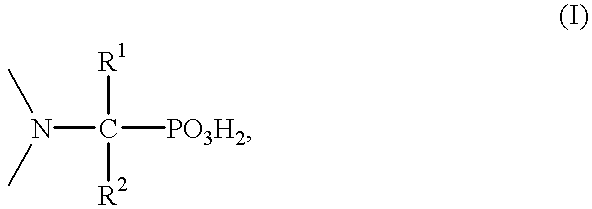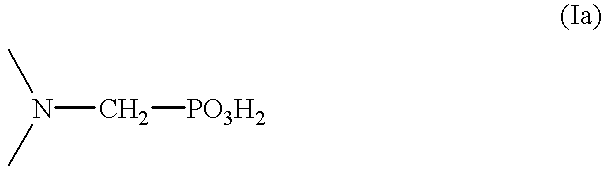Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
620results about "Zinc compounds" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
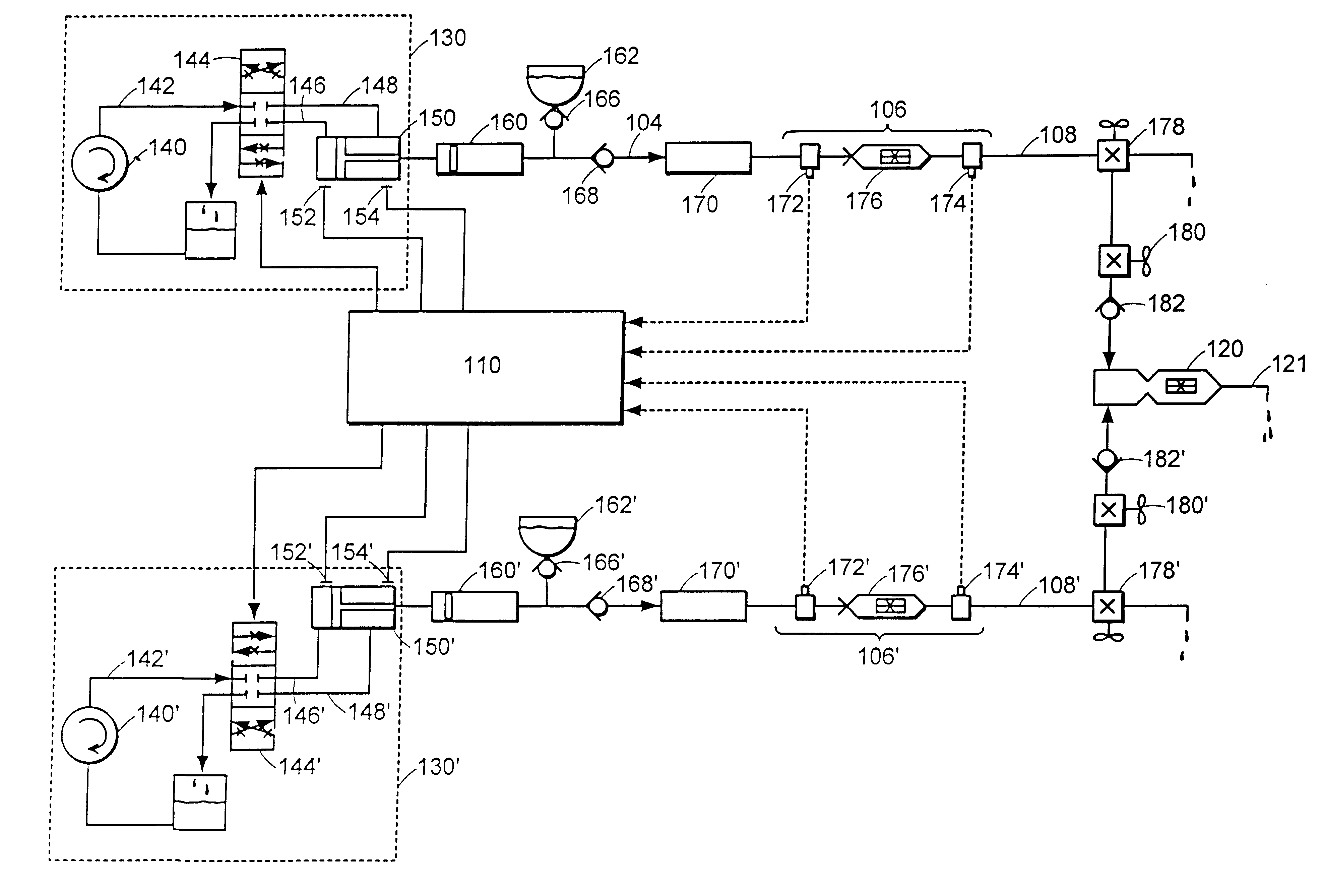
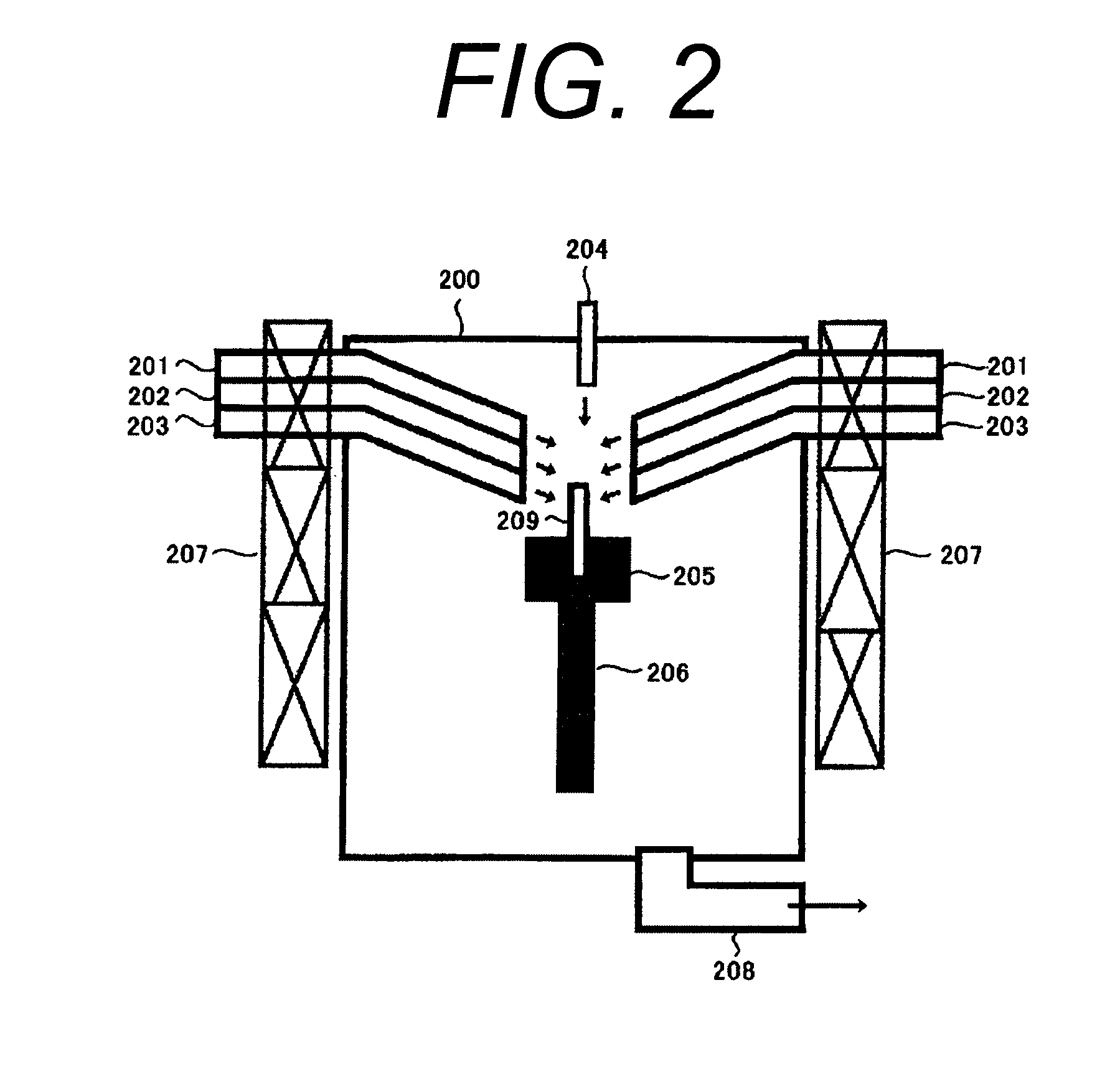
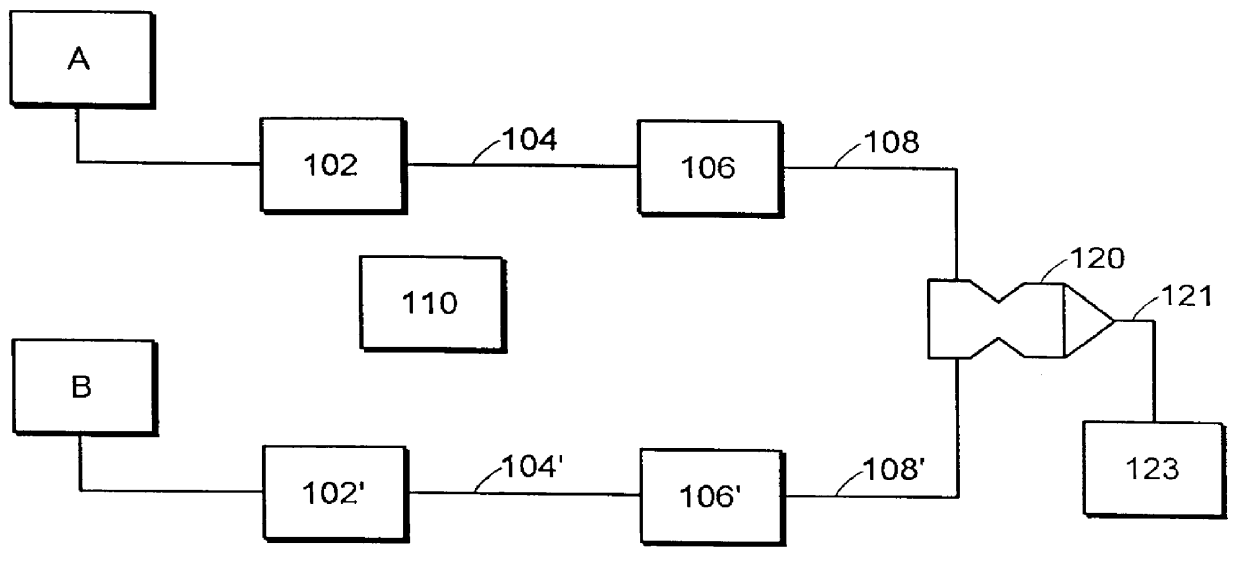
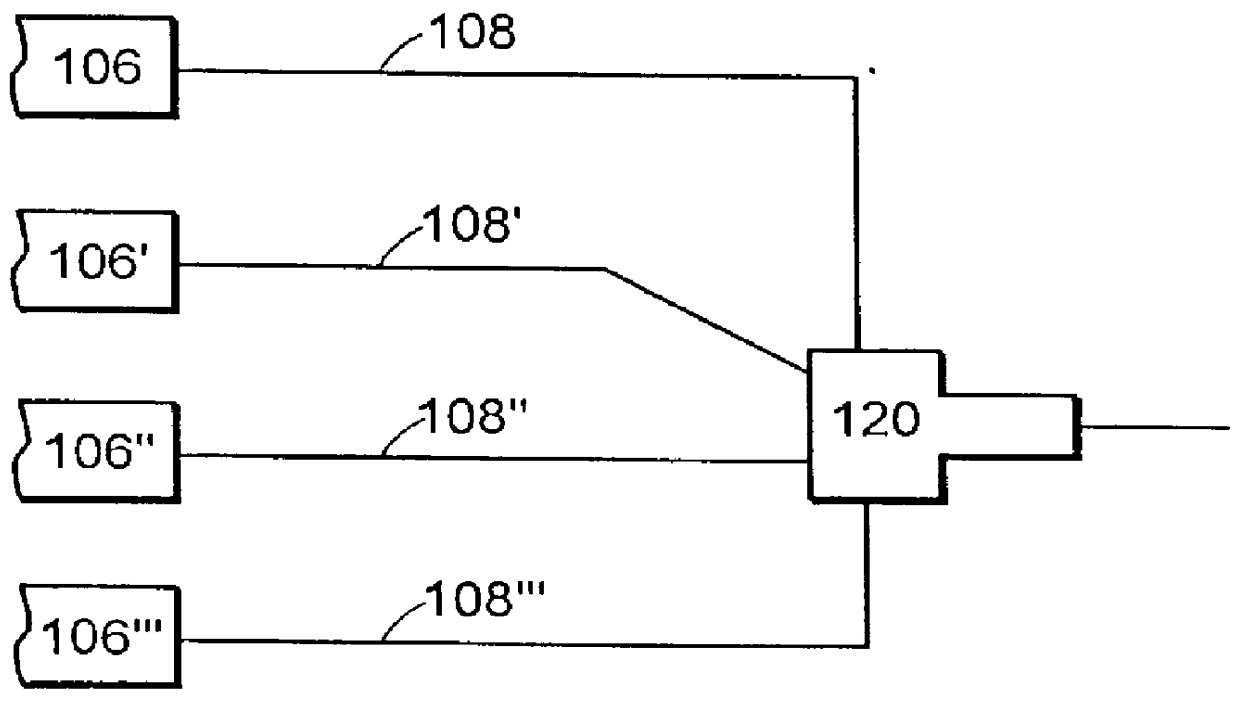
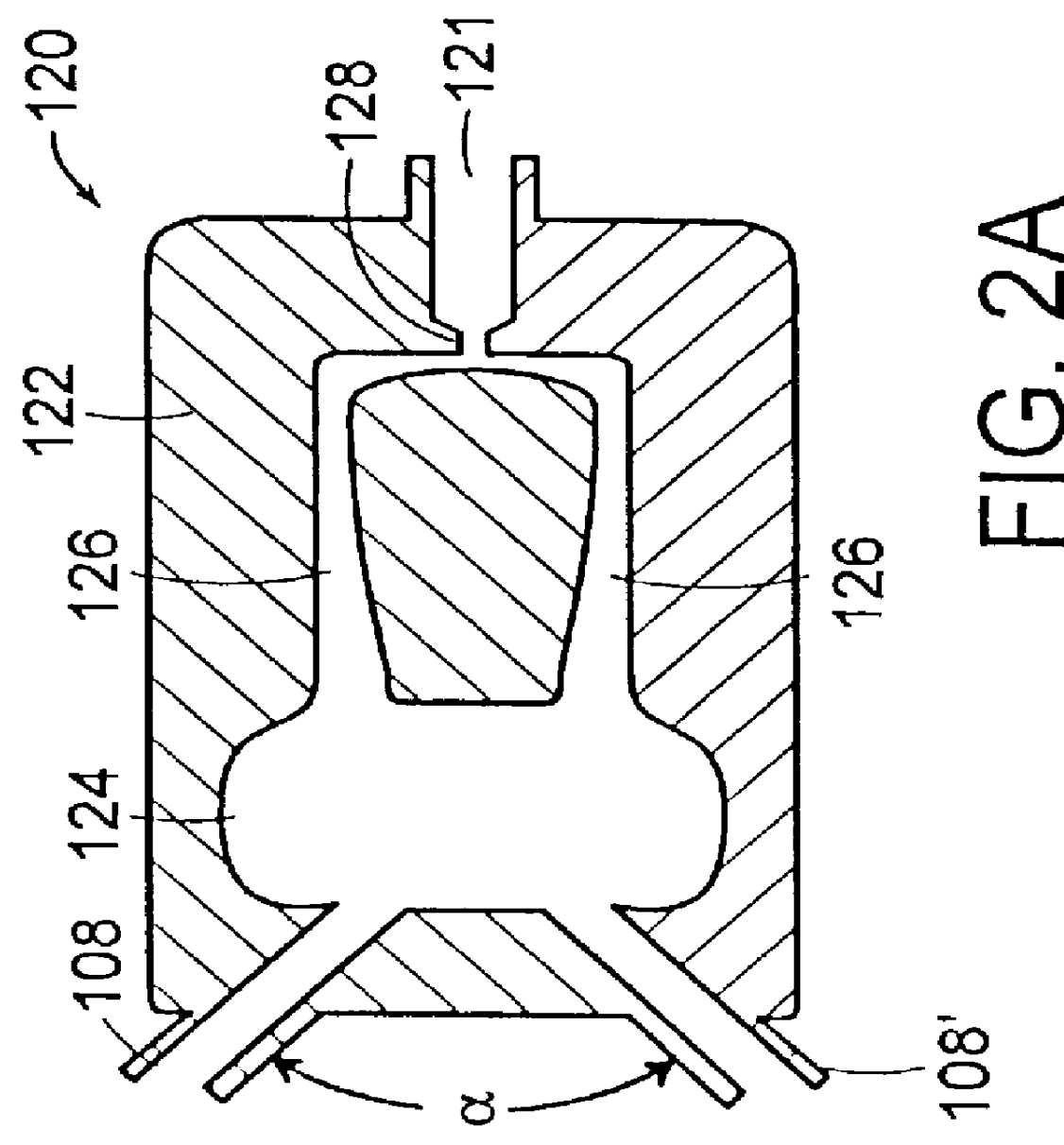
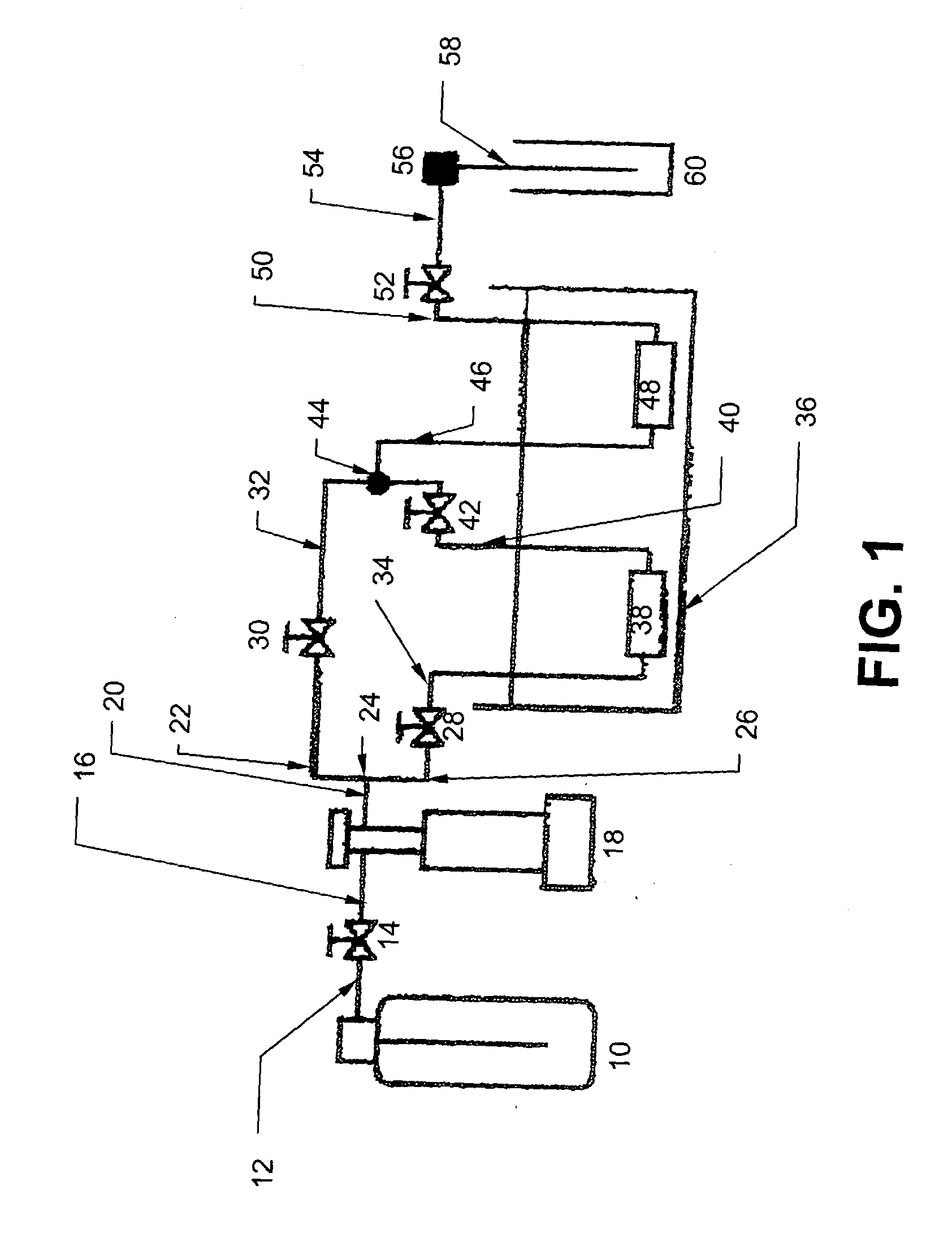
Multiple stream high pressure mixer/reactor
InactiveUS6221332B1Improve reliabilityFew and less partCalcium/strontium/barium carbonatesPressurized chemical processStream flowLow density
Enhanced macromixing, mesomixing, and micromixing of multiple discrete reactant streams, particularly for precipitation reactions of low density pumpable fluids, are obtained by controlled continuous high pressure multiple reactant streams flowing into a chemical mixer / reactor. Individual reactant streams are pressurized to about 8,000 to 50,000 psi and achieve velocities up to about 250 meters / second in the final stage of the chemical mixer / reactor. Reactant flows are controlled by a combination of a fixed restriction and a variable driving pump.
