Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
54 results about "Punctal plug" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
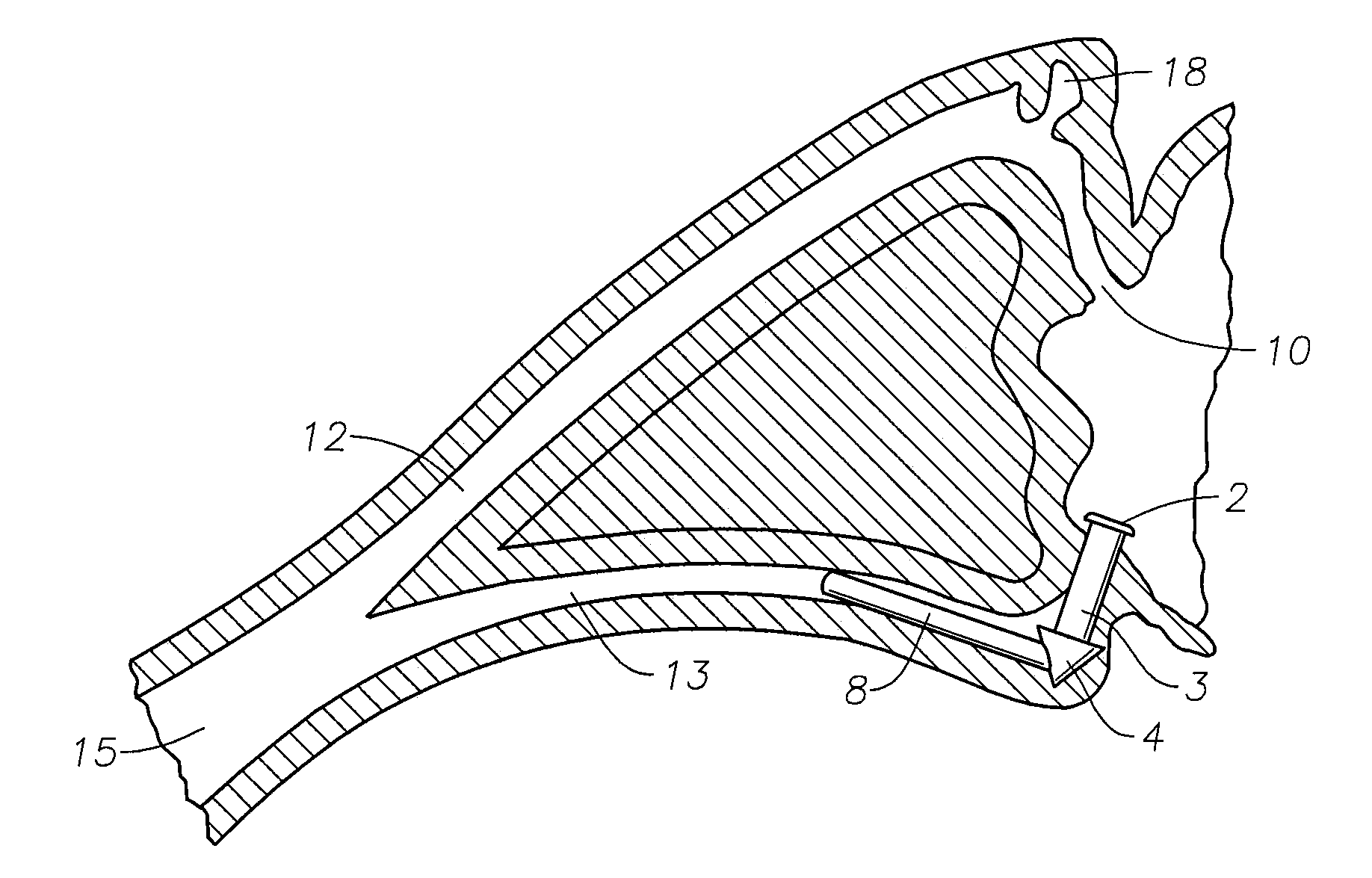
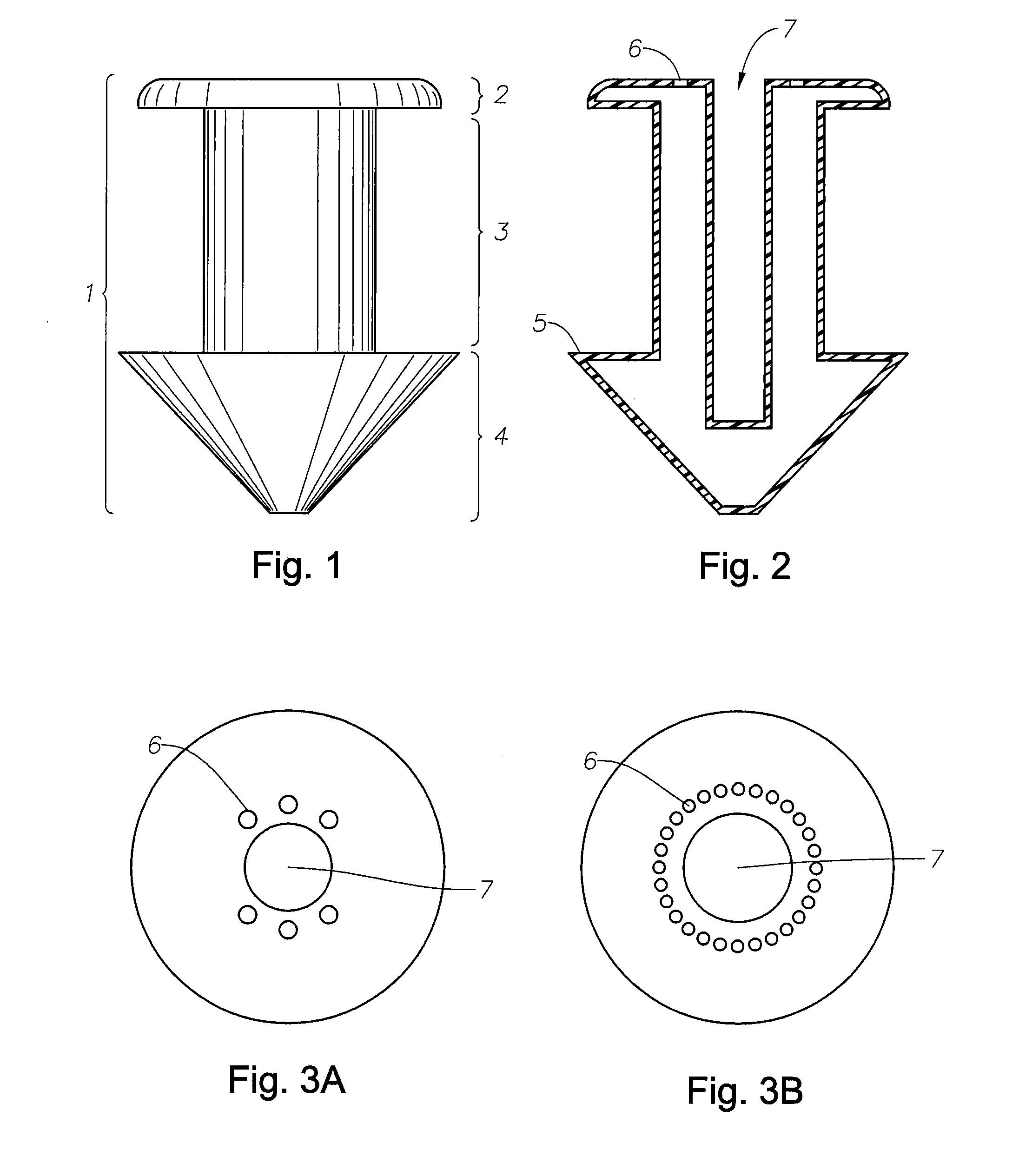
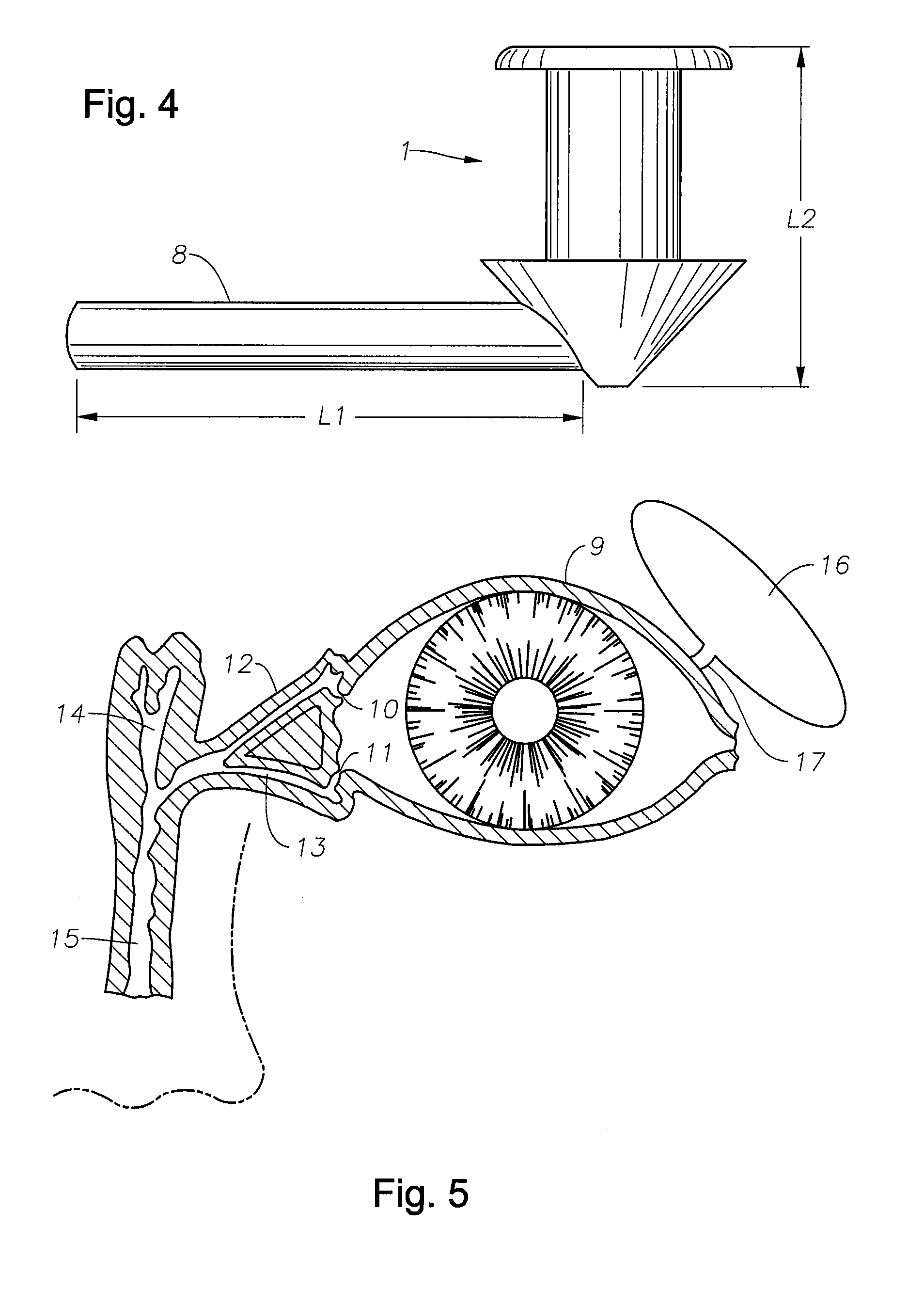
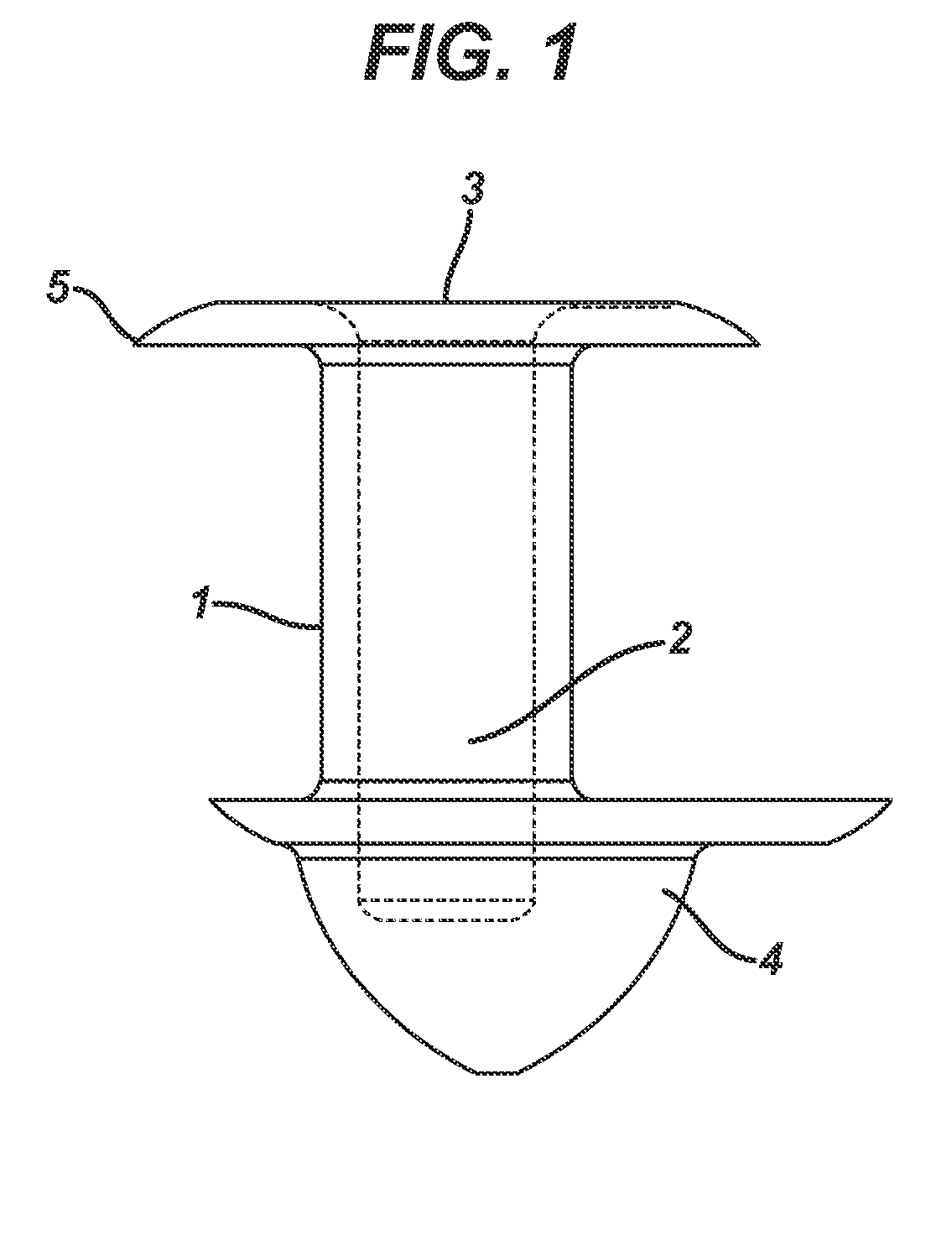
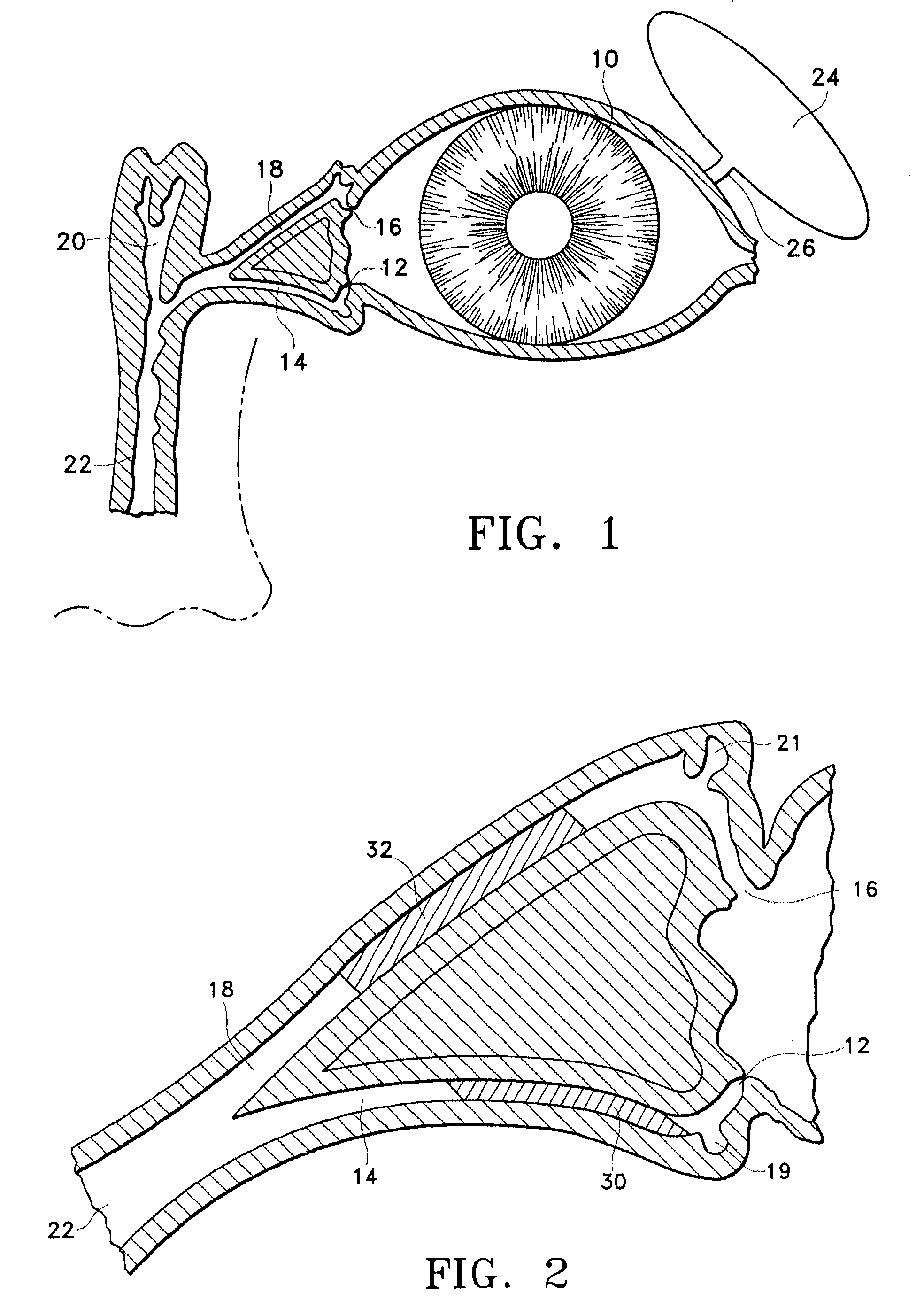
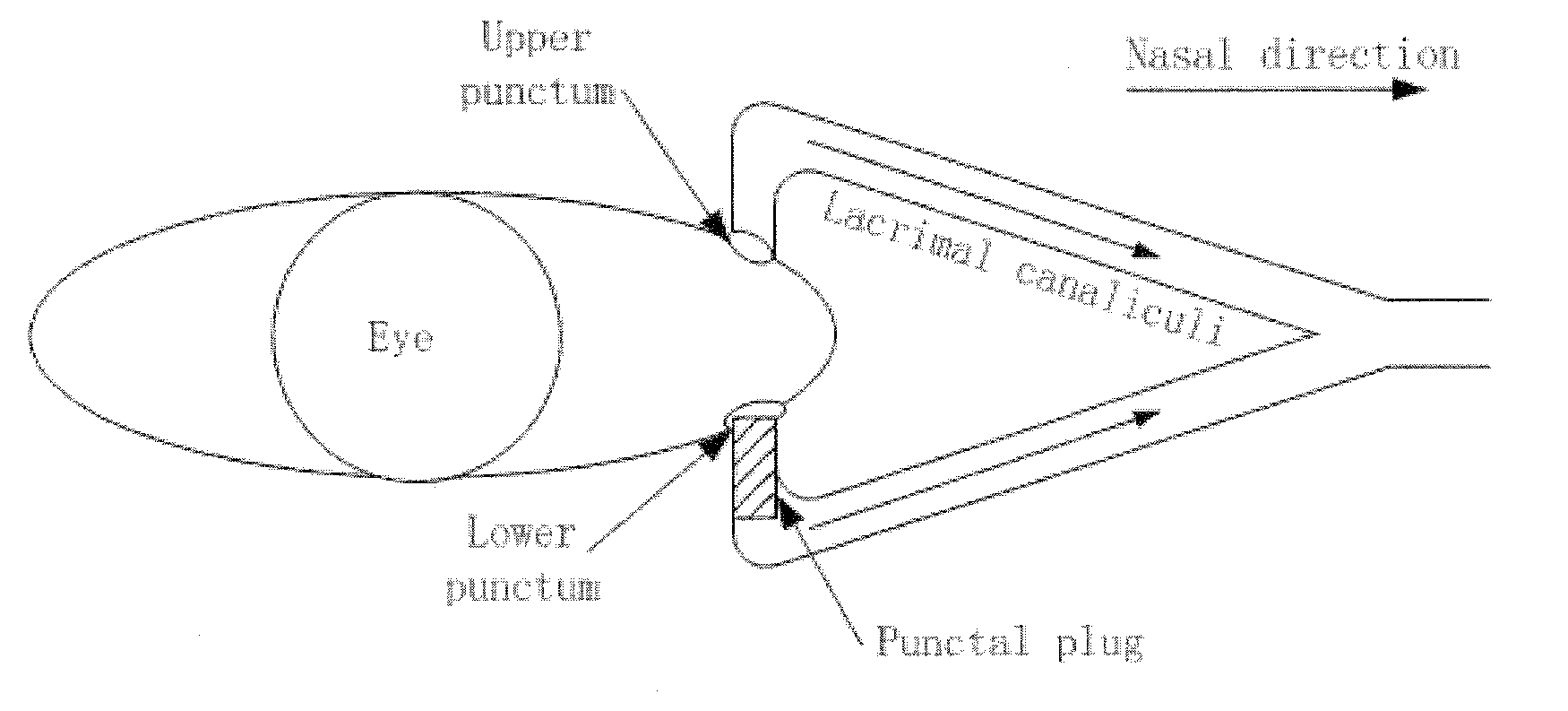
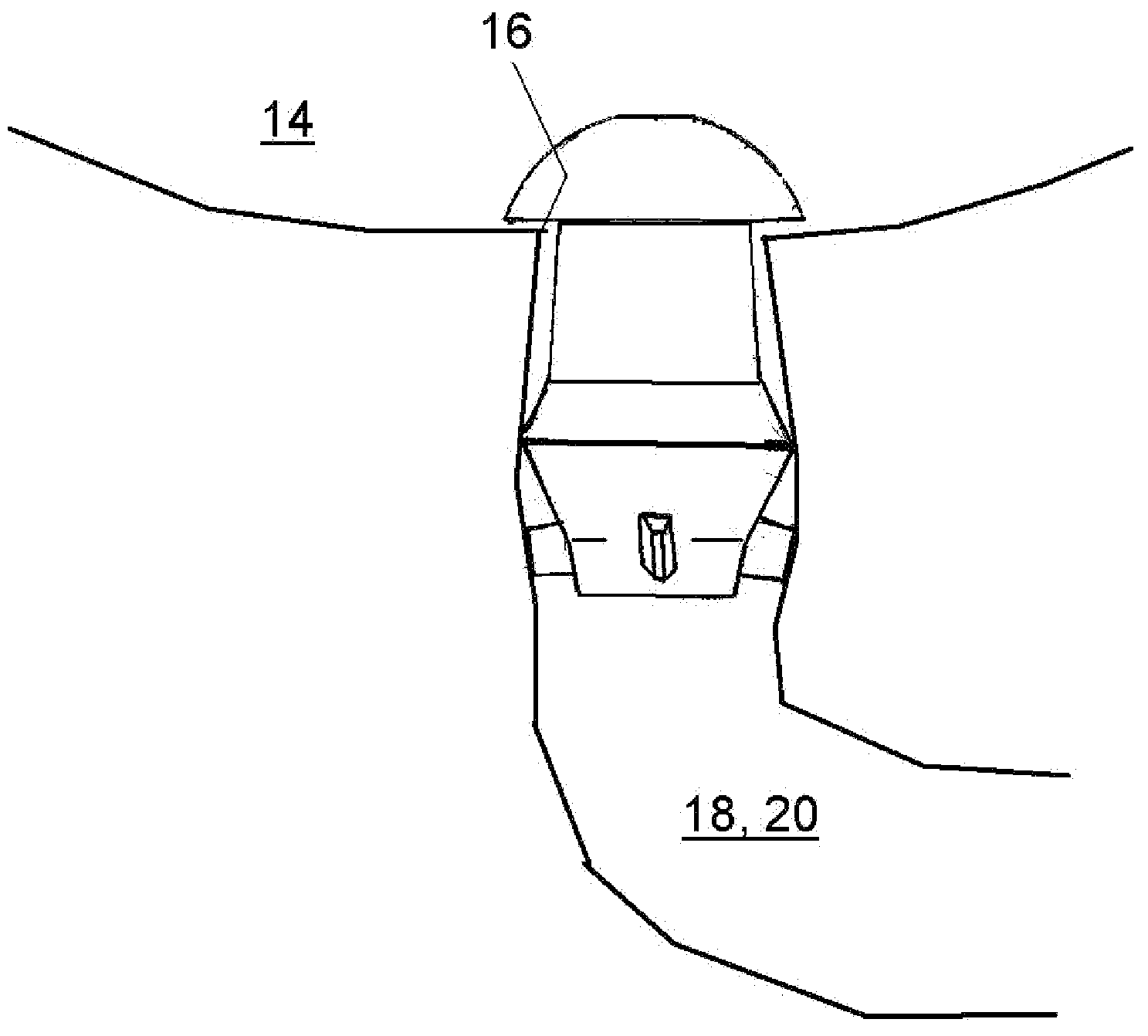
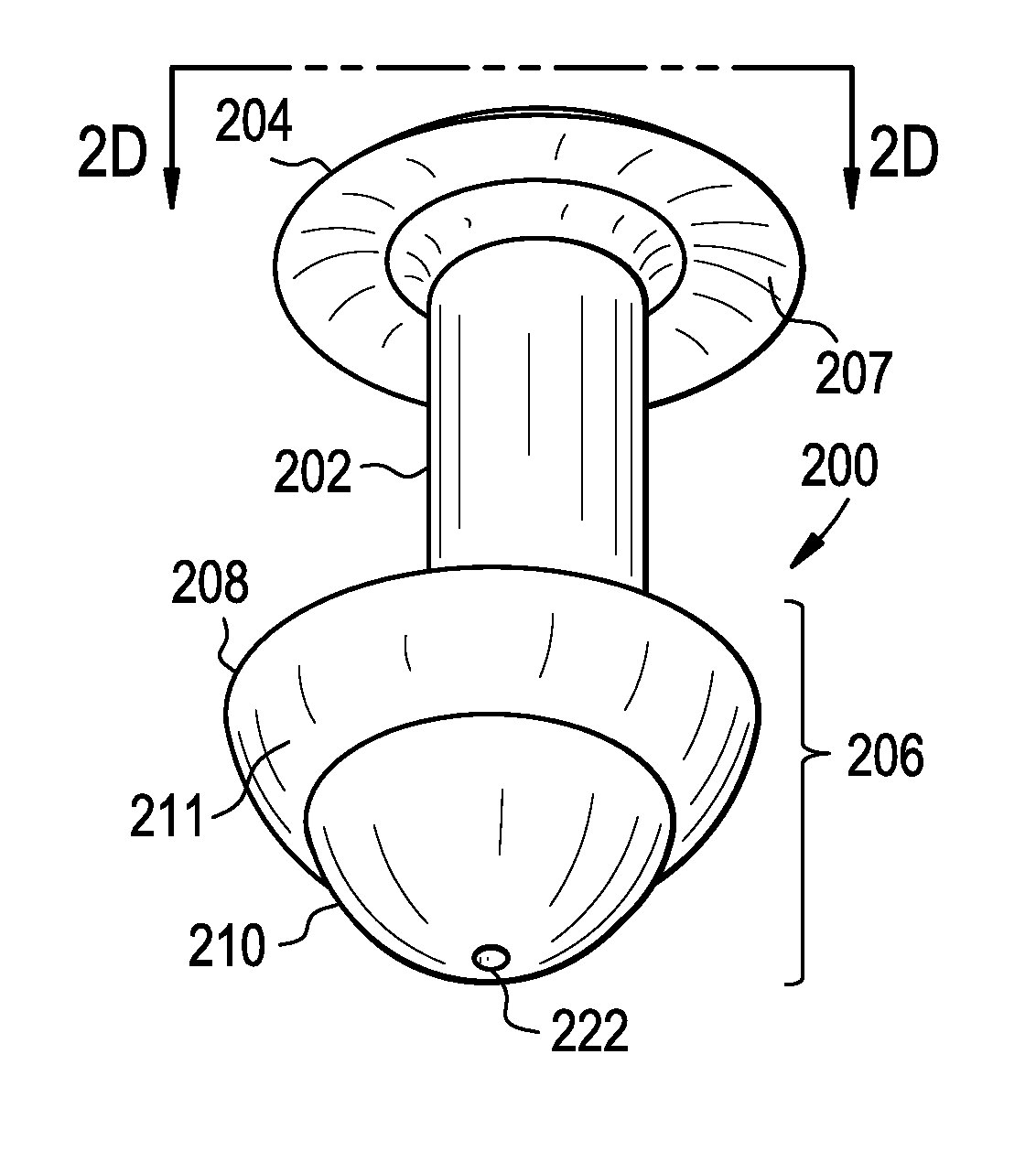
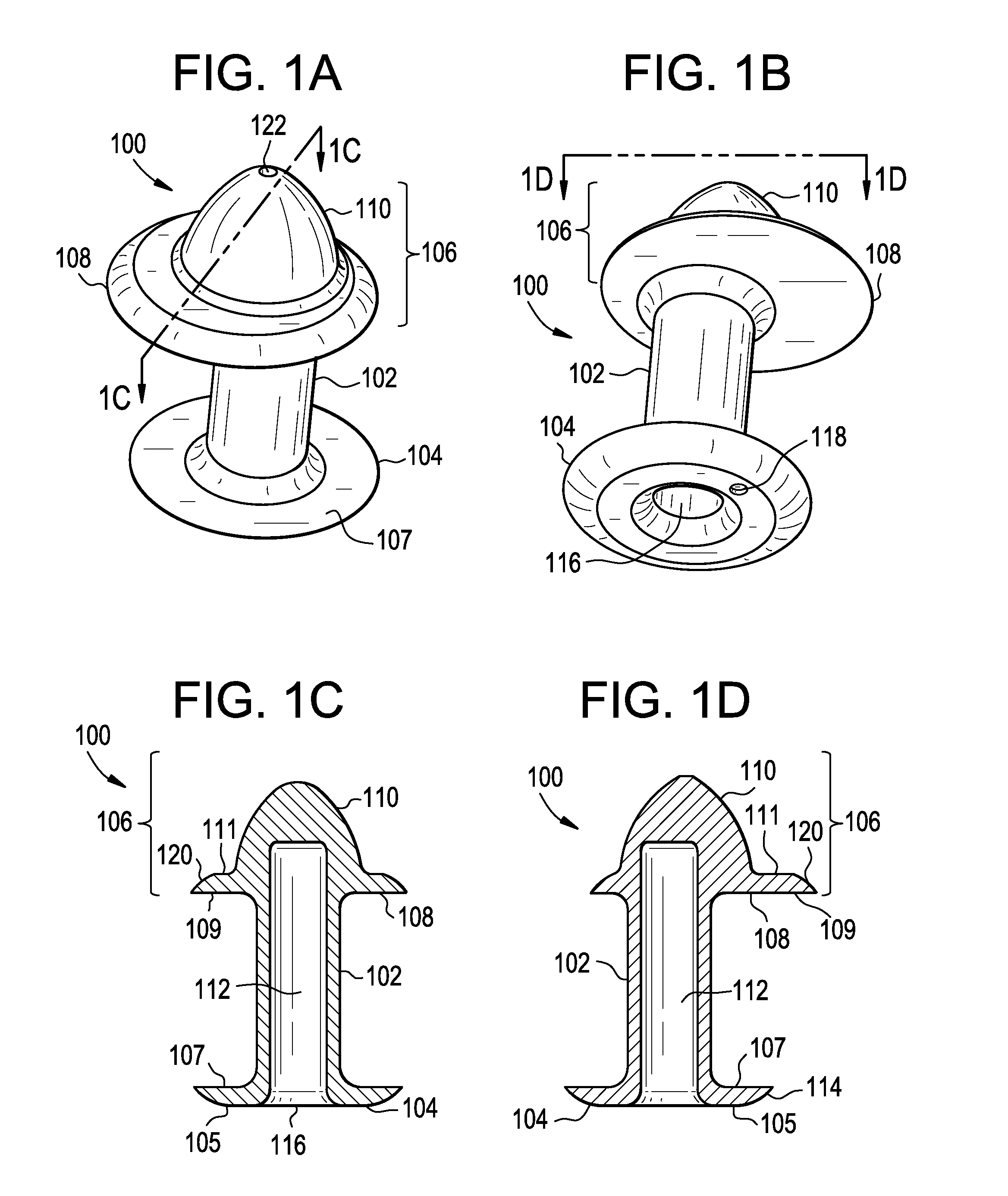
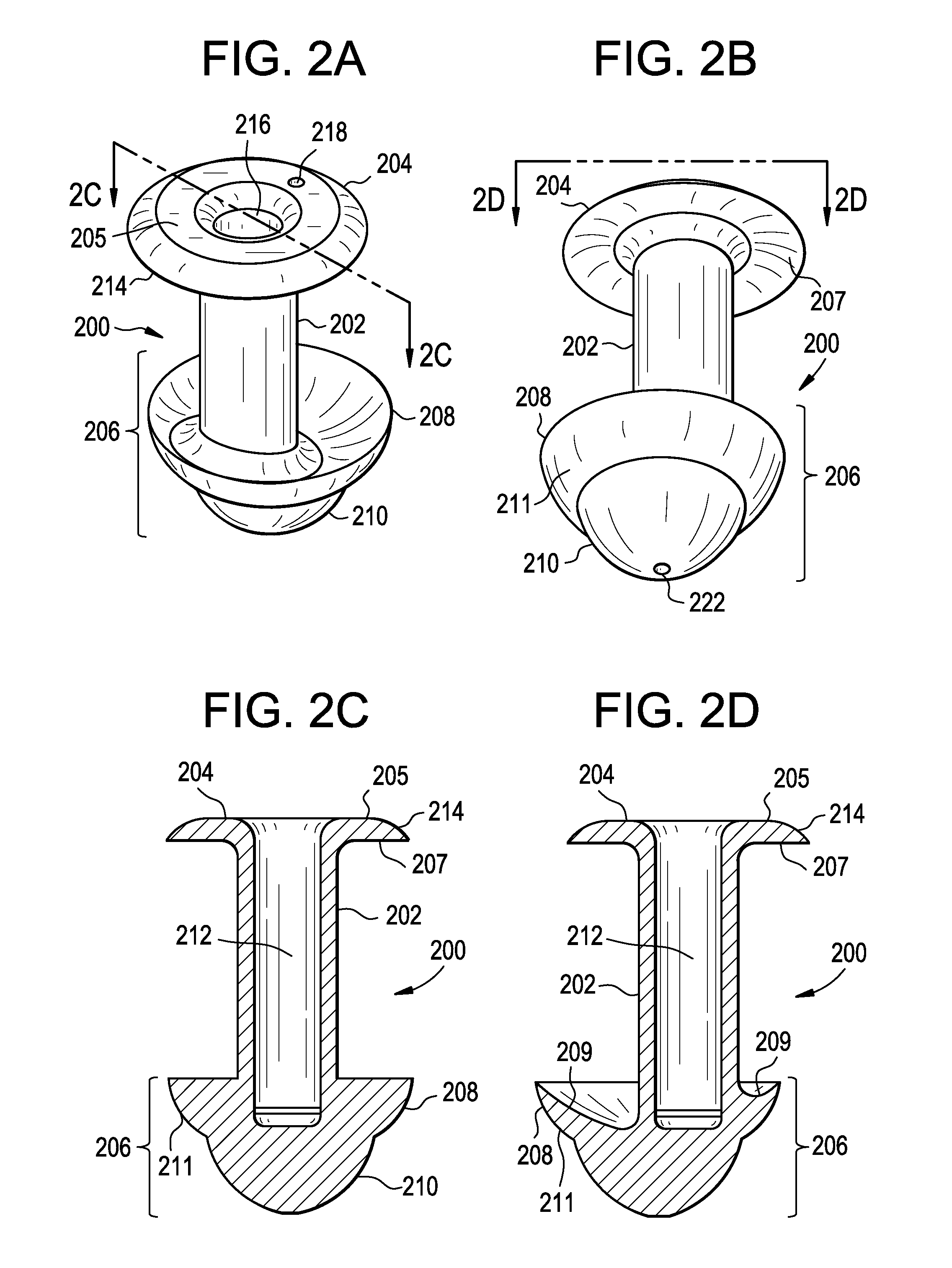
A punctal plug, also known as tear duct plug or lacrimal plug, is a small medical device that is inserted into the tear duct (puncta) of an eye to block the duct. This prevents the drainage of liquid from the eye. They are used to treat dry eye.
