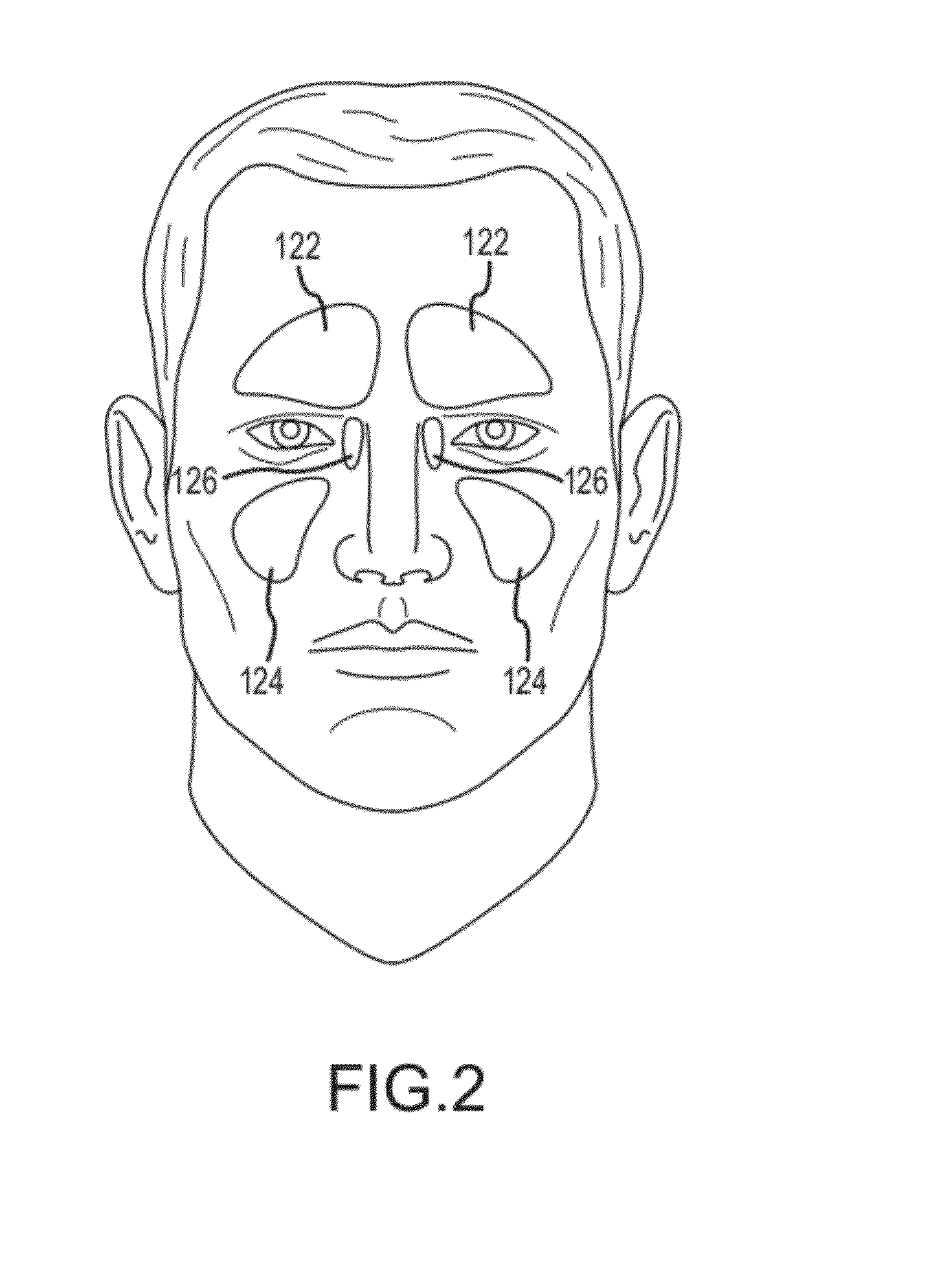
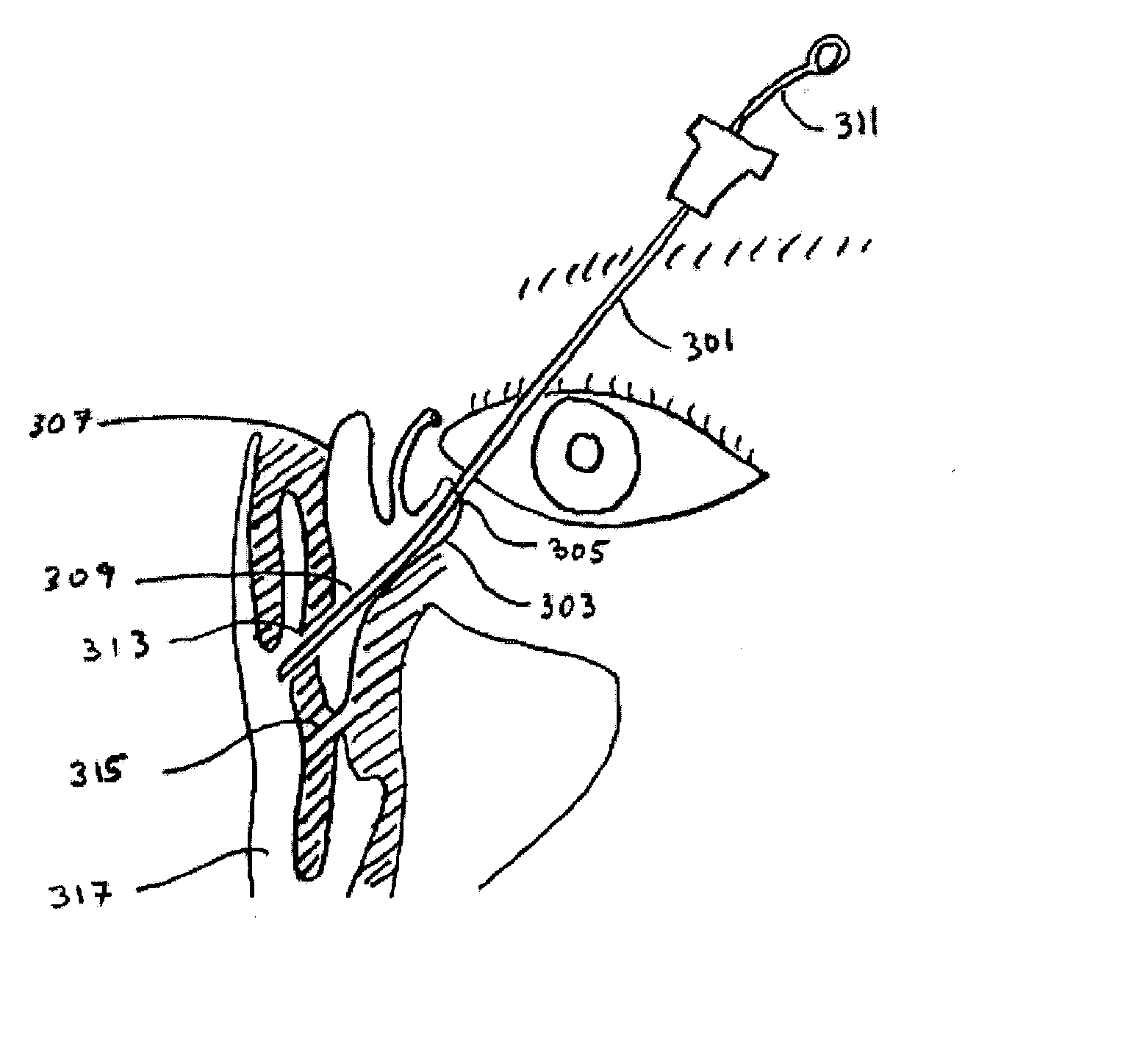
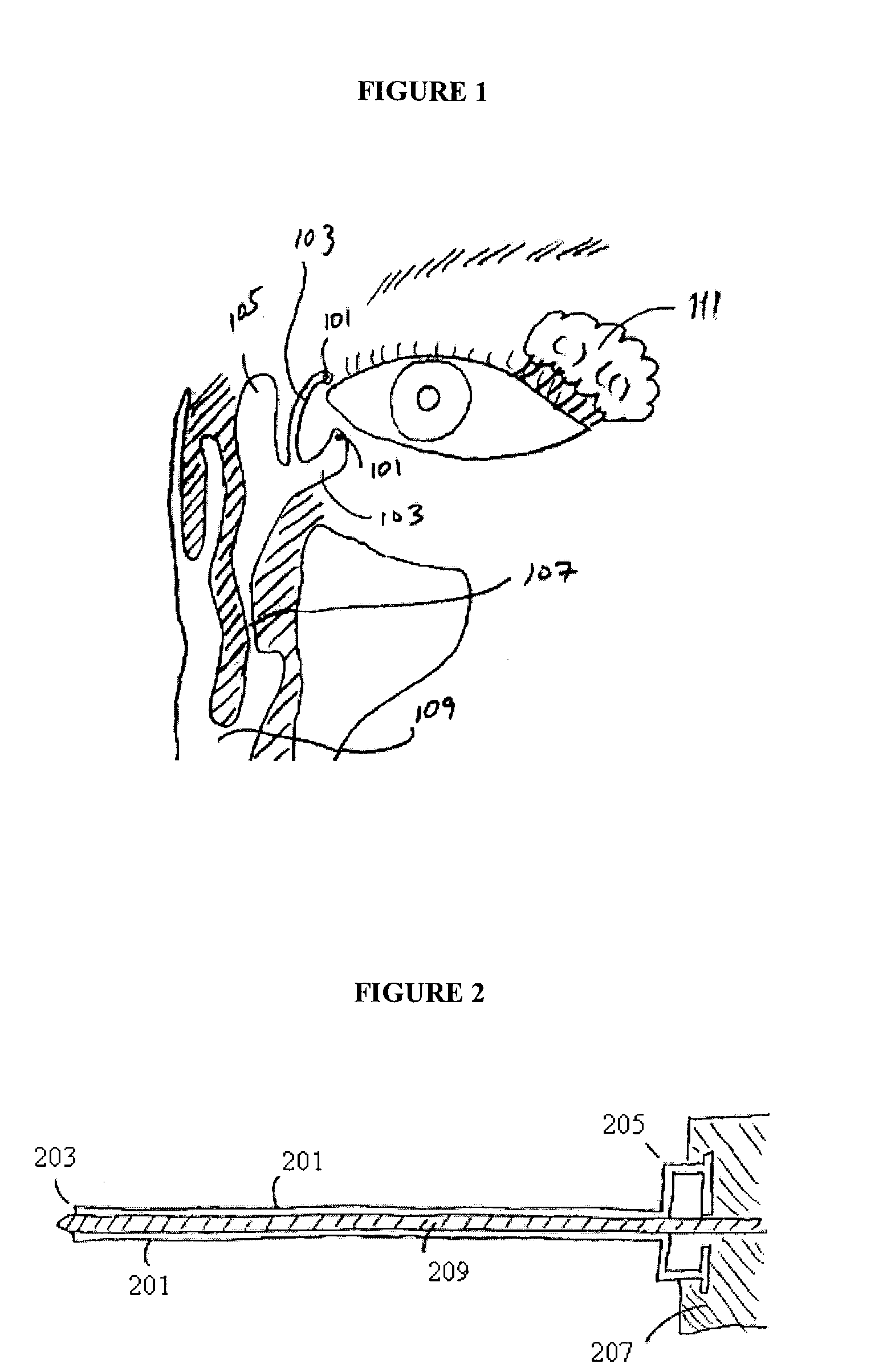
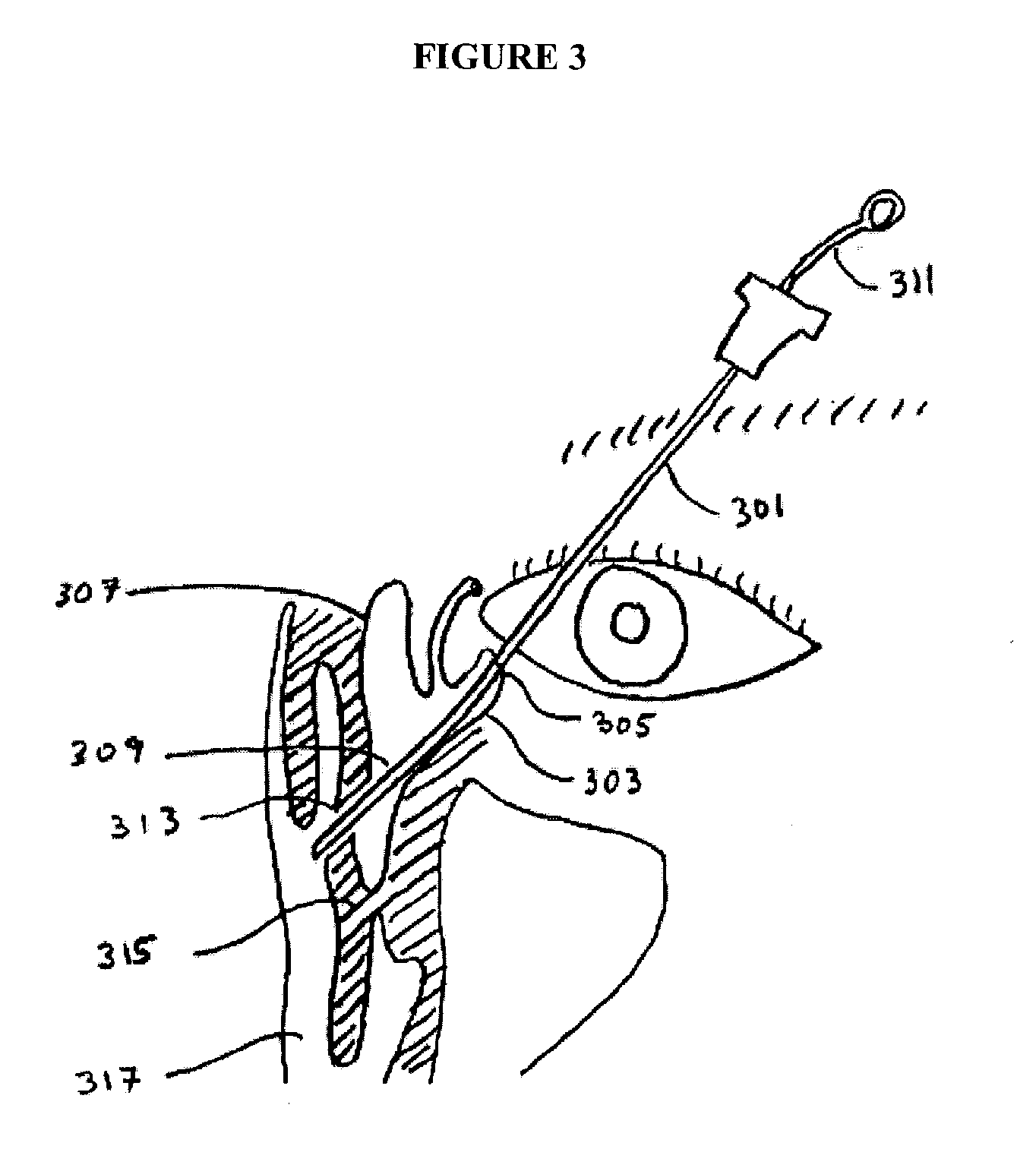
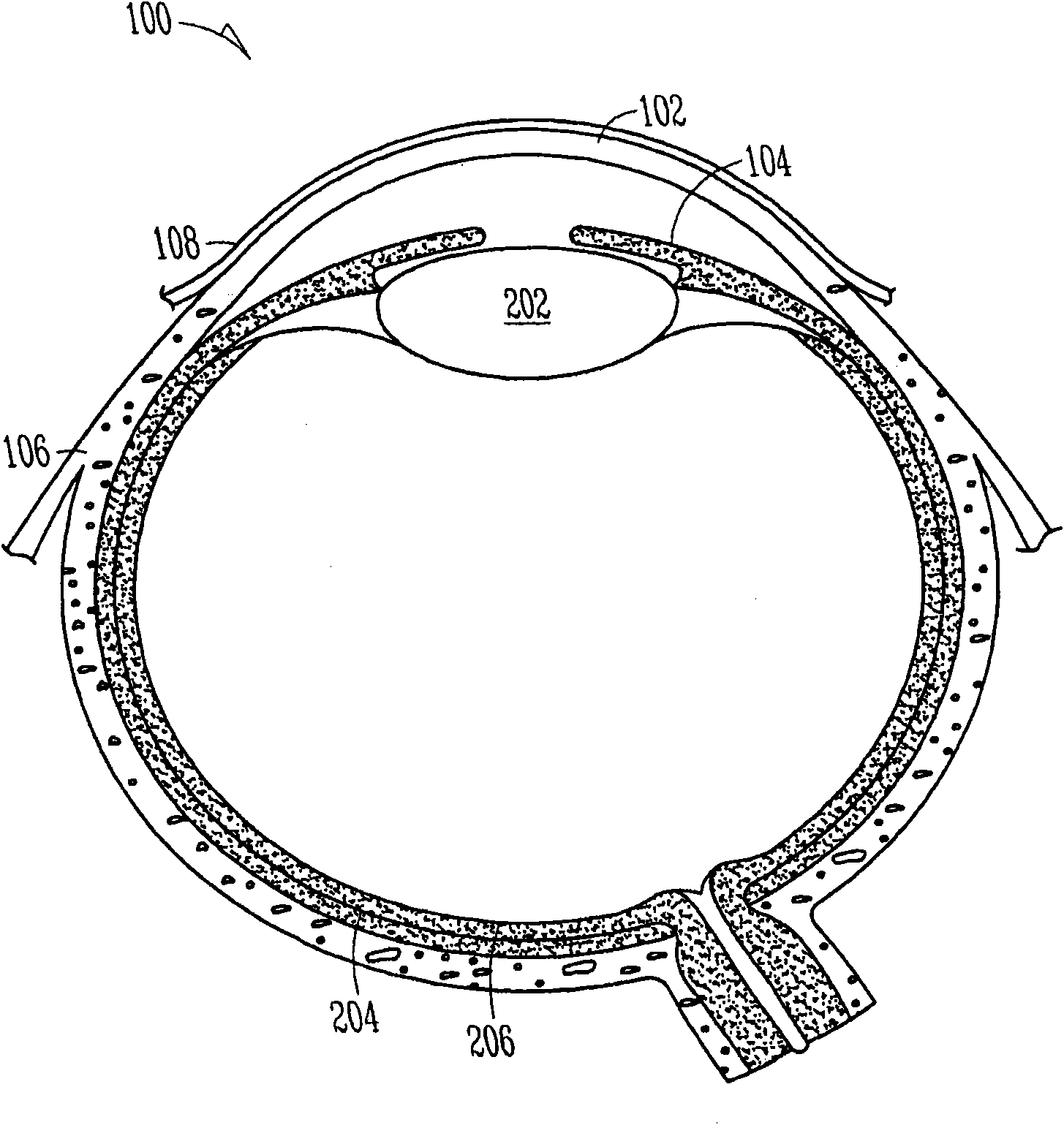
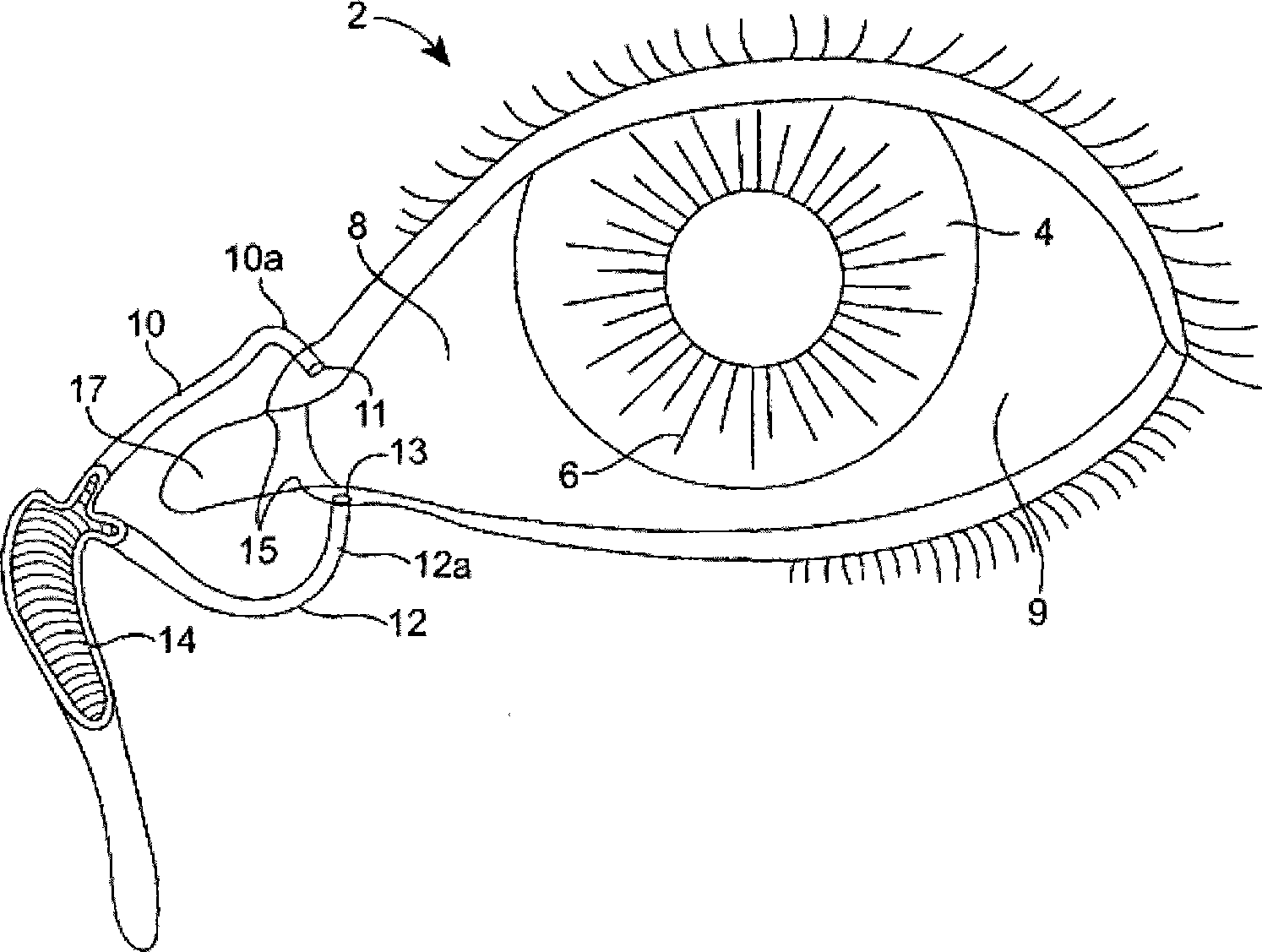
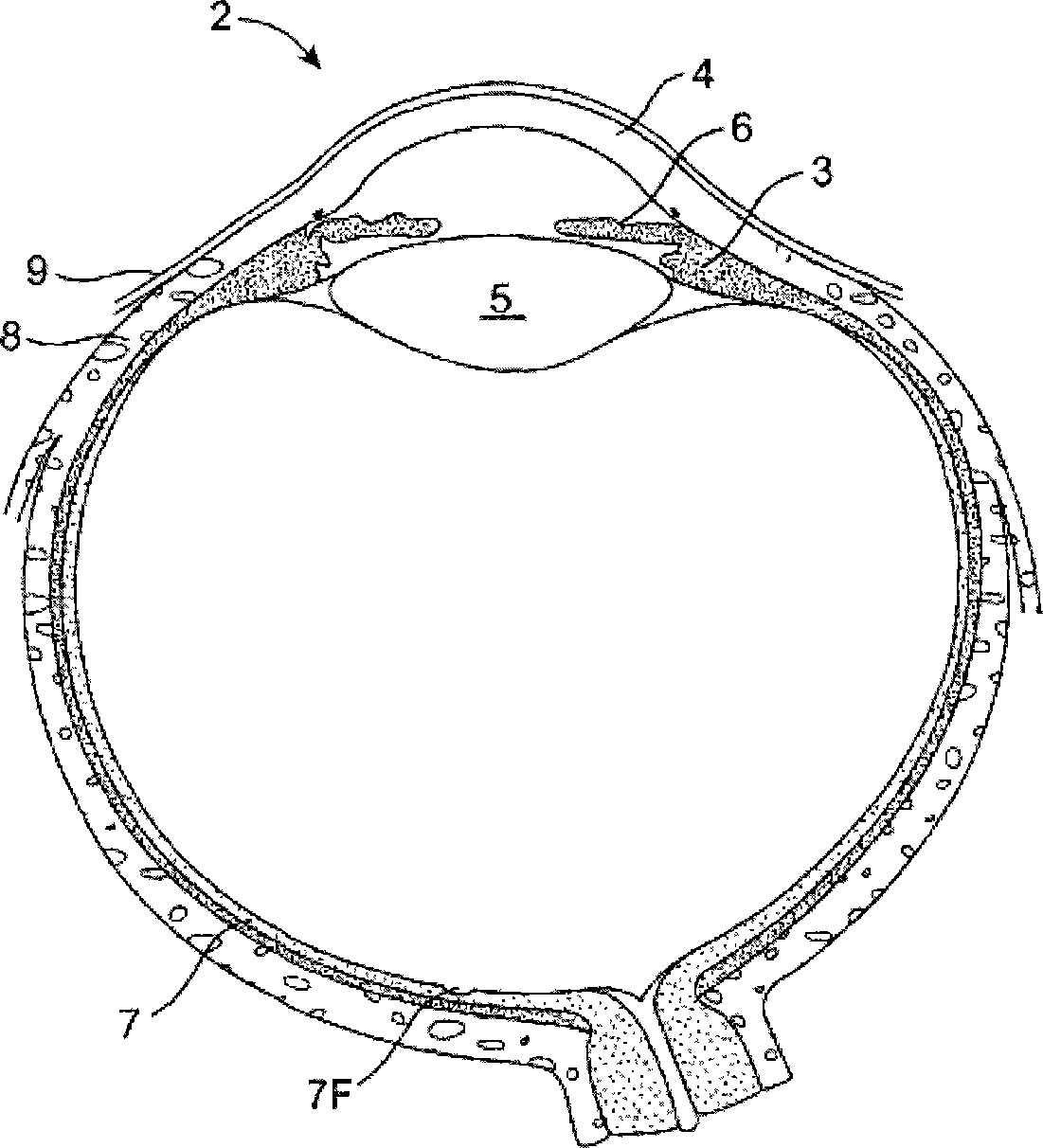
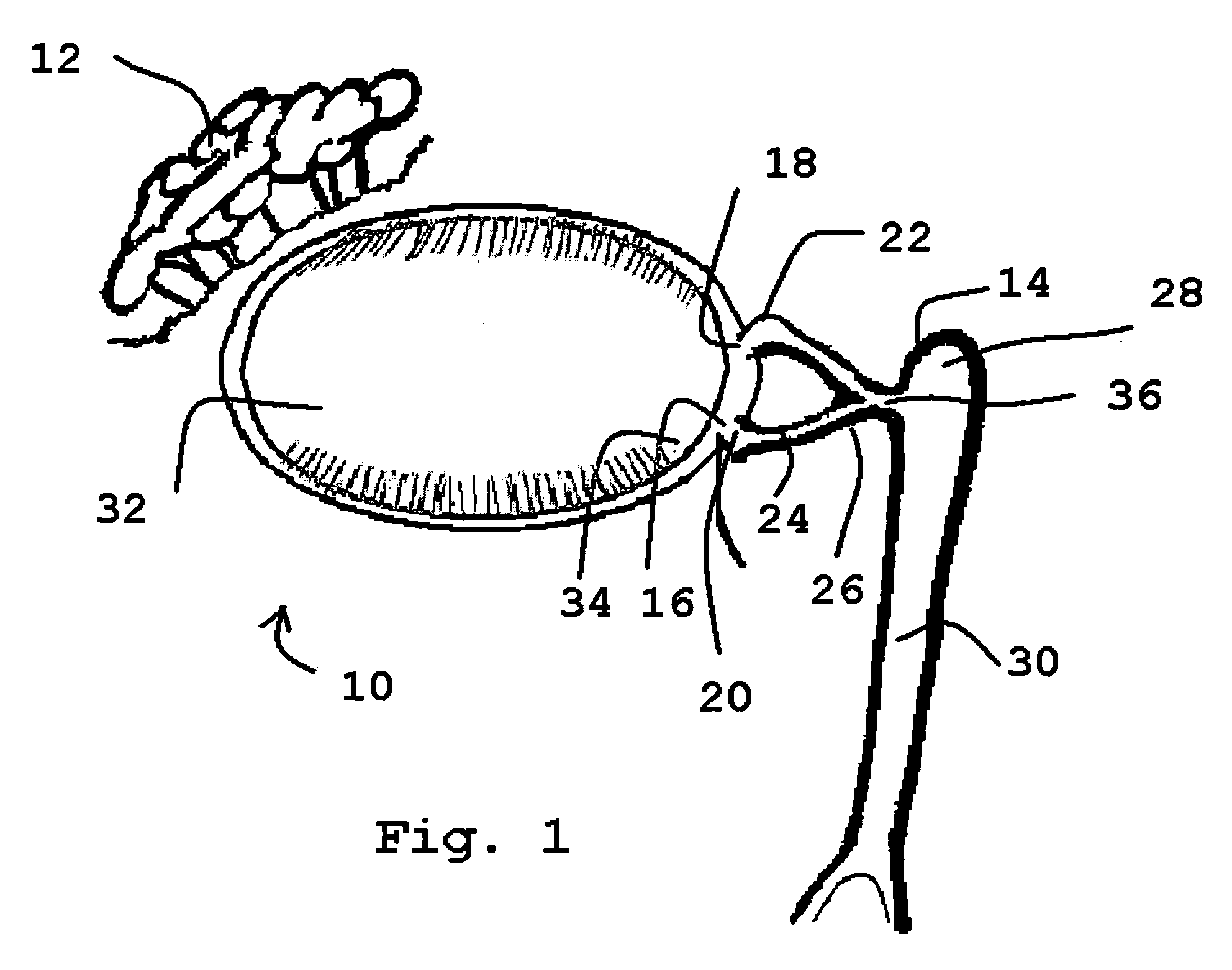
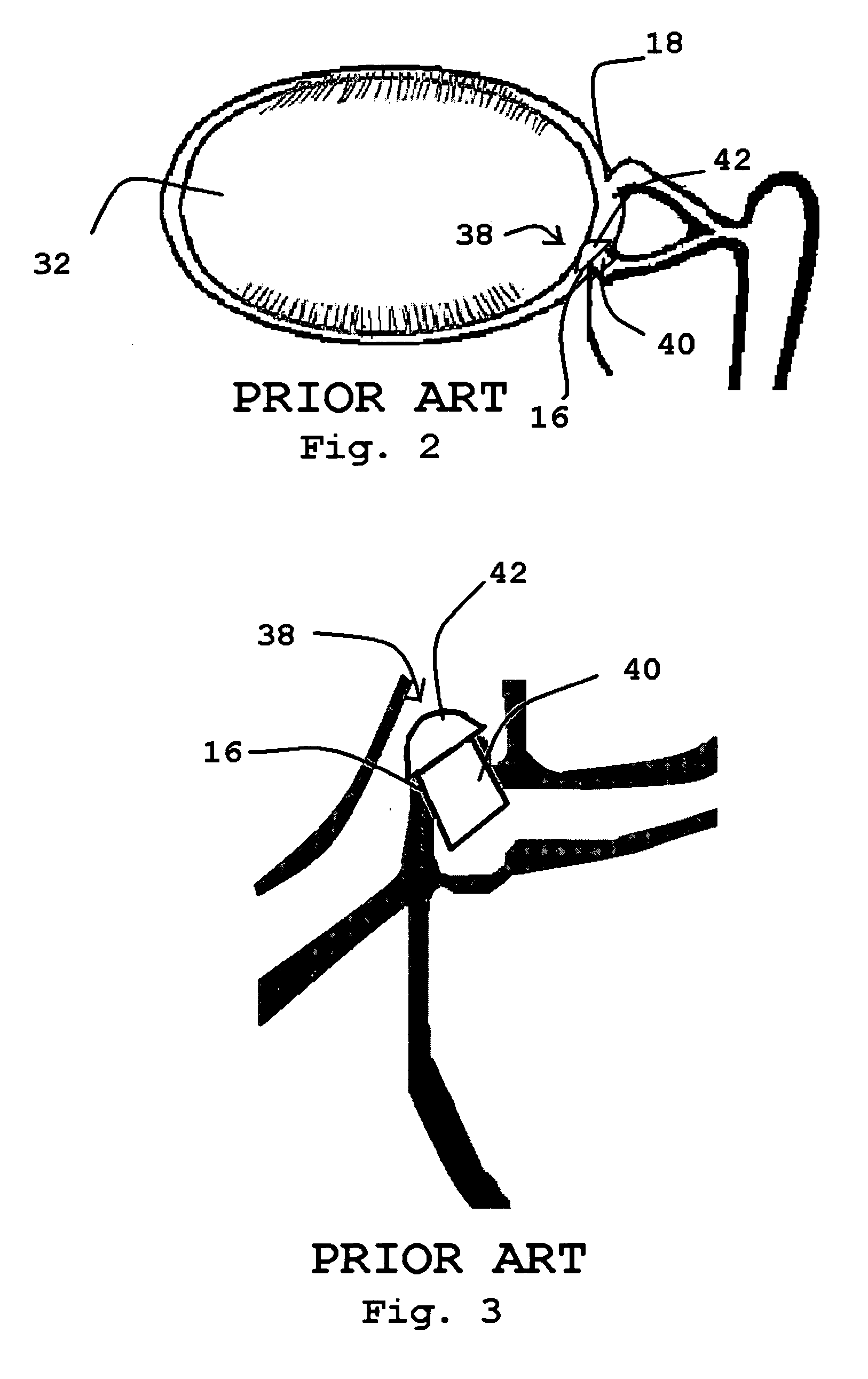