Owner:MICROFLUIDICS INT
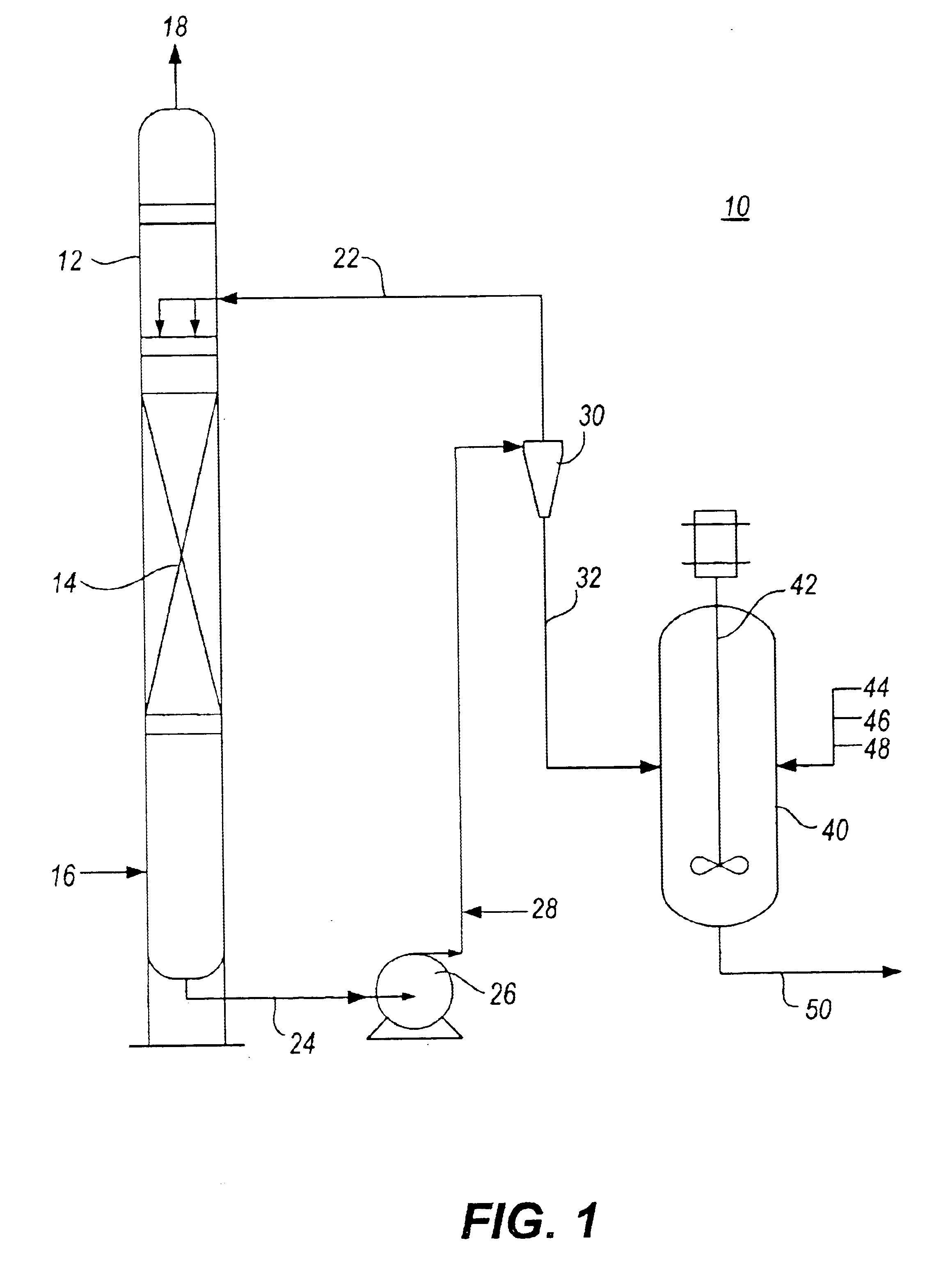
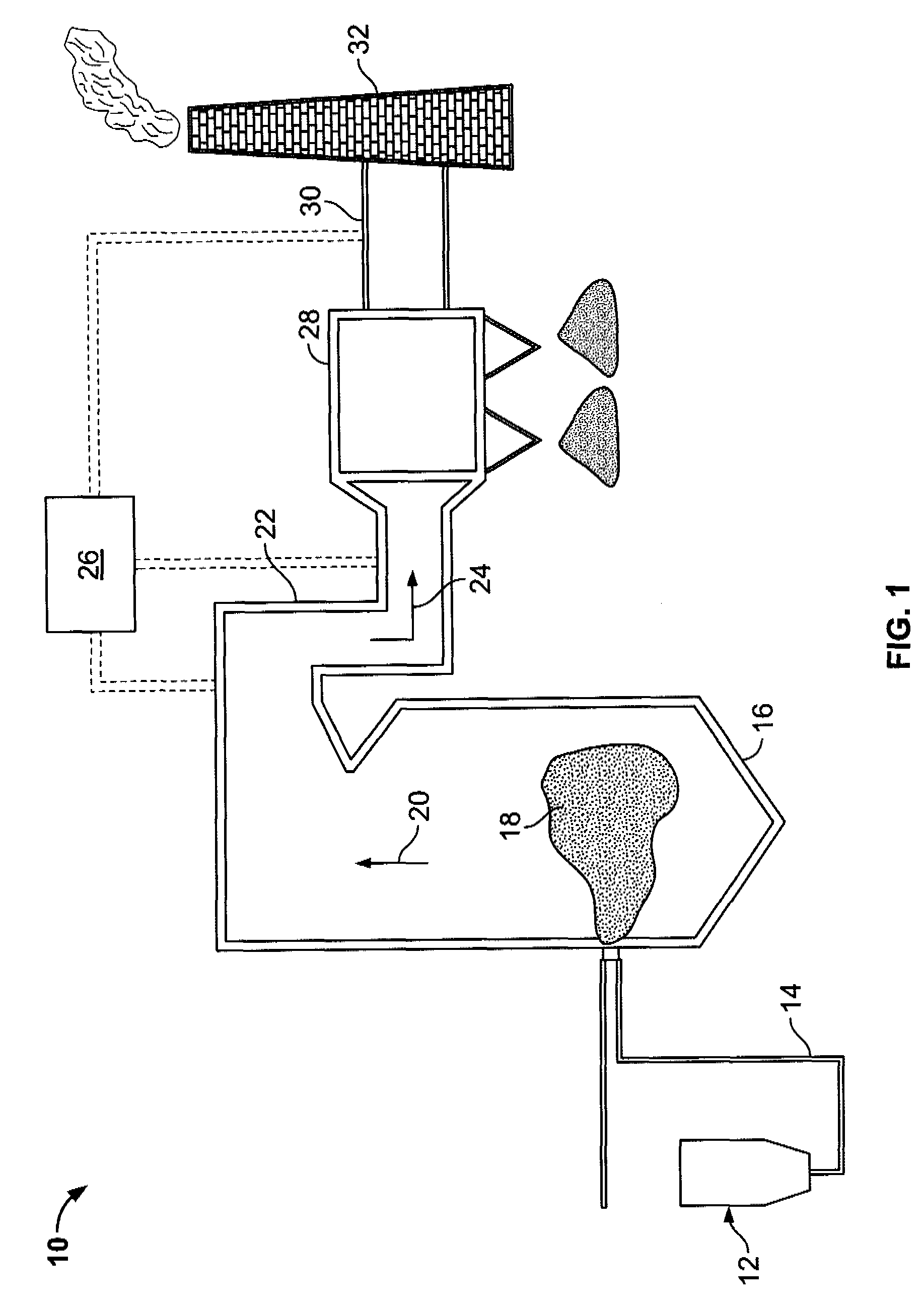
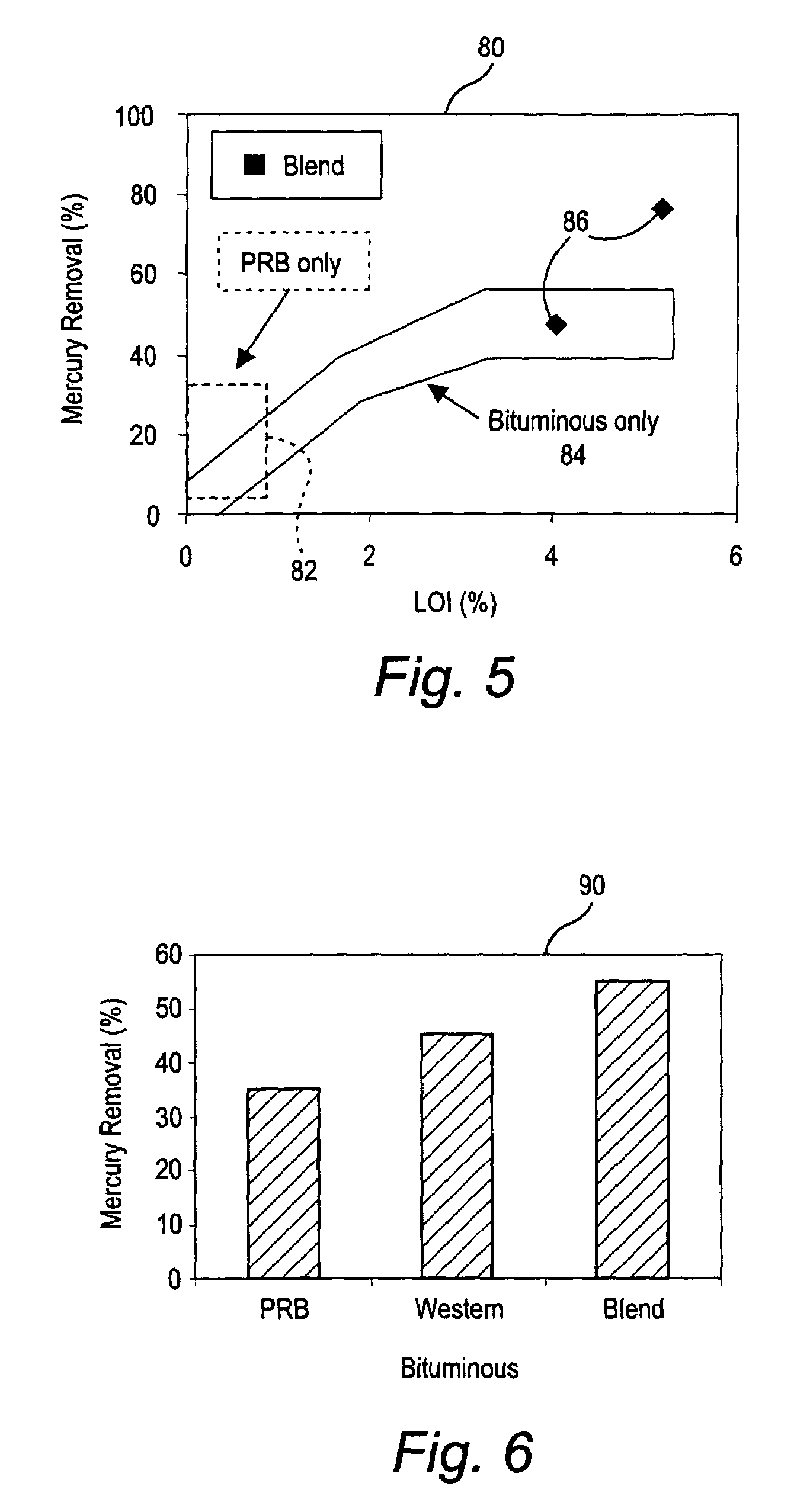
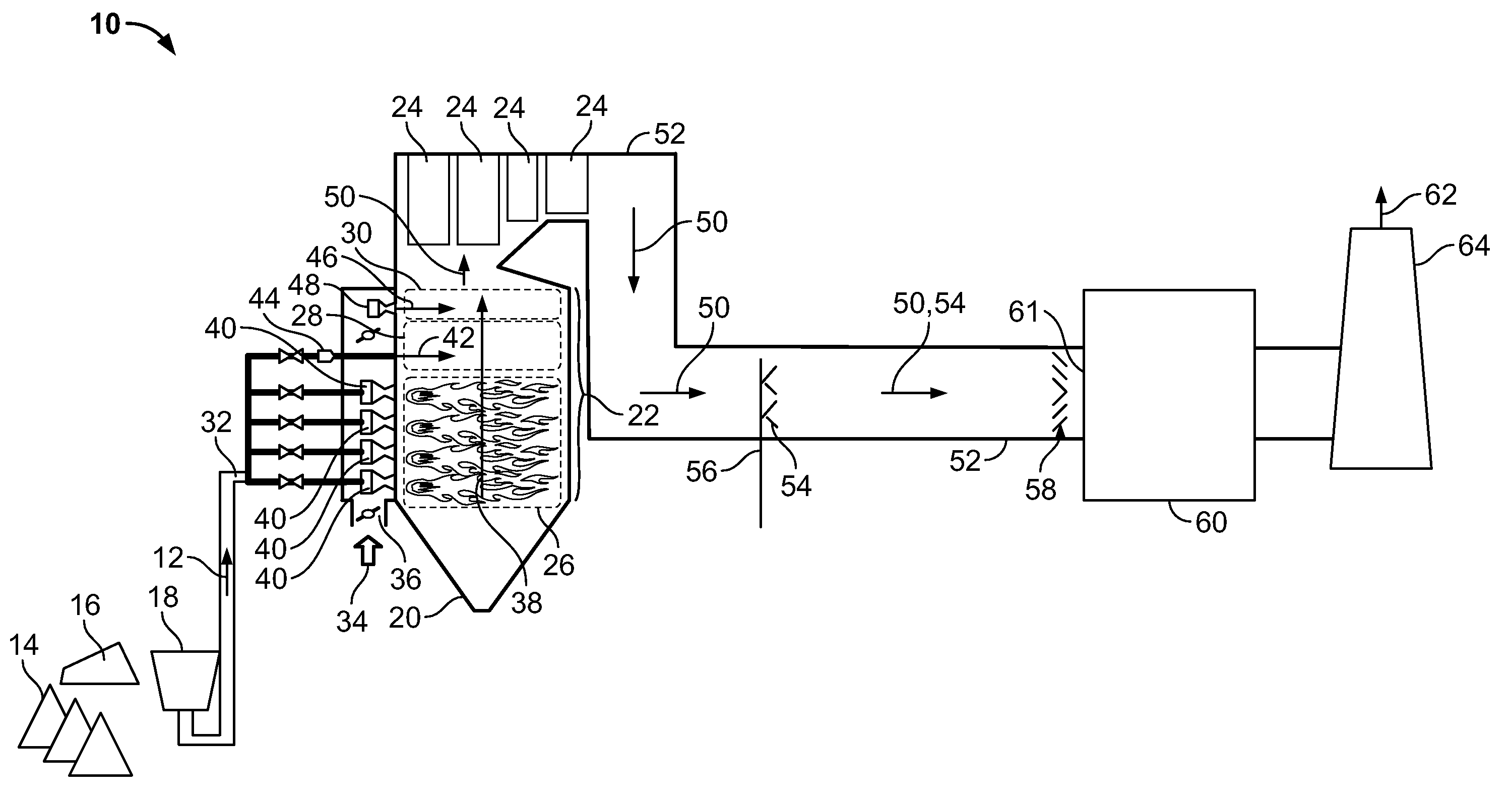
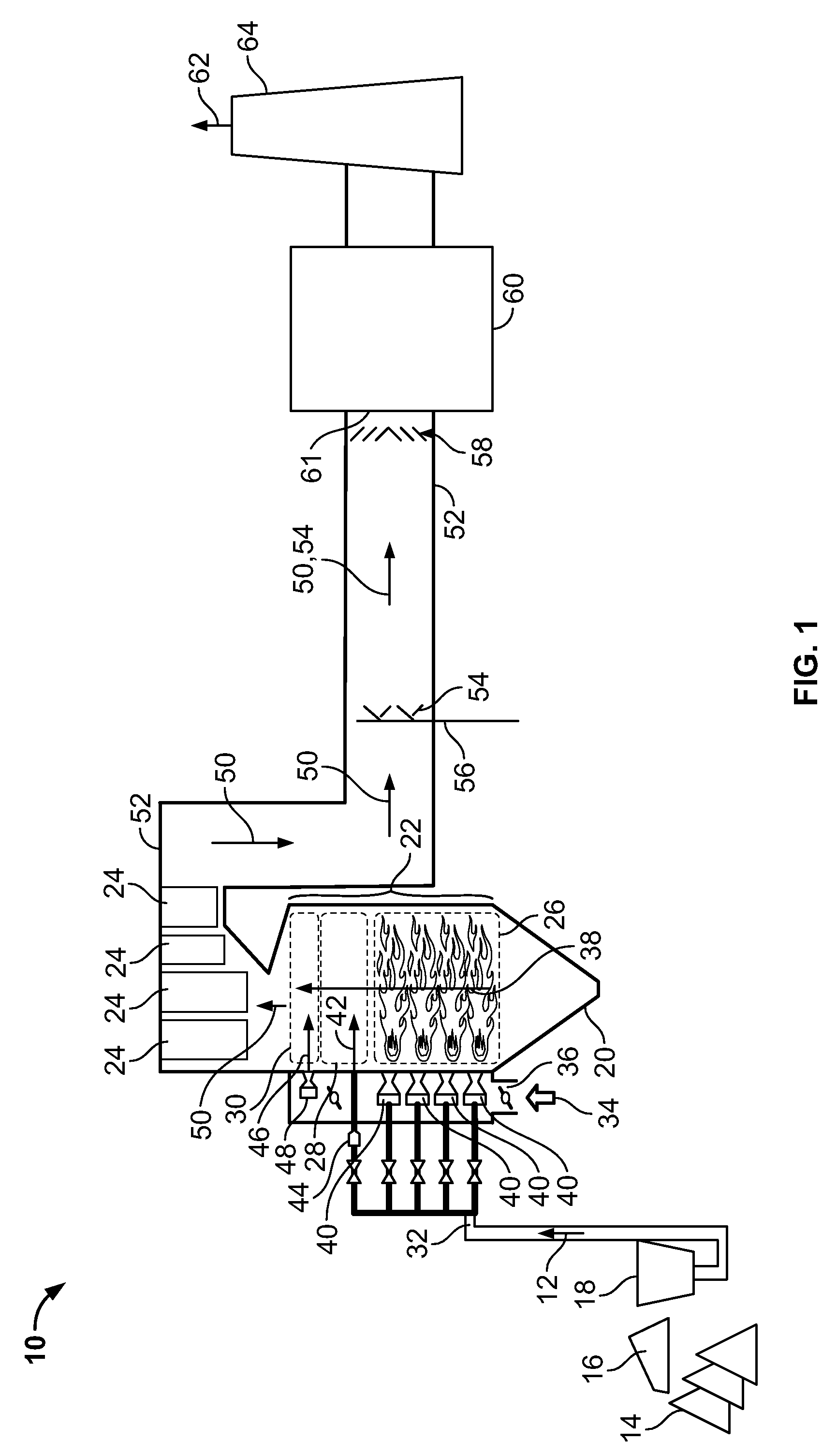
Method for removal and stabilization of mercury in mercury-containing gas streams
InactiveUS6942840B1Improve scalabilityCombination devicesDispersed particle filtrationOxygenVapor phase
The present invention is directed to a process and apparatus for removing and stabilizing mercury from mercury-containing gas streams. A gas stream containing vapor phase elemental and / or speciated mercury is contacted with reagent, such as an oxygen-containing oxidant, in a liquid environment to form a mercury-containing precipitate. The mercury-containing precipitate is kept or placed in solution and reacts with one or more additional reagents to form a solid, stable mercury-containing compound.