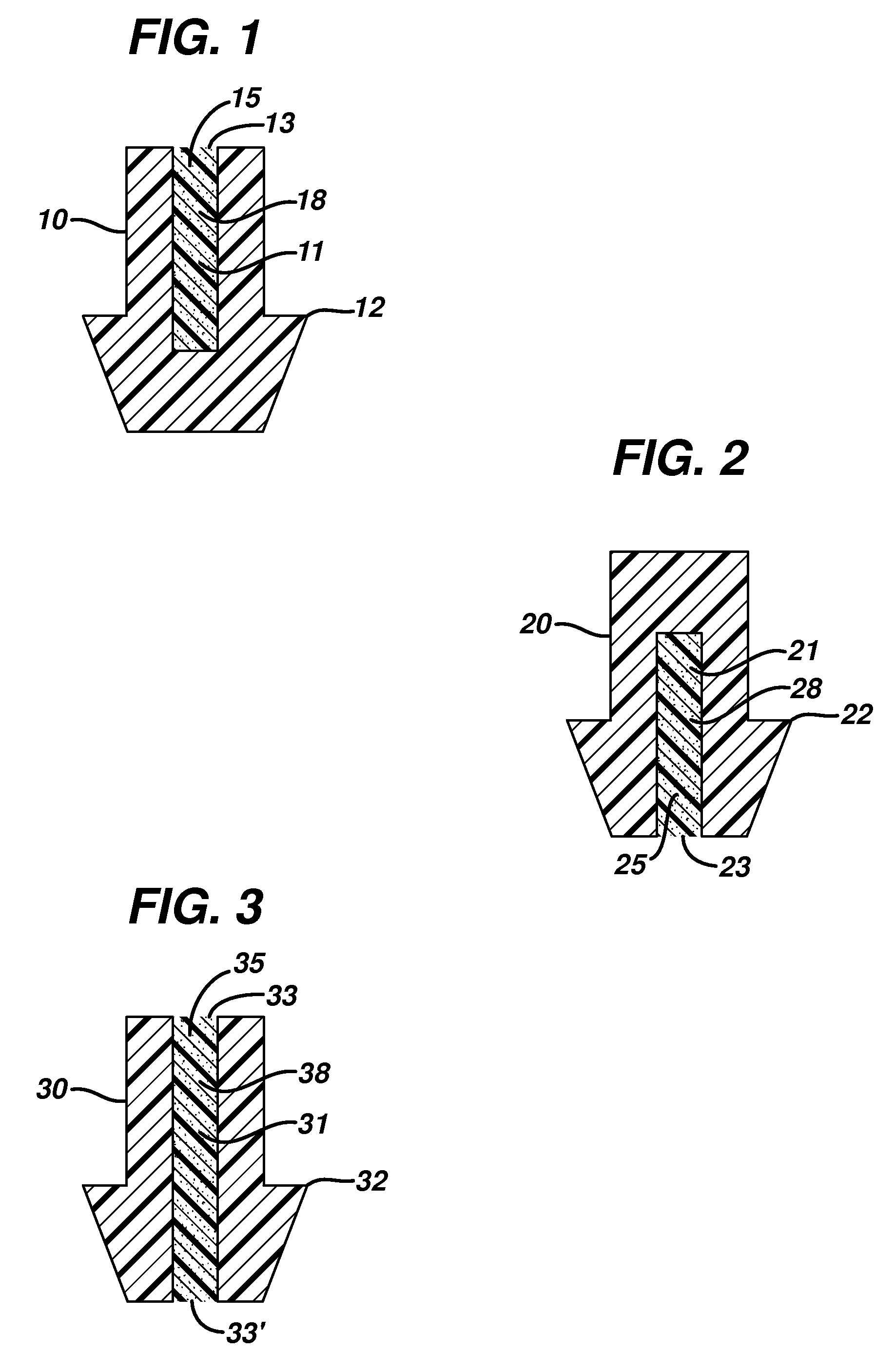
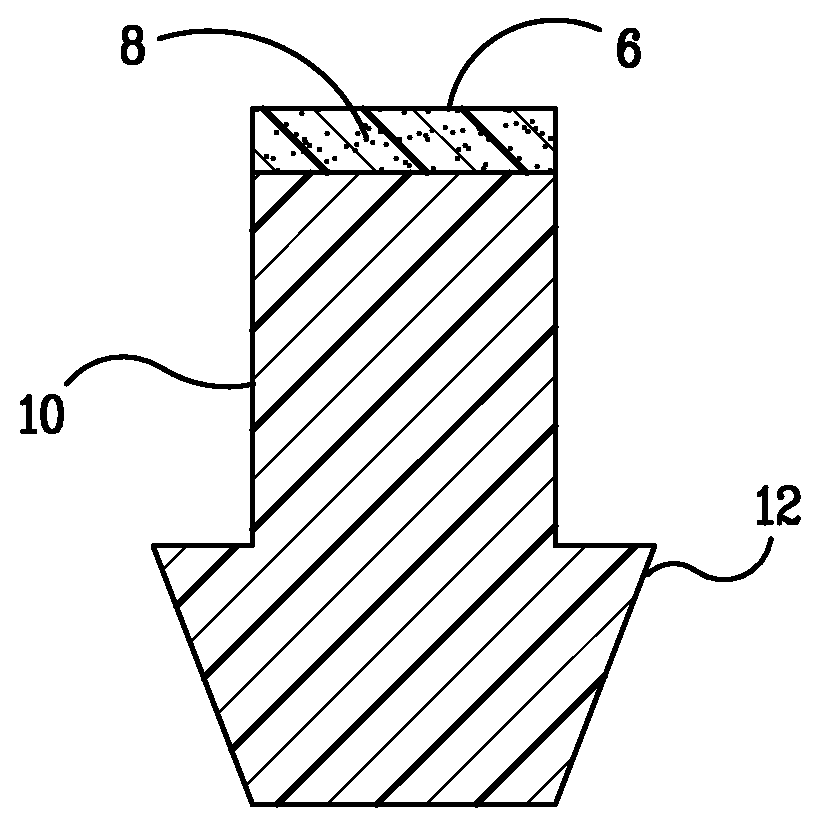
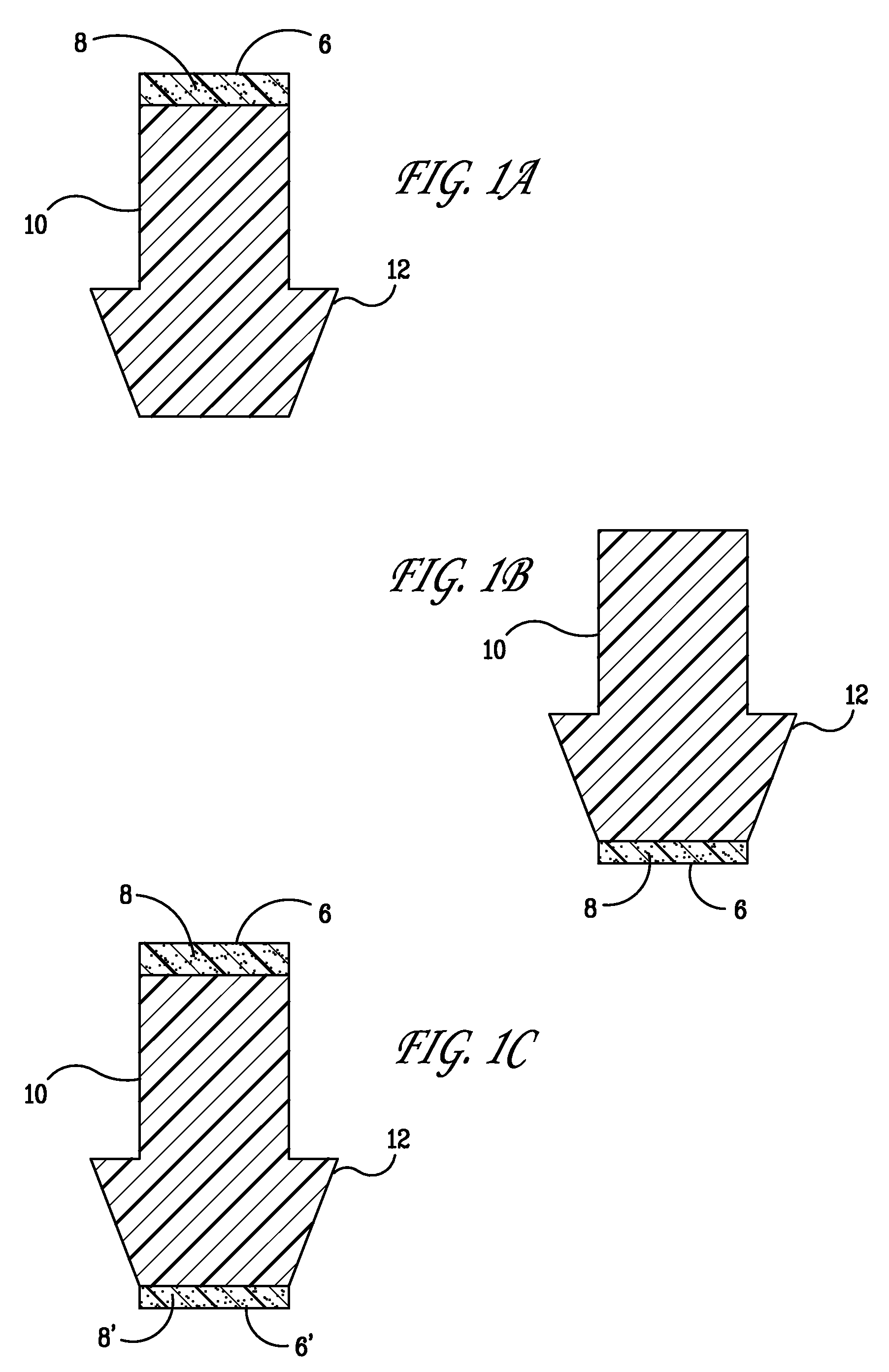
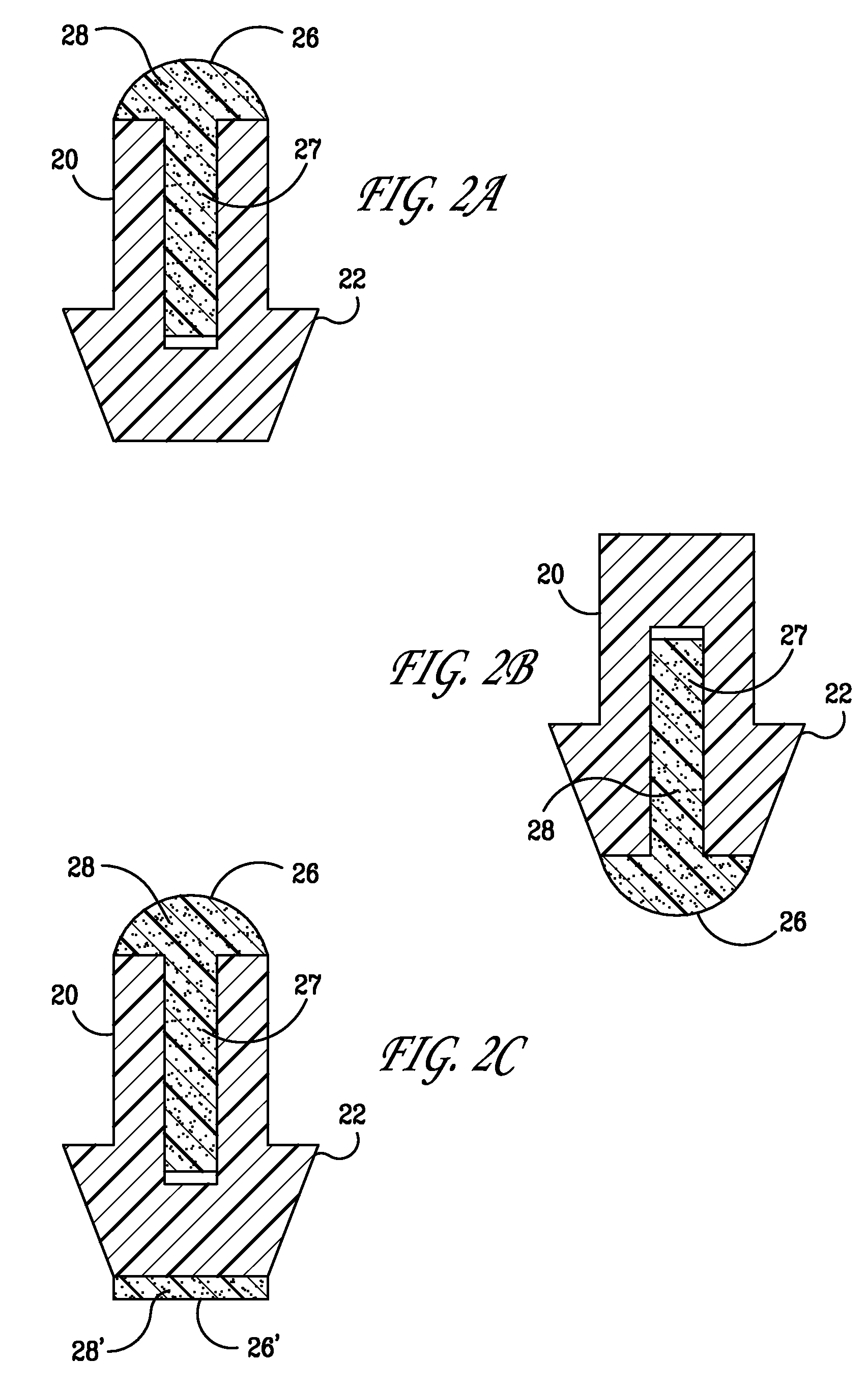
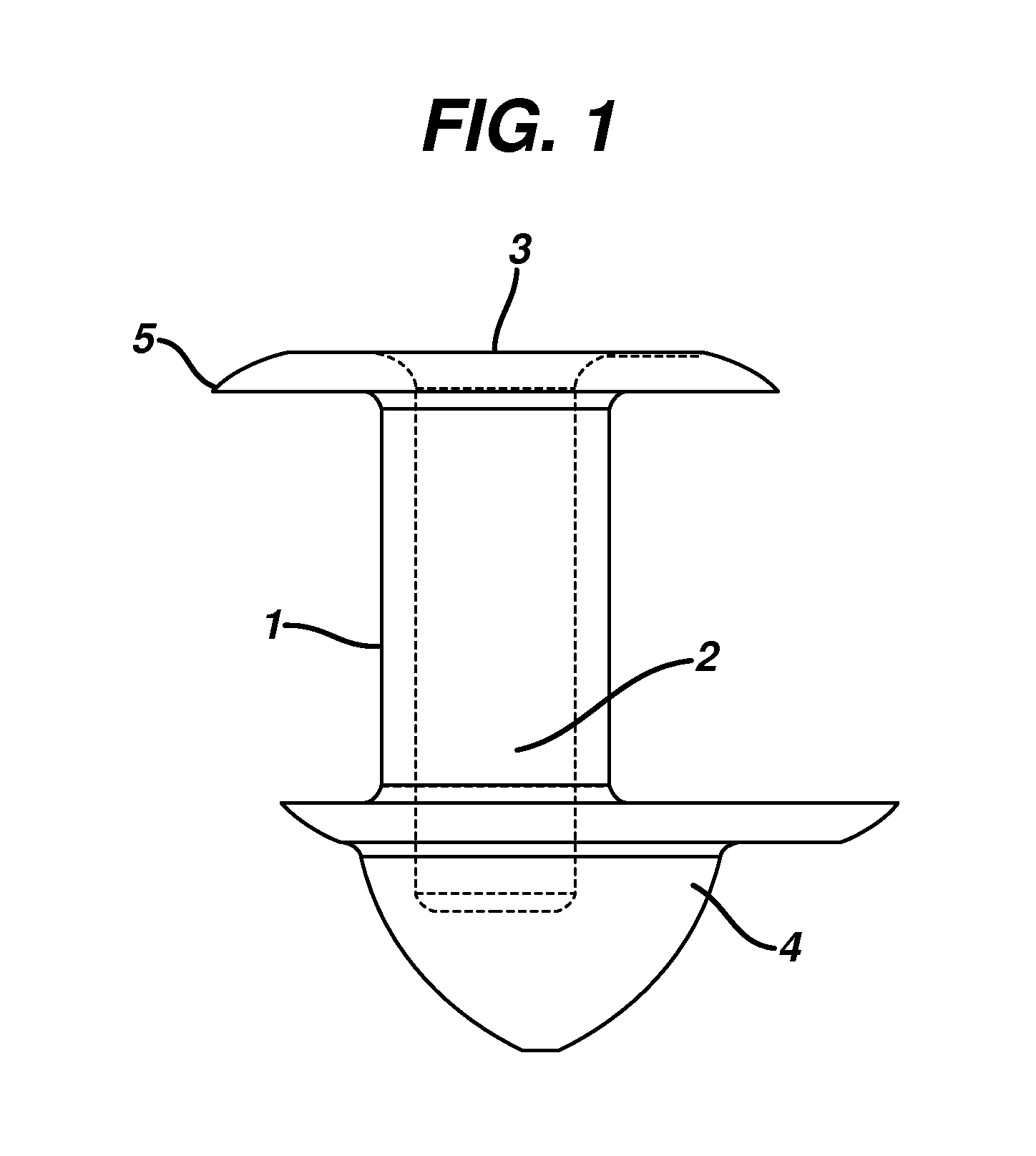
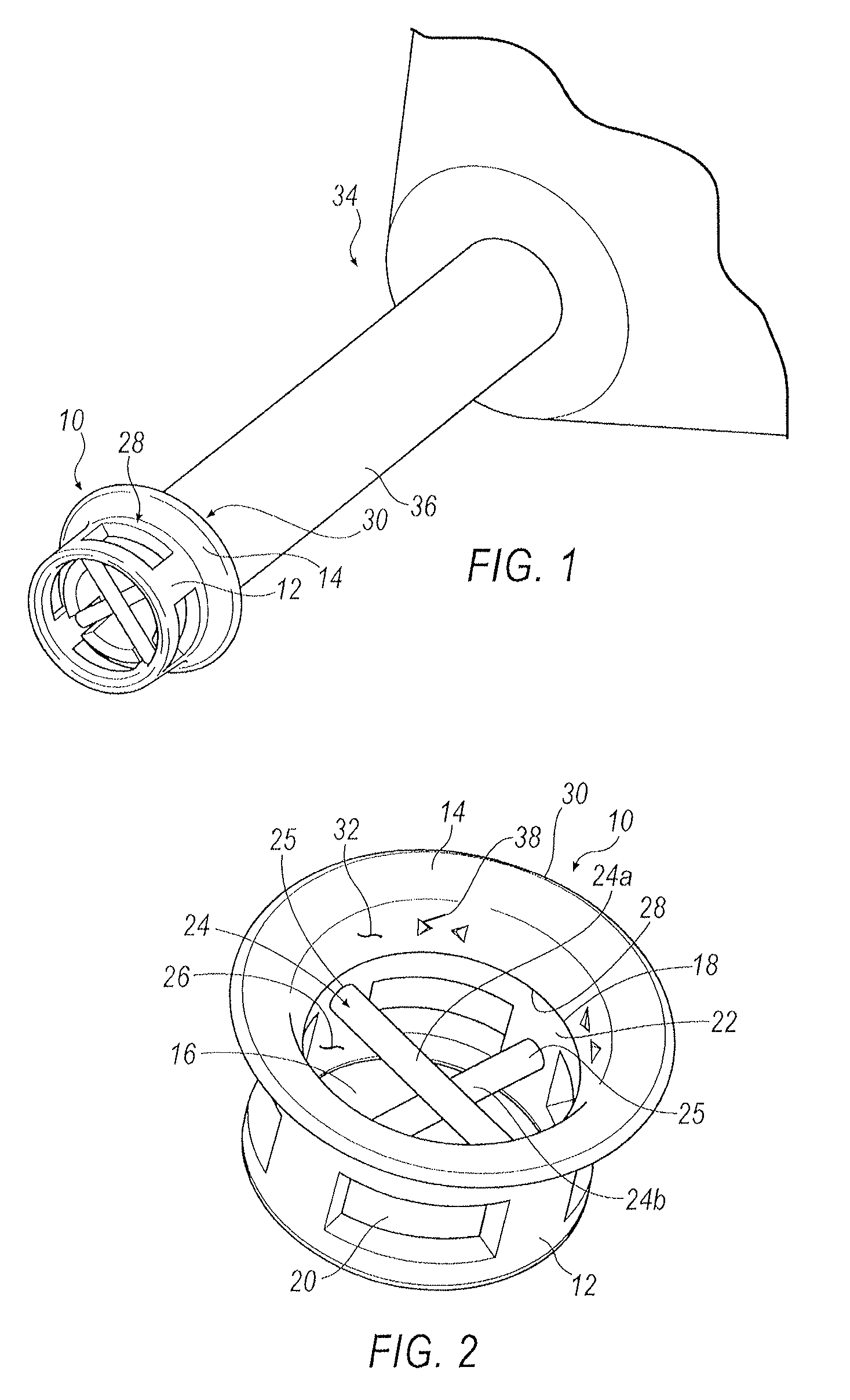
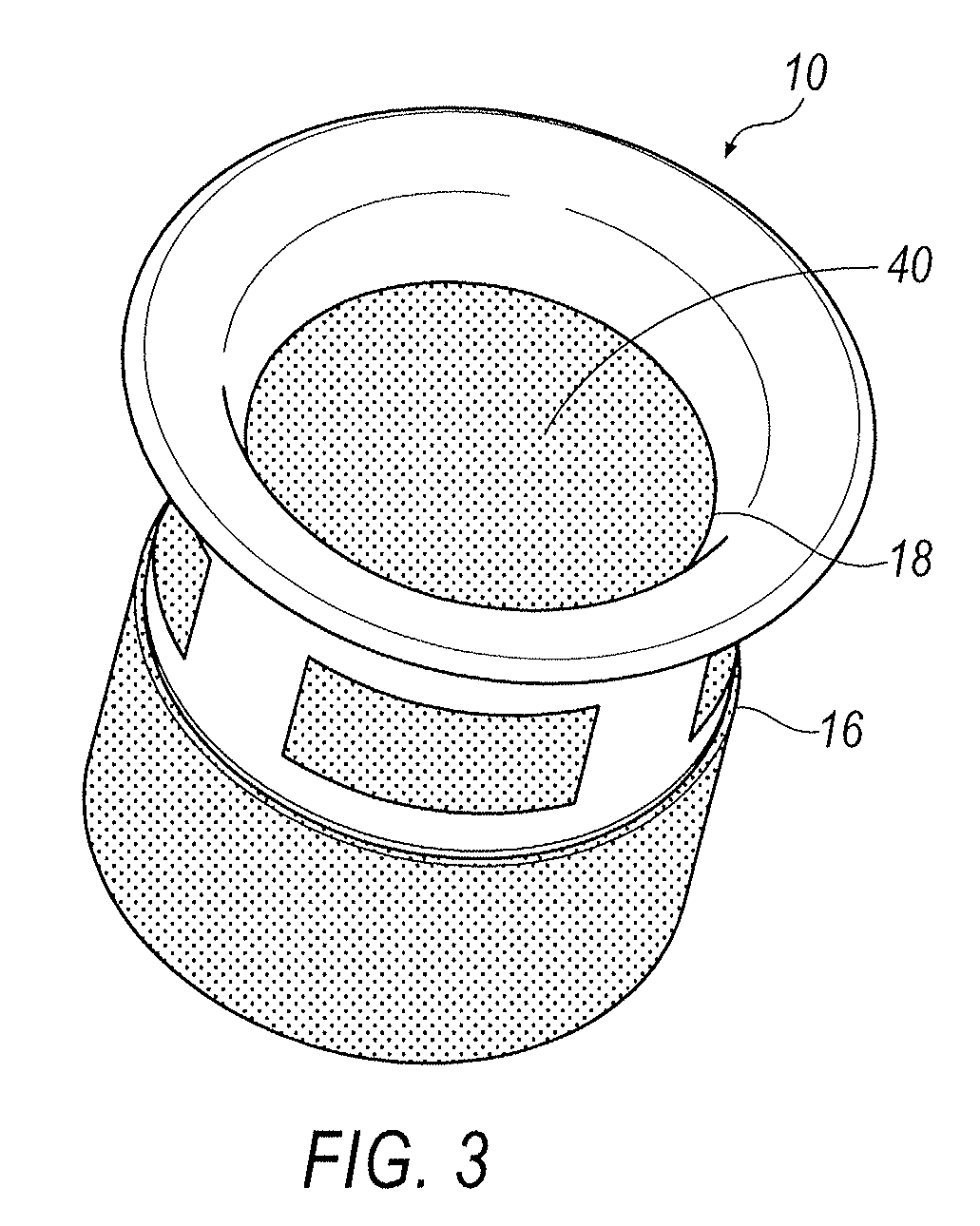
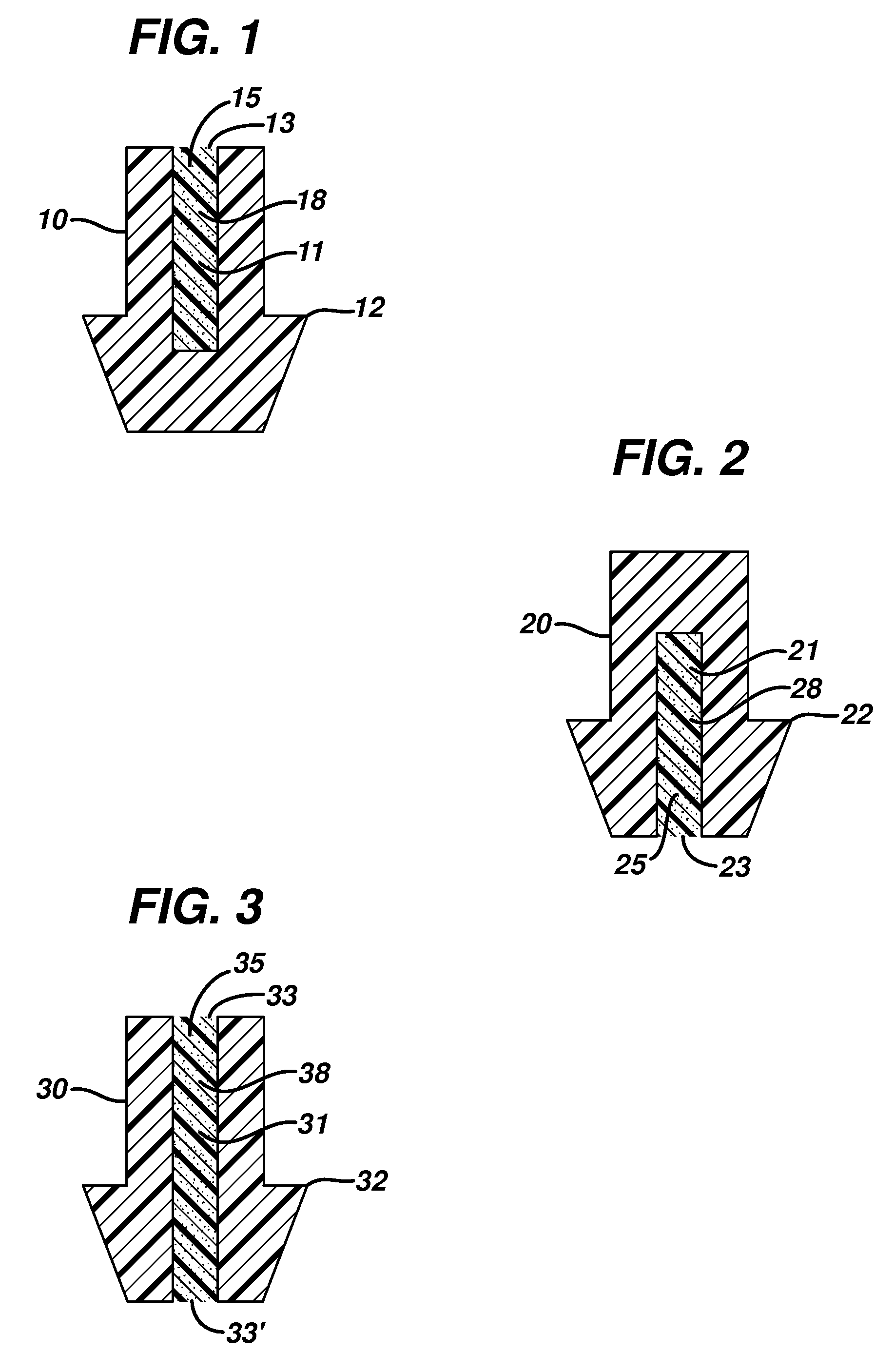
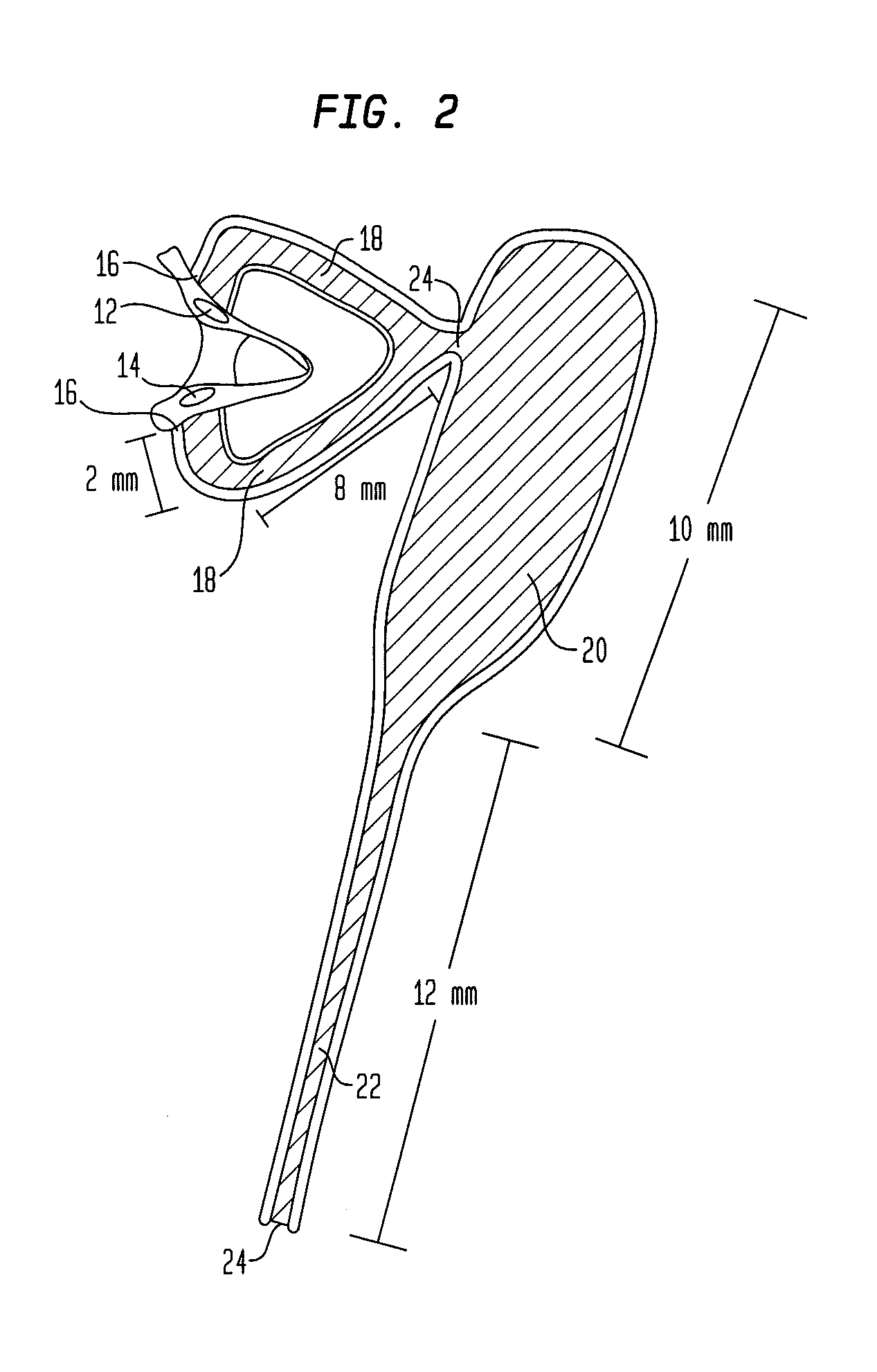
Punctal Plugs and Methods of Delivering Therapeutic Agents
ActiveUS20080181930A1High retention rateIncrease stiffnessBiocideSenses disorderCollagen Punctal PlugsParylene coating
The present invention concerns implantable ocular devices for the sustained release of medication to the eye, and methods for manufacturing and using such