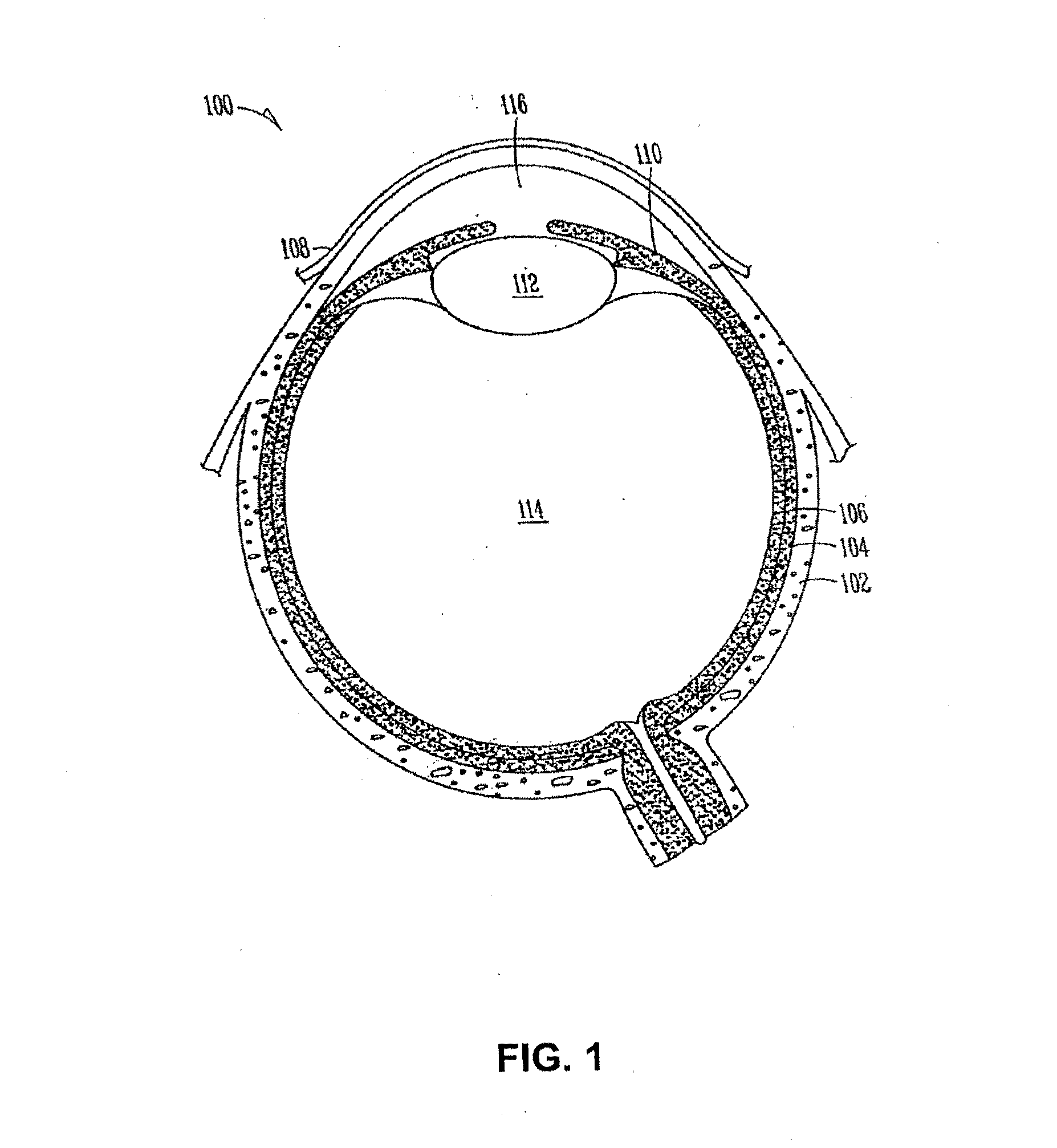
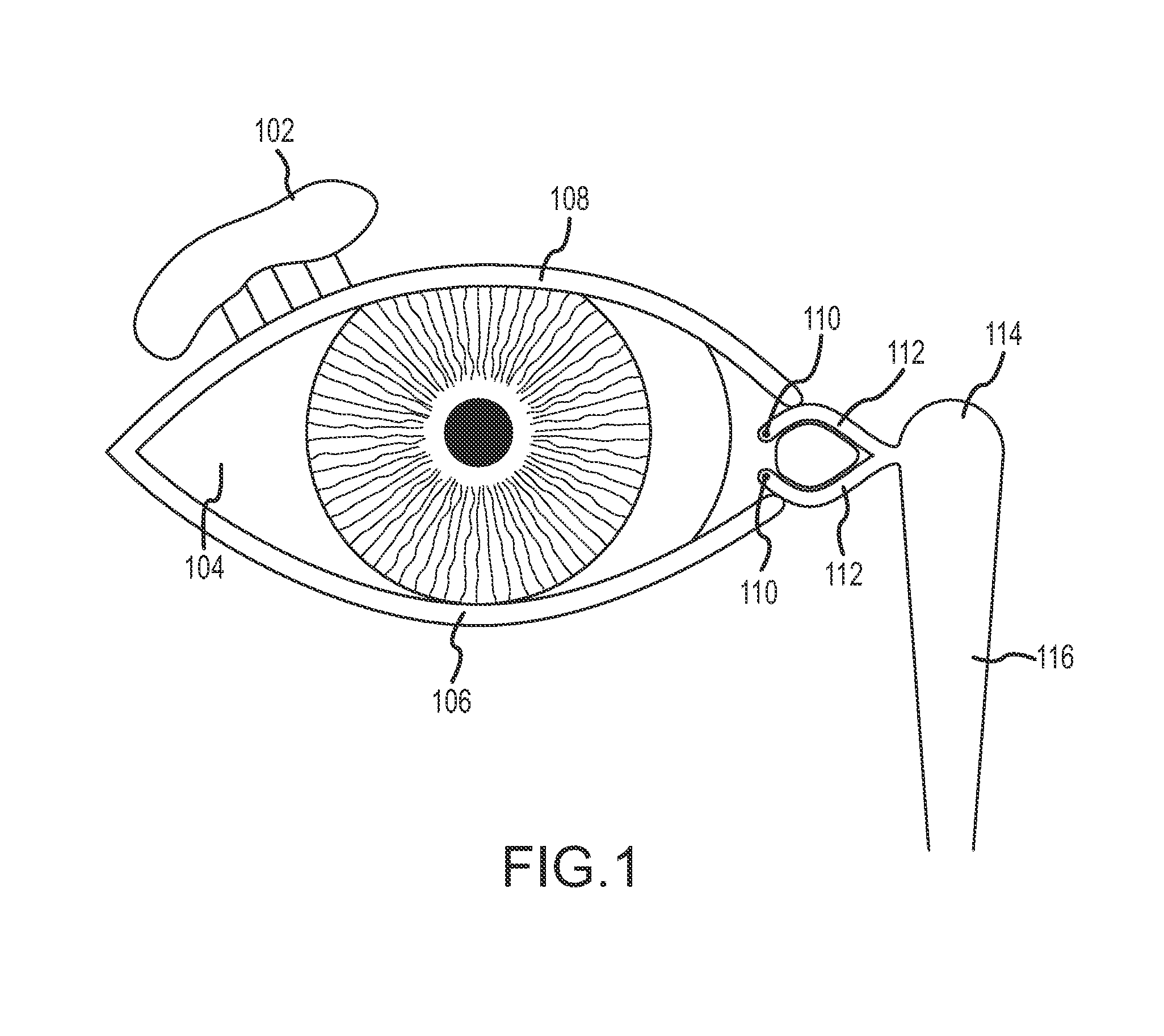
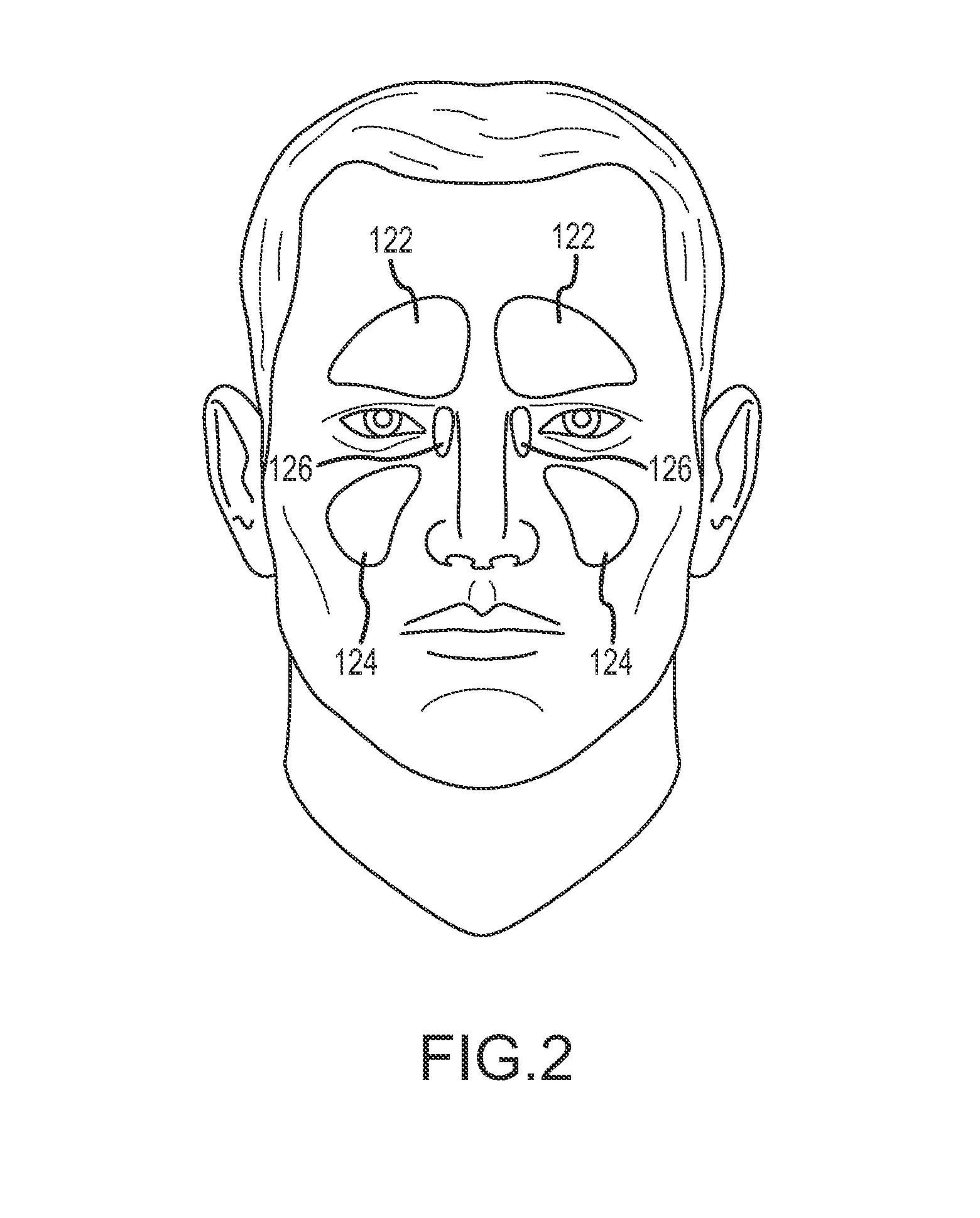
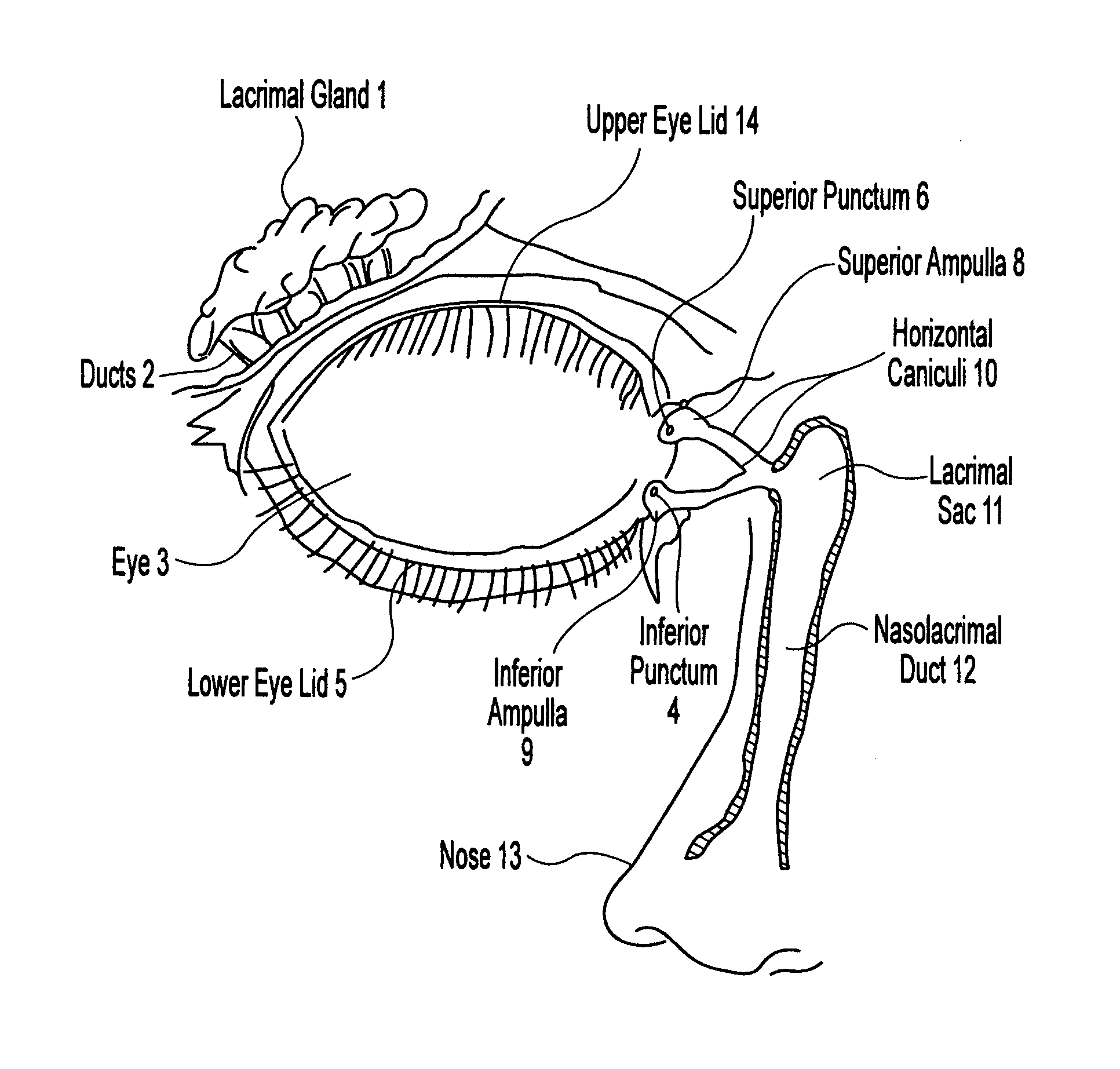
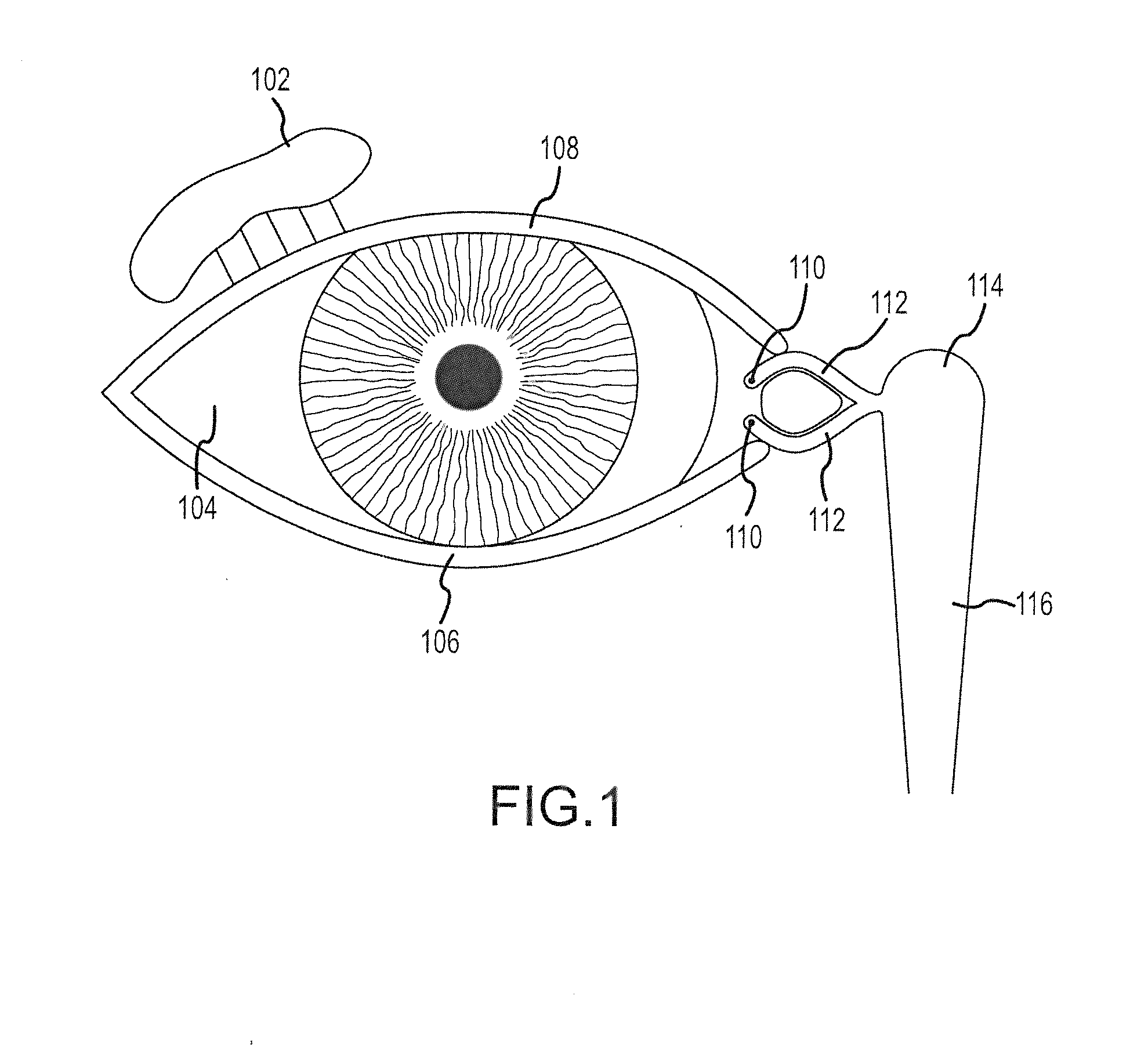
Organ with organ cavity which connects the vestibule of the conjunctival sac to the nasal cavity. Examples: There are only two lacrimal ducts, the right and the left lacrimal ducts.