Owner:MERCURY CONTROL TECH
Nitride semiconductor crystal and its production method
InactiveUS20110129669A1Easy to produceEfficient productionSemiconductor/solid-state device manufacturingGlass/slag layered productsCrystal growthSeed crystal
A method for efficiently producing a plate-like nitride semiconductor crystal having the desired principal plane in a simple method is provided. A raw material gas is fed to a seed crystal in which a ratio (L / W) of length L in a longitudinal direction and maximum width W, of a plane of projection obtained by projecting a crystal growth face on the seed crystal in a growth direction is from 2 to 400, and the maximum width W is 5 mm or less, thereby growing a plate-like semiconductor crystal on the seed crystal.
Owner:MITSUBISHI CHEM CORP
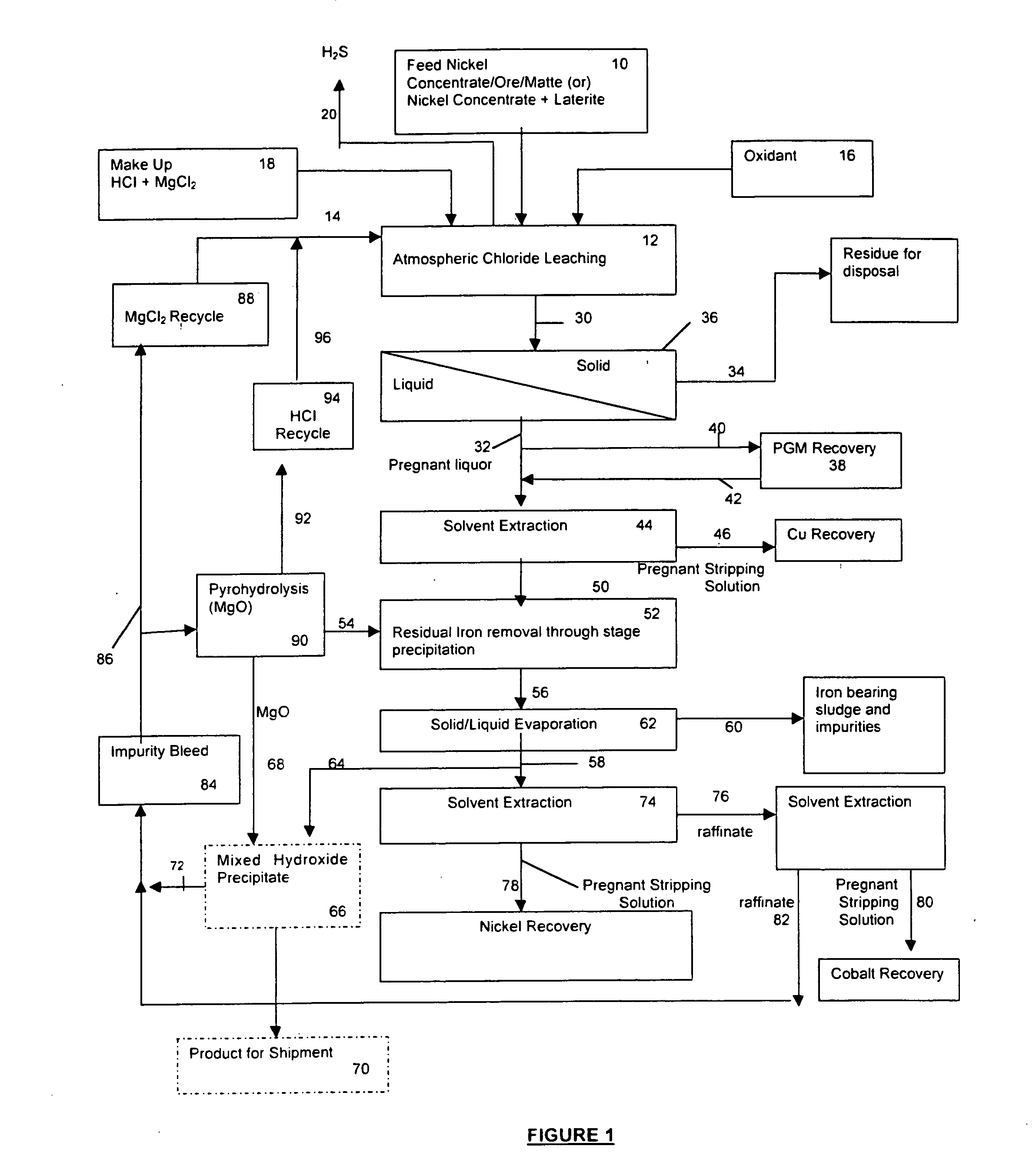
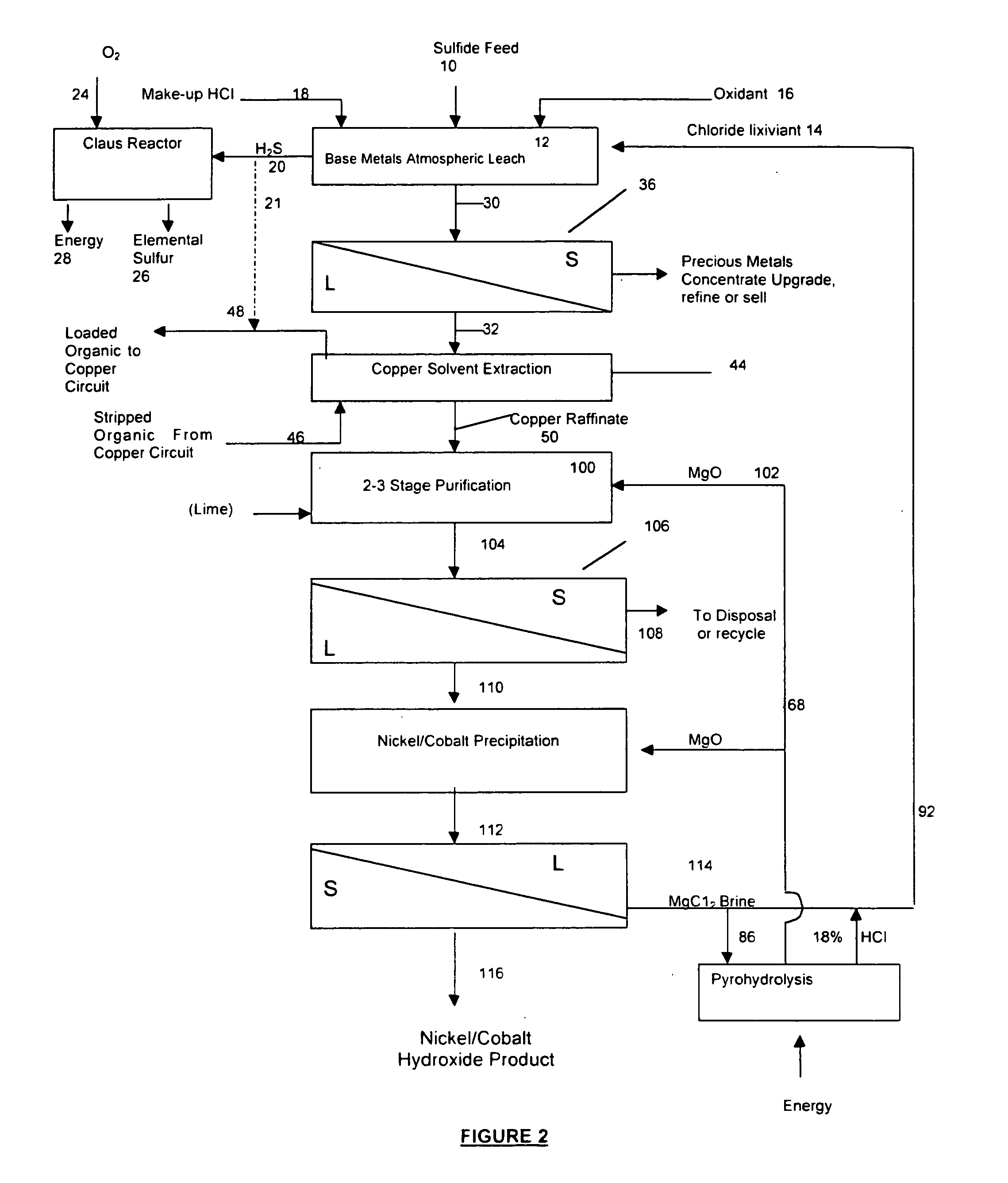
Process for the recovery of value metals from base metal sulfide ores
InactiveUS20050118081A1Simple gas/liquidReduce the amount requiredSulfur compoundsSolid sorbent liquid separationSulfideDissolution
A process for leaching a value metal from a base metal sulfide ore, comprising the step of leaching the ore with a lixiviant comprising a chloride, an oxidant and hydrochloric acid is disclosed. The leaching is controlled, by use of low concentrations of hydrochloric acid and a redox potential, to effect formation of hydrogen sulfide from the base metal sulfide ore. The hydrogen sulfide is stripped from the leach solution, thereby reducing the amount of sulfate generated in the leach to very low levels. The leaching may also be conducted to limit the co-dissolution of platinum group metals and gold with the base value metals. The leach forms a value metal-rich leachate and a solids residue. The solids residue may be subsequently leached to recover the platinum group metals and gold. The value metal-rich leachate can be is oxidized and neutralized to recover the value base metals. In an embodiment, the chloride is magnesium chloride and lixiviant solution is regenerated.