devices. In one embodiment, the present invention provides a device comprising: (a) a body comprising a matrix of a prostaglandin and a silicone; (b) a parylene coating on the outer surface of the body; and (c) one or more pores extending from the outer surface of the parylene coating to the outer surface of the body.
Owner:NOVARTIS AG
Punctal plugs for the delivery of active agents
The invention provides punctal plugs for the delivery of active agent to one or both of the tear fluid of the eye and to the nasolacrimal duct. The plugs of the invention have a body, a reservoir contained within the body, and optionally a collarette. The reservoir has at least one opening and contains a polymeric material and at least one active agent.
Owner:JOHNSON & JOHNSON VISION CARE INC
Punctal plugs for the delivery of active agents
The invention provides punctal plugs for the delivery of active agents. The plugs have a body throughout which at least one active agent is dispersed or that is coated with a polymeric material containing at least one active agent.
Owner:JOHNSON & JOHNSON VISION CARE INC
Punctal plugs for the delivery of active agents
The invention provides punctal plugs for the delivery of active agent to one or both of the tear fluid of the eye and to the nasolacrimal duct that comprise a body, at least one cap, and optionally a collarette.
Owner:JOHNSON & JOHNSON VISION CARE INC
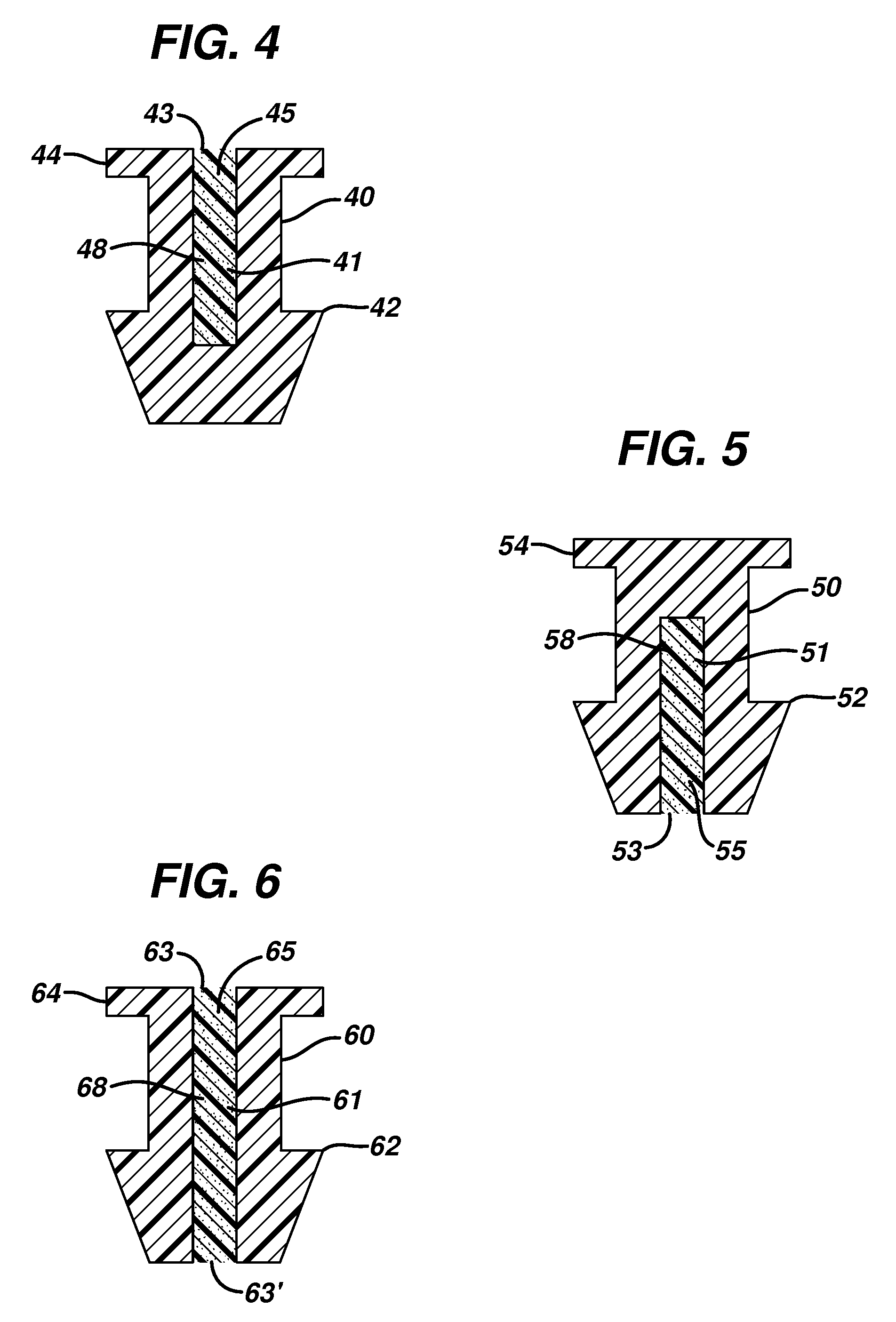
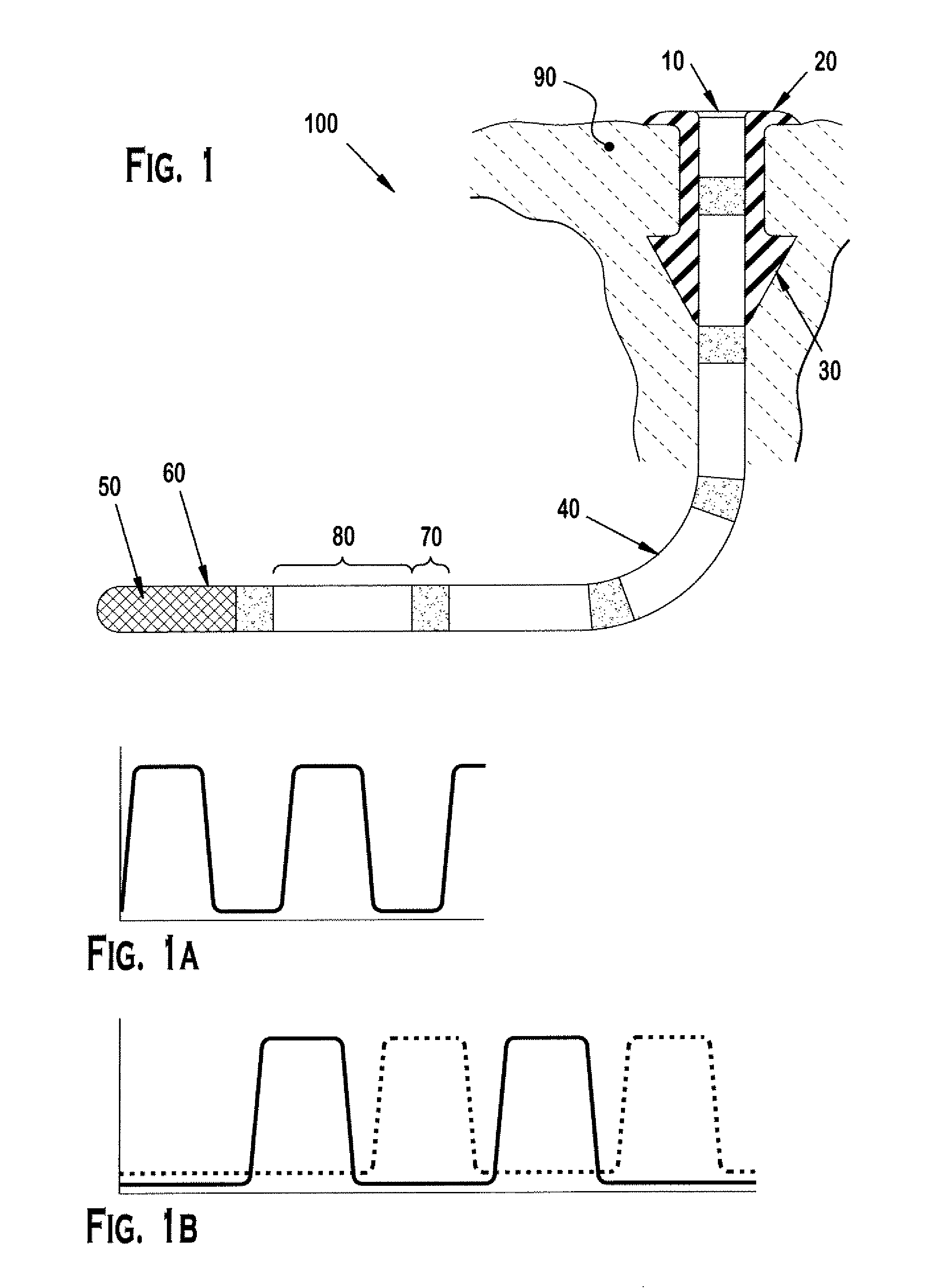