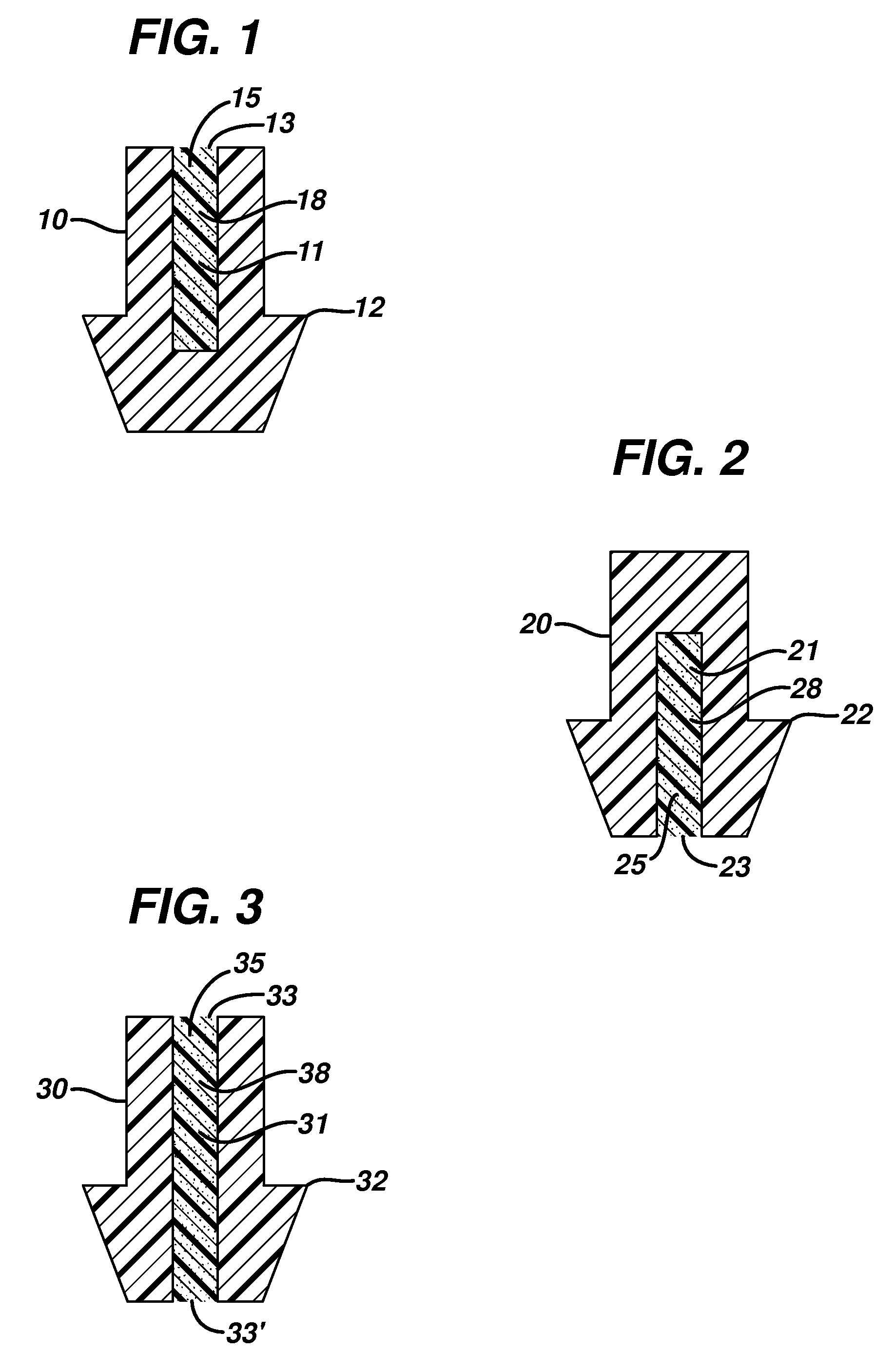
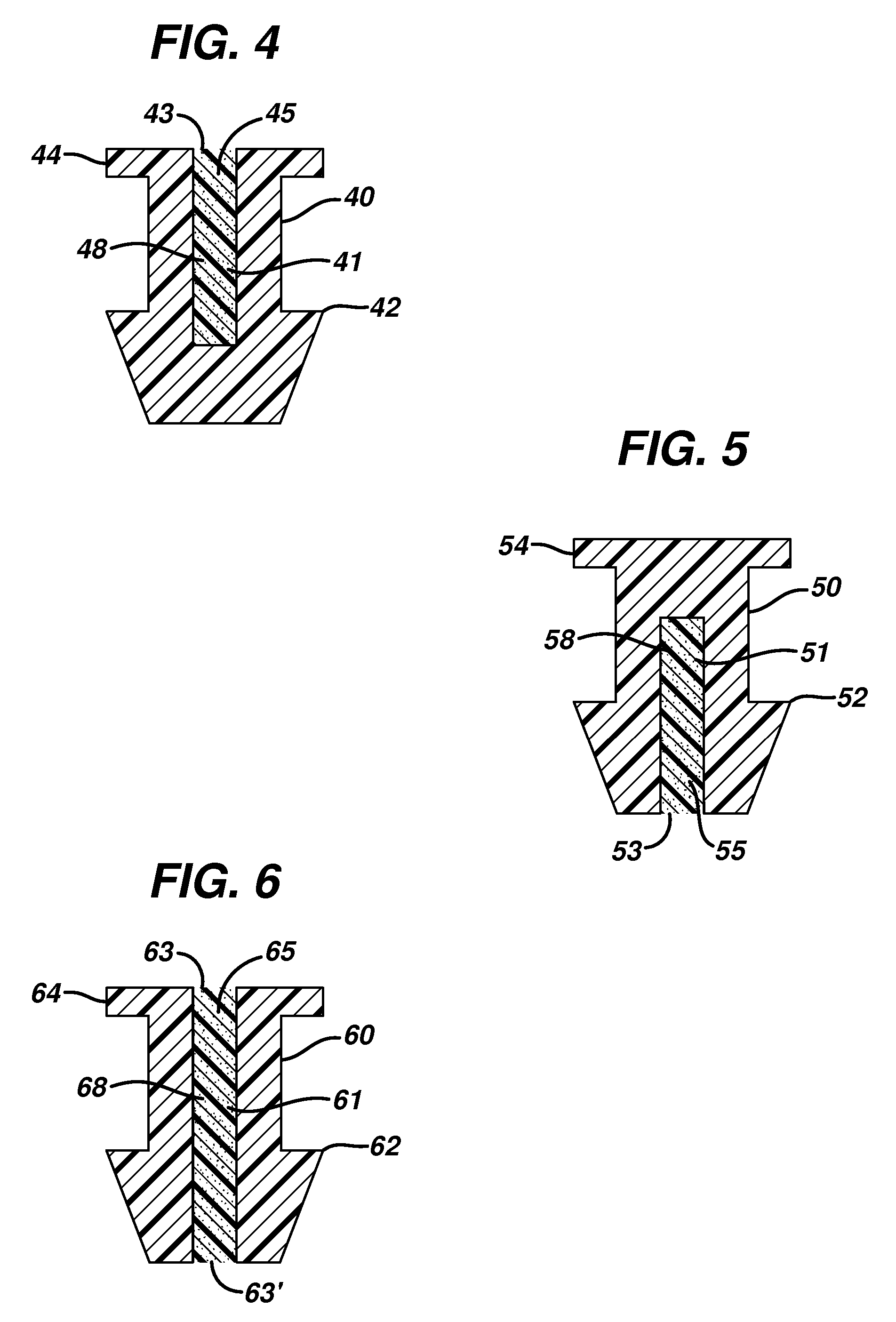
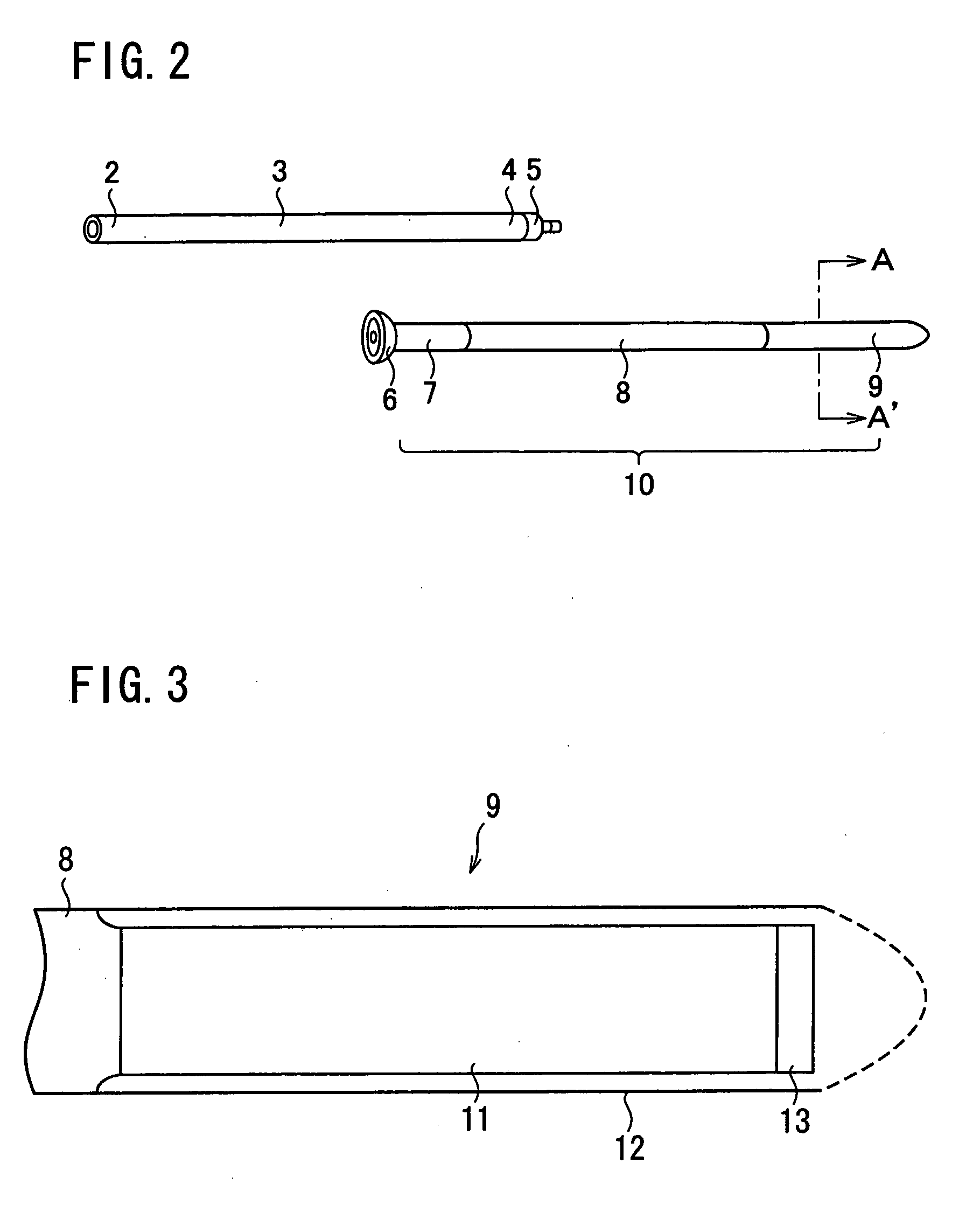
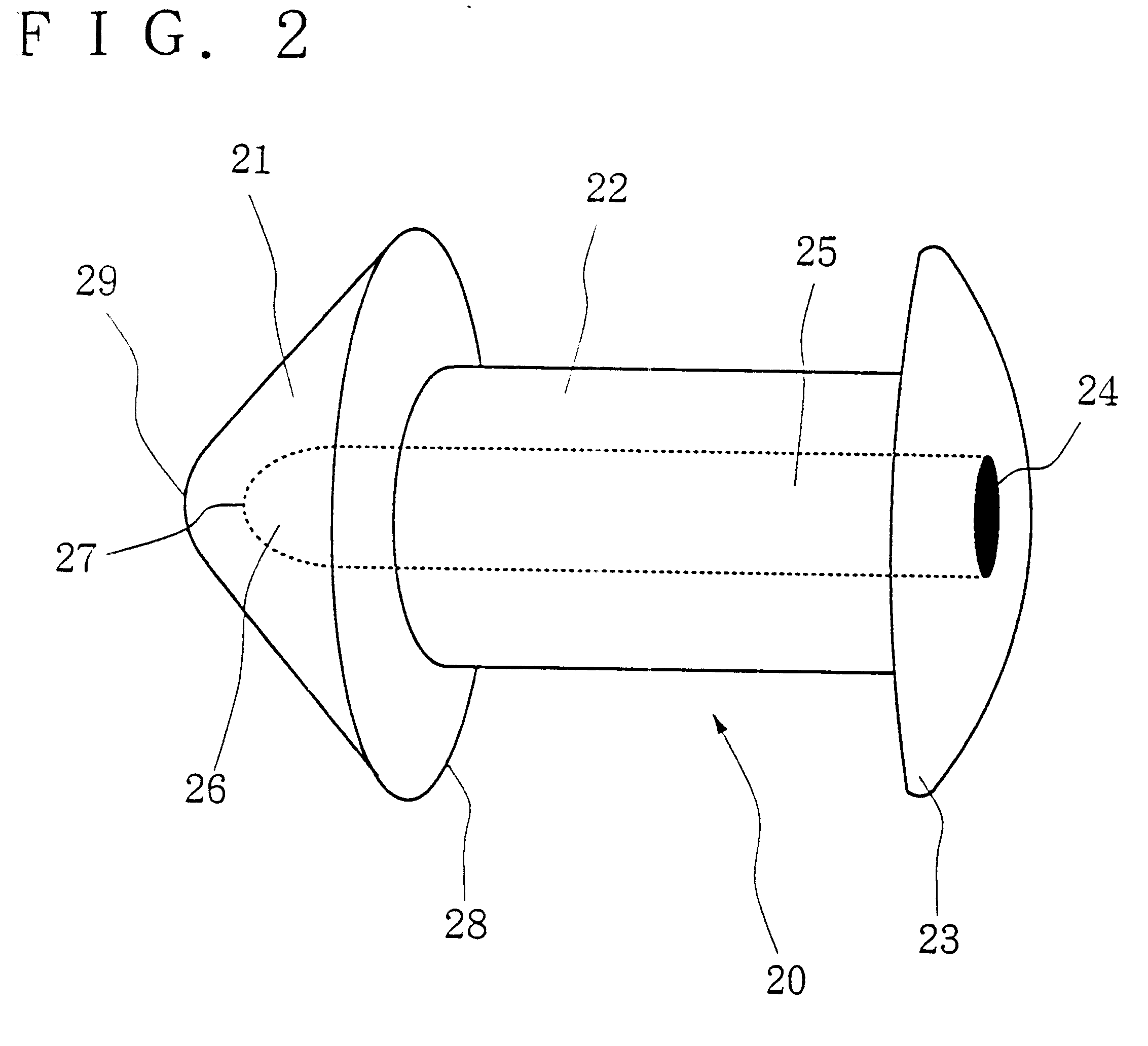
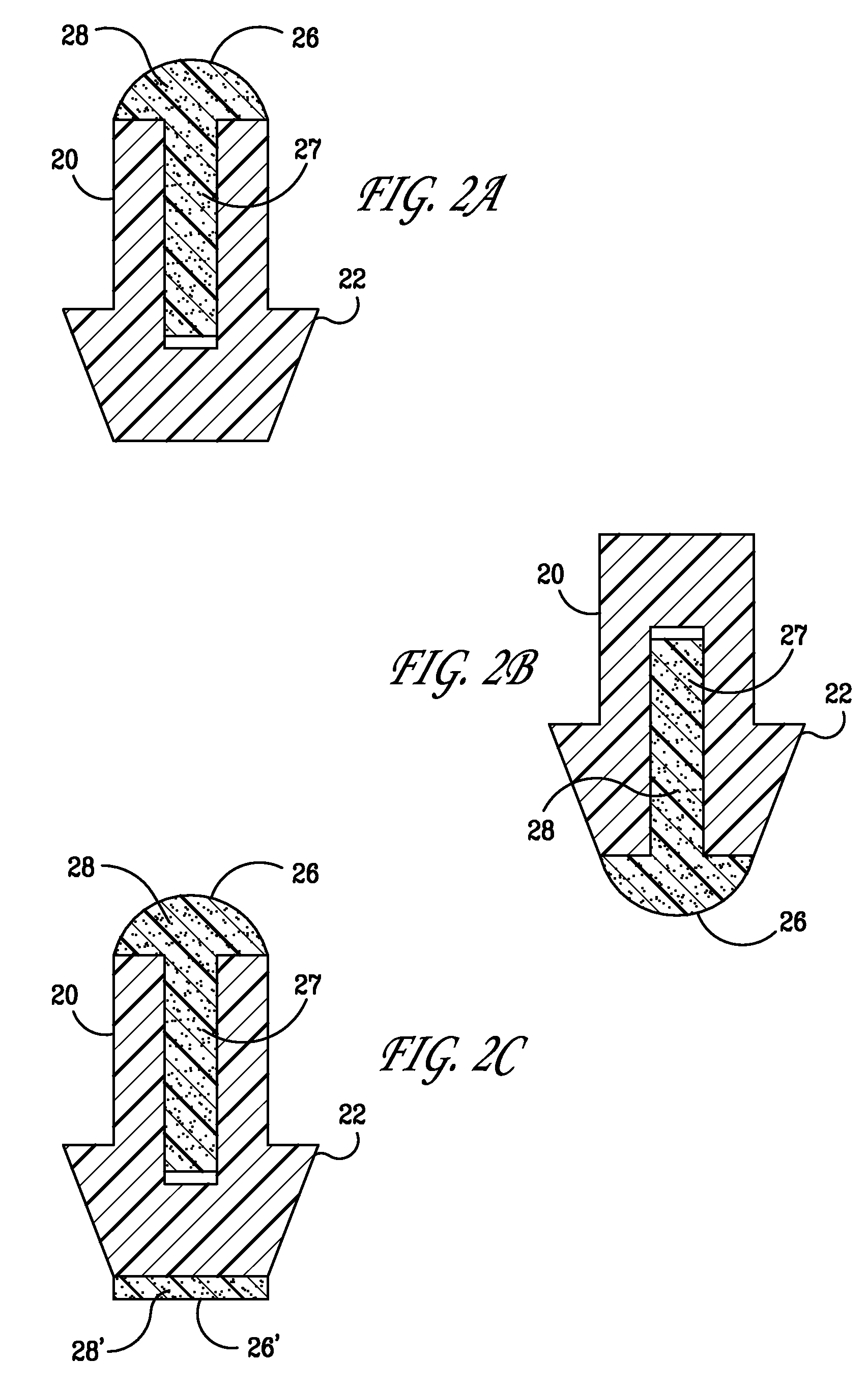
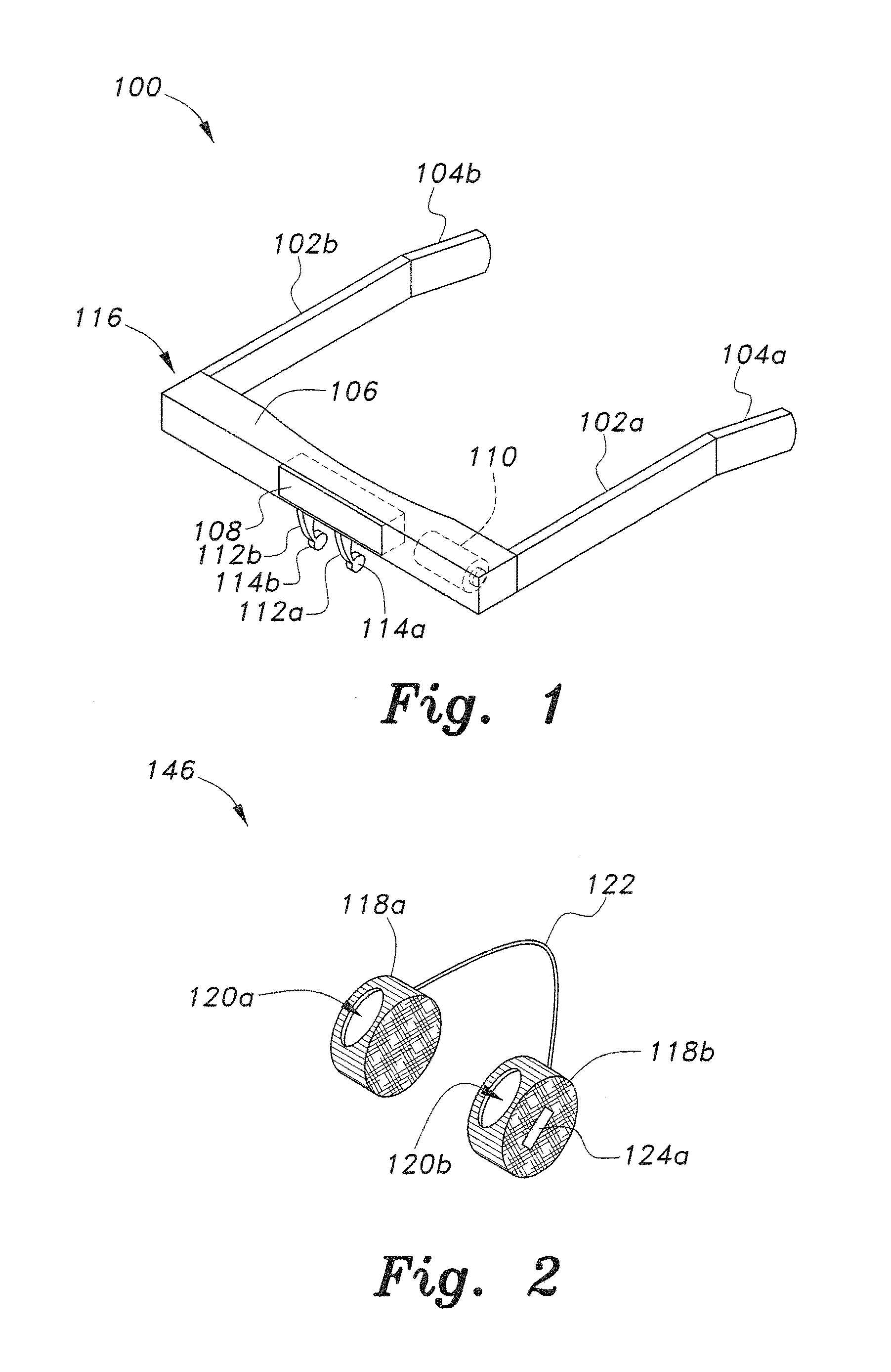
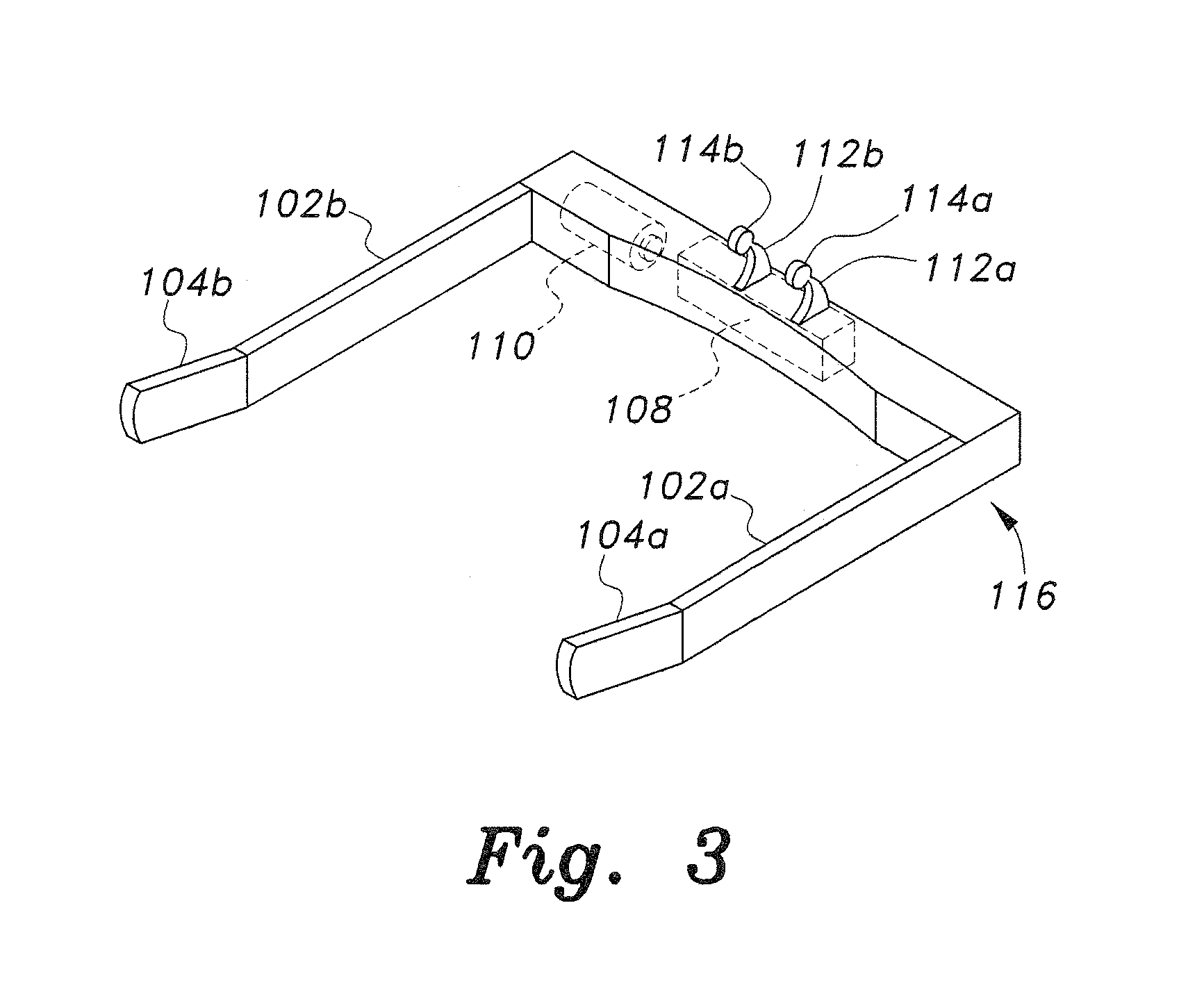
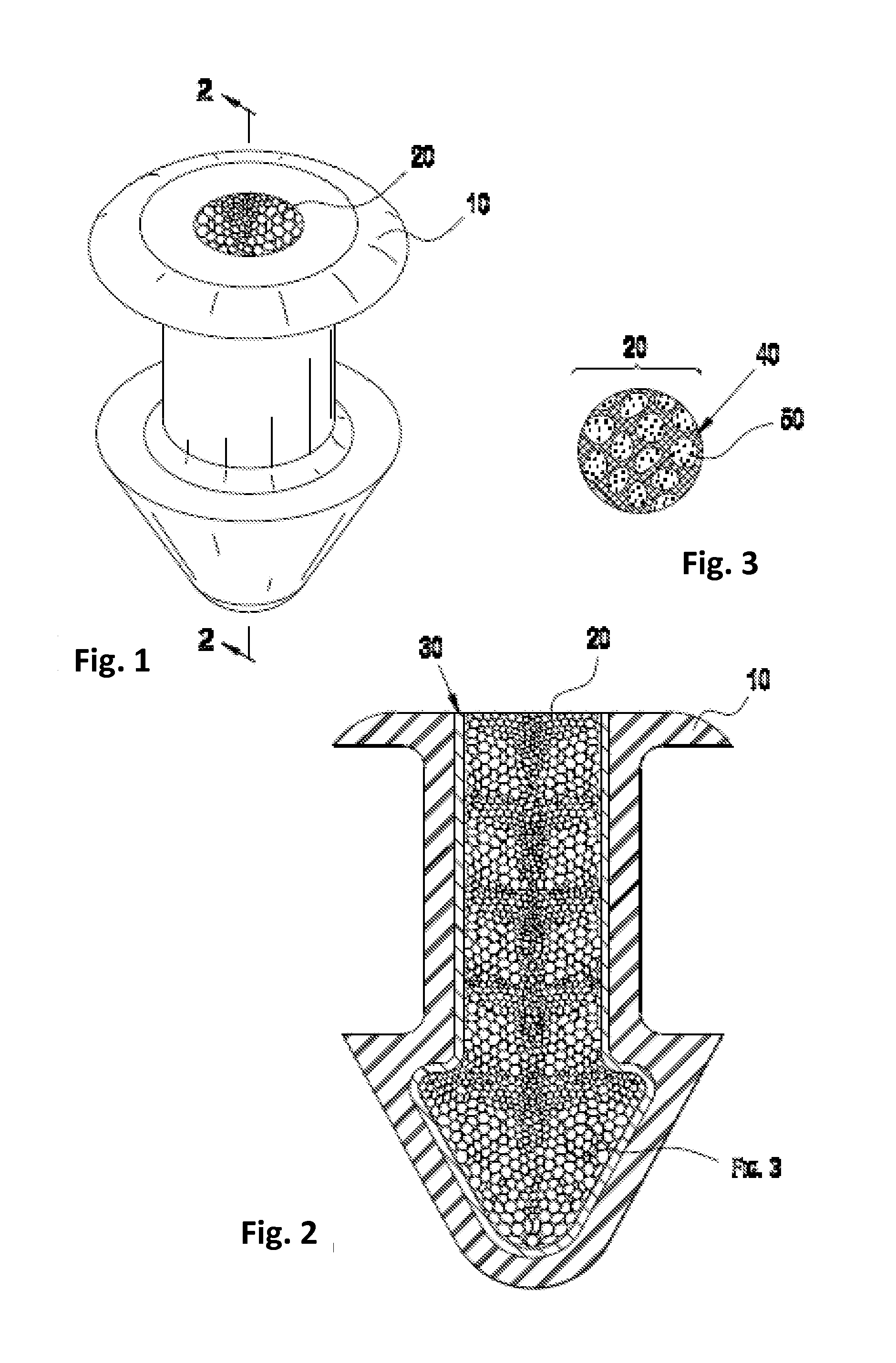
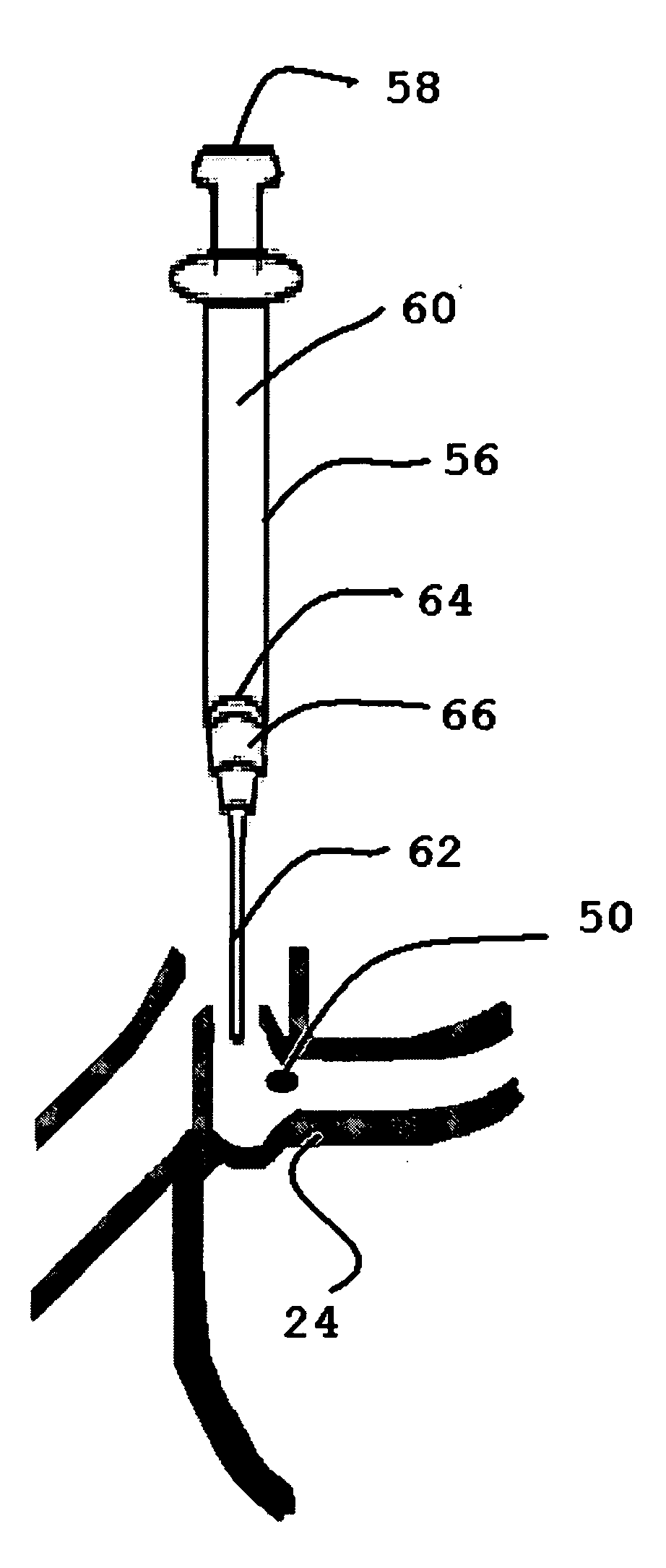
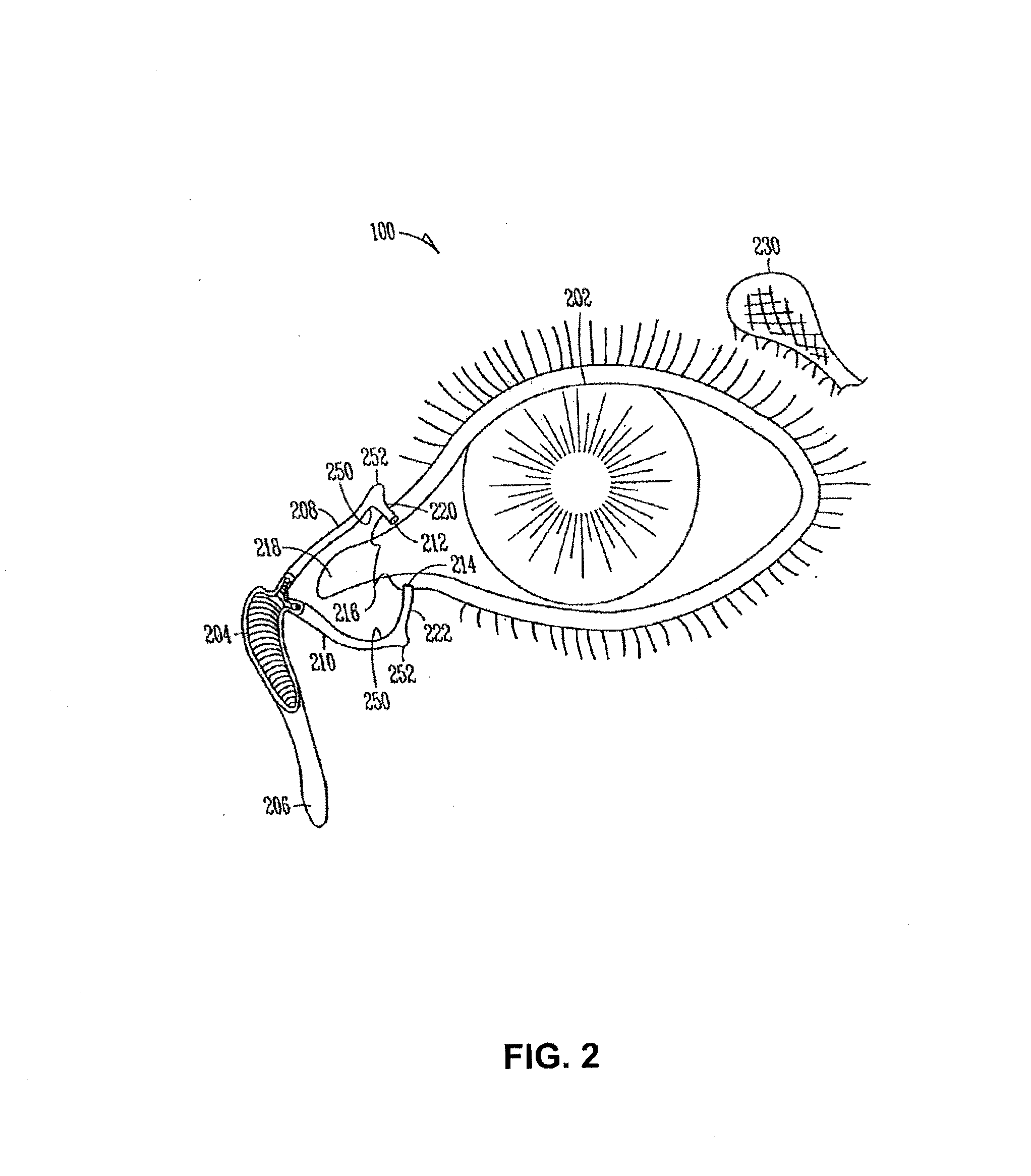
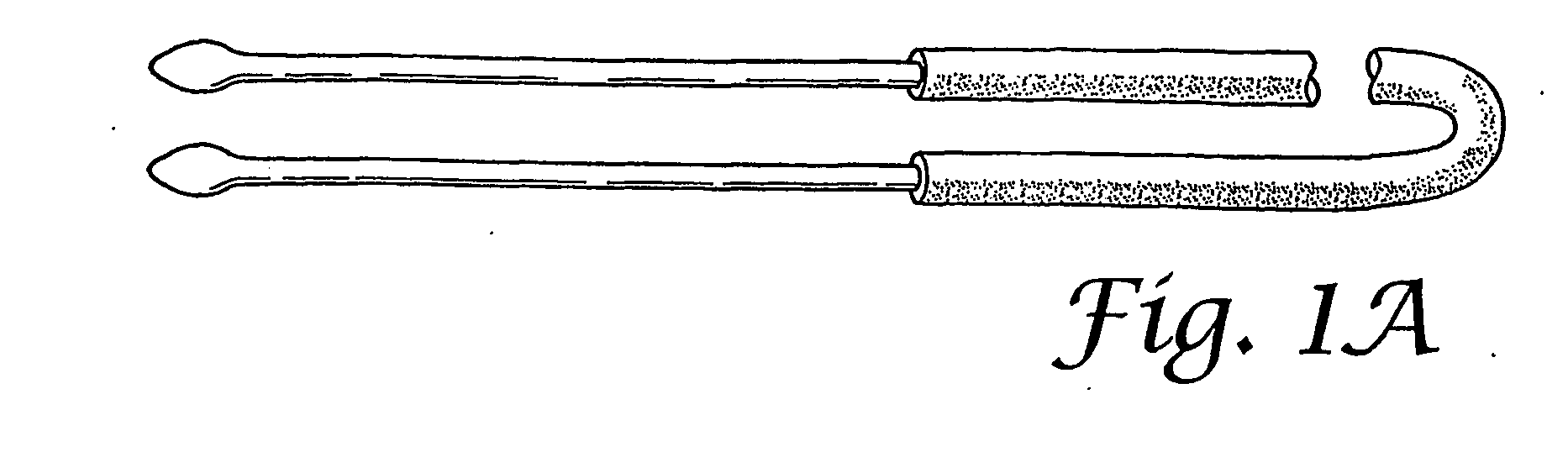
Insertion and extraction tools for lacrimal implants
Insertion and extraction tool, systems, and methods for use with lacrimal implants. An insertion tool is disclosed that includes a proximal end, a distal end, and a tool body therebetween. The distal end includes a mechanical coupling to receive a cartridge preloaded with a lacrimal implant, and a plunger configured to dispense the lacrimal implant from a preloaded cartridge.