Owner:JAGUAR NICKEL INC
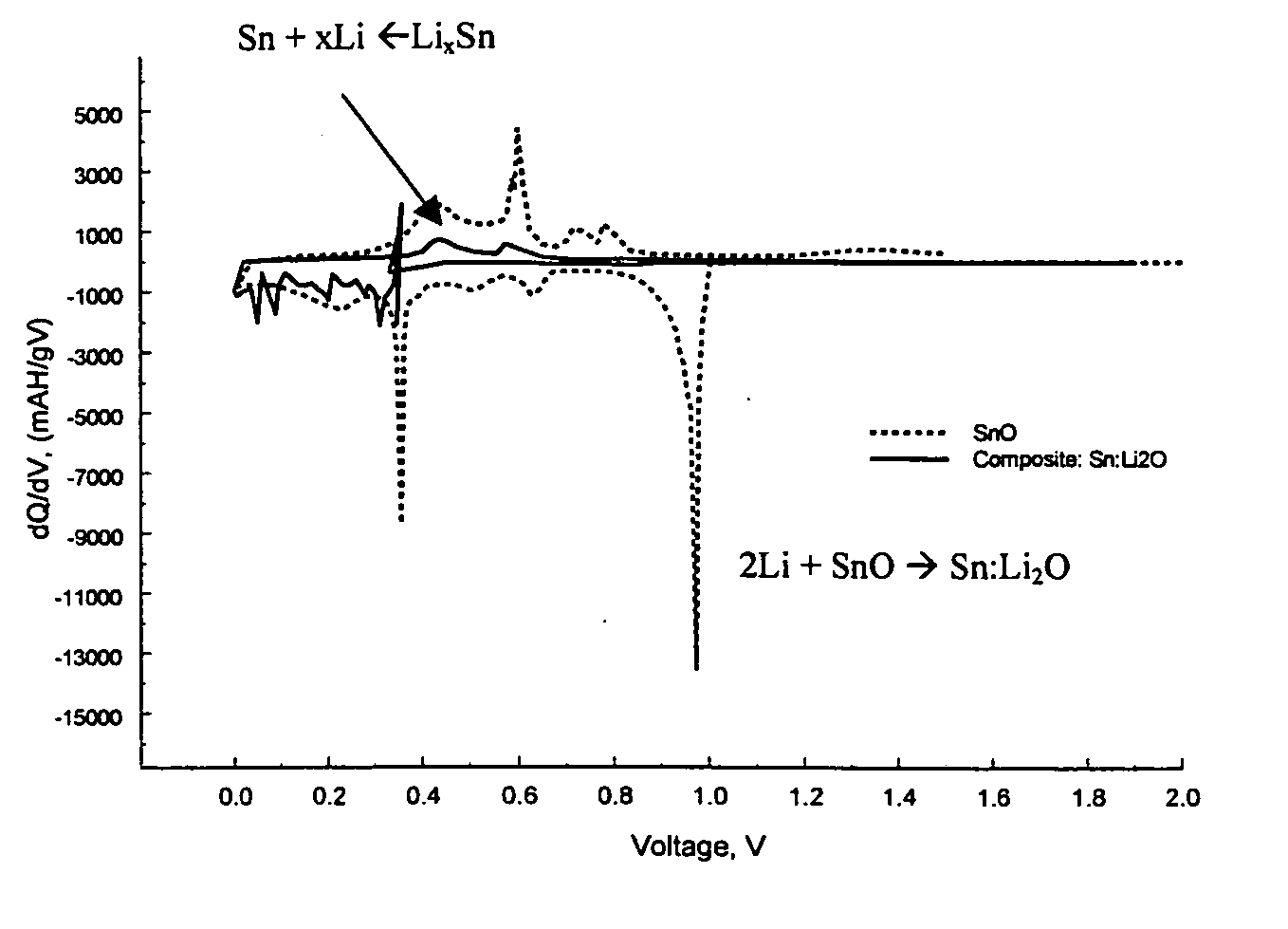
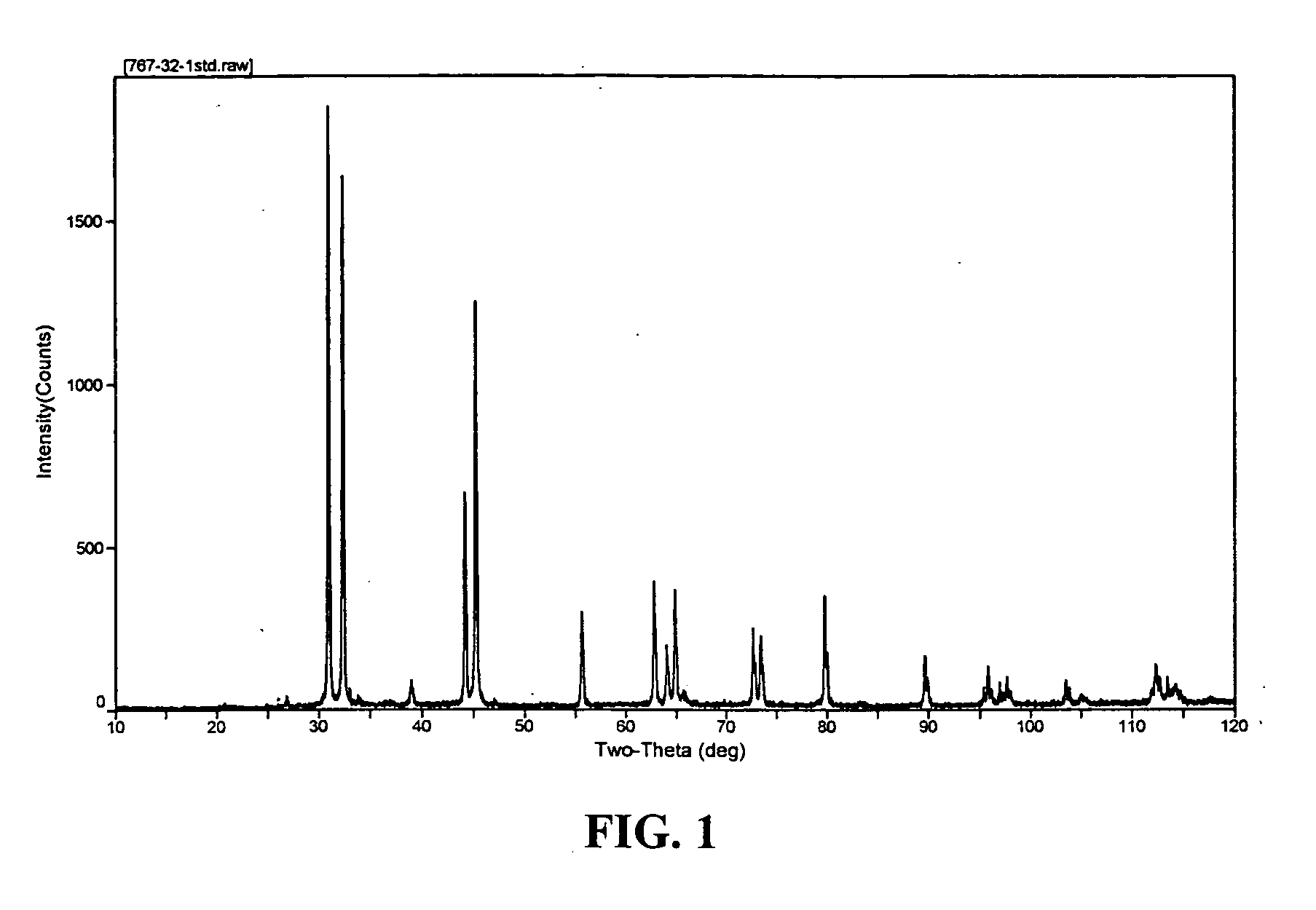
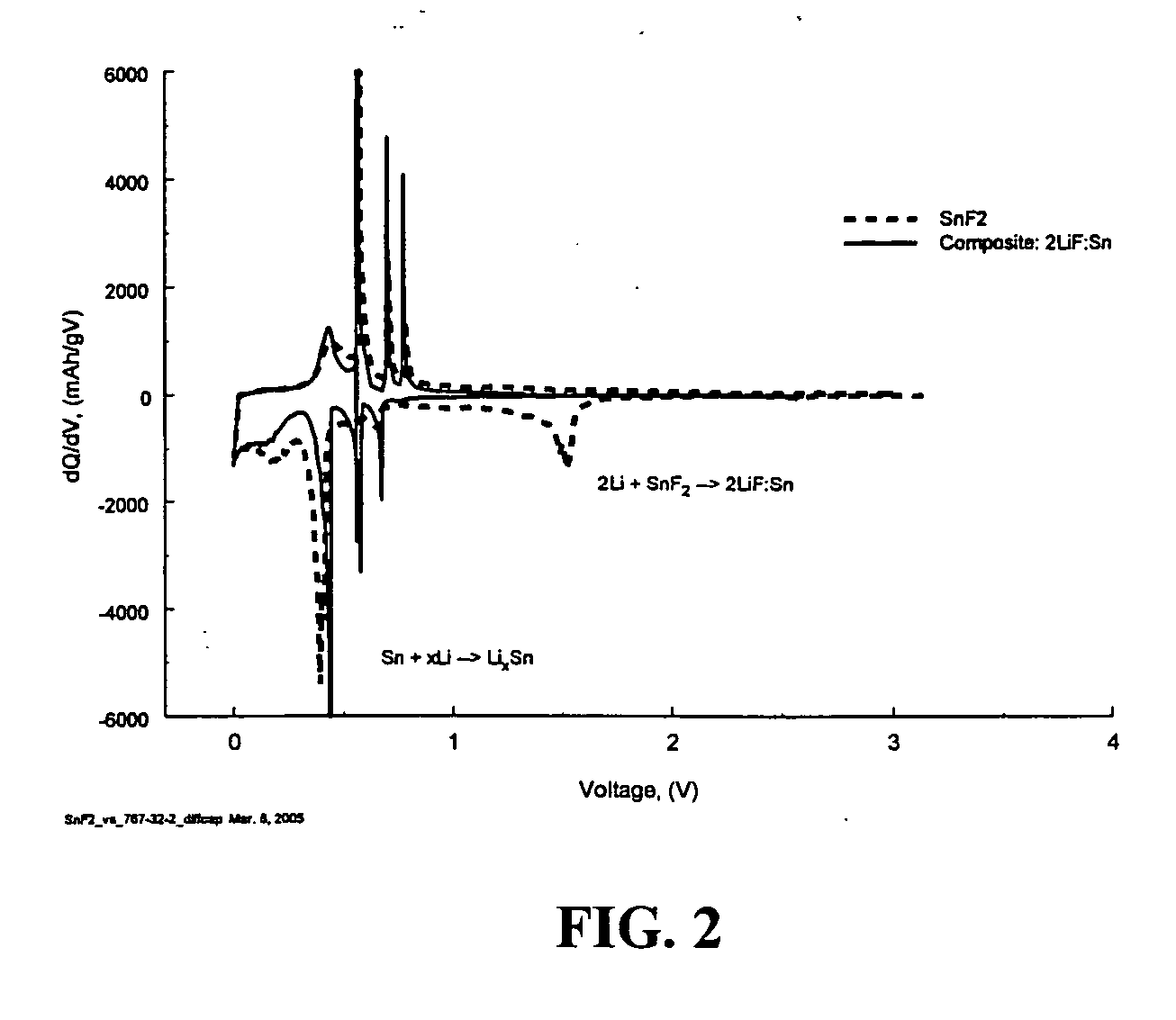
Composite materials of nano-dispersed silicon and tin and methods of making the same
Composite compounds of tin and lithium, silicon and lithium, or tin, silicon, and lithium having tin and silicon nano-dispersed in a lithium-containing matrix may be used as electrode materials and particularly anode materials for use with rechargeable batteries. Methods of making the composite compounds include the oxidation of alloys, the reaction of stabilized lithium metal powder with tin and silicon oxides, and the reaction of inorganic salts of lithium with tin and silicon containing compounds.