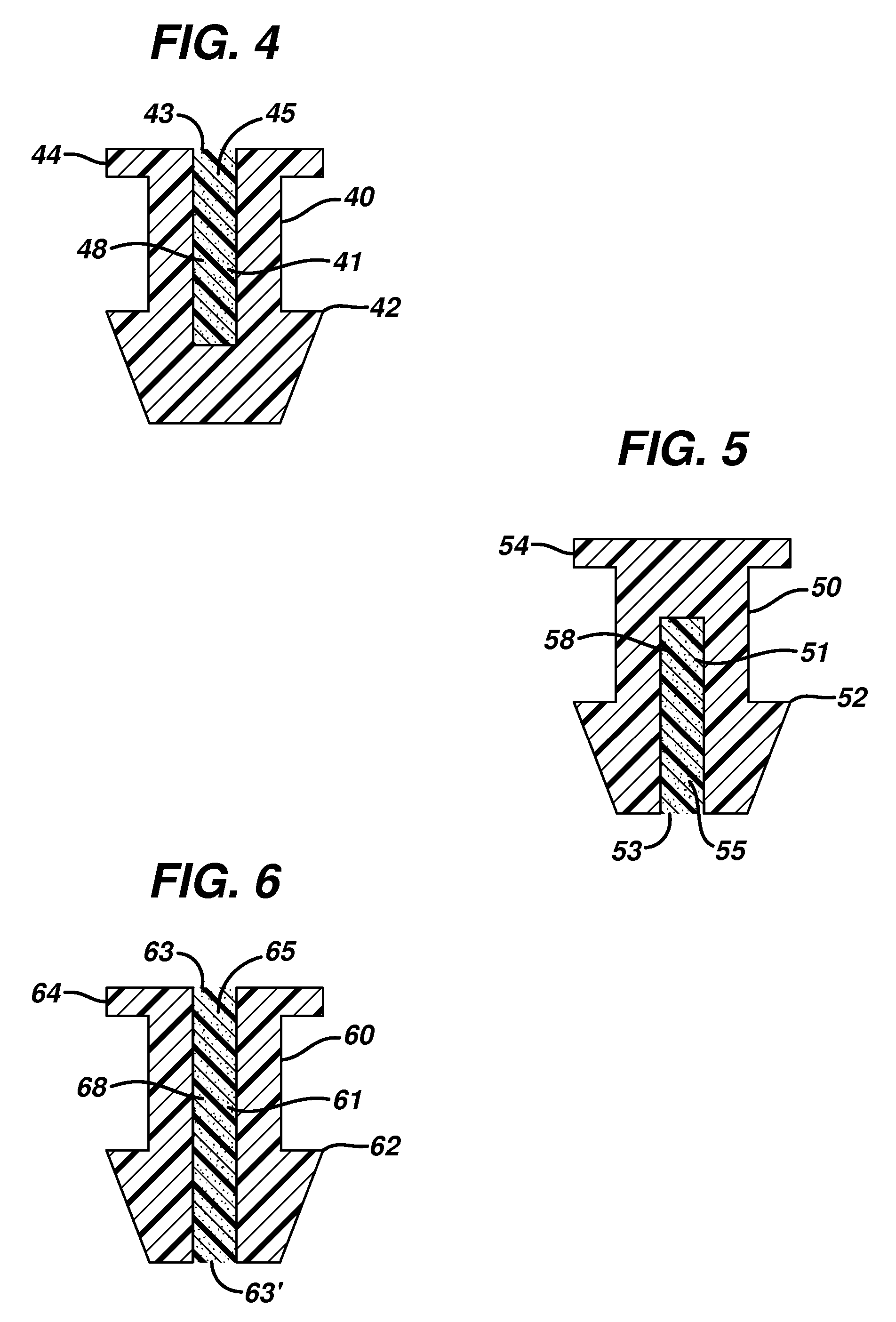
Punctal plug containing drug formulation
ActiveUS20120059338A1Easy insertion stabilityEasy long-term stabilityEye surgeryMedical devicesControlled releaseEyelid
Disclosed are lacrimal inserts and their method of use for delivery of medication to the eye. The plug includes a body portion sized to pass through a lacrimal punctum and be positioned within a lacrimal canaliculus of the eyelid. The plug may contain a core, or reservoir, at least partially within the body portion comprising a therapeutic agent that is configured to controlled release into the eye and is configured for release medication via a designated port, valve, or orifice in the insert housing and inhibits diffusion of medication via the housing itself.