Owner:MATI THERAPEUTICS
Punctal plugs for the delivery of active agents
The invention provides punctal plugs for the delivery of active agent to one or both of the tear fluid of the eye and to the nasolacrimal duct. The plugs of the invention have a body, a reservoir contained within the body, and optionally a collarette. The reservoir has at least one opening and contains a polymeric material and at least one active agent.
Owner:JOHNSON & JOHNSON VISION CARE INC
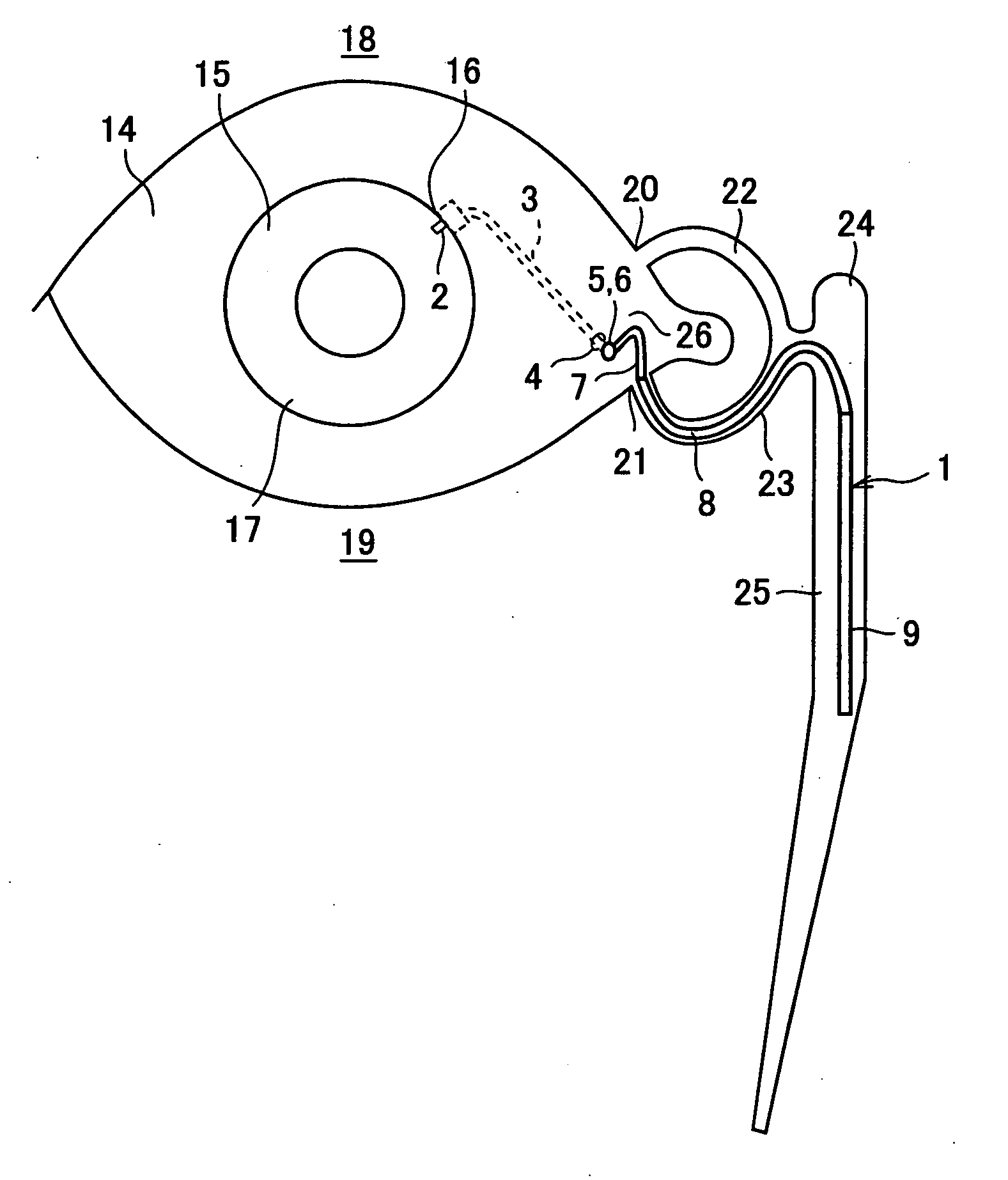
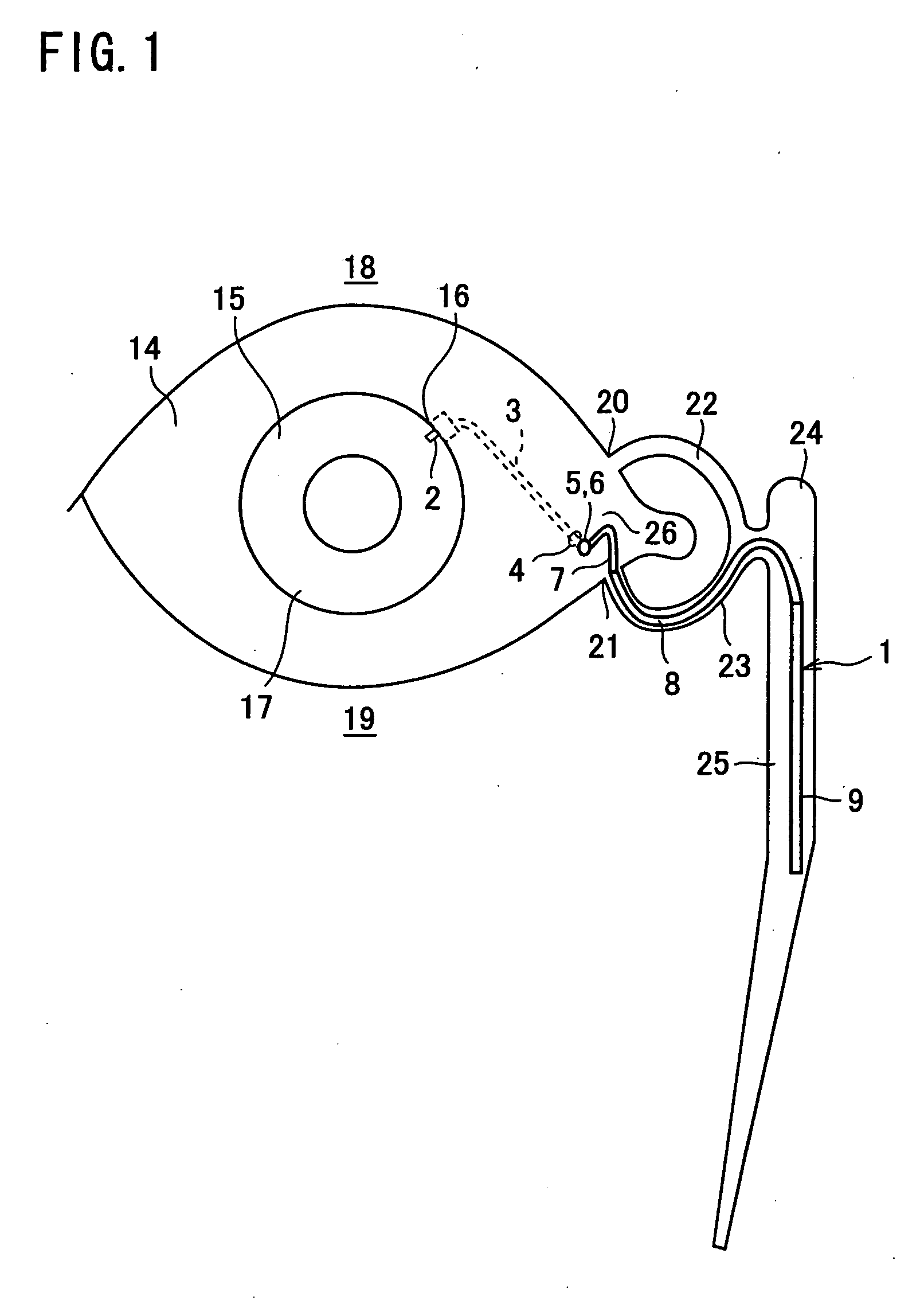
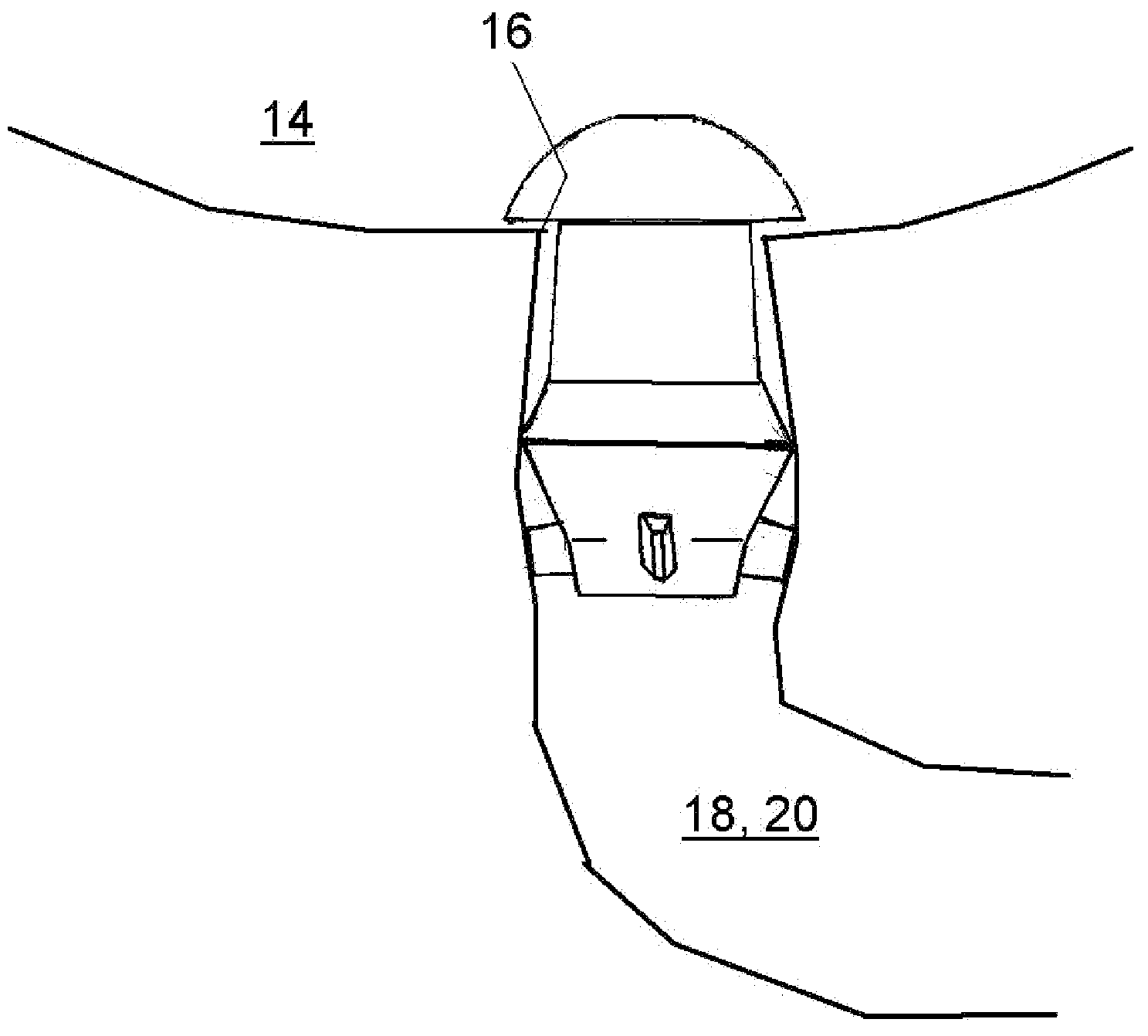
Aqueous humor drainage implant for treatment glaucoma
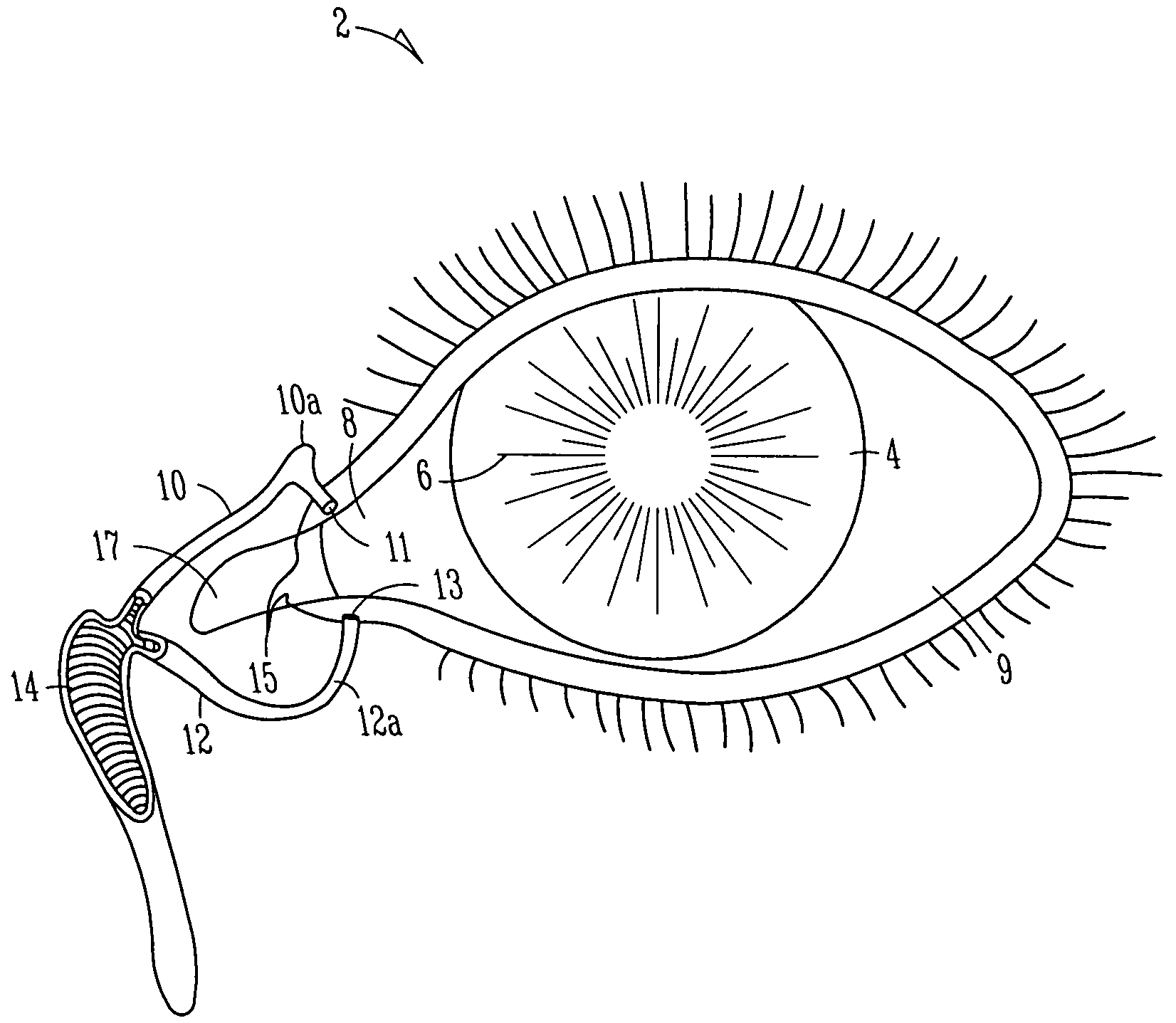
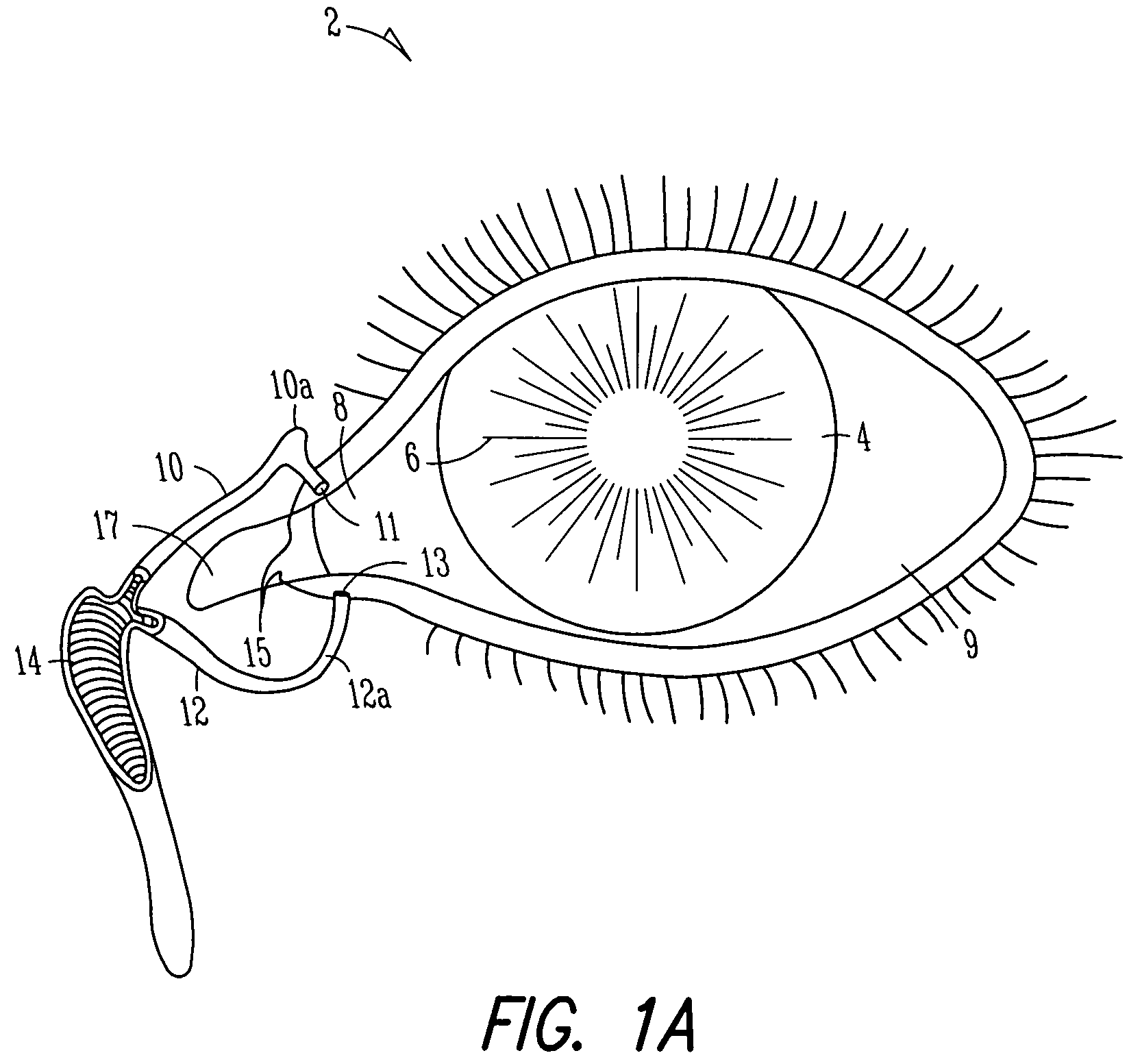
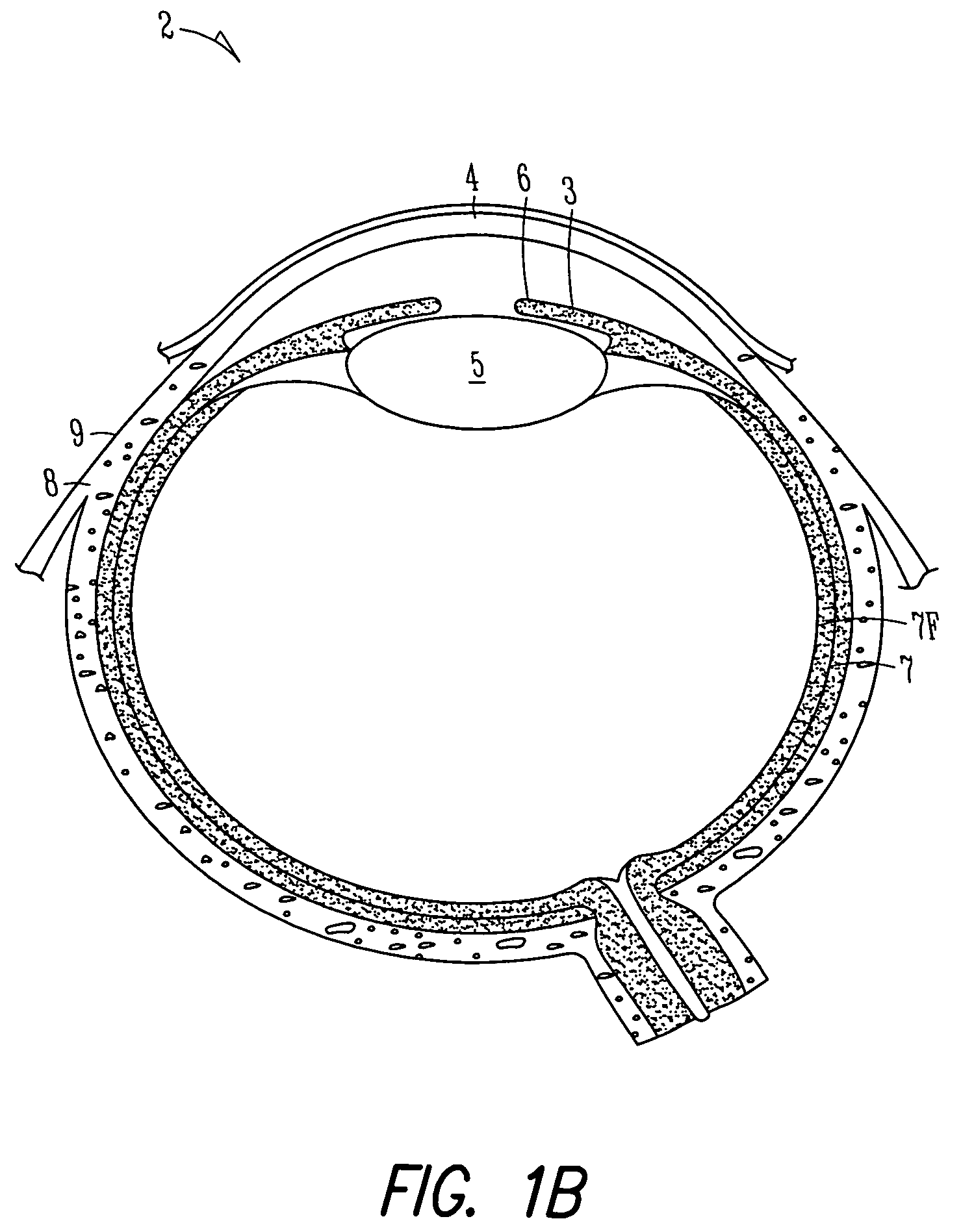
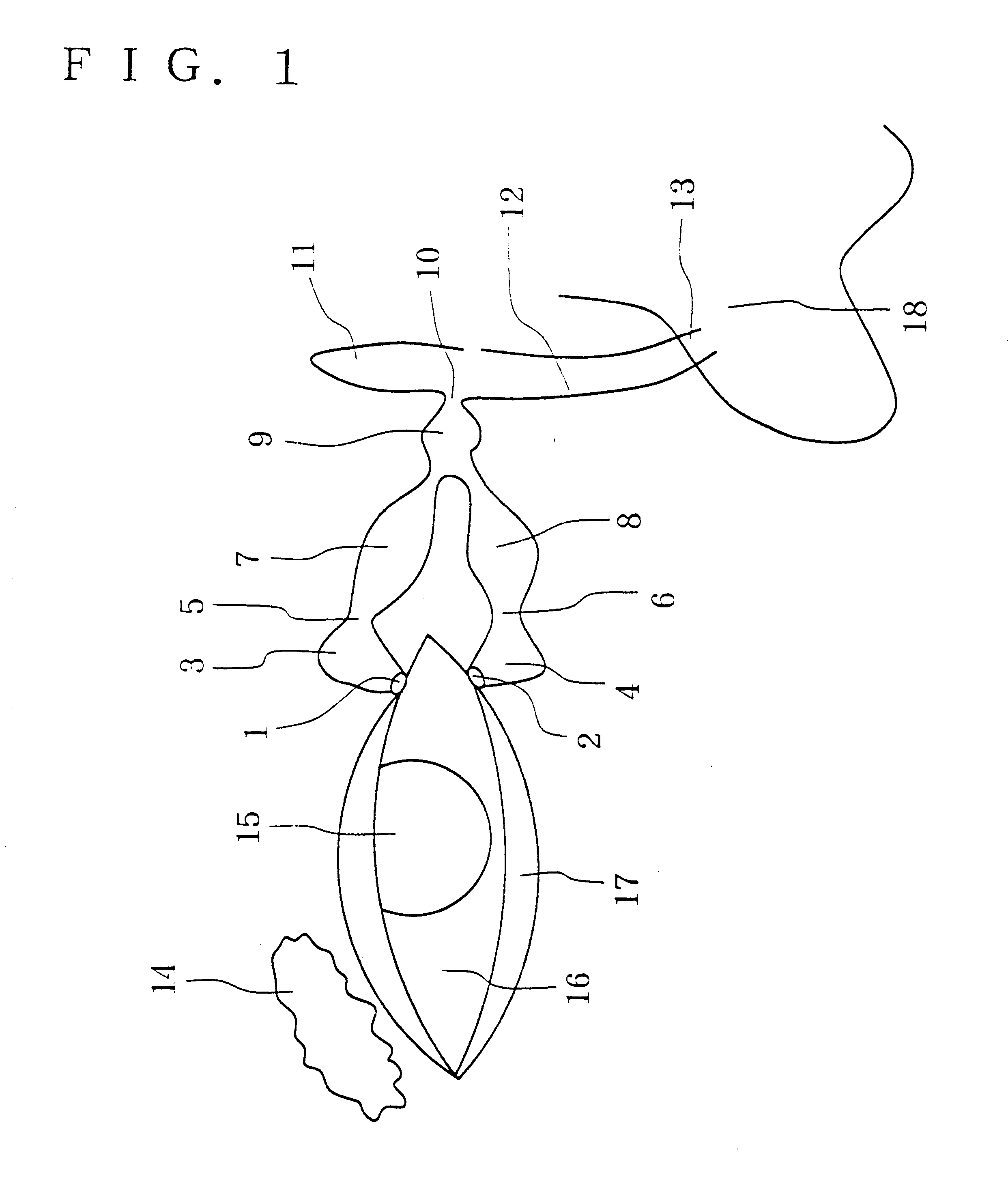
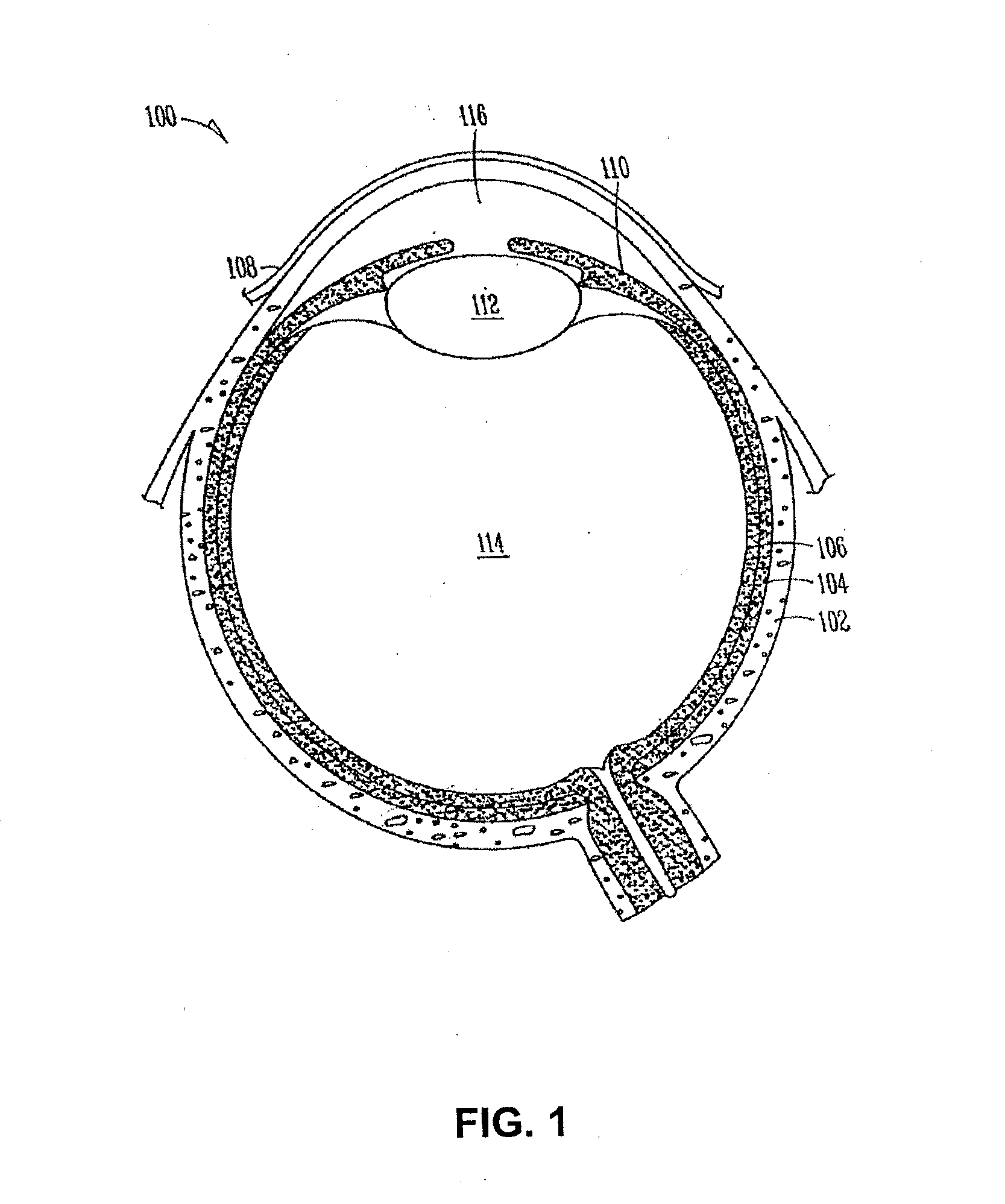
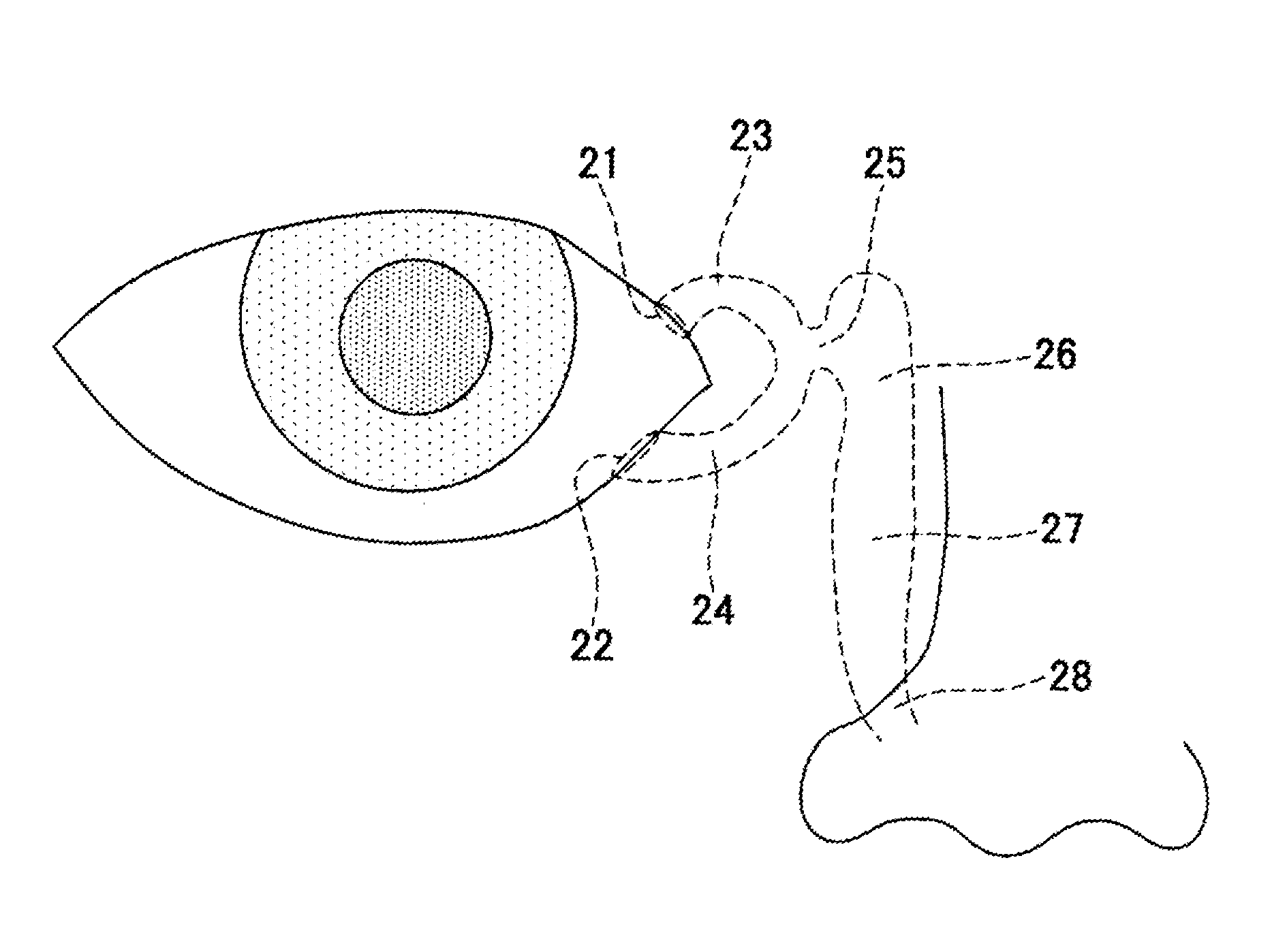
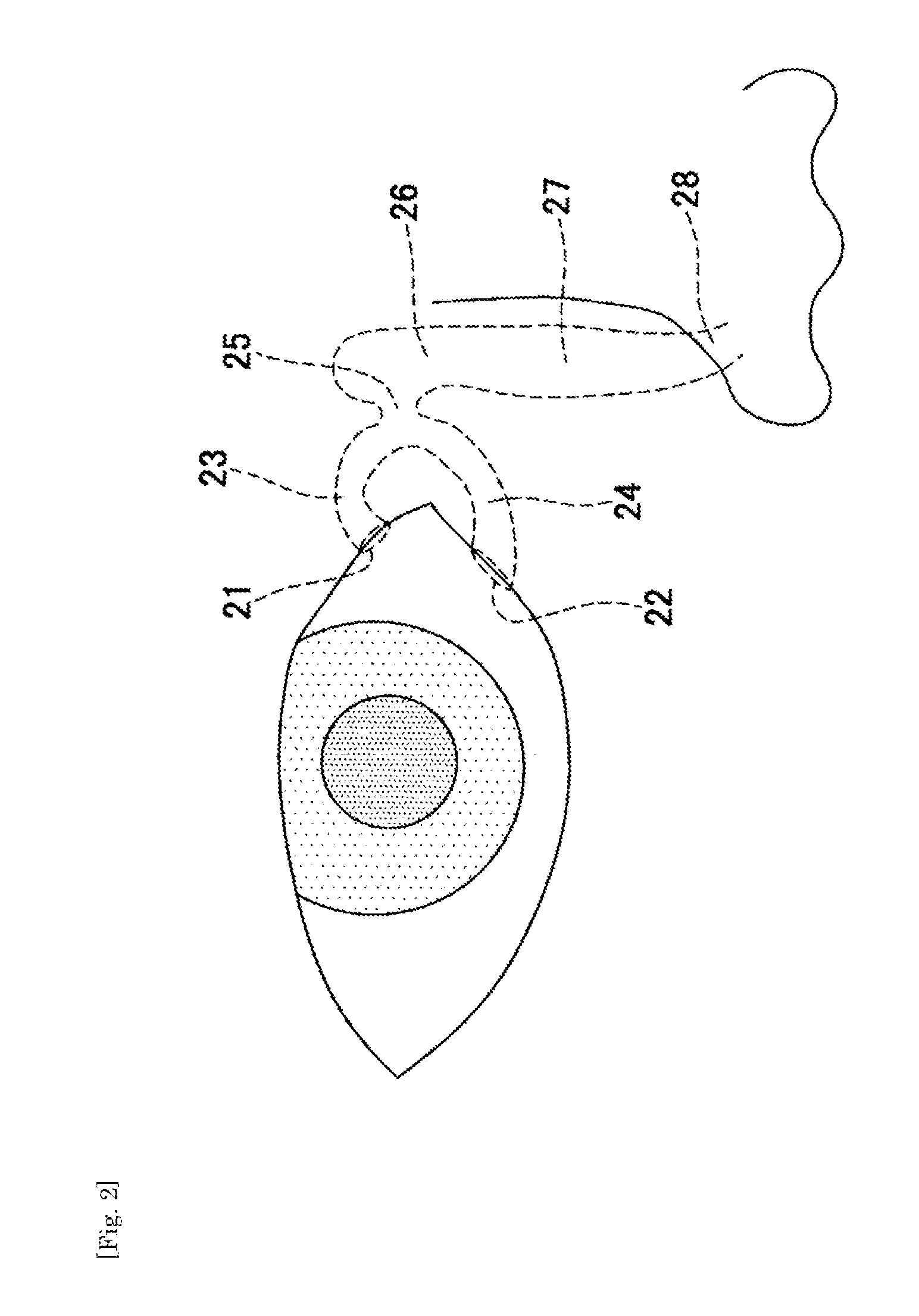
A first tube (3) and second tube (7) for guiding aqueous humor to the exterior of the eye are connected to each other in the vicinity of a surface of conjunctiva (14) via a first joint (5) and second joint (6). A filter part (9) provided to prevent reflux infection from the exterior to interior of the eye is connected to the second tube (7) via a third tube (8) positioned inside a lower lacrimal canaliculus (23). This enables an aqueous humor drainage implant (1) to be positioned in the eye and the exterior of the conjunctiva with reduced invasiveness. With the aqueous humor drainage implant for glaucoma treatment, the aqueous humor in the eye can be drained to the exterior of the conjunctiva while preventing reflux infection at the viral level, and the intraocular pressure reducing effect can be sustained for extended time periods over the lifespan of the patient. Further, the aqueous humor drainage implant can be readily positioned with reduced surgical invasiveness, while posing no danger of damaging the eye or nasolacrimal duct after the installation.