Owner:LIVENT USA CORP
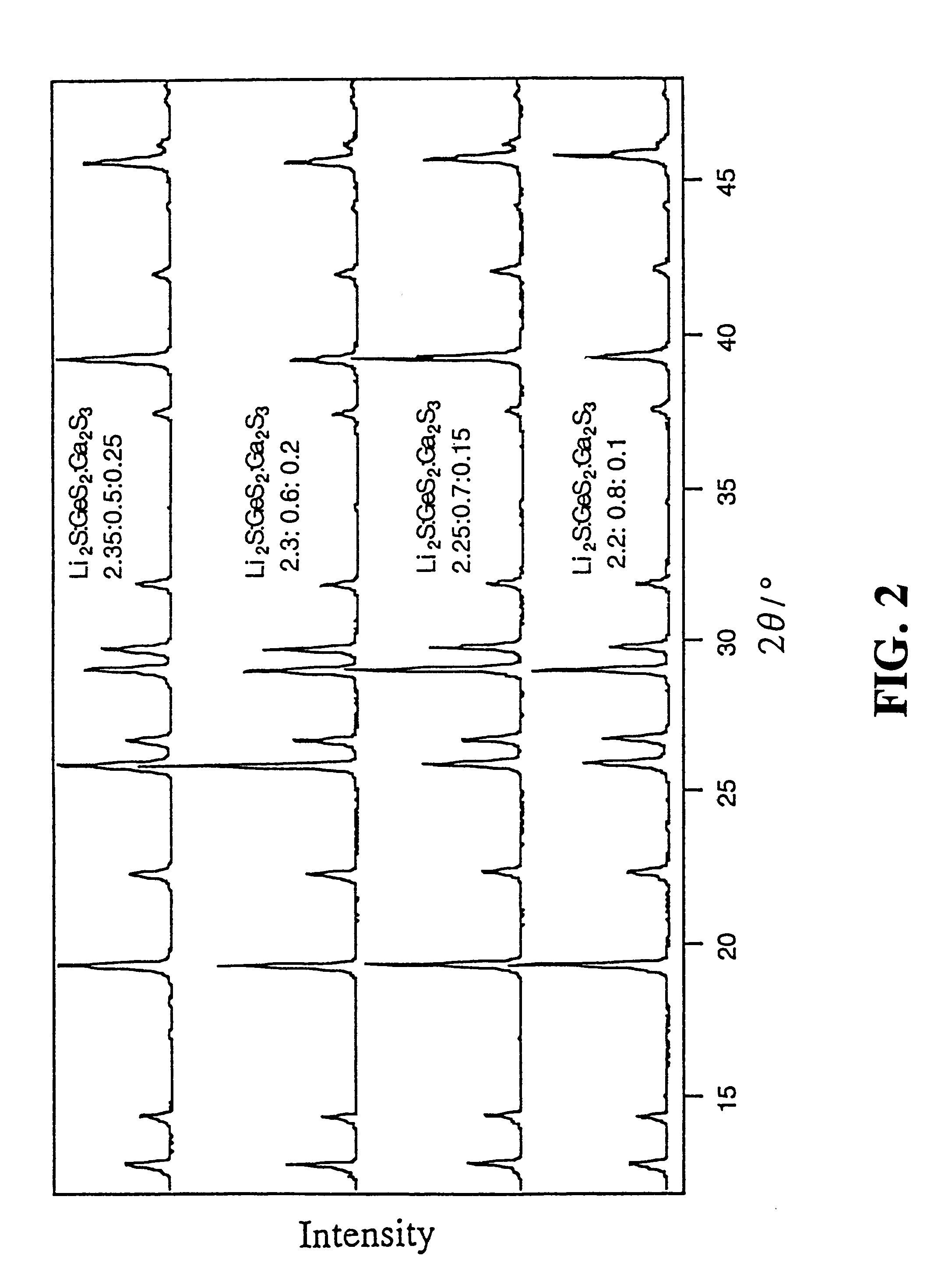
Lithium-ion-conductive solid electrolyte and solid-electrolyte lithium battery
InactiveUS6277524B1Non-metal conductorsGallium/indium/thallium compoundsPhysical chemistryElectrolyte
A lithium-ion-conductive solid electrolyte includes a lithium-ion-conductive substance expressed by a general formula Li2S-GeS2-X wherein "X" is at least one member selected from the group consisting of Ga2S3 and ZnS, or Li2S-SiS2-P2S5. It is superb in terms of stability and safety at elevated temperatures, since it is a crystalline solid of high ion conductivity. It can be applied to a solid electrolyte for lithium batteries.
Owner:TOYOTA JIDOSHA KK +1
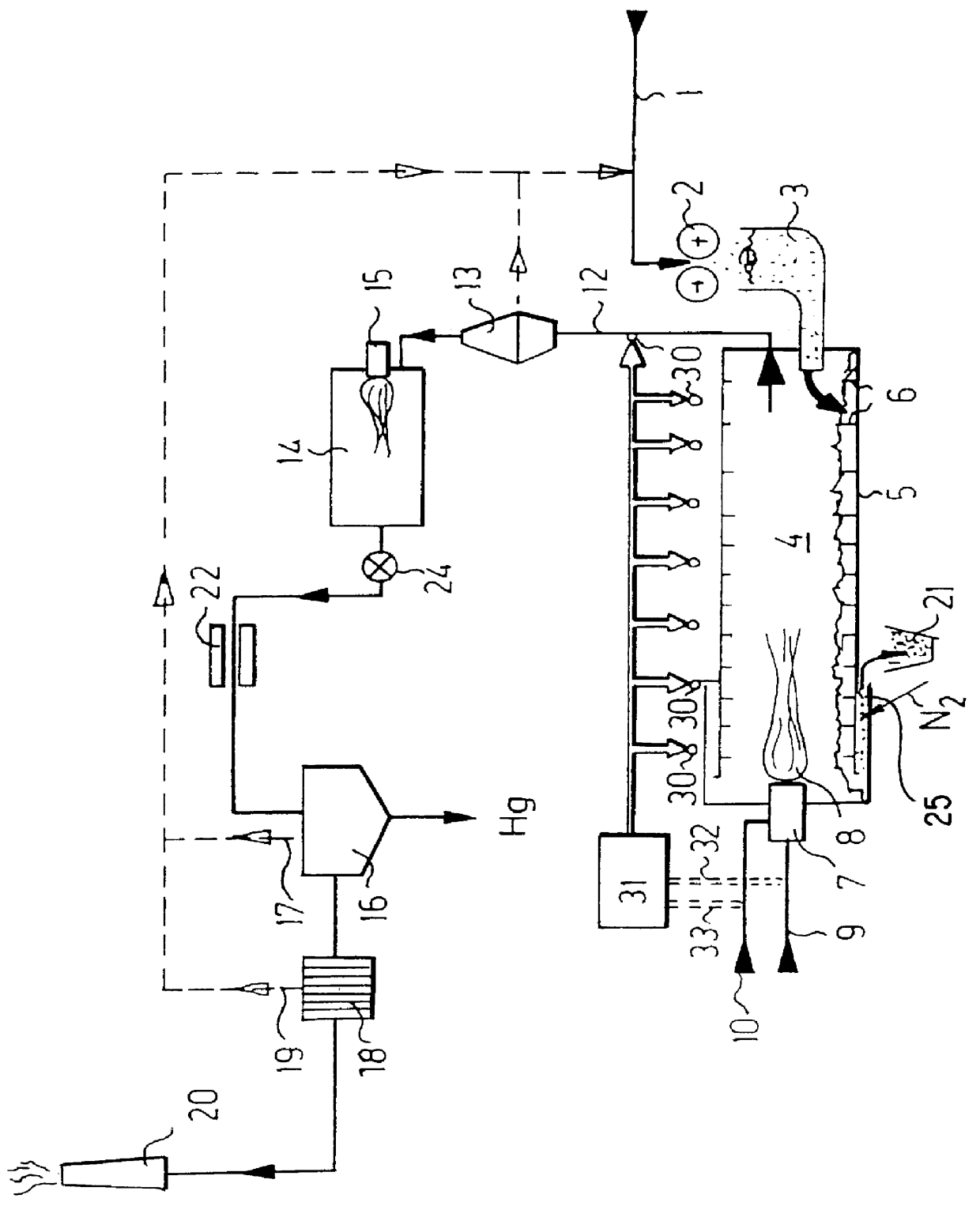
Process for removing mercury from mercury contaminated materials
PCT No. PCT / CH96 / 00252 Sec. 371 Date May 15, 1998 Sec. 102(e) Date May 15, 1998 PCT Filed Jul. 8, 1996 PCT Pub. No. WO97 / 02864 PCT Pub. Date Jan. 30, 1997Mercury-contaminated substances are fed through a shredder to a loader that loads a rotary tubular kiln, in which a mercury content is evaporated by a primary burner. A temperature of an unlined rotary kiln is monitored from an outside at various points over an entire length, by infrared sensors. The thus acquired data are supplied to a regulating unit that regulates fuel supply and primary air supply through various control lines. Burned gas is supplied through a duct to a cyclone separator in which solids are separated and returned to the process. The gaseous substances are fed through a postcombustion chamber of a quench to a washer in which mercury is removed. The residual gas reaches a chimney through an active coal filter. This process is particularly easy to control, economical and allows a particularly low residual mercury content to be reached, for example less than 0.1 ppm, in a residual combustion product.
Owner:DECO HANULIK
Use of multiple stream high pressure mixer/reactor
InactiveUS6159442AHigh strengthMaintain good propertiesCalcium/strontium/barium carbonatesPressurized chemical processStream flowHigh pressure
Owner:MICROFLUIDICS INT
Sorbents and sorbent composition for mercury removal
A system for removing mercury from combustion gas. The system includes a combustion device, a stack, and a duct system that couples the combustion device to the stack. The system further comprises an injection system that is coupled to the duct system. The injection system injects sorbents including alkali-based sorbents and carbon-based sorbents into the duct system.