Owner:JOHNSON & JOHNSON VISION CARE INC
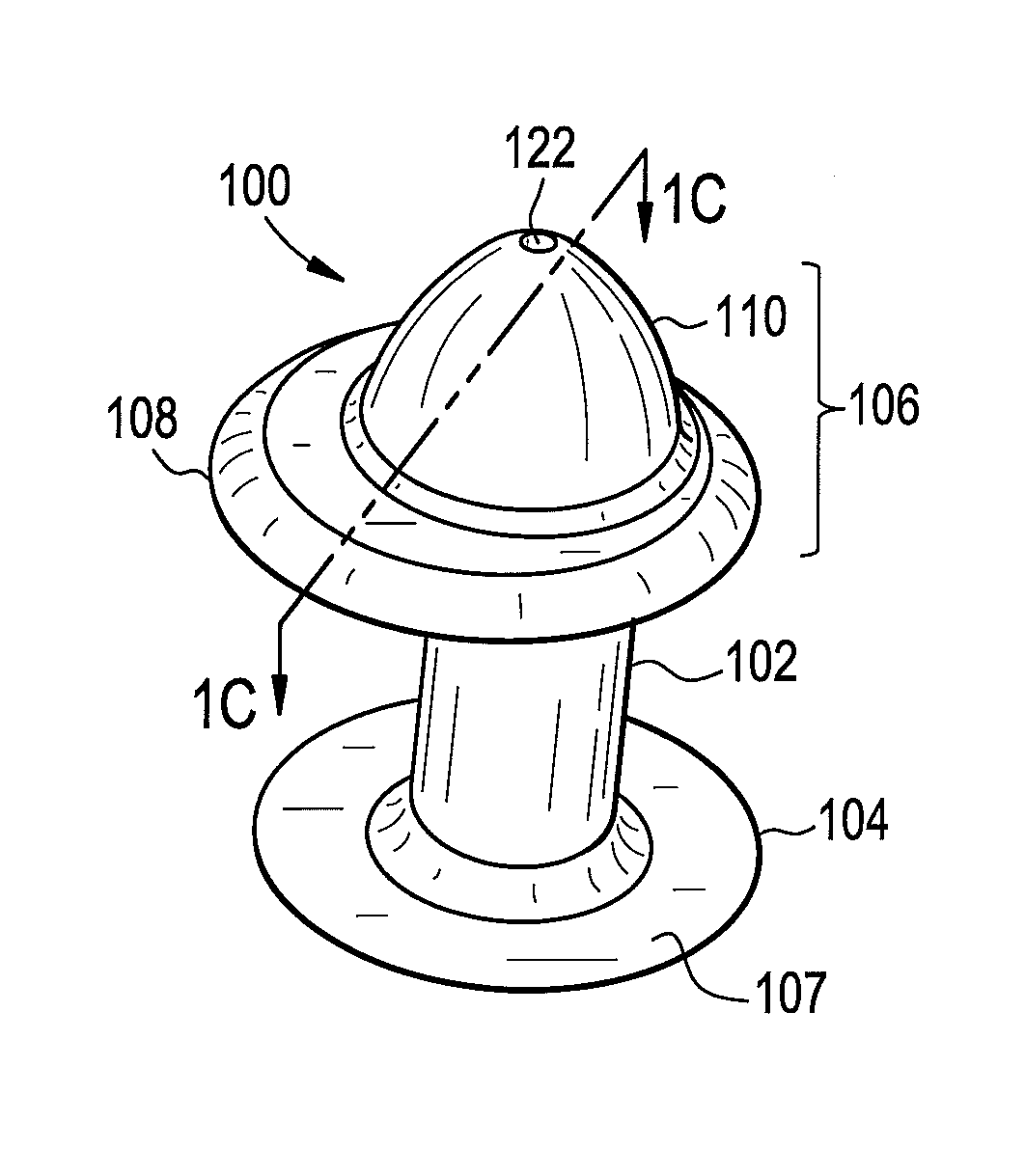
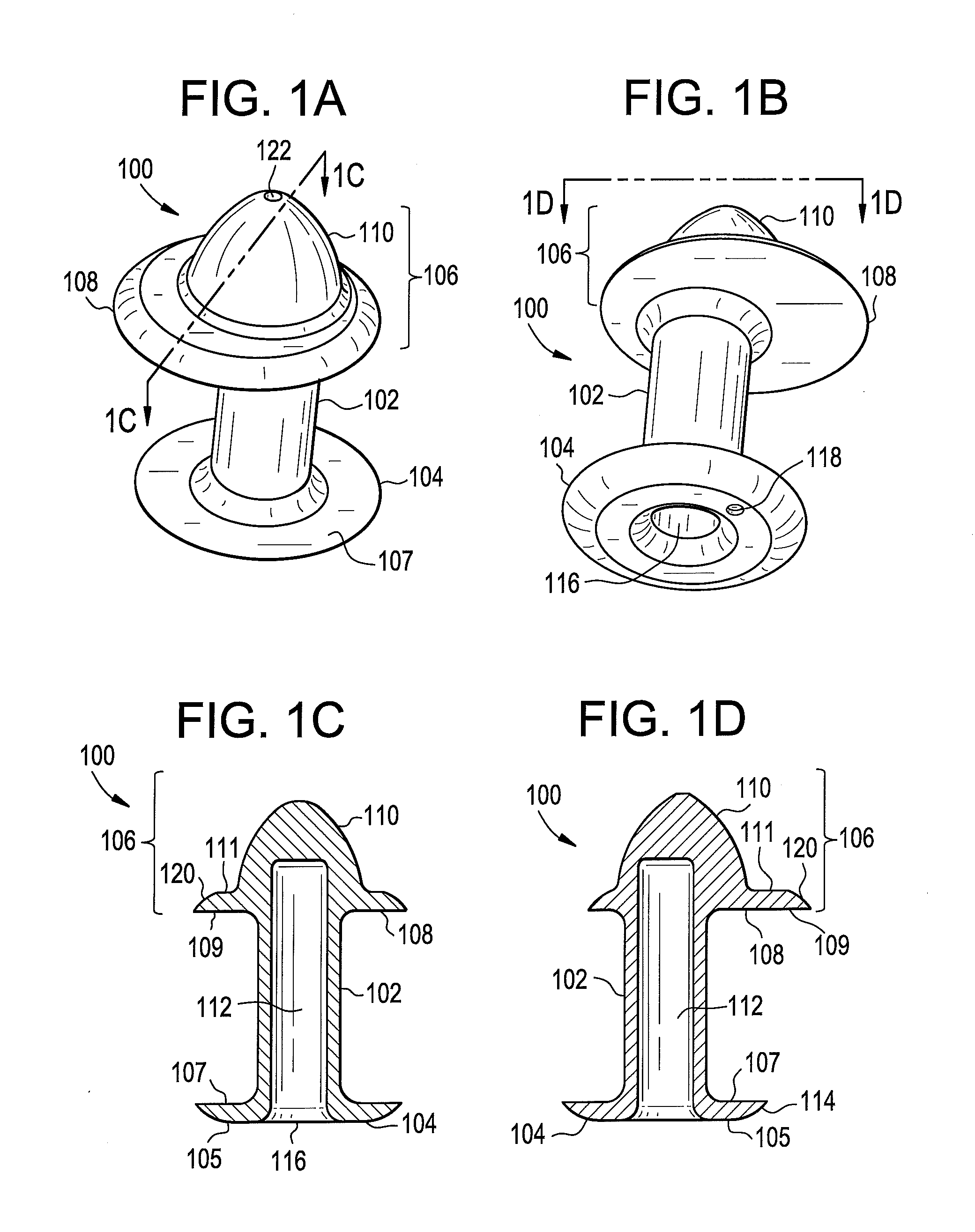
Punctal Plugs
Owner:JOHNSON & JOHNSON VISION CARE INC
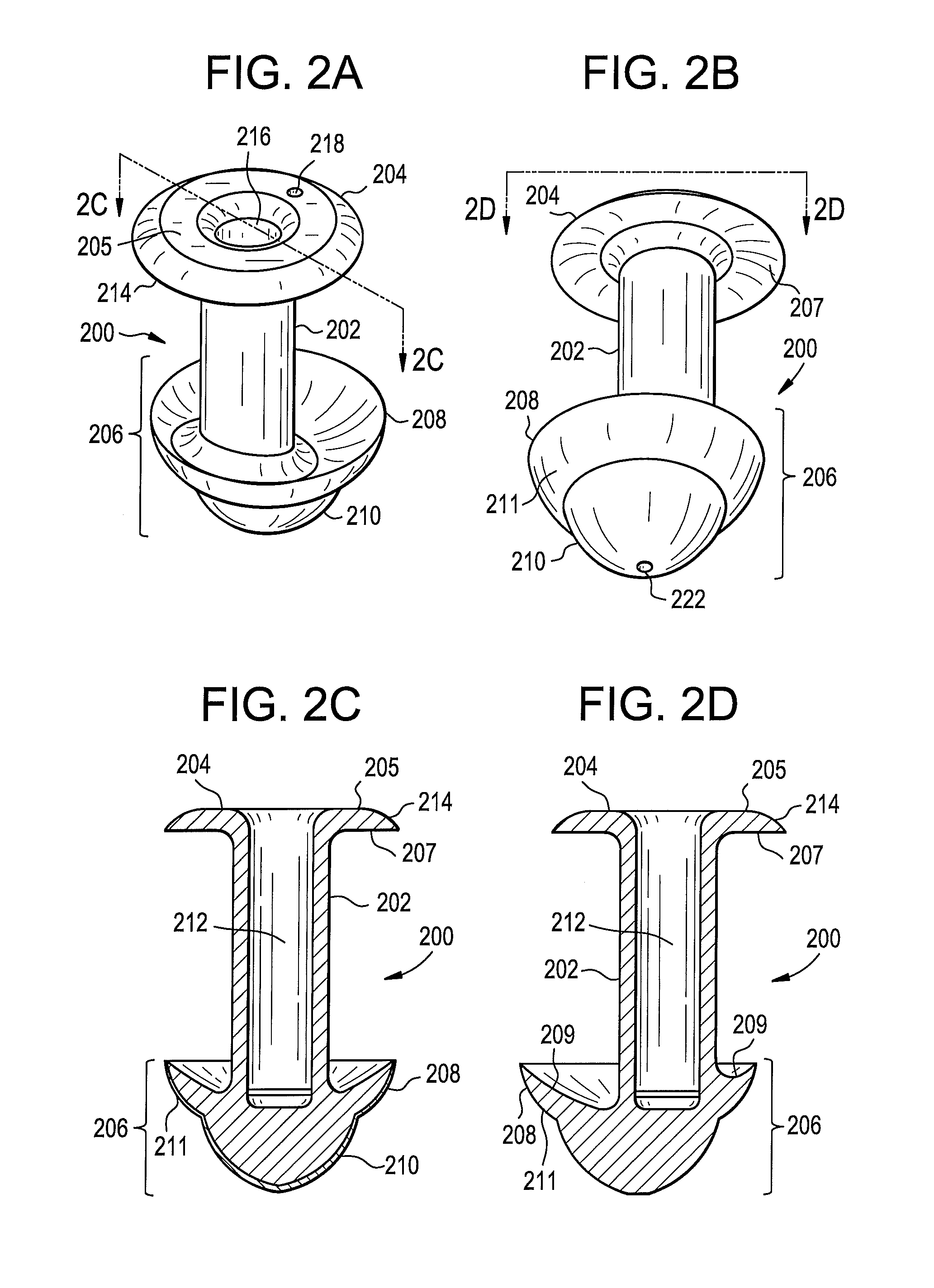
Punctal plugs
Owner:JOHNSON & JOHNSON VISION CARE INC
Punctal plugs for controlled release of therapeutic agents
Disclosed are lacrimal inserts and their method of use for delivery of medication to the eye. The plug includes a body portion sized to pass through a lacrimal punctum and be positioned within a lacrimal canaliculus of the eyelid. The plug may contain a core, or reservoir, at least partially within the body portion comprising a therapeutic agent that is configured for controlled release into the eye by means of an osmotic engine.