Owner:JAPAN SCI & TECH CORP
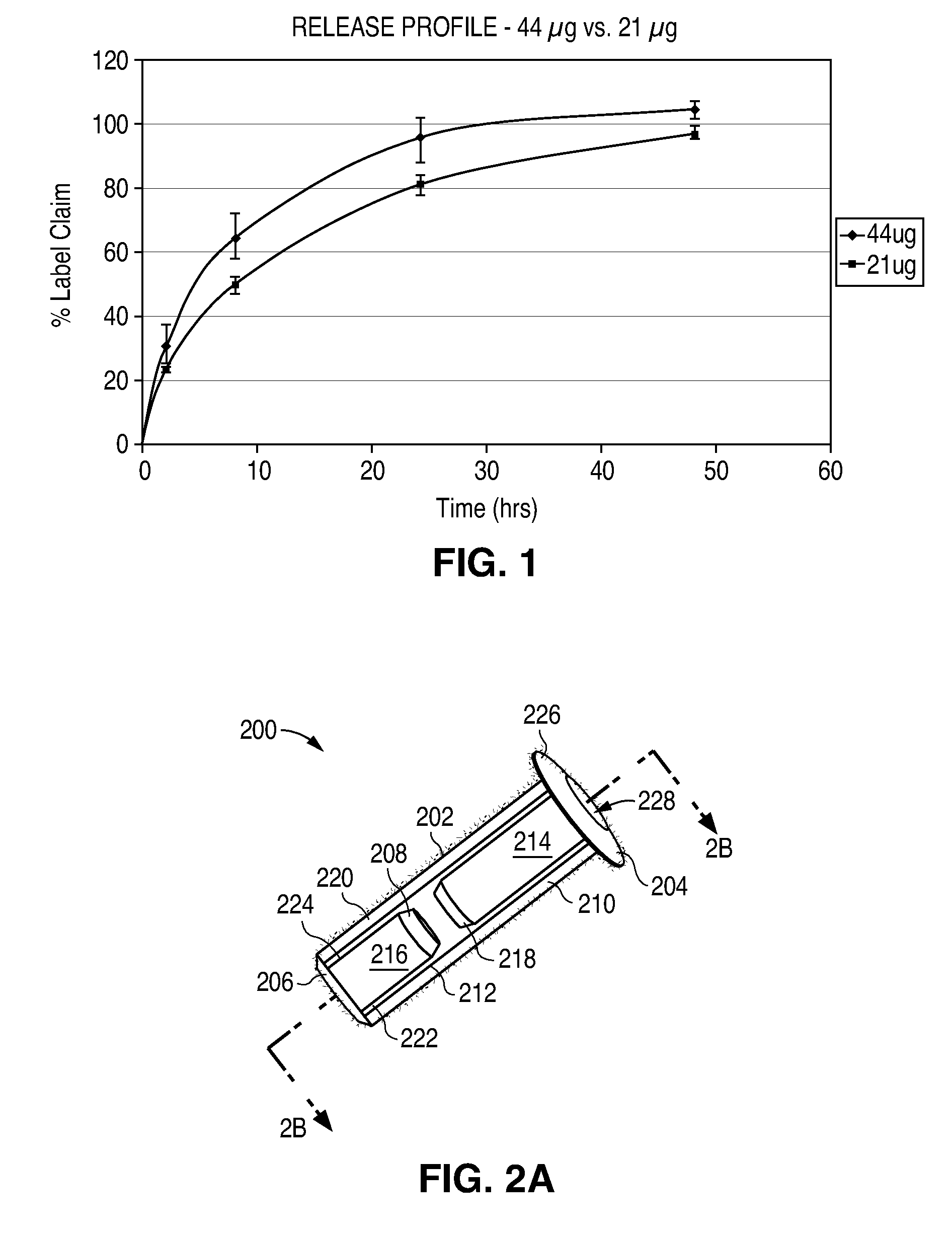
Sustained release delivery of one or more agents
InactiveUS20100209477A1Improve permeabilityReduce the amount requiredBiocideOrganic active ingredientsCross-linkExcipient
The lacrimal implant delivery systems and methods described herein provide for controlled release of a therapeutic agent for the treatment of disease, including the treatment of glaucoma, ocular hypertension, or elevated intraocular pressure with latanoprost or other anti-glaucoma agents. Treatment of disease, including glaucoma, ocular hypertension, or elevated intraocular pressure with latanoprost or other anti-glaucoma agent in conjunction with penetration enhancer, such as benzalkonium chloride, and / or artificial tears is also provided. Also provided are implants containing a drug core emplacable in a punctum adjacent to an eye of a patient for controlled release of a therapeutic agent such as latanoprost for the treatment of glaucoma, the drug core containing a polymer such as cross-linked silicone, a therapeutic agent, and an excipient, wherein the excipient can increase the rate of release of the agent from the drug core, or can increase the drug loading in the core without loss of desirable homogeneity of the agent within the core, or can improve retention of the agent in the eye or in tear fluid, or can increase corneal penetration of the agent into the eye.
Owner:MATI THERAPEUTICS
Certain dinucleotides and their use as modulators of mucociliary clearance and ciliary beat frequency
The present invention relates to certain novel dinucleotides and formulations thereof which are highly selective agonists of the P2Y2 and / or P2Y4 purinergic receptor. They are useful in the treatment of chronic obstructive pulmonary diseases such as chronic bronchitis, PCD, cystic fibrosis, as well as prevention of pneumonia due to immobility. Furthermore, because of their general ability to clear retained mucus secretions and stimulate ciliary beat frequency, the compounds of the present invention are also useful in the treatment of sinusitis, otitis media and nasolacrimal duct obstruction. They are also useful for treatment of dry eye disease and retinal detachment as well as facilitating sputum induction and expectoration.
Owner:MERCK SHARP & DOHME CORP
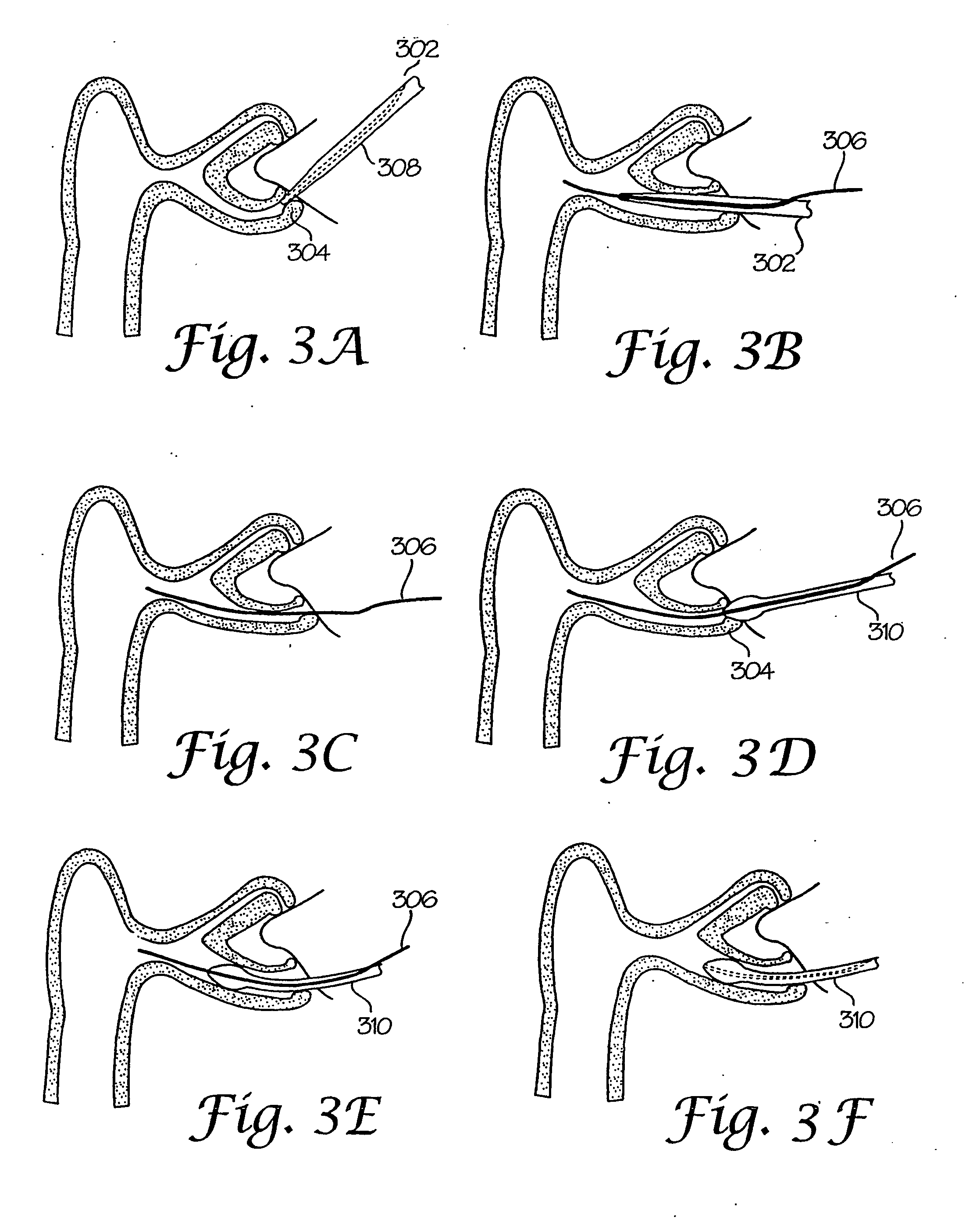
Apparatus for intubation of lacrimal drainage pathway
A device for intubation of a lacrimal duct is inserted into the lacrimal duct, having a smaller diameter tube or rod portion, and a larger diameter tube which is connected with one end of the smaller diameter portion, and a stopper which consists of a plug, brim, ring, etc., attached to the other end of the smaller diameter tube or rod. The tip of the larger diameter tube is a closed end.
Owner:MLC LIMITED
Punctal plugs for the delivery of active agents
The invention provides punctal plugs for the delivery of active agent to one or both of the tear fluid of the eye and to the nasolacrimal duct that comprise a body, at least one cap, and optionally a collarette.
Owner:JOHNSON & JOHNSON VISION CARE INC
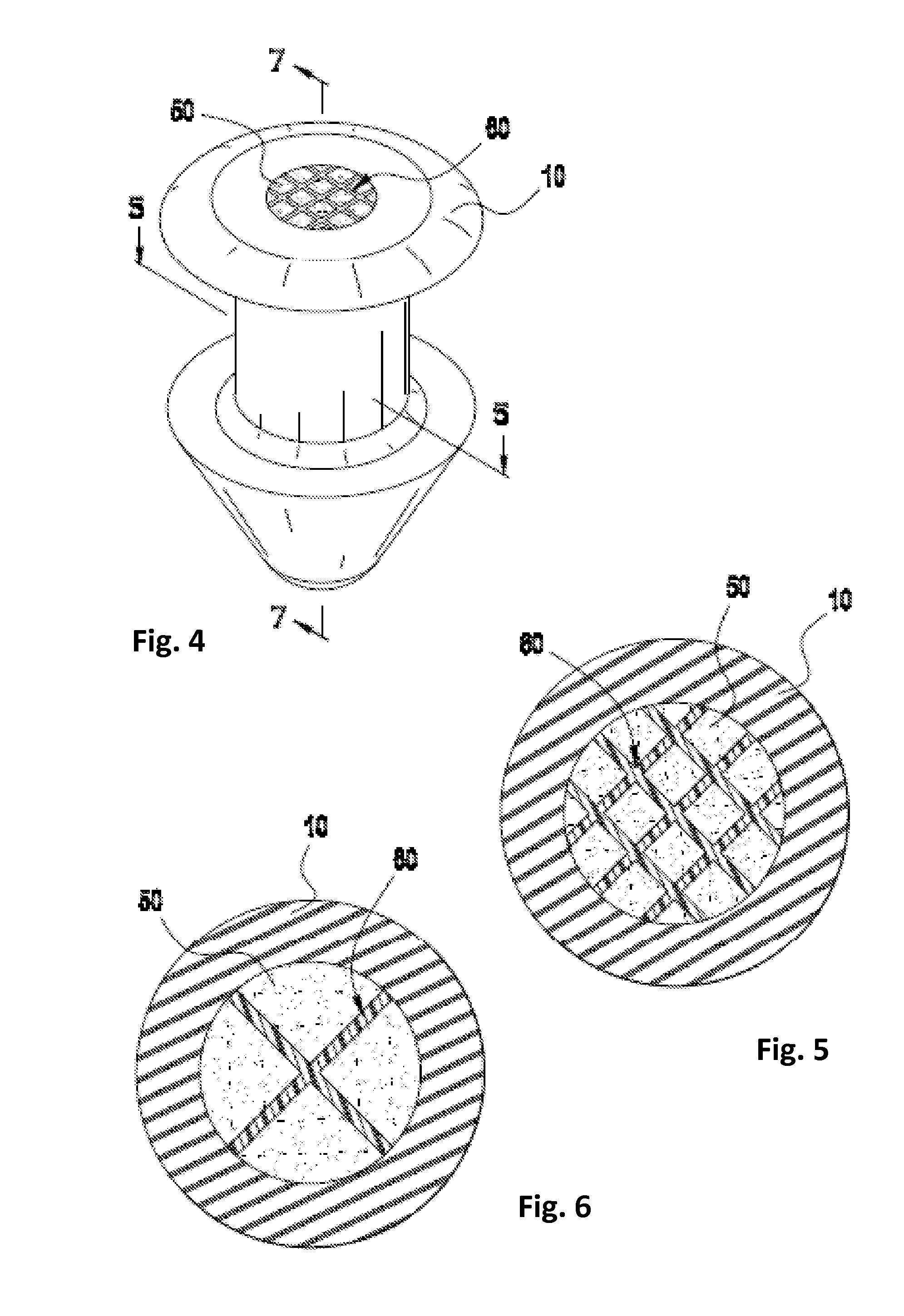
More easily visualized punctum plug configurations
An improved punctum plug is more easily visualized when positioned within a punctual canal of a recipient. The body of the plug features an outwardly exposed surface when properly positioned, and a substance causing at least the outwardly exposed surface to contrast with surrounding tissue, such that the use of the substance causes the plug to be more easily visualized than if the substance were not present. The substance, which may be disposed on the outwardly exposed surface or within the body of the plug, may include a saturated coloration, or may be phosphorescent, fluorescent or otherwise operative to reflect or re-radiate light to assist in visualization. For example, the substance may include an organic or inorganic phosphor or fluorescent material, reflective beads, quantum dots, a dye or pigment. Such reflection or re-radiation may occur at the same or different wavelength(s) compared to the illumination wavelength(s), whether or not either or both are within the visible part of the spectrum. If outside the visible region, a detector may be employed according to the invention for detecting the radiated light. A system for determining whether or not a punctum plug is positioned within the punctal canal of a person's eye is also enclosed, including at least one optical element permitting the eye to view itself, to be viewed by the other eye, or by a second person.
Owner:DIGGER MONTANA
Implant device, tool, and methods relating to treatment of paranasal sinuses
An implant device is configured to be implanted in a fistula to fluidly connect the lacrimal apparatus and a paranasal sinus. A surgical tool has an implant the implant device mounted on a carrier. Various methods involve a fistula between the lacrimal apparatus and a paranasal sinus. A kit includes an entry device for use to form a fistula and an implant tool for use to implant an implant device following formation of a fistula.
Owner:SINOPSYS SURGICAL
Sustained release delivery of active agents to treat glaucoma and ocular hypertension
InactiveUS20130053794A1Improved patient comfortImproved implant retentionOrganic active ingredientsSenses disorderActive agentOcular hypertension
A method of decreasing intraocular pressure (IOP) in an eye of a patient in need thereof includes implanting a first lacrimal implant through a firsts punctum and into a first lacrimal canaliculus of the eye of the patient. The method may further comprise implanting a second lacrimal implant through a second punctum and into a second lacrimal canaliculus of the eye of the patient, and releasing, on a sustained basis a therapeutically effective amount of an intraocular pressure-reducing therapeutic agent.
Owner:MATI THERAPEUTICS
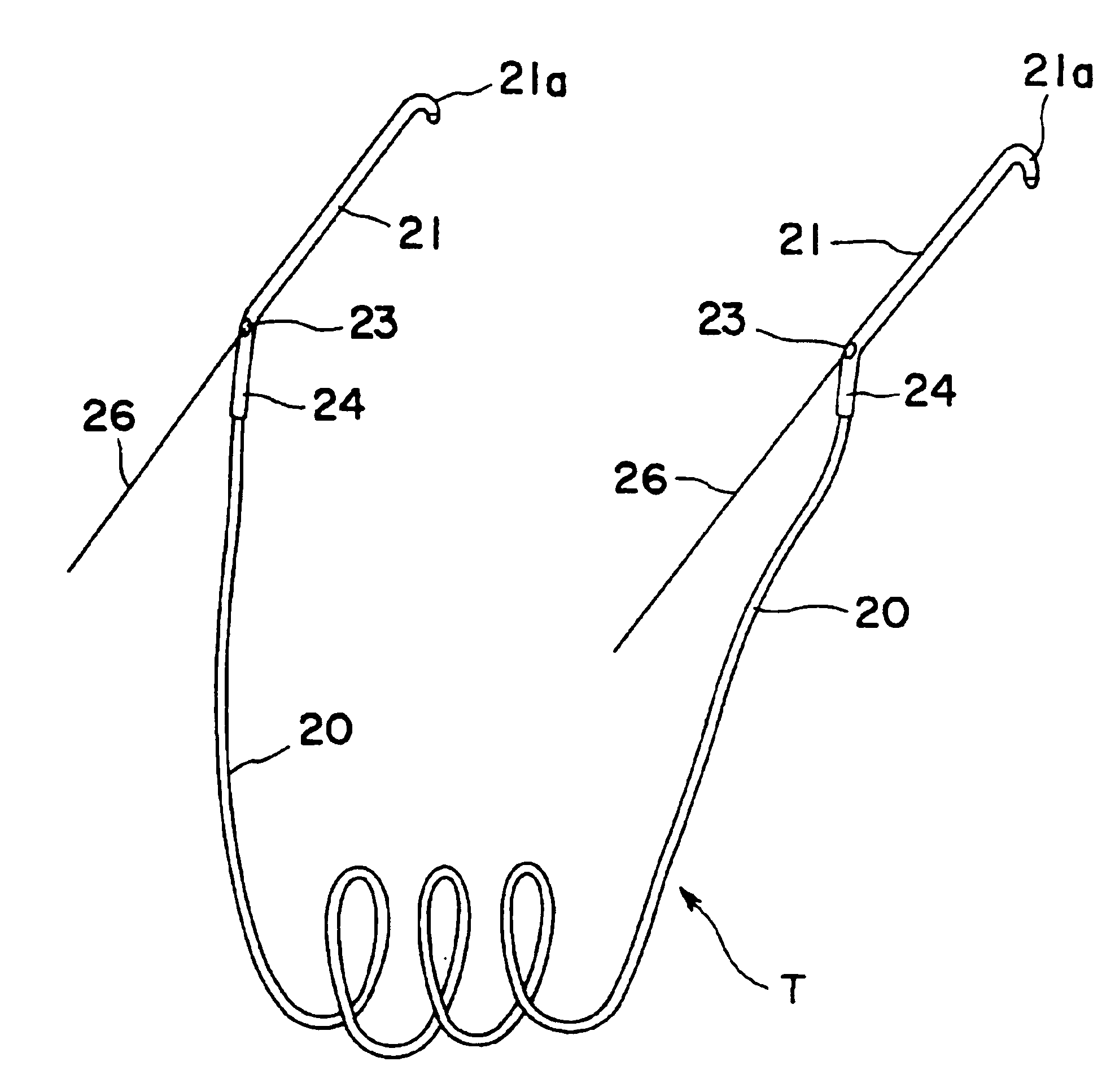
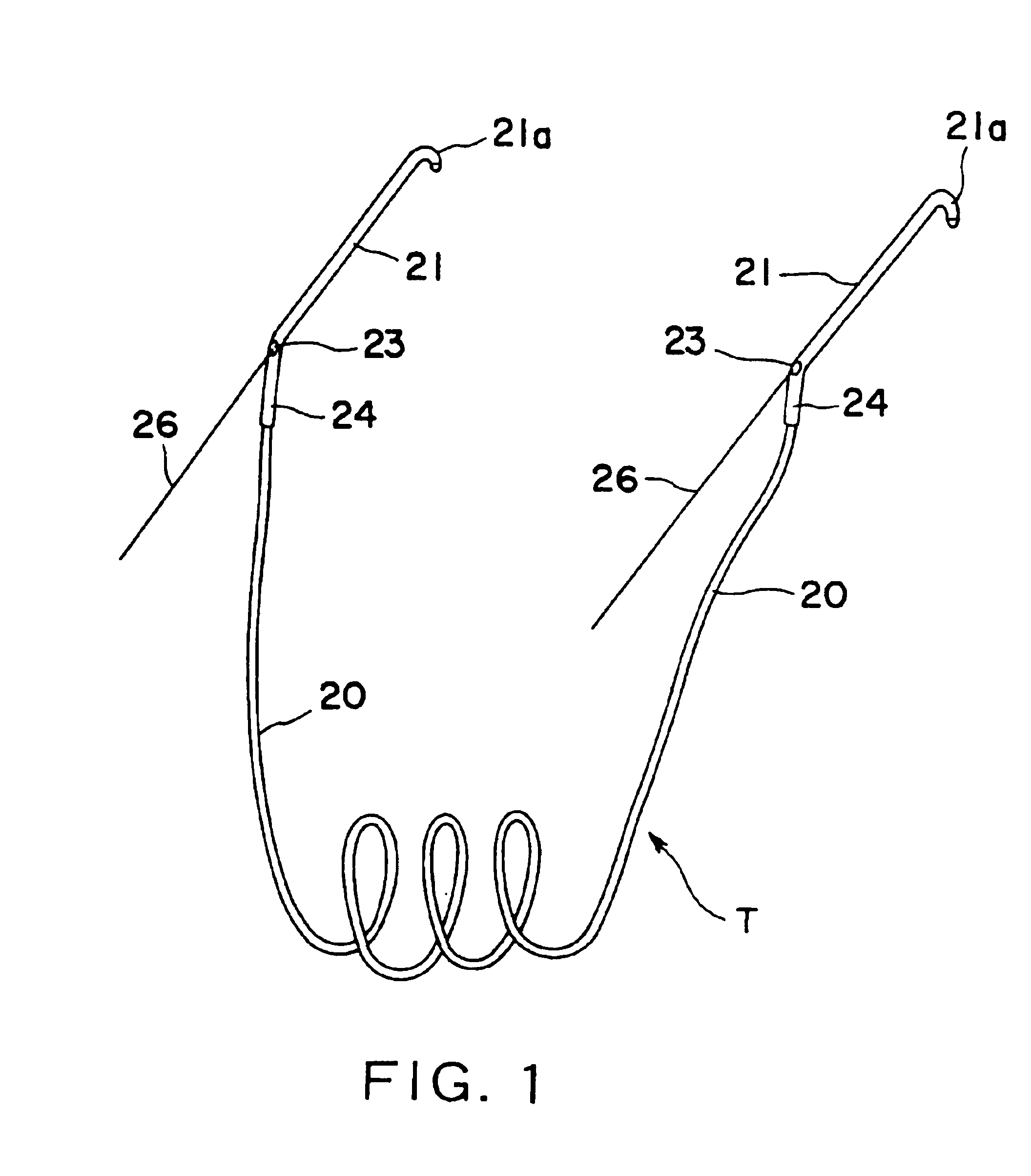
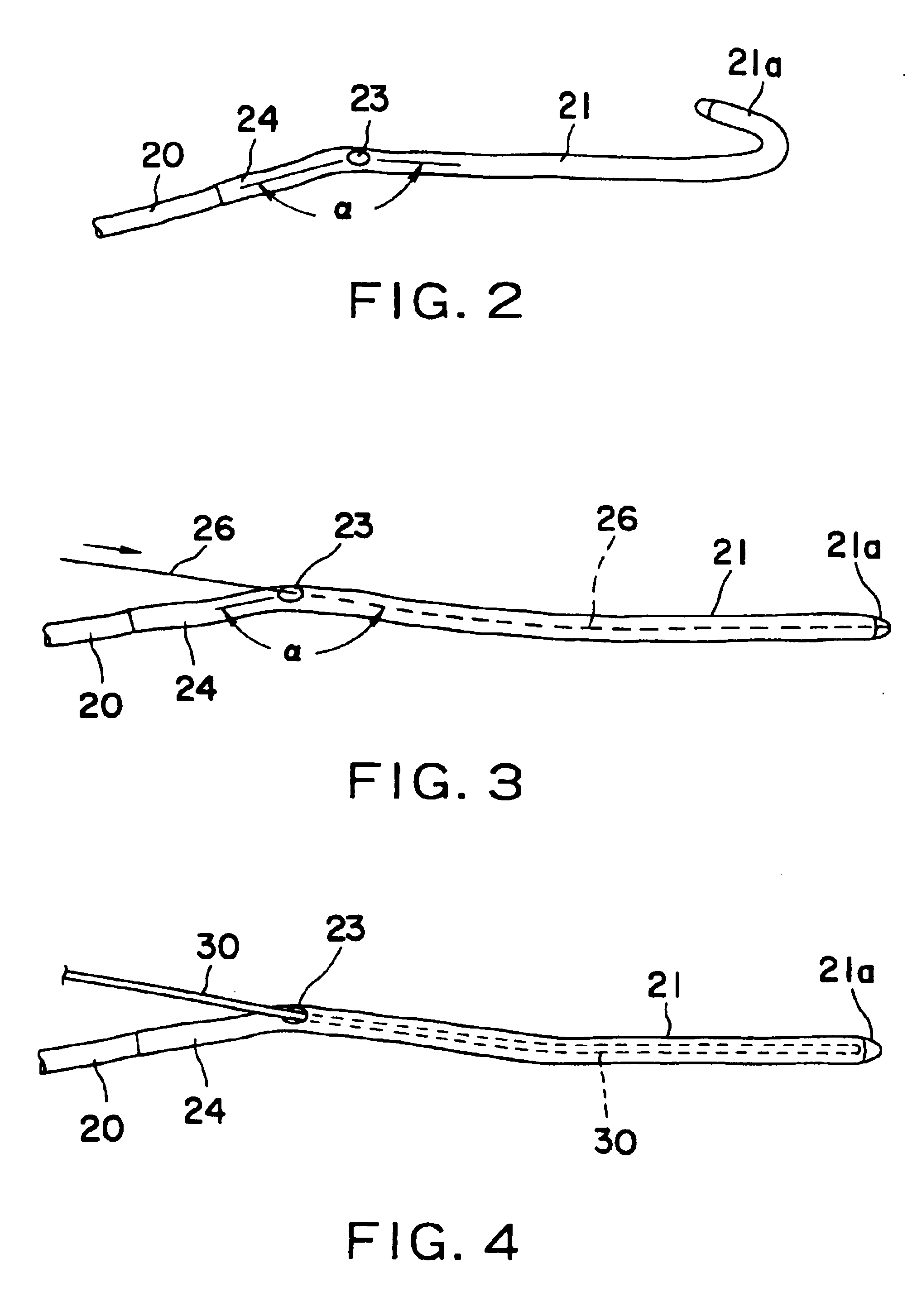
Nasolacrimal duct tube used for lacrimal duct reformation operation, and nasolacrimal duct tube instrument
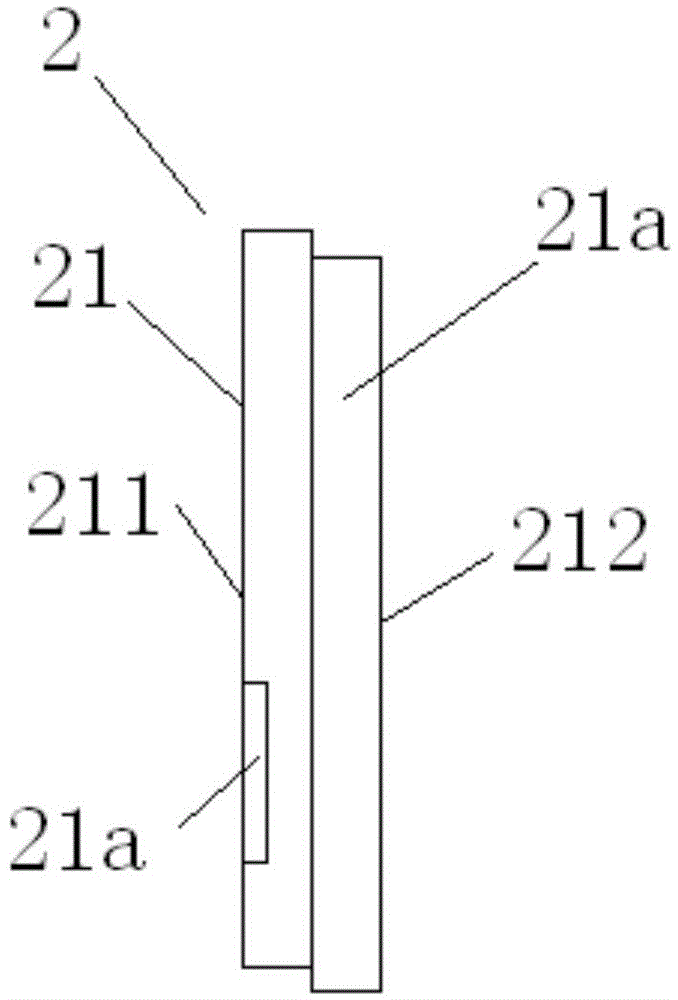
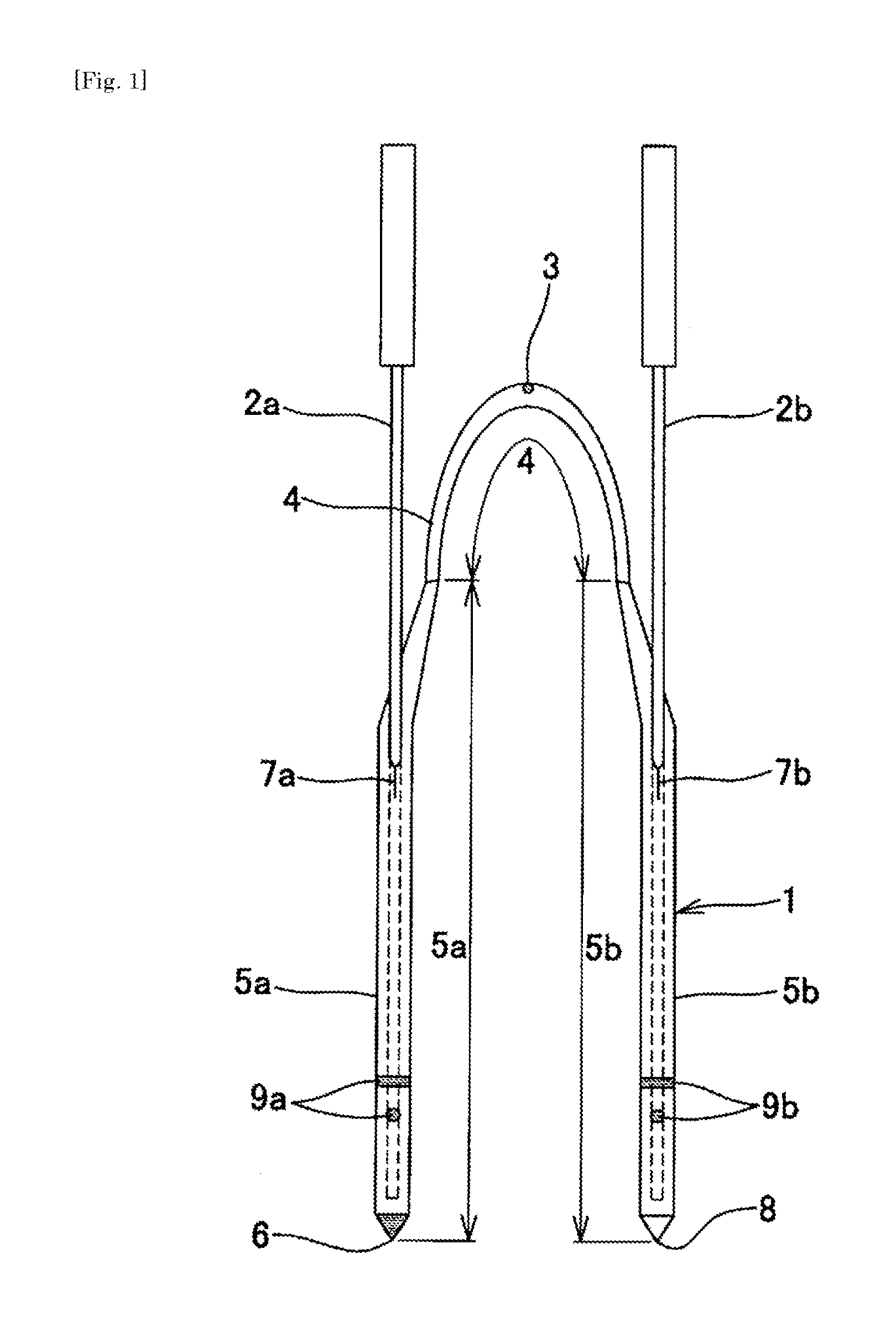
A nasolacrimal stent device illuminates a dark nasal cavity so that the interior of the nasal cavity can be directly observed to facilitate and ensure the correct insertion of a nasolacrimal stent into the nasal duct. Flexible, transparent probe tube segments (21) are connected to the opposite ends of a flexible detention tube segment (2) having a diameter that permits the flexible detention tube segment (2) to be inserted in a lacrimal passage, respectively. The respective extremities of distal end parts (21a, 21b) of the probe tube segments (21) are closed. The probe tube segments (21) are provided in their base end parts with openings (23), respectively. When inserting each probe tube segment (21) into the nasal cavity, an illuminating device, such as an optical fiber (30) is inserted through the opening (23) into the probe tube segment (21) to illuminate the interior of the nasal cavity through the transparent probe tube segment (21). The probe tube segment (21) can be surely caught with a hook and can be pulled out from the nasal cavity. An ultrasonic probe or an endoscope may be used instead of the illuminating device to find the position of the nasolacrimal stent in the nasal cavity.
Owner:TORAY IND INC +1
Method of intracanalicular laser dacryocystorhinostomy
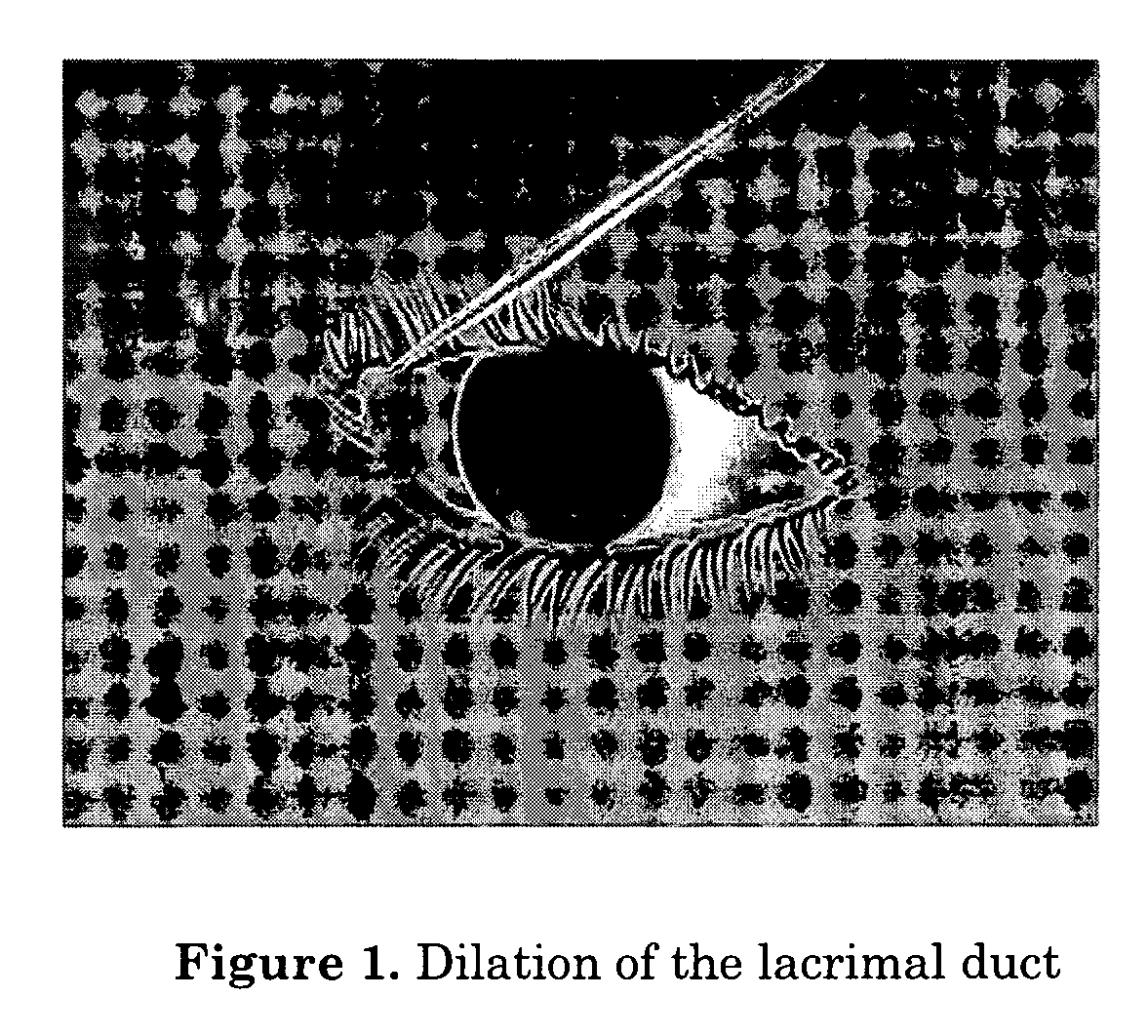
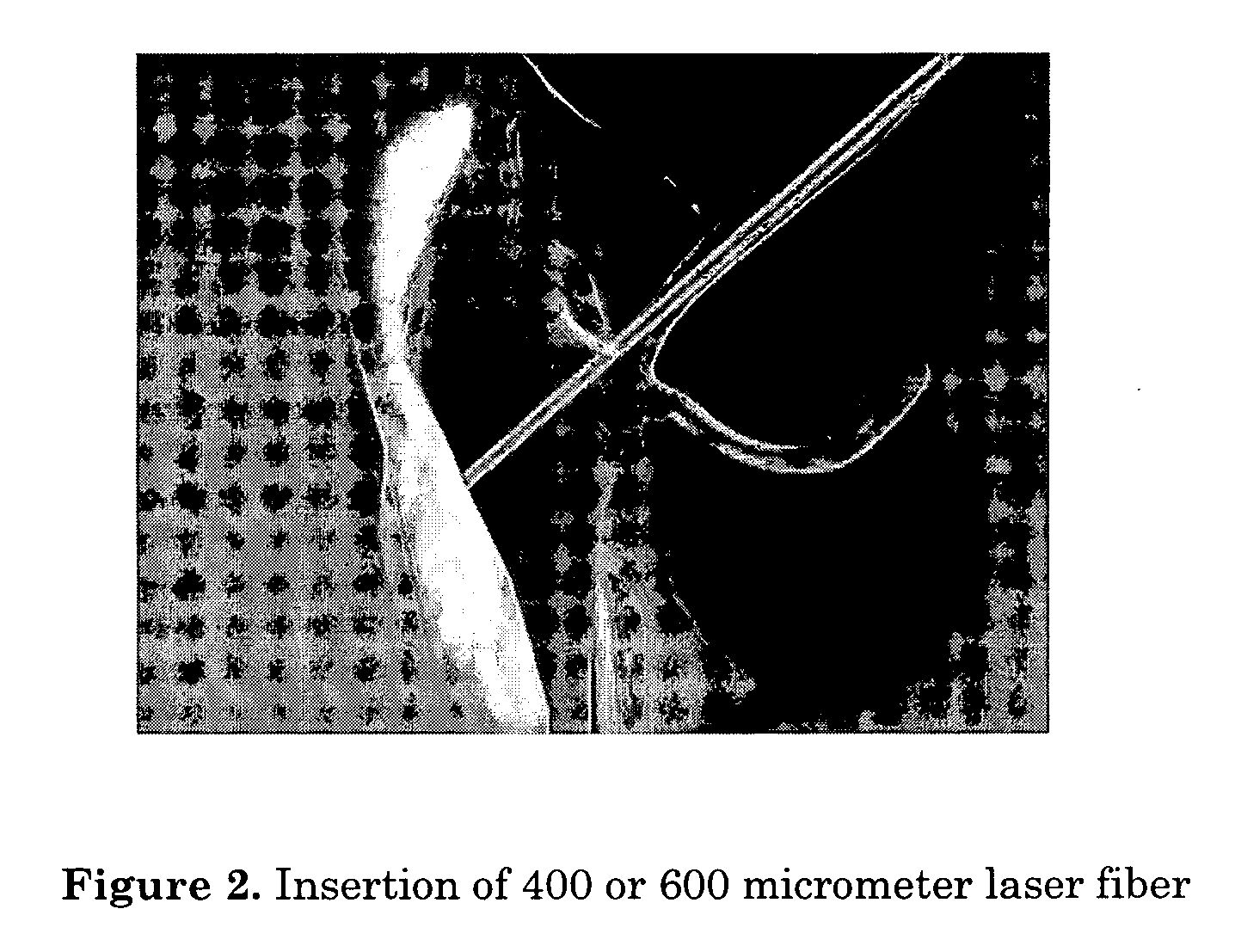
Method for performing transcanalicular dacryocystorhinostomy which comprises the steps of inserting of a suitable fiber optic which is connected to a solid state laser that emits a laser beam with a wavelength of about 700 nm to about 1600 nm through a lacrimal duct, using an endonasal endoscope to position an end of the fiber optic to a location where perforation is to be formed, firing the laser until perforation to form an osteotomy, and optionally enlarging of the osteotomy.
Owner:EQUIPSA
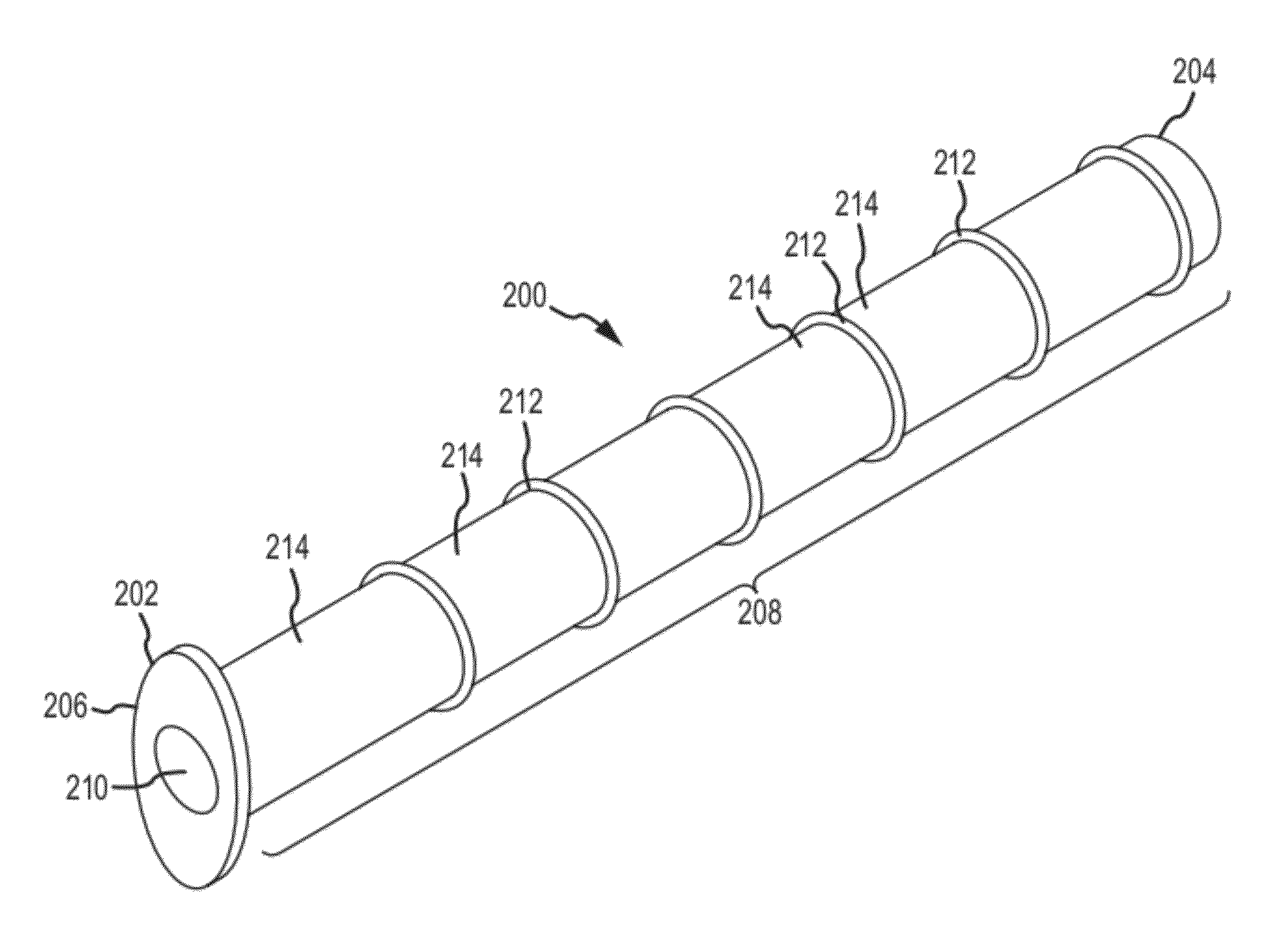
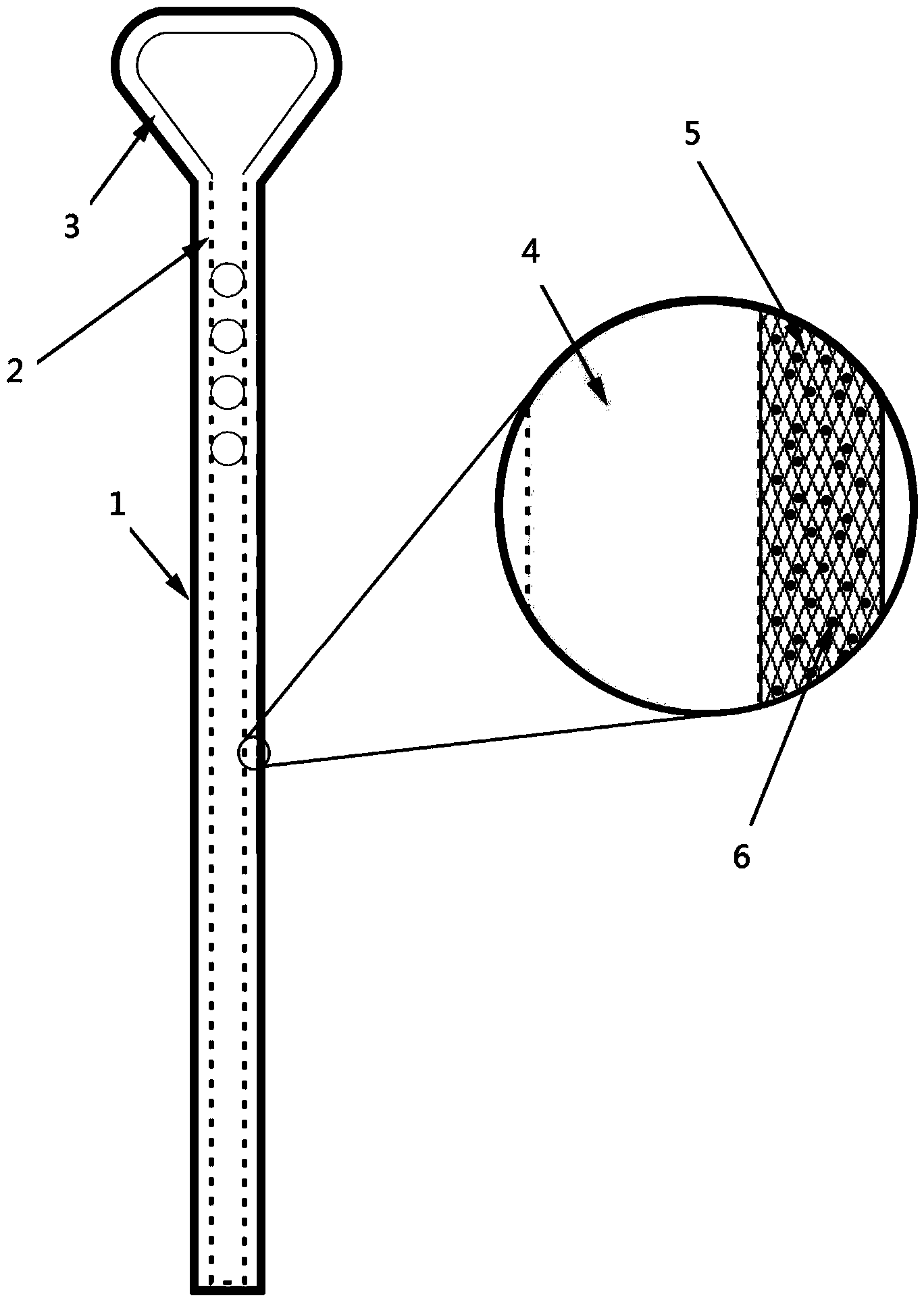
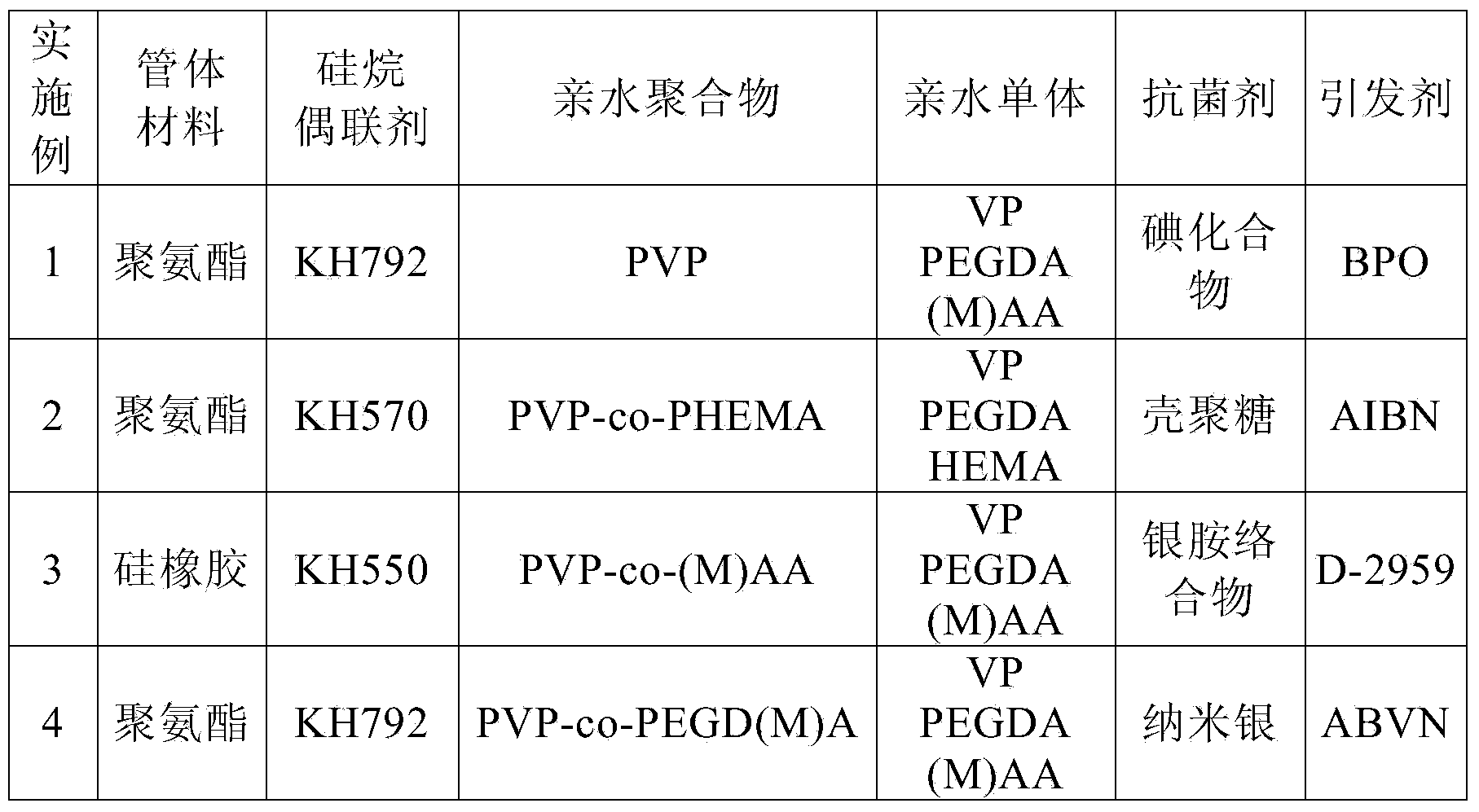
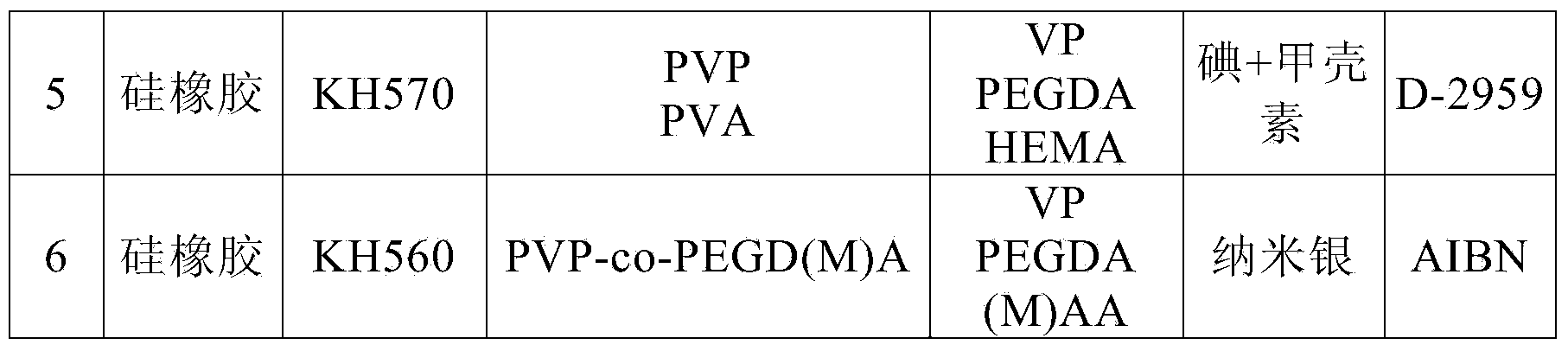
Hydrophilic lubricating antibacterial lacrimal duct tube and manufacturing method thereof
ActiveCN104042295AImprove push effectReduce coefficient of frictionStentsCatheterComposite functionEngineering
The invention relates to a hydrophilic lubricating antibacterial lacrimal duct tube and a manufacturing method of the hydrophilic lubricating antibacterial lacrimal duct tube. The lacrimal duct tube comprises a main tube and a lifting ring. The upper end of the main tube is connected with the lower end of the lifting ring. The main tube and the lifting ring respectively comprise a tube body and a composite function layer attached to the tube wall, wherein the composite function layer is used for resisting bacterial infection and reducing adhesion of the tube body to the lacrimal duct. A basic lacrimal duct tube is processed through plasma and subjected to silane coupling agent surface treatment sequentially and then treated through an active reaction solution, a polymerization reaction is triggered, the composite function layer is formed on the tube wall of the basic lacrimal duct tube, and then the hydrophilic lubricating antibacterial lacrimal duct tube is obtained. The hydrophilic lubricating antibacterial lacrimal duct tube has a uniform surface function coating and thus has durable lubricity, tissue adhesion resistance and a good antibacterial property in a moist state.