Owner:JOHNSON & JOHNSON VISION CARE INC
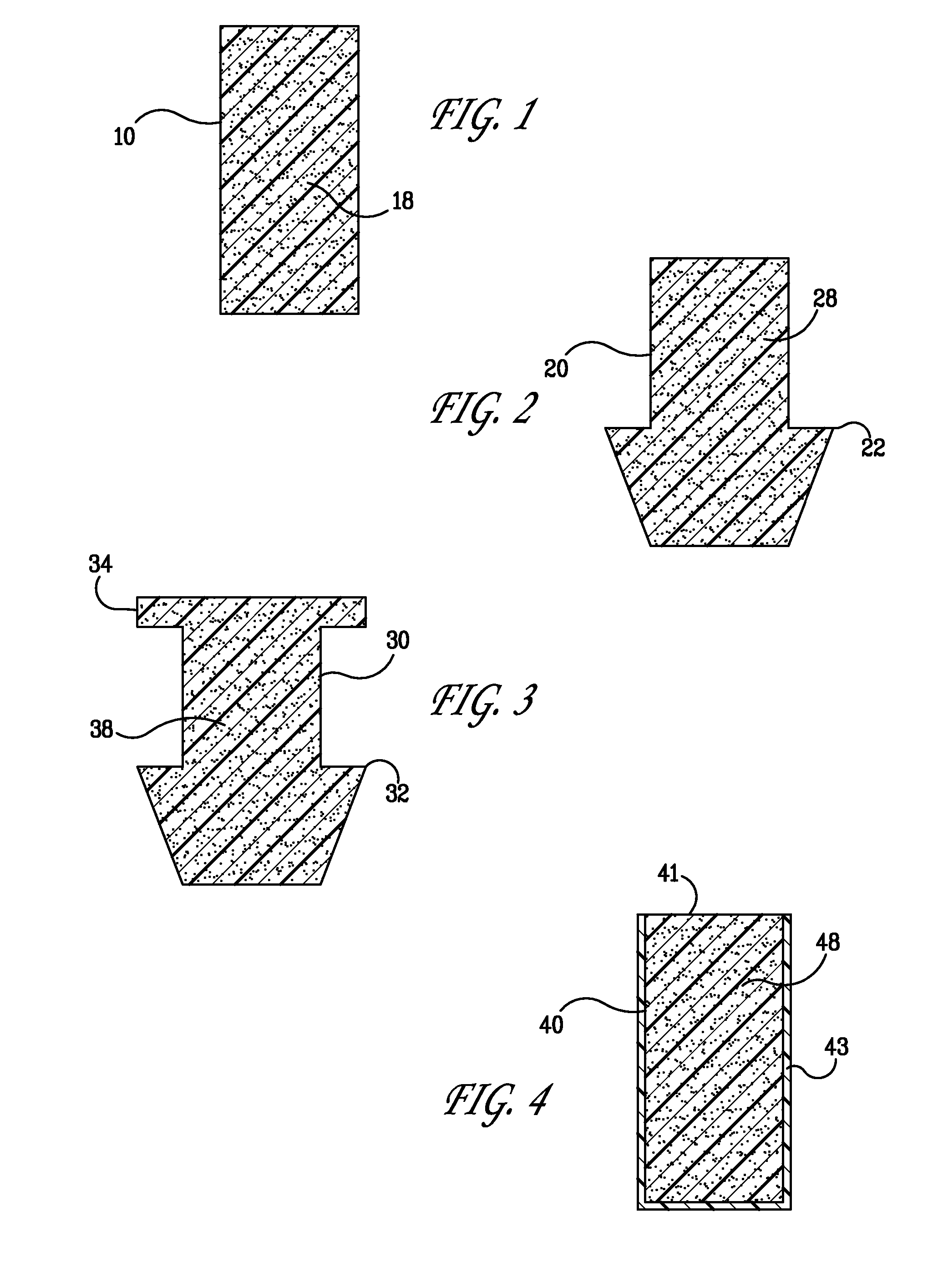
Punctal plug comprising a water-insoluble polymeric matrix
InactiveUS20080114076A1Senses disorderPharmaceutical delivery mechanismCollagen Punctal PlugsPolyester
Disclosed is a pharmaceutical composition comprising a water insoluble polymer matrix that comprises a bioerodable polyester polymer or a fatty acid based polyester polymer, or a mixture of both polymers, wherein the polymer matrix has a melting point of less than 60° C., and wherein the composition is liquid or paste at room temperature and is formulated to occlude a punctual channel in a subject and conforms to the shape of the canalicular or punctal channel.
Owner:ALCON RES LTD
Punctal plug comprising a water-insoluble polymeric matrix
InactiveUS20140128478A1Senses disorderPharmaceutical delivery mechanismPolyesterCollagen Punctal Plugs
Owner:ALCON RES LTD
Implantable punctal plug
Owner:ALCON RES LTD
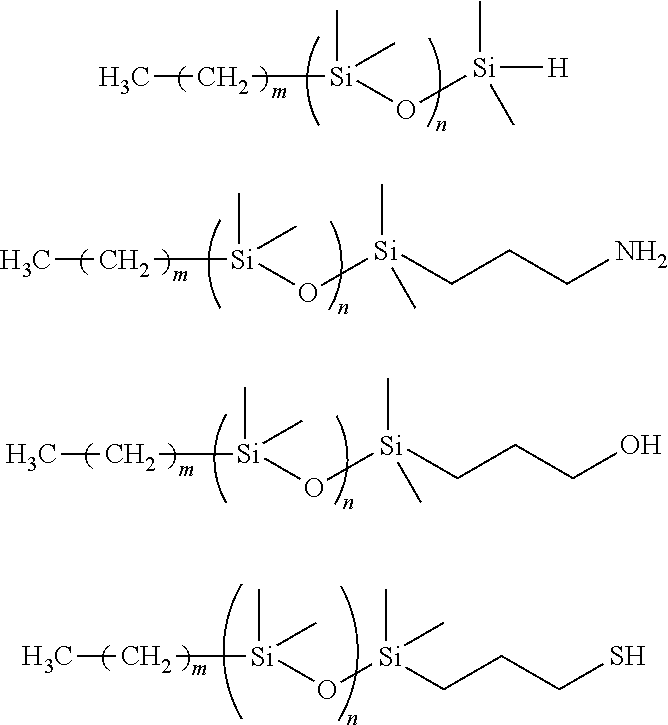
Macroinitiator containing hydrophobic segment
The present invention relates to macroinitiators comprising at least one hydrophobic segments in a molecule, wherein a molecular weight of the hydrophobic segment is 300 to 1800. The present invention further relates to block copolymers, wetting agent and polymeric materials having the block copolymers of the present invention associated with, which is suitable for medical devices, particularly for ophthalmic devices, including contact lenses, ophthalmic lenses, punctal plugs and artificial corneas.
Owner:JOHNSON & JOHNSON VISION CARE INC
Punctal plugs
ActiveUS20130131612A1Easy insertion stabilityEasy long-term stabilityEye surgeryMedical devicesCollagen Punctal PlugsPunctal plug
Owner:JOHNSON & JOHNSON VISION CARE INC
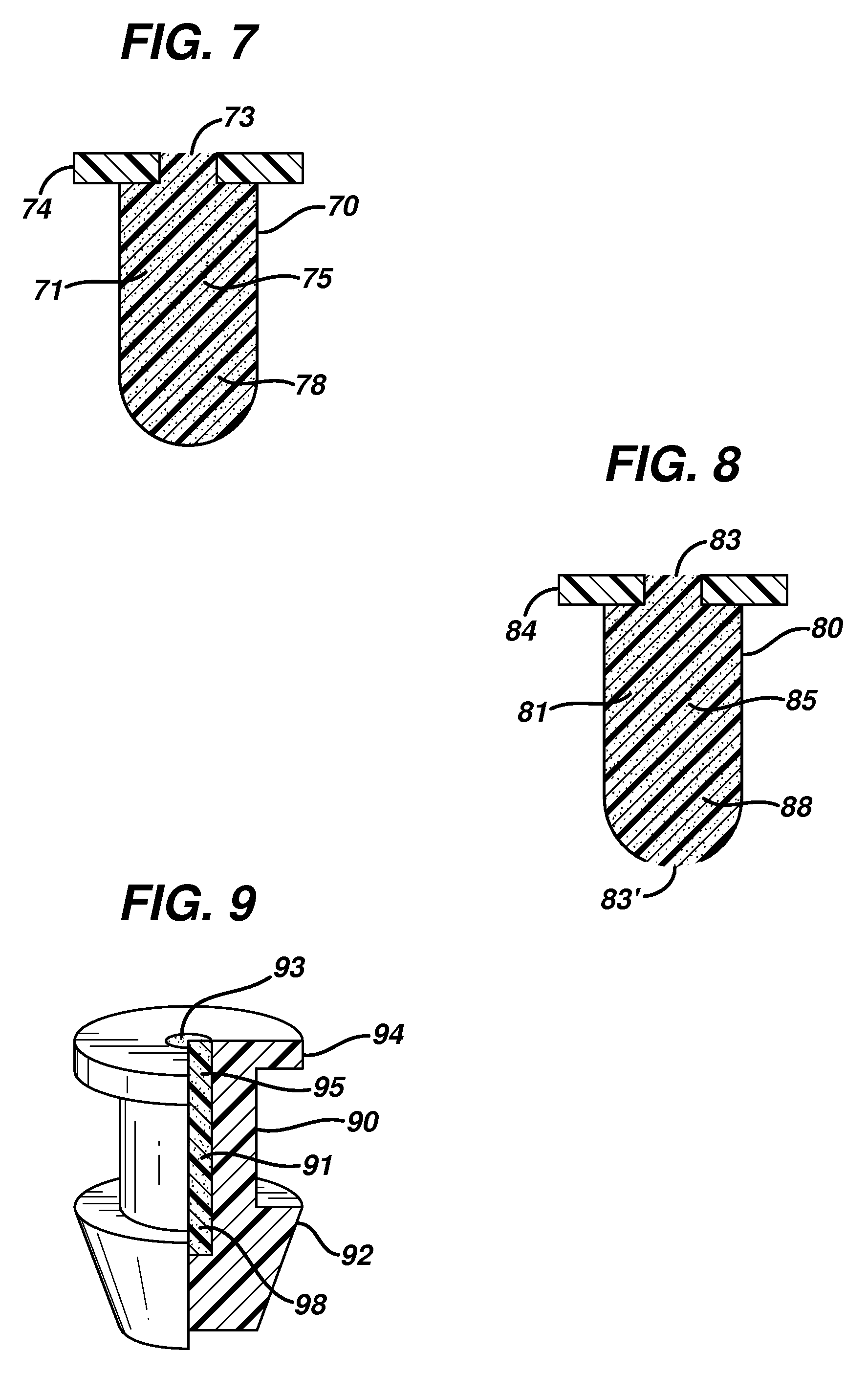
Dry eye treatment by puncta plugs
InactiveUS20100310622A1Effectively impermeableIncrease delivery rateOrganic active ingredientsSenses disorderCollagen Punctal PlugsMedicine
A punctal plug and method of treating dry eyes are provided. The punctal plug has two or three layer structure and contains at least one drug, for treating conditions such as dry eyes contained in a core, a potion of which is covered by a drug impermeable shell such that drug can radially diffuse from the core. The punctal plug can be inserted into a patient's upper punctum, lower punctum, or both to deliver the drug for an extended period of time. The drug for treating dry eyes can be, for example, cyclosporine A. The plug can also be used for extended delivery of ophthalmic drugs.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
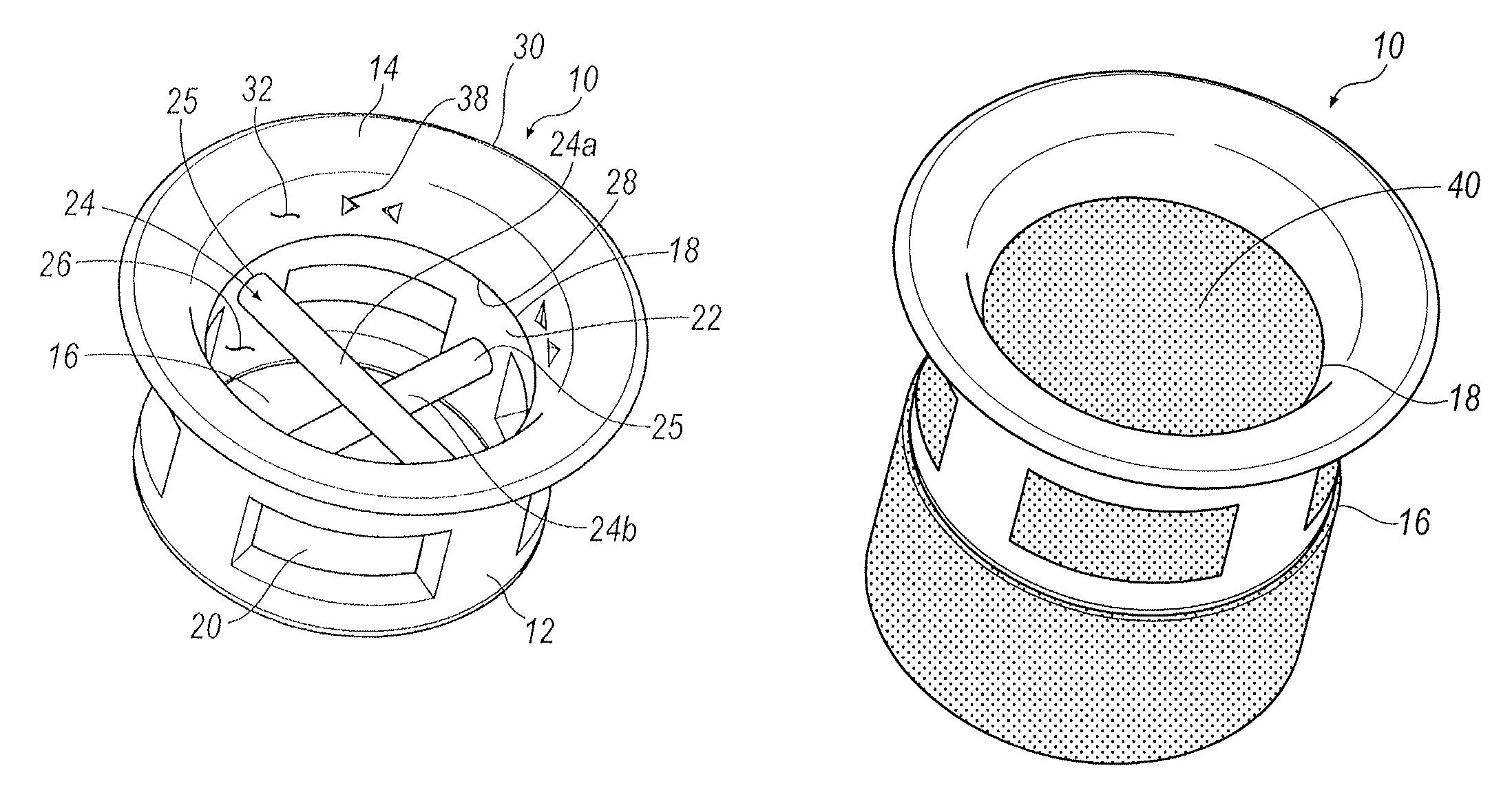
Multi-piece punctal plug
A multi-piece punctal plug has one or more superficial plugs connected to an anchor plug deeper in the naso-lacrimal canaliculus. The anchor is fixed to the wall of the lacrimal duct while the superficial plugs are axially and detachably secured to the anchor plug so that the multi-plug complex may be inserted or removed together as one, or the superficial plugs may be detached separately and replaced securely without disturbing the anchor. Removal and insertion is accomplished by a specific tool having an end for engaging the plugs.
Owner:ORDICH STEVEN
Eye drug delivery system
ActiveUS20130184661A1Easy to manufactureEasy to useEye surgeryMedical applicatorsCollagen Punctal PlugsActive agent
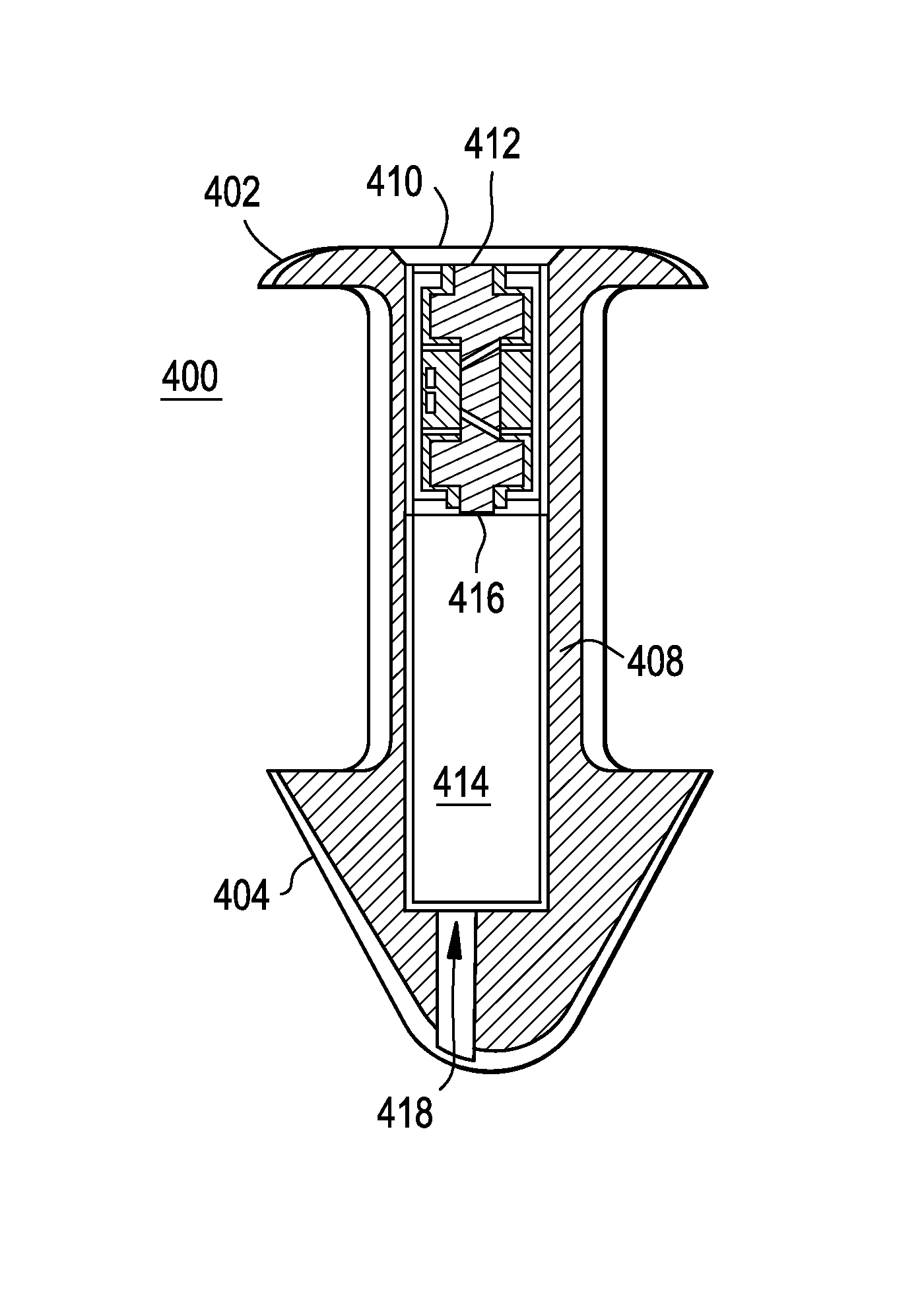
A punctal plug or lacrimal insert comprising a microelectromechanical system pump and associated reservoir may be utilized to deliver precise dosages of an active agent into the eye though the tear film. The microelectromechanical system pump comprises four main components; namely, a reservoir, a pump, a series of valves and a vent. The microelectromechanical system pump is positioned within a cavity in the punctal plug. The microelectromechanical system pump is positioned with a cavity in the punctal plug.