Owner:EYE & ENT HOSPITAL SHANGHAI MEDICAL SCHOOL FUDAN UNIV
Device and method for dacryocystorhinostomy
InactiveUS20040204704A1Reducing trauma to the nasolachrymal ductReduce insertionEye surgerySurgical instrument detailsFiber bundleMedicine
An introducer and system and method for using the introducer in endoscopic combined laser assisted DCR for treatment of nasolachrymal drainage obstruction (NLDO) is disclosed. An introducer comprising a hollow outer tube and an atraumatic inner mandril is inserted into the lachrymal sac section. The atraumatic inner mandril is removed and (one or more) optical fibers or fiber bundles are inserted for illumination to determine proper position and for ablating a drainage channel. The fiber or bundle is then removed and a DCR intubation set can then be introduced to maintain the drainage channel. One advantage of this device and method is that all aspects of the procedure can be performed through the introducer, thus only requiring a single insertion point, reducing trauma to the lachrymal duct, and reducing the complexity and risk of complication or infection. Another advantage of this device and method is that it can be used with many pre-existing intubation sets used for current DCR procedures, thus the present device and method can easily and cost effectively be introduced as a first line procedure.
Owner:CERAMOPTEC IND INC
Implant device, tool, and methods relating to treatment of paranasal sinuses
An implant device is configured to be implanted in a fistula to fluidly connect the lacrimal apparatus and a paranasal sinus. A surgical tool has an implant the implant device mounted on a carrier. Various methods involve a fistula between the lacrimal apparatus and a paranasal sinus. A kit includes an entry device for use to form a fistula and an implant tool for use to implant an implant device following formation of a fistula.
Owner:SINOPSYS SURGICAL
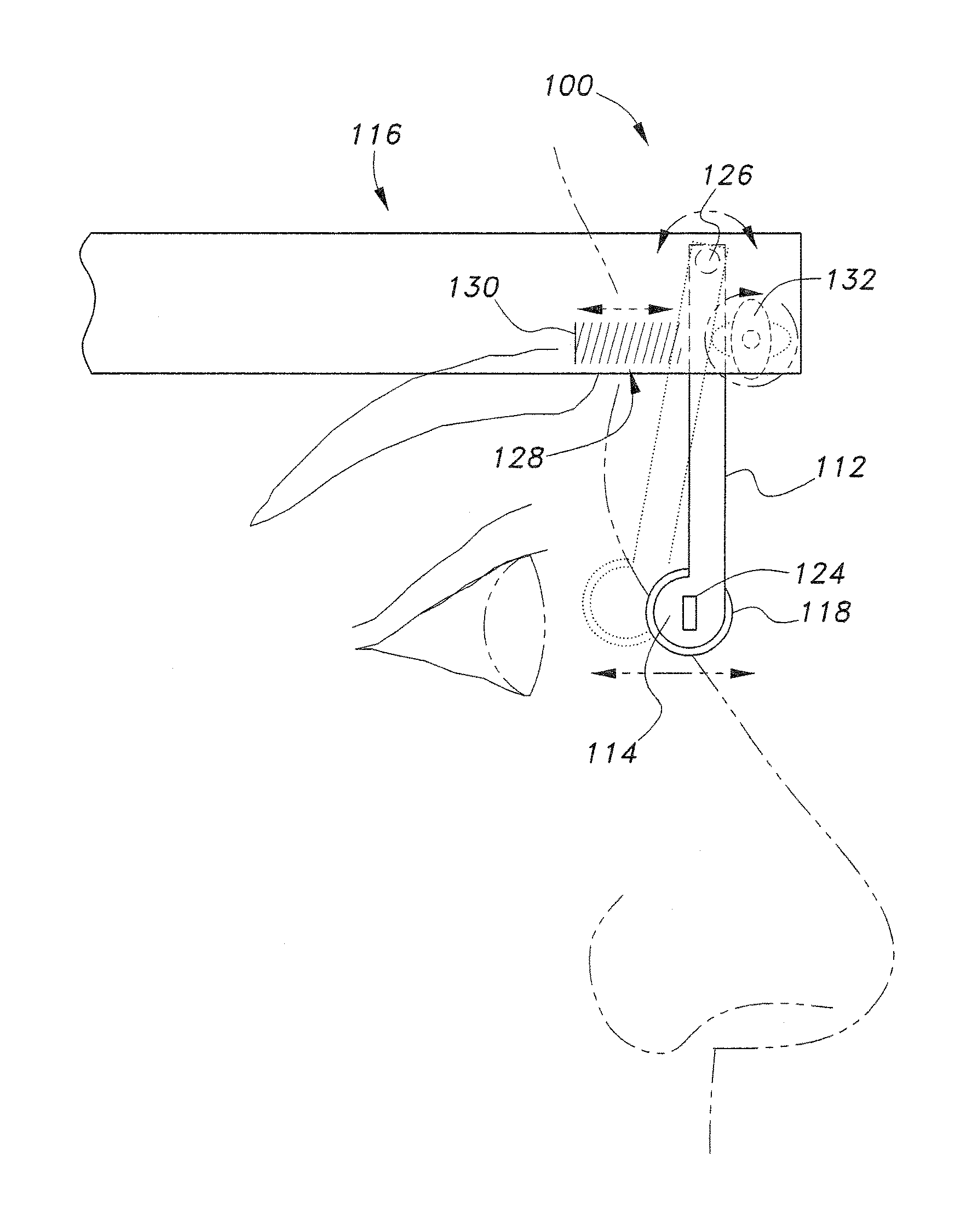
Lacrimal duct therapy device
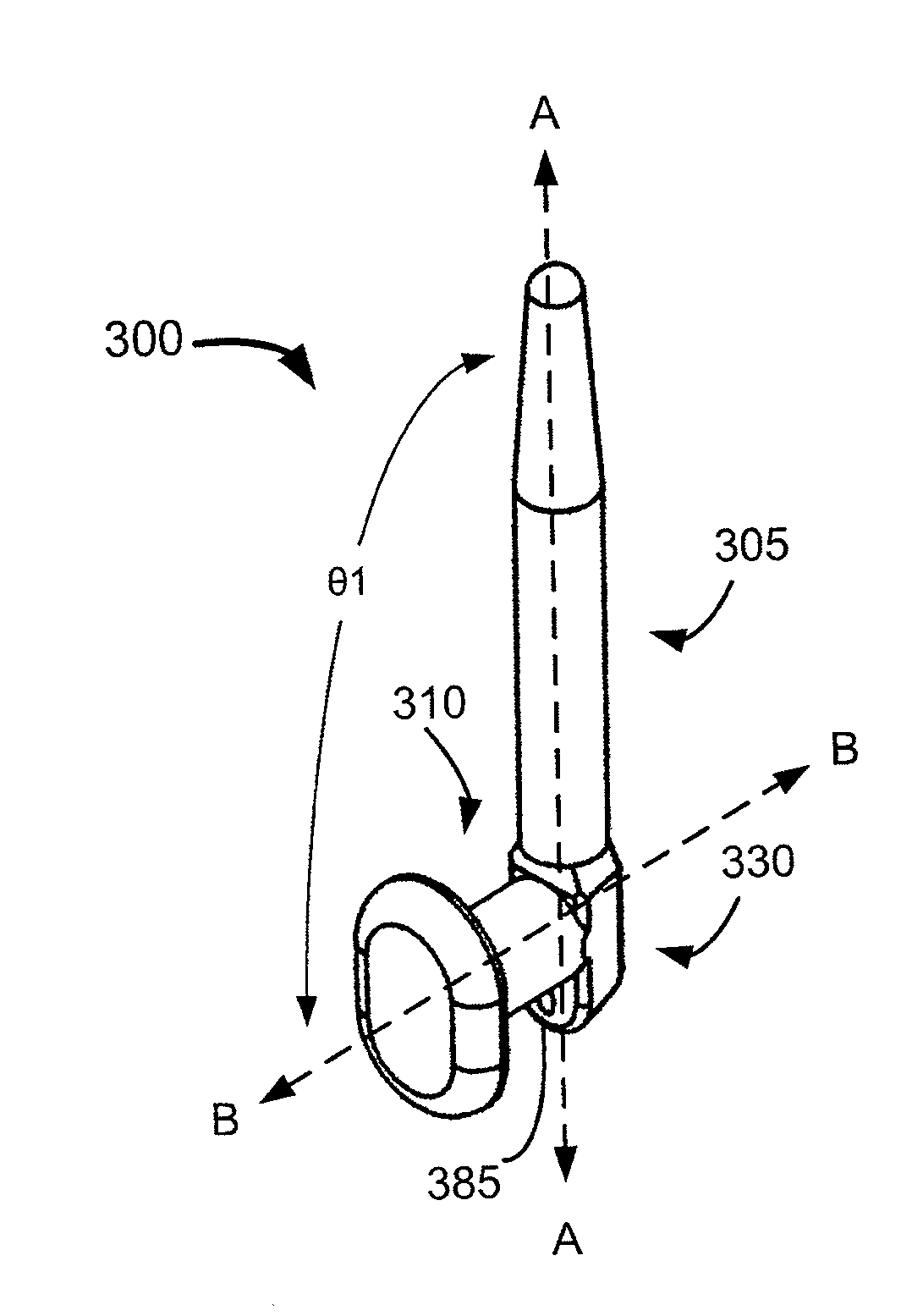
The lacrimal duct therapy device includes a frame assembly and an actuator assembly mounted on the frame assembly. The actuator assembly includes a contact assembly and a motor assembly configured to drive the contact assembly. The contact assembly includes at least one cam member having a base portion and a lobe portion and at least one arm member. The at least one cam member is mounted on a rod member attached to the motor assembly for rotation therewith. The at least one cam member bears against a leg member pivotally attached to the frame assembly, the leg member terminating in a knob bearing against the user's lacrimal duct region. Actuation of the motor periodically pivots the leg member so that the knob massages the lacrimal duct.
Owner:ADAIE HAMDAH J S
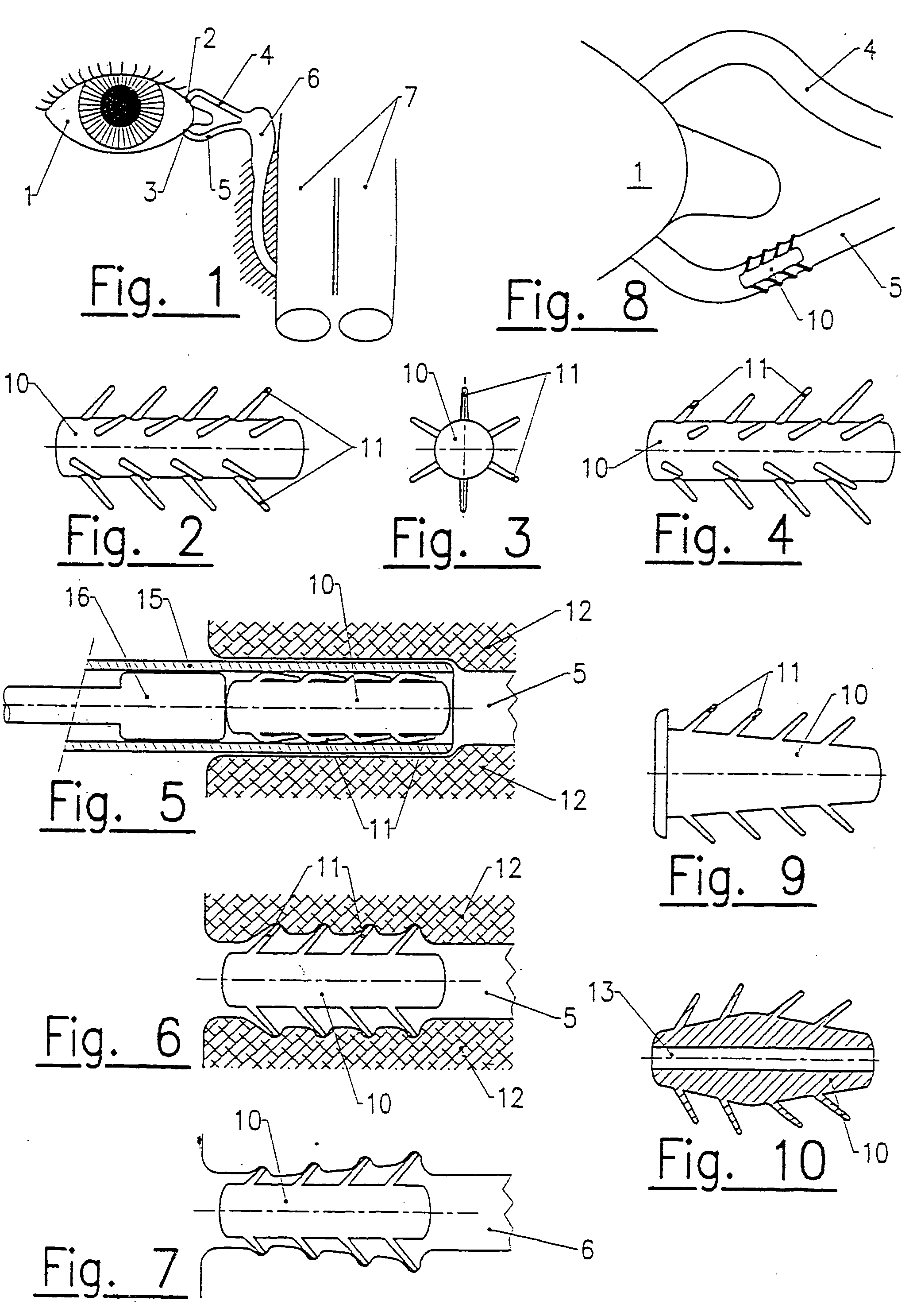
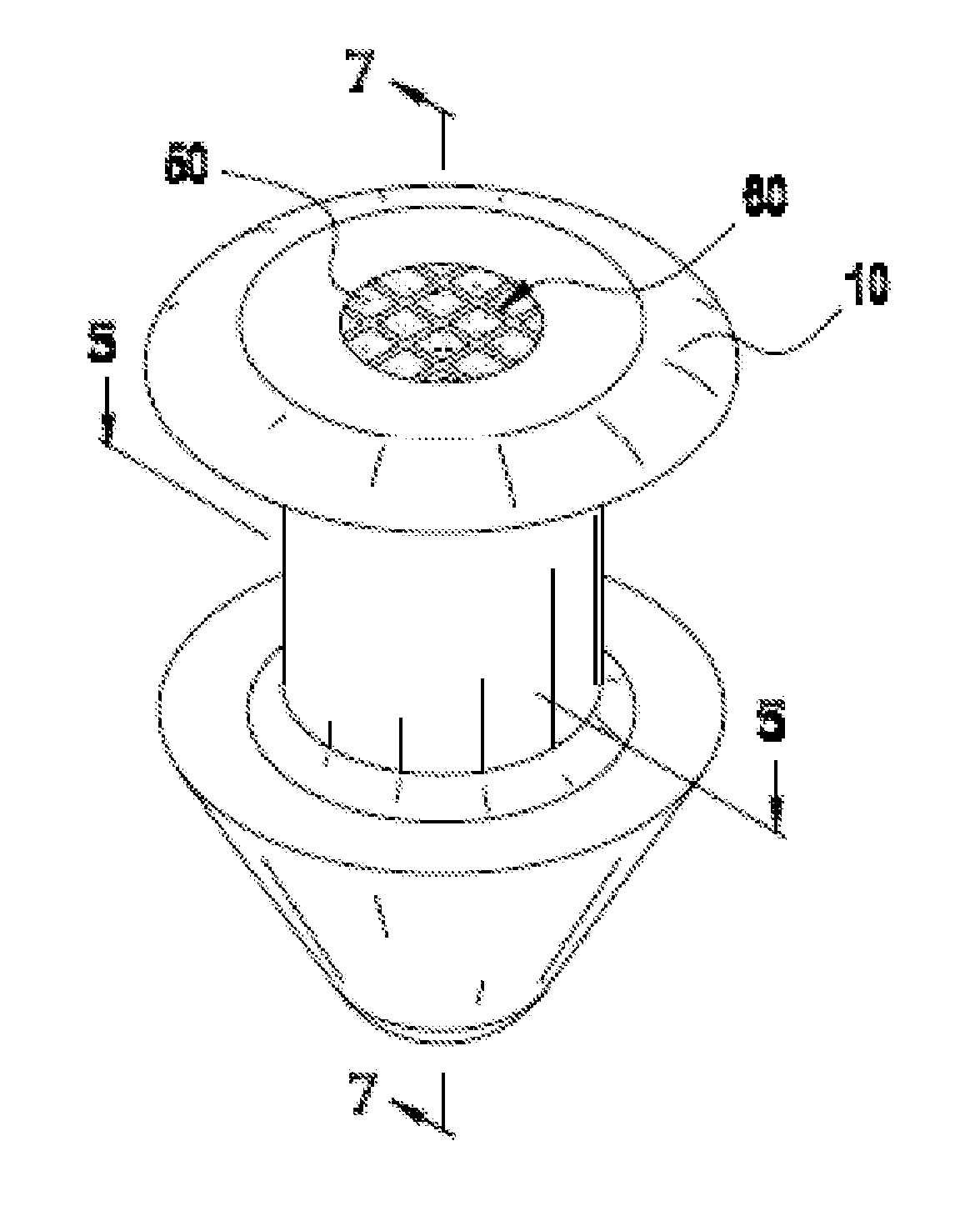
Lachrymal plugs and methods for setting same
InactiveUS20040210182A1Solving the Insufficiency of ElasticityReduce passageEye surgerySurgeryNasal cavityEngineering
The invention concerns lachrymal plugs, and methods for setting said devices. It concerns a lachrymal plug (10) designed to be inserted in the lachrymal canaliculi (5), said plug, optionally provided with an axial duct, comprising on its outer walls flexible elements (11) foldable on said walls to enable the plug to be inserted in the lachrymal duct and adapted to straighten up once the lachrymal plug is set, so as to maintain the latter in position. The invention generally concerns means for controlling the flow of tears running from the surface of the eye towards the nasal cavity.
Owner:FOUERE ALAIN +1
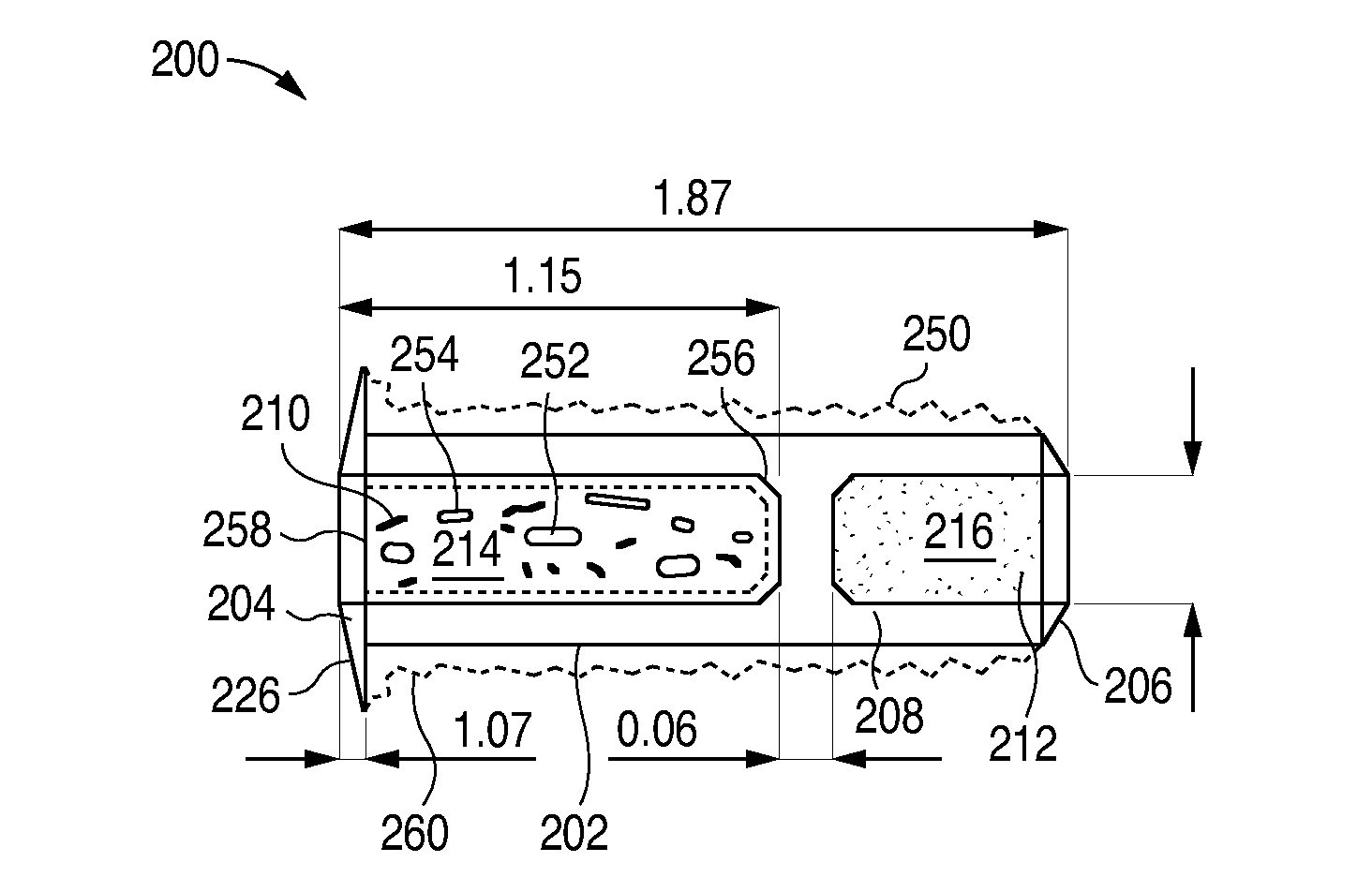
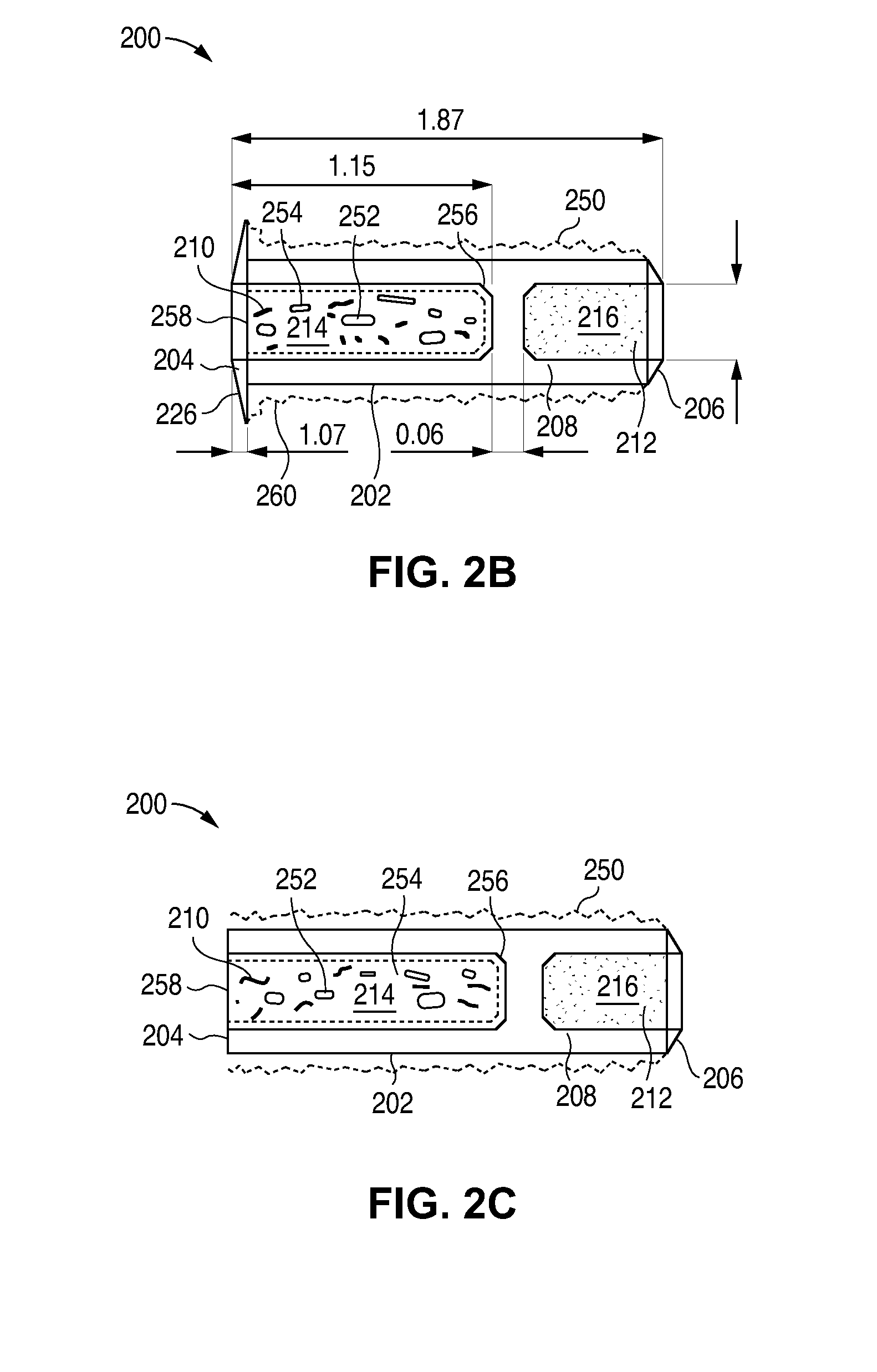
Porous matrix drug core for lacrimal insert device
Disclosed are novel structures and methods for drug cores containing therapeutic agents. Such cores may be inserted or manufactured in lacrimal insert devices for treating a variety of ocular conditions by eluding therapeutic agents according to a release profile determined by the structure and composition of the drug core.
Owner:JOHNSON & JOHNSON VISION CARE INC
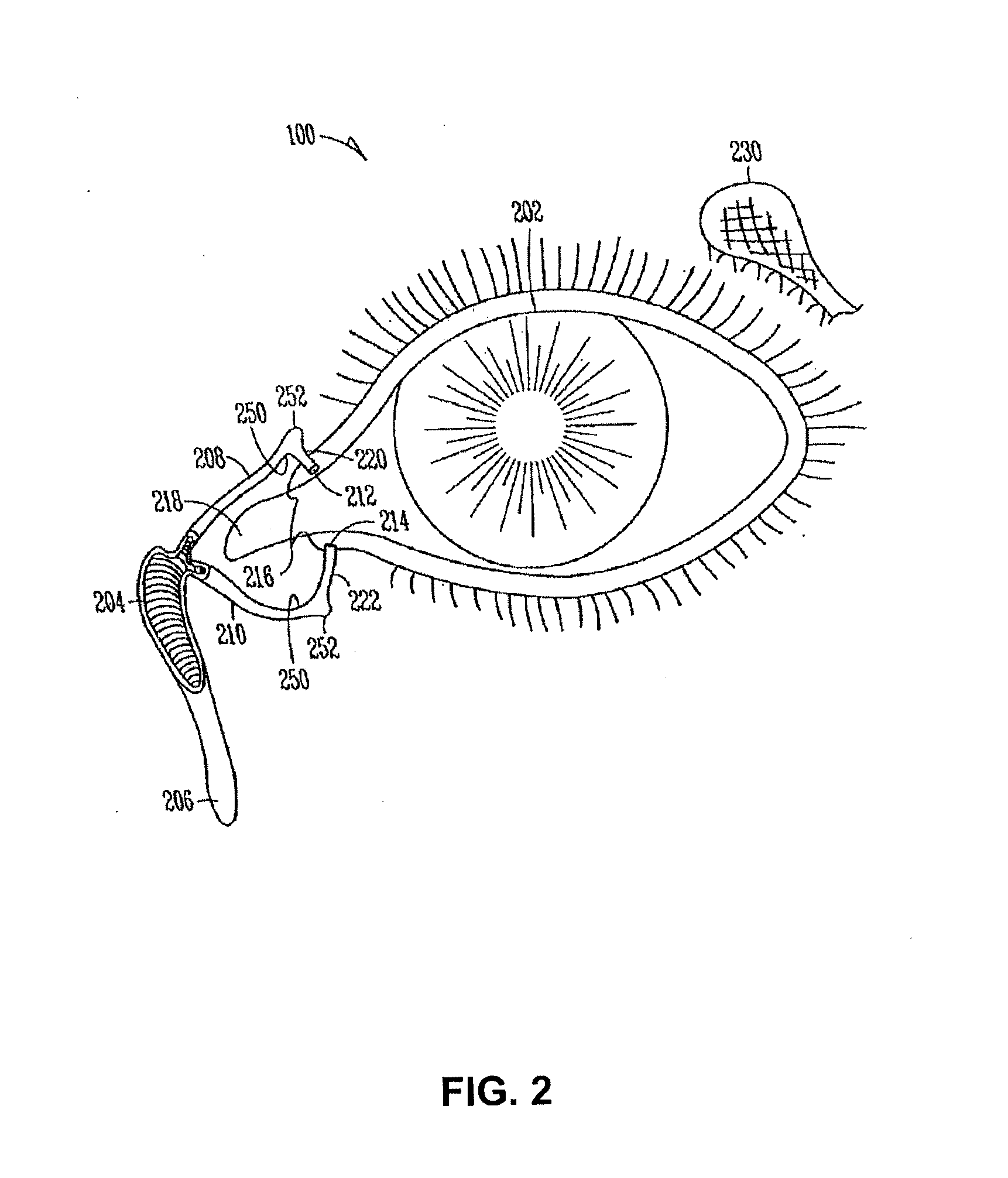
Lacrimal implants and related methods
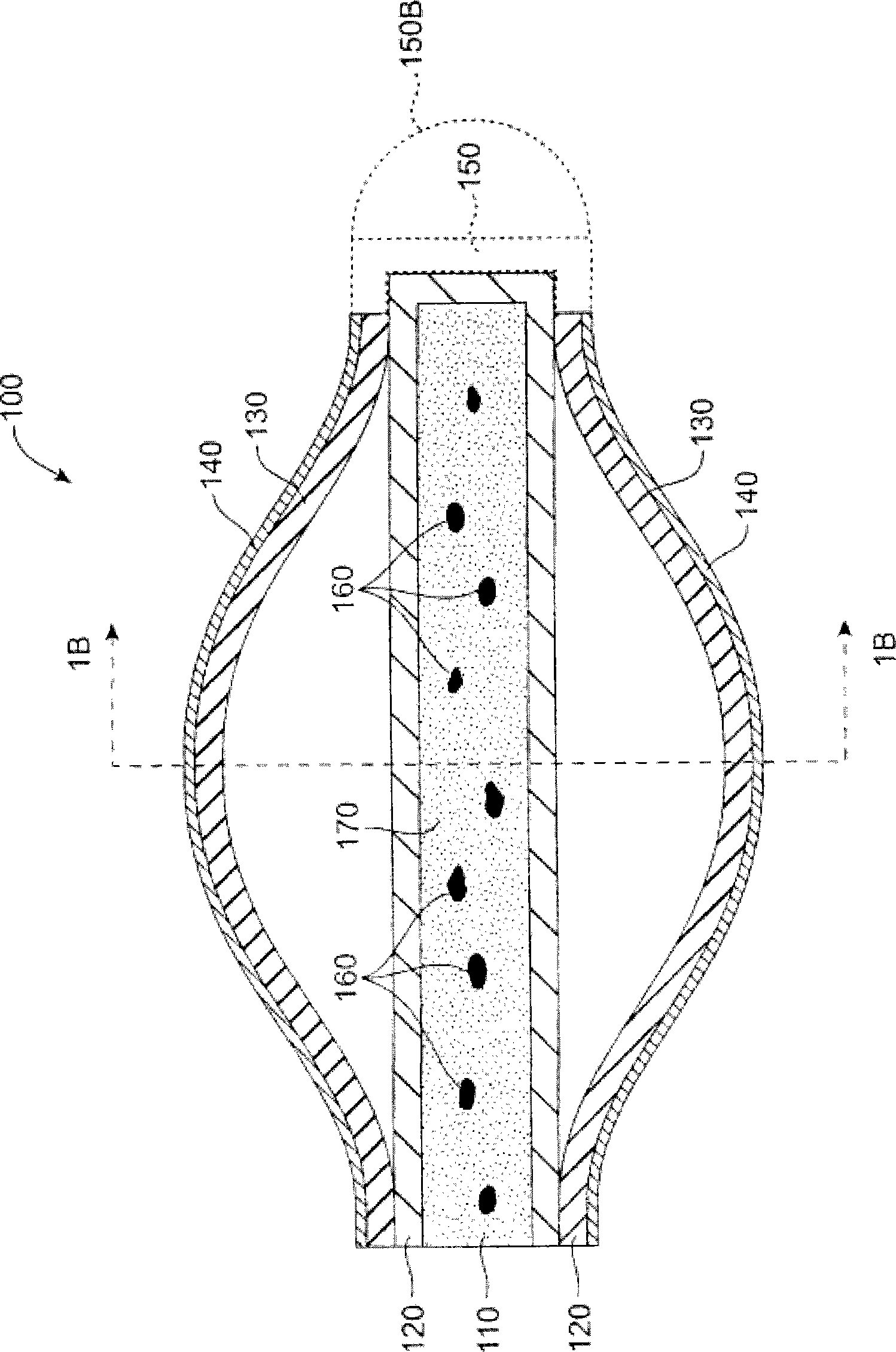
Lacrimal implants and related methods providing secure retention within the lacrimal punctum of an eye are described. The lacrimal implants can comprise a implant body configured for at least partial insertion through the lacrimal punctum and into a lacrimal canaliculus. The implant body can include a deformable retention structure that can be configured to substantially encapsulate an expandable retention element. In some examples, the expandable retention element can include a fluid absorbing material, which can be exposed to fluid such as via a fluid permeable retainer or a fluid permeable aperture. As the fluid absorbing material retains fluid (i.e., upon acceptance of fluid into the retention structure), its size increases and its shape can change to urge one or more portions of the retention structure outward, such as against a wall of the lacrimal canaliculus, thereby securely retaining the lacrimal implant within the punctum.
Owner:MATI THERAPEUTICS
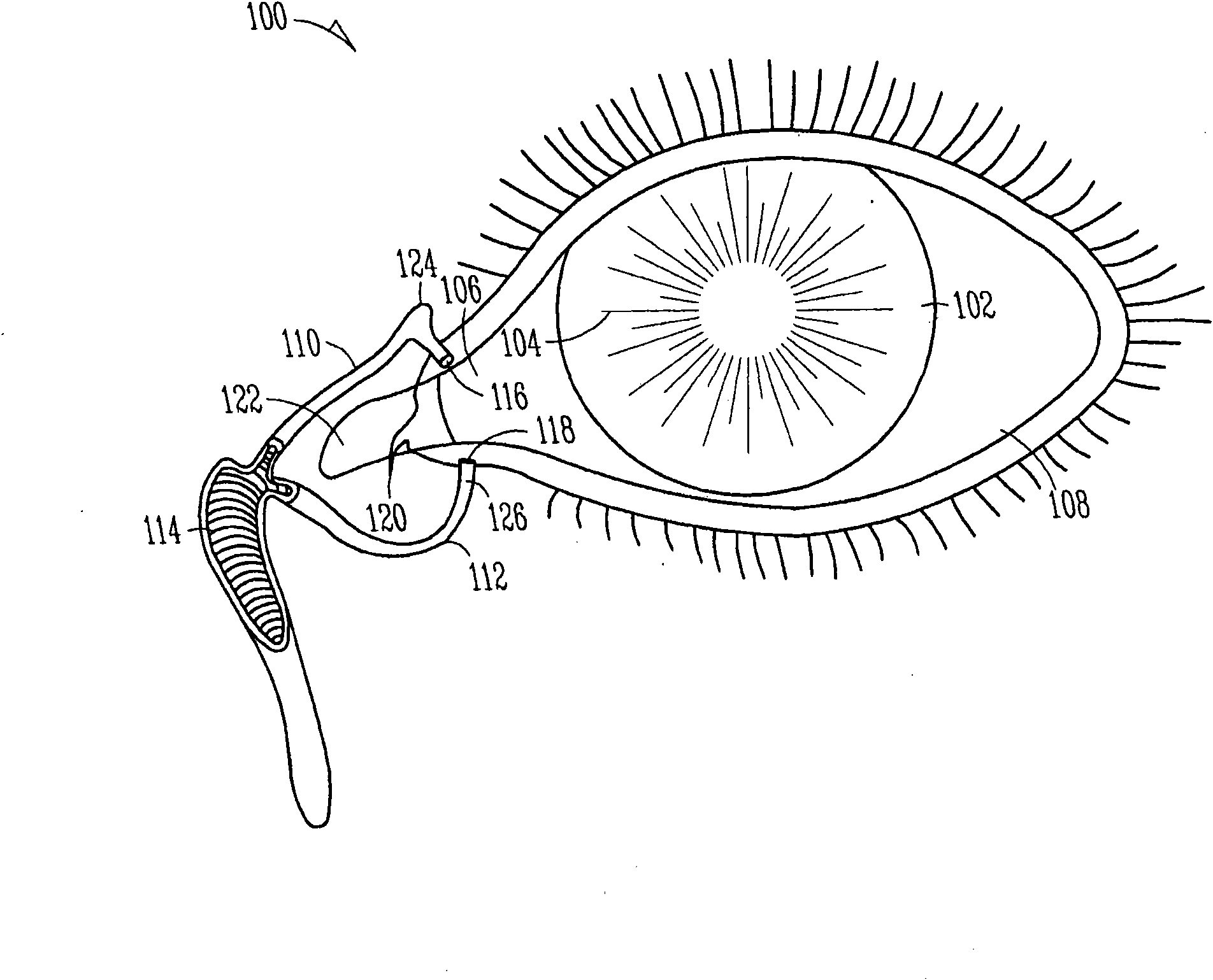
Drug delivery methods, structures, and compositions for nasolacrimal system
An implant which is provided by the invention for being inserted into the lacrimal punctum of patient comprises a main body. The main body comprises a distant end, a near end and a shaft between the distant end and the near end. The distant end of main body can be inserted into the inner cavity of lacrimal duct to the distant end through the lacrimal punctum. The main body comprises a therapeuticagent included in the flux core of pharmaceutical base. The exposing of pharmaceutical base to the tear causes that the effective therapeutic agent is released into the tear in duration. The main body is provided with a sheath which is configured on the pharmaceutical base for inhibiting the releasing of agent from the near end. The body is also provided with an outer surface which is combined with the tissue of inner cavity wall that restricts the discharging of tissue of inner cavity wall. In a specific execution mode, the pharmaceutical base comprises a non-biological absorptive polymer, such as the silicone in the non-homogeneous phase compound comprising the agent.
Owner:MATI THERAPEUTICS
Lacrimal filler
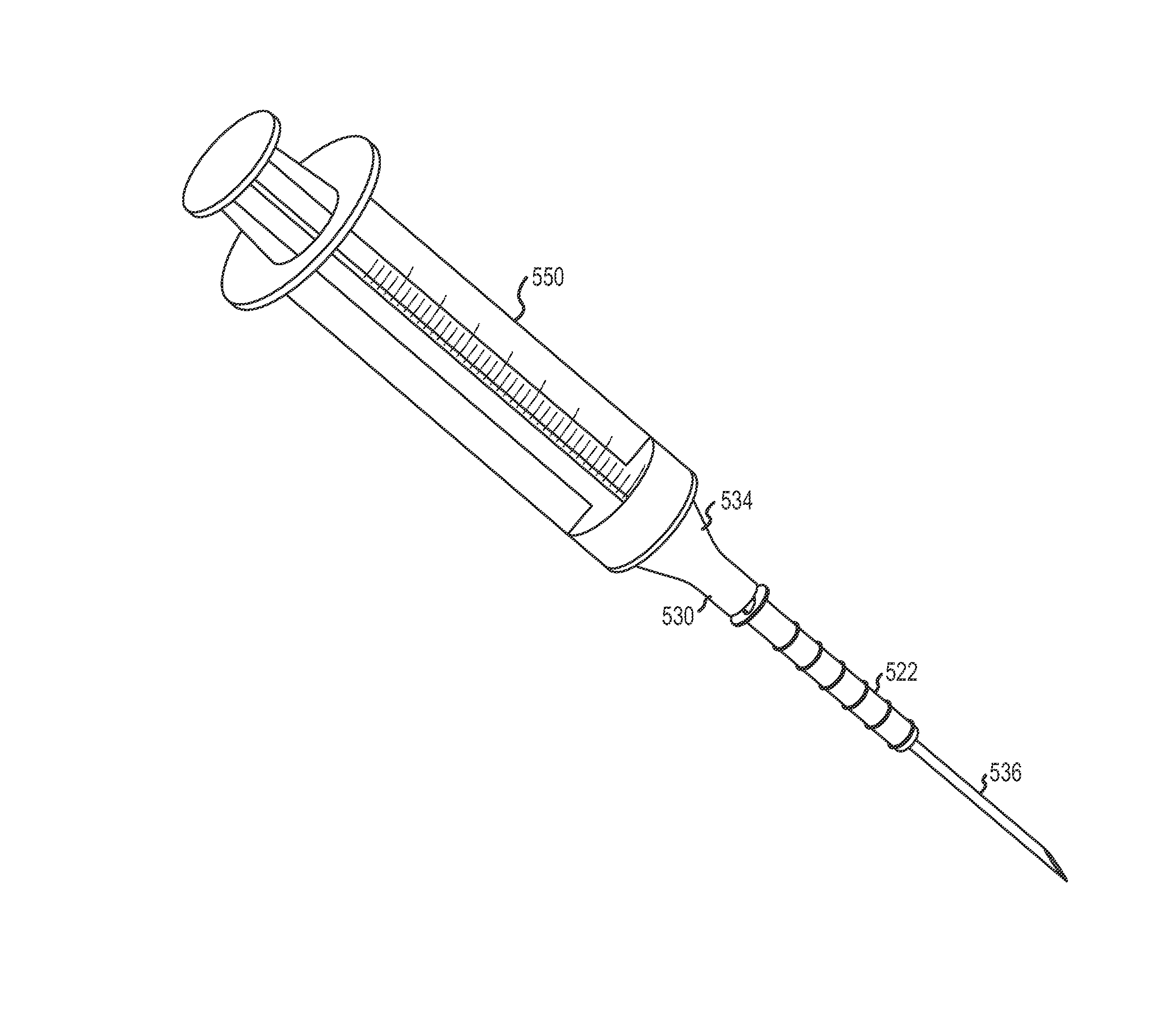
ActiveUS20110066138A1Avoid erosionPrevent outflowSurgical adhesivesEye surgeryDilatorBiological materials
A biomaterial mass is inserted through a punctum into a canaliculus in the lacrimal outflow system with a syringe. The mass has an outer cross section that is less than the inner cross section of the canaliculus. The mass absorbs liquid to swell to form a lacrimal filler or sealing mechanism. The lacrimal filler has an external cross section that conforms to the internal cross section of the canaliculus and a soft outer surface relative to the surrounding tissues to prevent erosion of the canaliculus lining. The lacrimal filler forms an occlusion that prevents the outflow of liquid through the lacrimal outflow system to retain tears within the eye to maintain eye lubrication and wetness. The mass and syringe are provided in kit with a rubber stopper and, optionally, a punctal dilator, an injecting catheter, and an enzyme for dissolving or degrading the lacrimal filler at a later time.
Owner:FEZZA FAMILY PROPERTIES
Drug delivery system and methods of treating open angle glaucoma and ocular hypertension
ActiveUS20140025022A1Improved patient comfortImproved implant retentionOrganic active ingredientsEye surgeryOpen angle glaucomaOcular hypertension
A method of decreasing intraocular pressure (IOP) in an eye of a patient in need thereof includes implanting a first lacrimal implant through an upper punctum and into an upper lacrimal canaliculus of the eye of the patient. The method may further comprise implanting a second lacrimal implant through a lower punctum and into a lower lacrimal canaliculus of the eye of the patient, and releasing, on a sustained basis a therapeutically effective amount of an intraocular pressure-reducing therapeutic agent.
Owner:MATI THERAPEUTICS
Implant device, tool, and methods relating to treatment of paranasal sinuses
An implant device is configured to be implanted in a fistula to fluidly connect the lacrimal apparatus and a paranasal sinus. A surgical tool has an implant the implant device mounted on a carrier. Various methods involve a fistula between the lacrimal apparatus and a paranasal sinus. A kit includes an entry device for use to form a fistula and an implant tool for use to implant an implant device following formation of a fistula.
Owner:SINOPSYS SURGICAL
Completely-depredated medicine carrying nasolacrimal stent and implantation system thereof
InactiveCN104398328AAvoid postoperative removalImplantation is convenient and easyStentsMedical devicesNasal cavityNose
The invention relates to a completely-depredated medicine carrying nasolacrimal stent. The completely-depredated medicine carrying nasolacrimal stent comprises a sleeve-shaped drainage tube made of biodegradable high polymer materials; a medicine carrying groove is formed in the outer surface of the drainage tube; the drainage tube is of a structure capable of being compressed or expanded; and the diameter of at least a part of the drainage tube in the expanded state is larger than that of the part of drainage tube in the compressed state. After lumen rebuilding is completed, water and carbon dioxide are finally generated by the completely-depredated medicine carrying nasolacrimal stent to be discharged through the nasal cavity, and secondary operations are avoided, so that pains of a patient are omitted. The nasolacrimal stent is suitable for approaching the body from the nasal cavity, so that injuries, caused by the fact that the nasolacrimal stent approaches the body from the positions above the eyes, to the lacrimal ductile and the nasolacrimal canal inner membrane are avoided. According to the completely-depredated medicine carrying nasolacrimal stent, medicines are loaded through the medicine carrying groove, the medicine targeting release function is achieved, and the nasolacrimal canal is accordingly treated in a fixed point manner.
Owner:PUYI (SHANGHAI) BIOTECHNOLOGY CO LTD
Method for preventing nasolacrimal duct obstruction
The invention is directed to a method for preventing nasolacrimal duct obstruction (NLDO) in a patient receiving high dose radioactive iodine for treatment of cancer. The method includes administering an effective amount of perchlorate anion to the eyes of the patient.
Owner:KEM HLDG
Completely-degradable net-shaped nasolacrimal stent and implantation system thereof
InactiveCN104398329AAvoid postoperative removalEffective treatmentStentsMedical devicesNasal cavityNose
The invention relates to a completely-degradable net-shaped nasolacrimal stent. The completely-degradable net-shaped nasolacrimal stent comprises a sleeve-shaped net-shaped body made of biodegradable high polymer materials and provided with holes; a medicine coating is arranged on the surface of the net-shaped body; the net-shaped body is composed of a first net-shaped body part and a second net-shaped body part which are connected; in the compression state, the first net-shaped body part and the second net-shaped body part are the same in diameter; and in the expansion state, the diameter of the first net-shaped body part is larger than the diameter of the second net-shaped body part. After lumen rebuilding is completed, water and carbon dioxide are finally generated by the completely-degradable net-shaped nasolacrimal stent to be discharged through the nasal cavity; and second operations and pains of a patient are avoided. The nasolacrimal stent is suitable for approaching the body from the nasal cavity, so that injuries, caused by the fact that the nasolacrimal stent approaches the body from the positions above the eyes, to the lacrimal ductile and the nasolacrimal canal inner membrane are avoided. By means of the completely-degradable net-shaped nasolacrimal stent, the medicine slow release effect is achieved through the medicine coating; and continuous treatment on the nasolacrimal canal for a long time is accordingly achieved.
Owner:PUYI (SHANGHAI) BIOTECHNOLOGY CO LTD
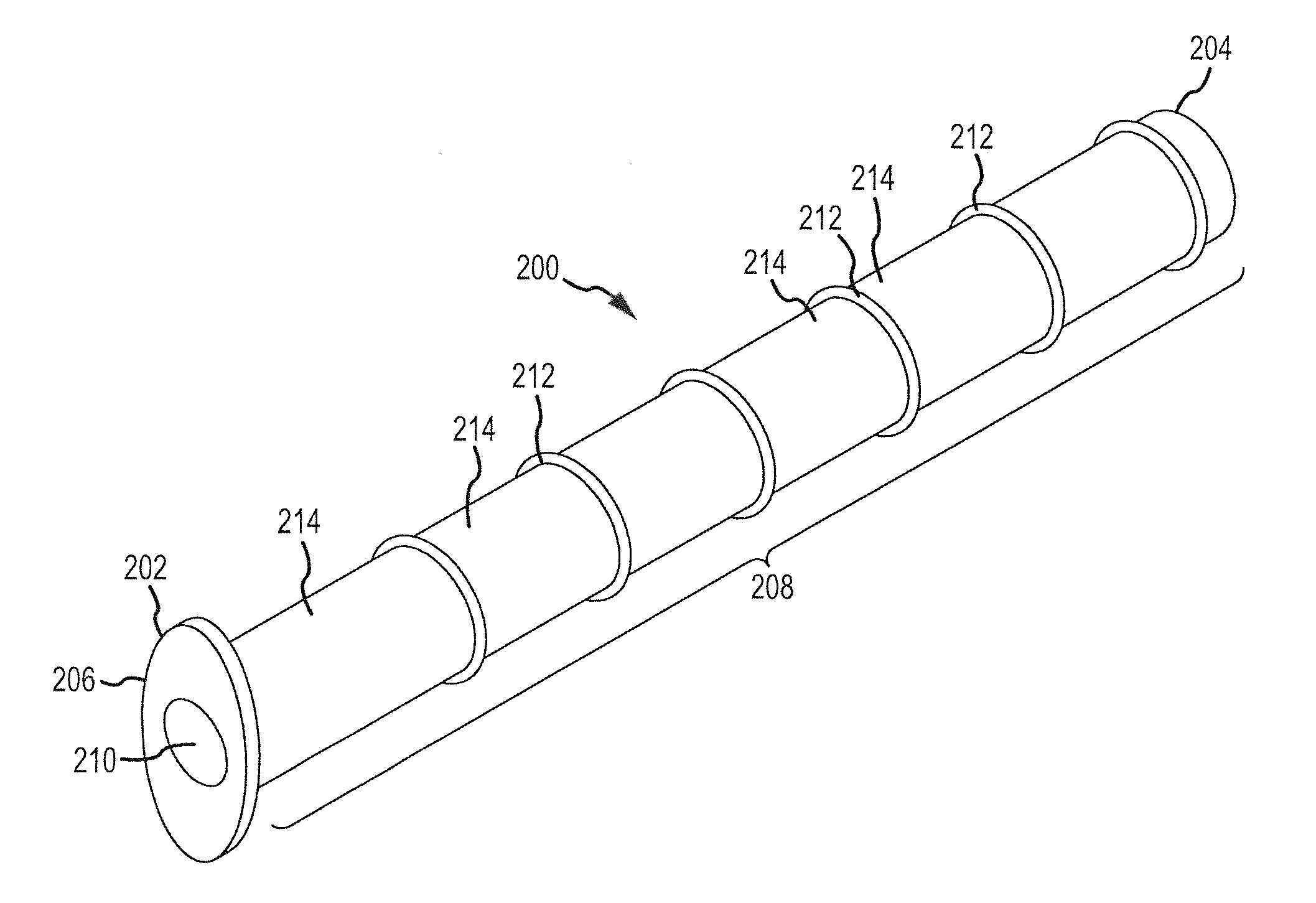
Lacrimal duct tube
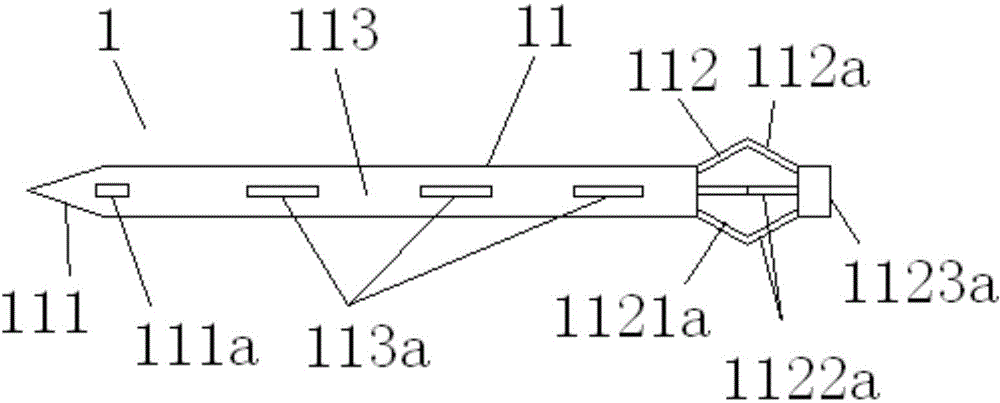
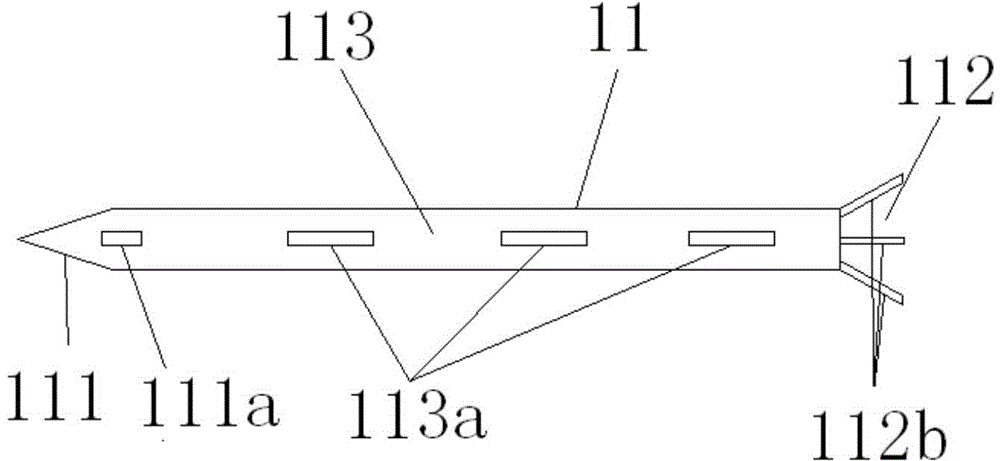
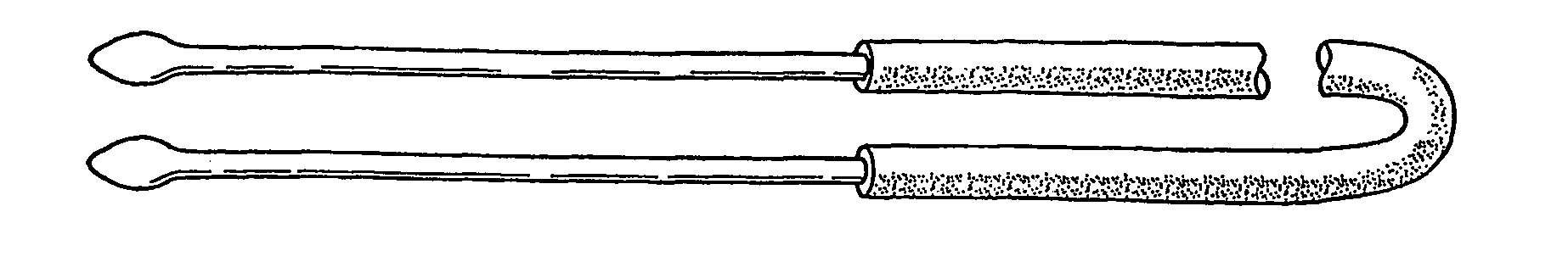
A lacrimal duct tube (31) includes an integrated tube that is placed in a lacrimal duct and capable of insertion of an insertion aid (36) in a detachable manner. The tube (31) has a terminal end opening (33) at a terminal end. In the vicinity of the terminal end opening (33), a stopper part (32a, 32b, 32c, and 32d) for stopping the insertion aid (36) is inserted in the tube (31). The stopper part (32a, 32b, 32c, and 32d) is opened at both ends in an axial direction of the tube and has a cylindrical structure including an inner wall surface (37a, 37b, 37c, and 37d) to guide and stop the insertion aid (36).
Owner:KANEKA CORP
Device for insertion of lacrimal stents
Disclosed herein is system for inserting lacrimal stents utilizing a rigid tapered probe and a flexible, blunt-tipped probe, wherein both probes provide a channel for directing a guidewire. The guidewire assists in efficiently positioning lacrimal stents, while minimizing possible injury to the lacrimal apparatus structures.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Multi-piece punctal plug
A multi-piece punctal plug has one or more superficial plugs connected to an anchor plug deeper in the naso-lacrimal canaliculus. The anchor is fixed to the wall of the lacrimal duct while the superficial plugs are axially and detachably secured to the anchor plug so that the multi-plug complex may be inserted or removed together as one, or the superficial plugs may be detached separately and replaced securely without disturbing the anchor. Removal and insertion is accomplished by a specific tool having an end for engaging the plugs.
Owner:ORDICH STEVEN
Implantation tools, tool assemblies, kits and methods
An implant device to provide an artificial fluid path in fluid communication with the lacrimal apparatus is implanted through a fistula opening into the lacrimal apparatus using a working member disposed through the implant device and a break-away sheath. Implantation kits and tool assemblies including the working member, implant device and break-away sheath facilitate implantation of the implant device.
Owner:SINOPSYS SURGICAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com