Owner:JOHNSON & JOHNSON VISION CARE INC +1
Punctal plugs
ActiveUS20130123718A1Easy insertion stabilityEasy long-term stabilityEye surgeryMedical devicesCollagen Punctal PlugsPunctal plug
Owner:JOHNSON & JOHNSON VISION CARE INC
Punctal plugs
ActiveUS20130090611A1Easy insertion stabilityEasy long-term stabilityEye surgeryPharmaceutical delivery mechanismCollagen Punctal PlugsPunctal plug
Owner:JOHNSON & JOHNSON VISION CARE INC
Punctal plugs for the delivery of active agents
The invention provides punctal plugs for the delivery of active agent to one or both of the tear fluid of the eye and to the nasolacrimal duct. The plugs of the invention have a body, a reservoir contained within the body, and optionally a collarette. The reservoir has at least one opening and contains a polymeric material and at least one active agent.
Owner:JOHNSON & JOHNSON VISION CARE INC
Punctal plugs
InactiveCN102448412ARaise the possibilityEye surgeryPharmaceutical delivery mechanismCollagen Punctal PlugsPunctal plug
Owner:JOHNSON & JOHNSON VISION CARE INC
Punctal plugs with continuous or pulsatile drug release mechanism
Disclosed are lacrimal inserts and their method of use for delivery of of medication to the eye. The plug includes a body portion sized to pass through a lacrimal punctum and be positioned within a lacrimal canaliculus of the eyelid. The plug may contain a core, or reservoir, at least partially within the body portion comprising a therapeutic agent that is configured to controlled release into the eye.
Owner:JOHNSON & JOHNSON VISION CARE INC
Punctal plugs for controlled release of therapeutic agents
ActiveUS20120283669A1Simple and inexpensive to manufactureEye surgeryMedical devicesCollagen Punctal PlugsControlled release
Lacrimal inserts such as punctal plugs may be utilized for delivery of medication to the eye. The plug includes a body portion sized to pass through a lacrimal punctum and be positioned within a lacrimal canaliculus of the eyelid. The plug may contain a core, or reservoir, at least partially within the body portion comprising a therapeutic agent that is configured for controlled, pulsatile release into the eye.
Owner:JOHNSON & JOHNSON VISION CARE INC
Punctal plugs with directional release
ActiveUS20120197217A1Easy insertion stabilityEasy long-term stabilityEye surgeryMedical applicatorsControlled releaseEyelid
Disclosed are lacrimal inserts and their method of use for delivery of medication to the eye. The plug includes a body portion sized to pass through a lacrimal punctum and be positioned within a lacrimal canaliculus of the eyelid and includes means for directional release of medication into the lacrimal fluid. The plug may contain a core, or reservoir, at least partially within the body portion comprising a therapeutic agent that is configured to controlled release into the eye.
Owner:JOHNSON & JOHNSON VISION CARE INC
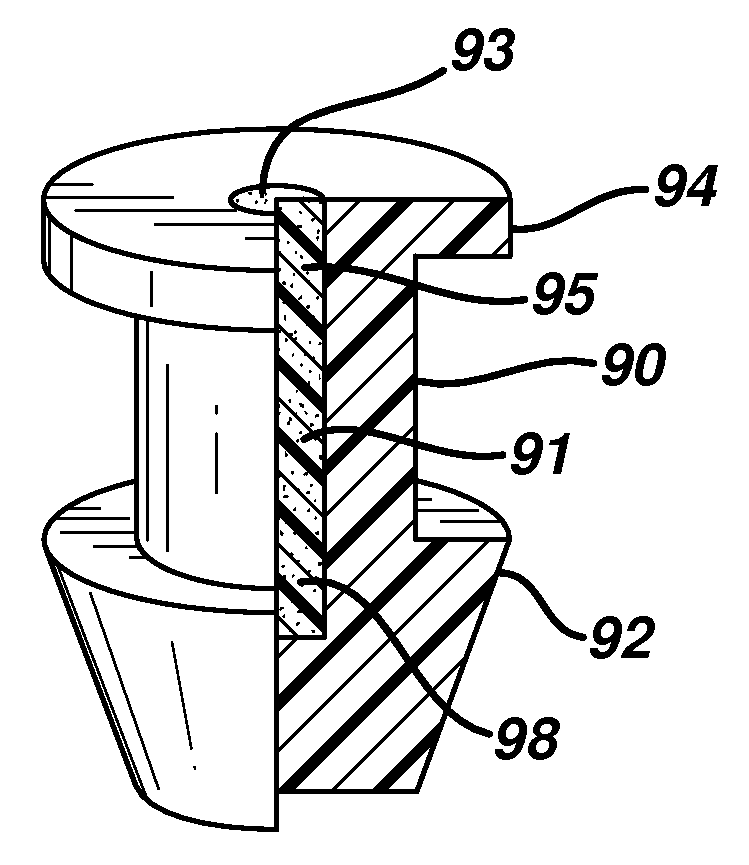
Balloon punctal plug
InactiveCN103300968ALow risk of accidental removalPrecise positioningEye surgeryProsthesisCollagen Punctal PlugsPunctal plug
Owner:JOHNSON & JOHNSON VISION CARE INC
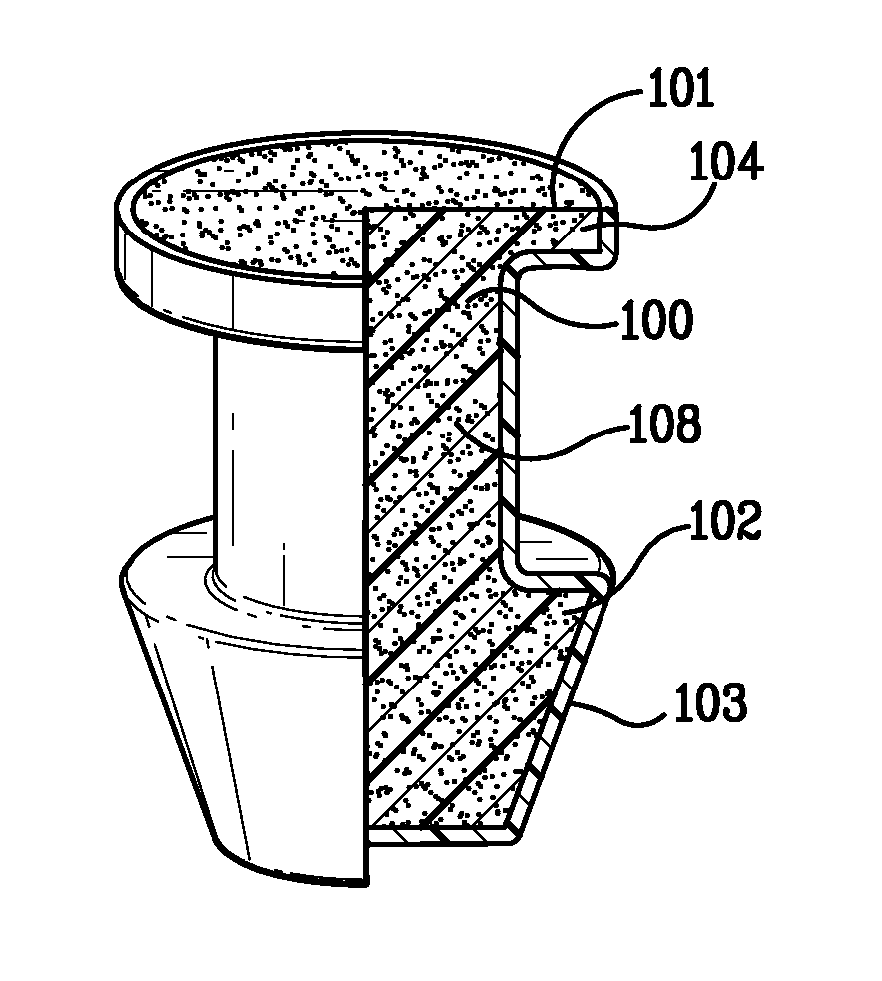
Eye drug delivery system
ActiveUS8808256B2Easy to manufactureEasy to useEye implantsEye surgeryCollagen Punctal PlugsActive agent
A punctal plug or lacrimal insert comprising a microelectromechanical system pump and associated reservoir may be utilized to deliver precise dosages of an active agent into the eye though the tear film. The microelectromechanical system pump comprises four main components; namely, a reservoir, a pump, a series of valves and a vent. The microelectromechanical system pump is positioned within a cavity in the punctal plug. The microelectromechanical system pump is positioned with a cavity in the punctal plug.
Owner:JOHNSON & JOHNSON VISION CARE INC +1
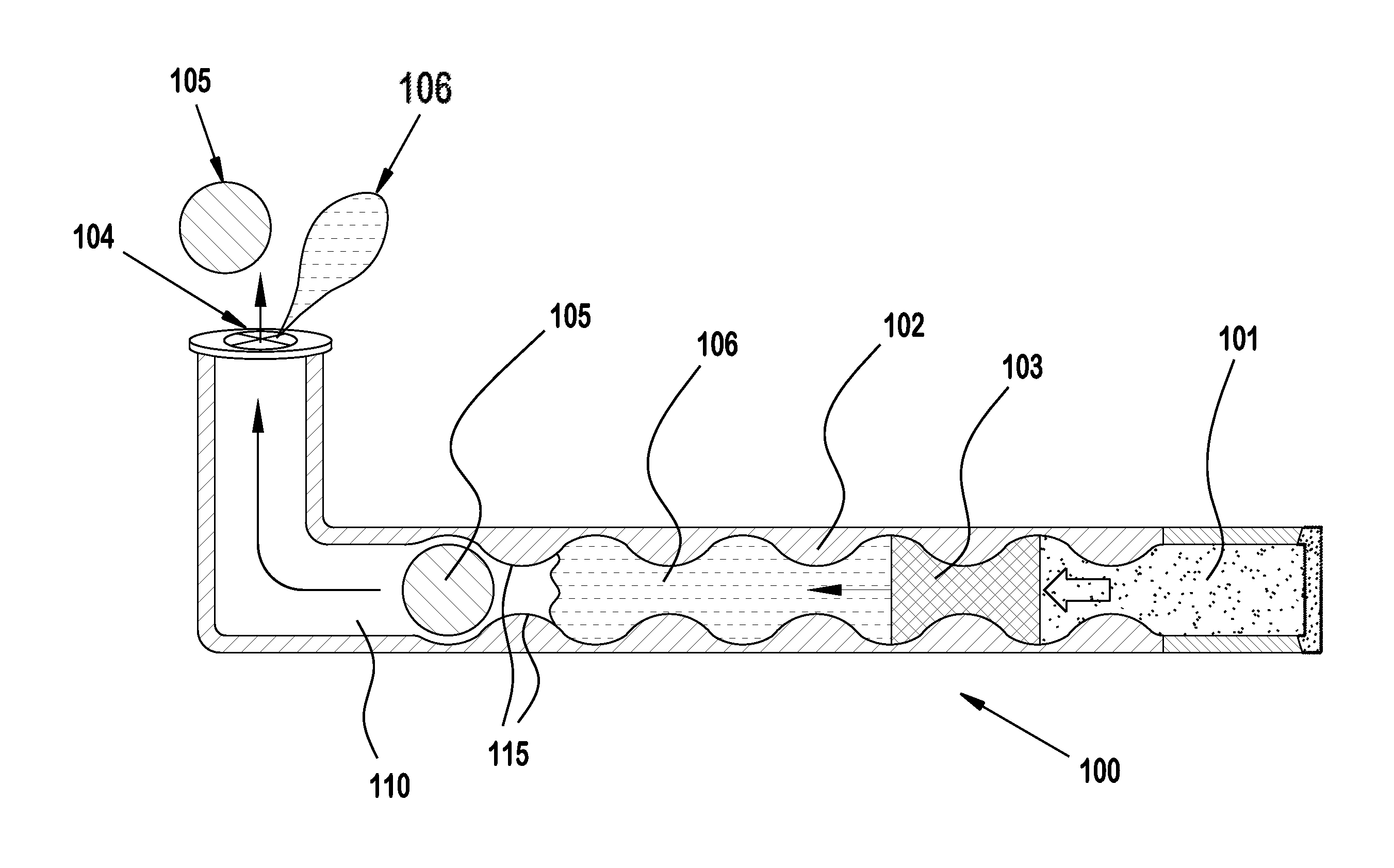
Super-lubricating serum albumin punctal plug and preparation method thereof
InactiveCN105879126AAvoid damageImprove the lubrication effectEye surgerySurgeryChemical reactionPolymer solution
The invention discloses a super lubricating serum albumin punctal plug. The punctal plug of the present invention has a main body (2), a first segment (1), a second segment (3) and a super lubricating coating (4). The surface of the serum albumin punctal plug is soaked in a hydrophilic polymer solution to form a hydrophilic polymer coating through a chemical reaction. When in contact with water, the hydrophilic group of the water-soluble polymer combines with water to swell rapidly , has excellent lubricating properties, can reduce the damage to soft tissue when the punctal plug is implanted, relieve pain, and prevent complications, so it is more suitable for clinical application.
Owner:HANGZHOU YAHUI BIOTECH CO LTD
Punctal plug with drug core retention features
Owner:JOHNSON & JOHNSON VISION CARE INC
Punctal plugs
ActiveUS9259352B2Easy to insertImprove stabilityEye surgeryMedical devicesCollagen Punctal PlugsPunctal plug
Owner:JOHNSON & JOHNSON VISION CARE INC
Microelectronic biosensor plug
ActiveUS20110282171A1Easy and quick insertionEasier for a patient to tolerateEye surgeryCatheterTearsLive organisms
A plug capable of providing information relating to a physical or chemical property of a body fluid, or the presence or amount of a molecular component therein in a living organism is disclosed. Specifically, one embodiment plug is capable of being inserted into a portion of a human eyelid in order to provide information relating to tear fluid is disclosed. This embodiment plug includes a body having a passage which allows for the natural flow of tear fluid therethrough. In addition, a sensing mechanism is provided which is capable of measuring, for example, glucose levels in the body of a patient through the analysis of the tear fluid. Such plug may further be designed so as to double as a punctal plug useful in preventing dry eye. Methods of utilizing and implanting such plugs are also disclosed.
Owner:FELDER ROBIN A
Punctal plugs
ActiveUS9259351B2Easy to insertImprove stabilityEye surgeryPharmaceutical delivery mechanismCollagen Punctal PlugsPunctal plug
Owner:JOHNSON & JOHNSON VISION CARE INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com