Owner:GENERAL ELECTRIC CO
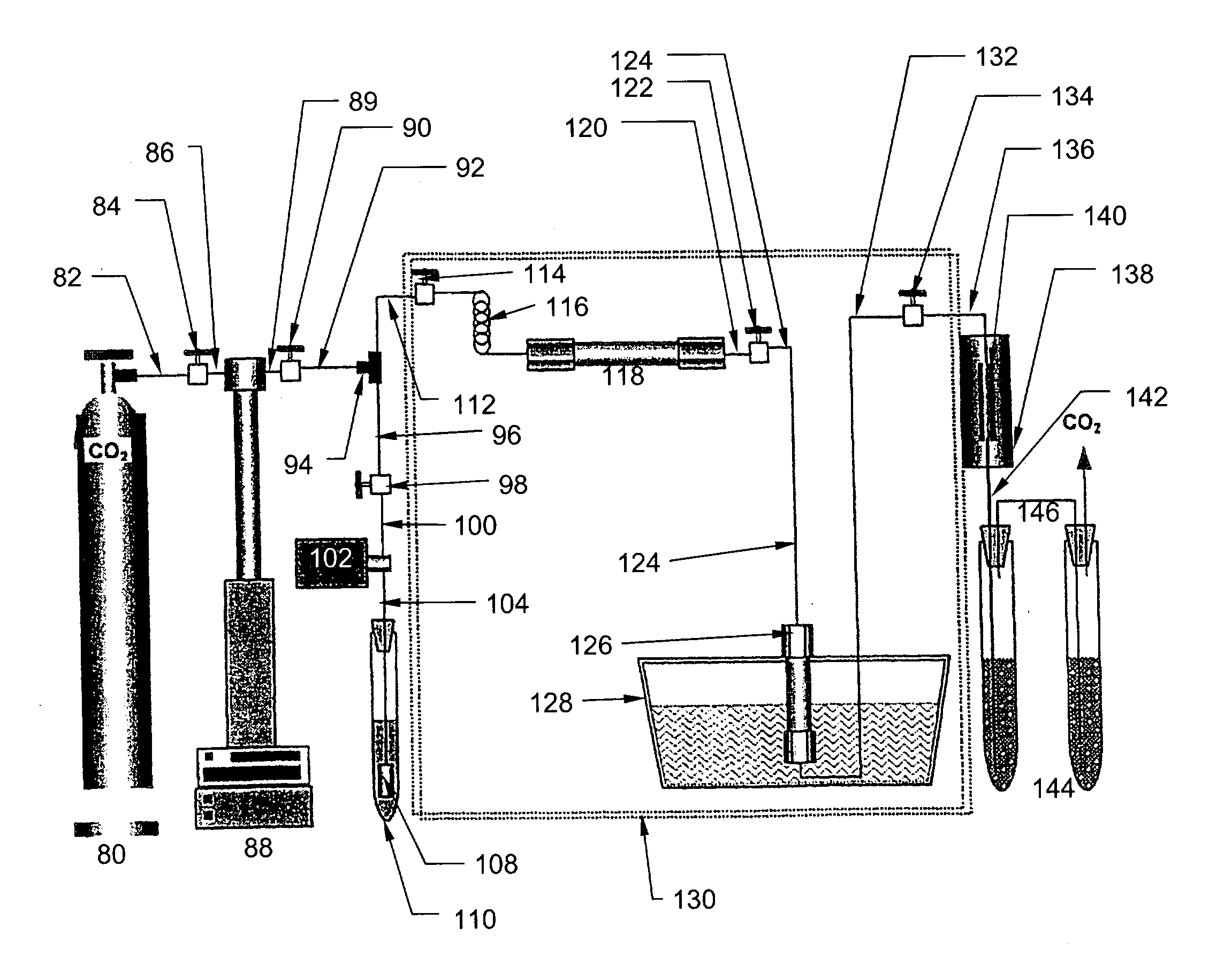
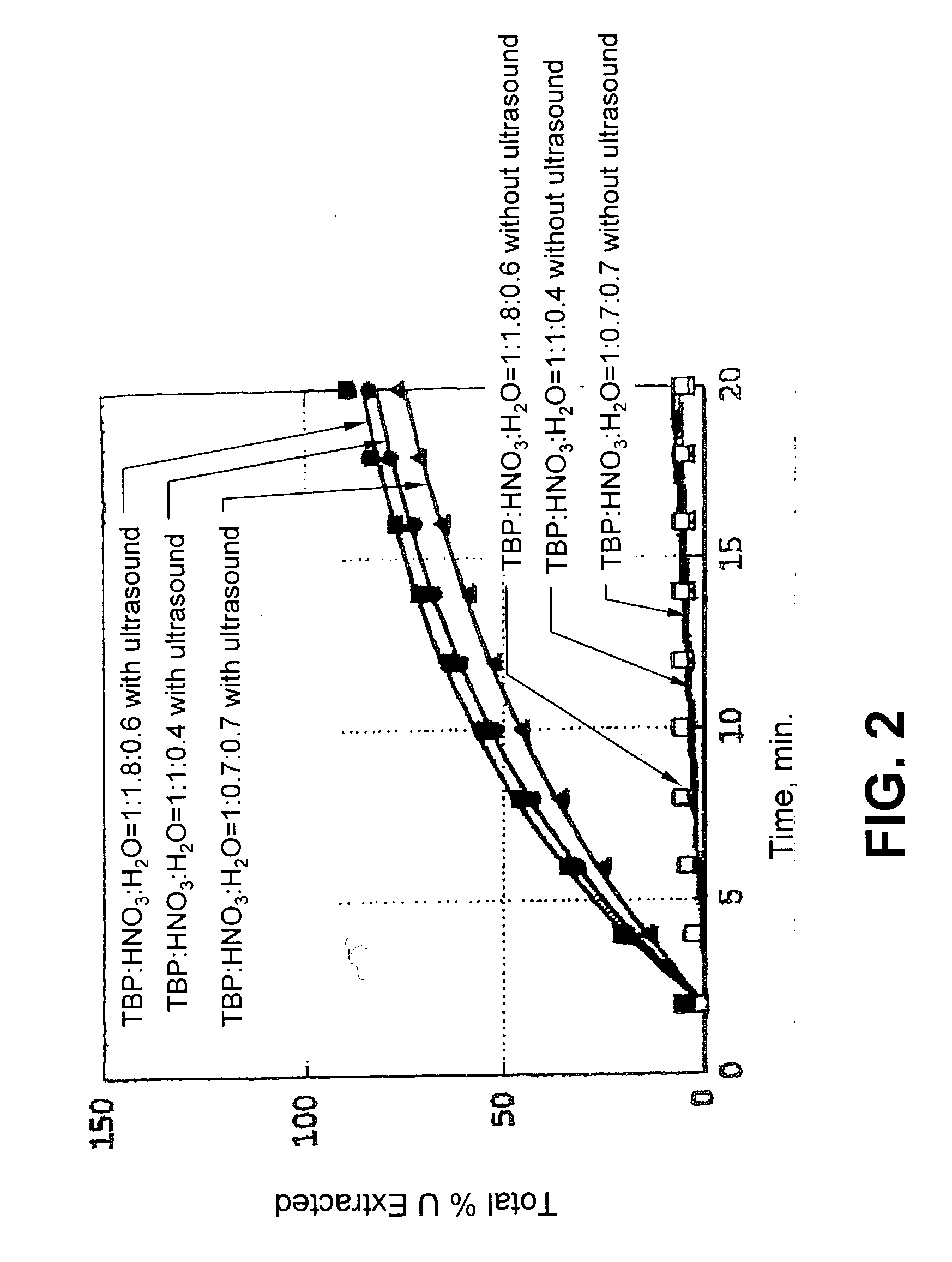
Ultrasound enhanced process for extracting metal species in supercritical fluids
InactiveUS20030183043A1Enhances rate and efficiencyReduce probabilitySolid sorbent liquid separationGold compoundsUranium oxidePresent method
Improved methods for the extraction or dissolution of metals, metalloids or their oxides, especially lanthanides, actinides, uranium or their oxides, into supercritical solvents containing an extractant are disclosed. The disclosed embodiments specifically include enhancing the extraction or dissolution efficiency with ultrasound. The present methods allow the direct, efficient dissolution of UO2 or other uranium oxides without generating any waste stream or by-products.
Owner:NAGOYA INDUSTRIAL SCIENCE RESEARCH INST +1
Rapid metal organic framework molecule synthesis method
InactiveUS20090131643A1Rapidly synthesizing MOF moleculesSynthesis fastGroup 8/9/10/18 element organic compoundsCopper organic compoundsSynthesis methodsMetal-organic framework
A rapid, simple and versatile metal organic framework molecule (MOF) synthesis method particularly adapted to make non-linear MOFs includes heating MOF precursors, such as a metal or metal oxide and an organic ligand, in a microwave oven for a period sufficient to achieve crystallization. Microwave-assisted MOF synthesis yields high quality MOF crystals in a reaction time ranging from about 5 seconds to about 2.5 minutes, compared to hours and days required in conventional solvothermal and hydrothermal methods. In addition, microwave assisted methods provide MOF materials with uniform crystal size and well-defined shape. Further, microwave synthesis of MOFs allows the size and shape of MOF crystals to be tailored for use in a wide range applications by manipulating reaction conditions. Secondary growth processes may also be employed to grow larger crystals using seeds obtained from microwave-assisted synthesis methods.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Nano-size layered double hydroxide and step-by-step precipitation preparation method thereof
InactiveCN103159238ARich sourcesLow costMaterial nanotechnologyTitanium compoundsPhysical chemistryAdsorption separation
The invention provides a nano-size layered double hydroxide and a step-by-step precipitation preparation method thereof. The preparation method comprises the following steps: based on a soluble salt of a metal and an alkali as raw materials, respectively precipitating metal ions constituting an LDH (layered double hydroxide) layer plate through step-by-step precipitation reaction and generating the LDH during the second-step precipitation process. As the activity of the hydroxide prepared by the first-step precipitation reaction is high, the LDH can fast nucleate and grow during the second-step precipitation, an obtained LDH sheet is thinner, the thickness of the layer plate is 5-15nm, the length of the layer plate is 50-150nm, and the specific surface area of a BET (Brunauer-Emmett-Teller) is 140-280m<2> / g, which is much larger than the specific surface area of the common LDH. By adopting the method, the shortcoming of small specific surface area during the preparation of the LDH through a co-precipitation method can be overcome. The preparation method adopted by the invention is simple and convenient, high temperature and high pressure are not required, special equipment is not required, sources of the raw materials are rich, and the cost is low. The layered double hydroxide can be widely applied to the fields of adsorption separation, catalysis, high polymer materials and the like.
Owner:BEIJING UNIV OF CHEM TECH
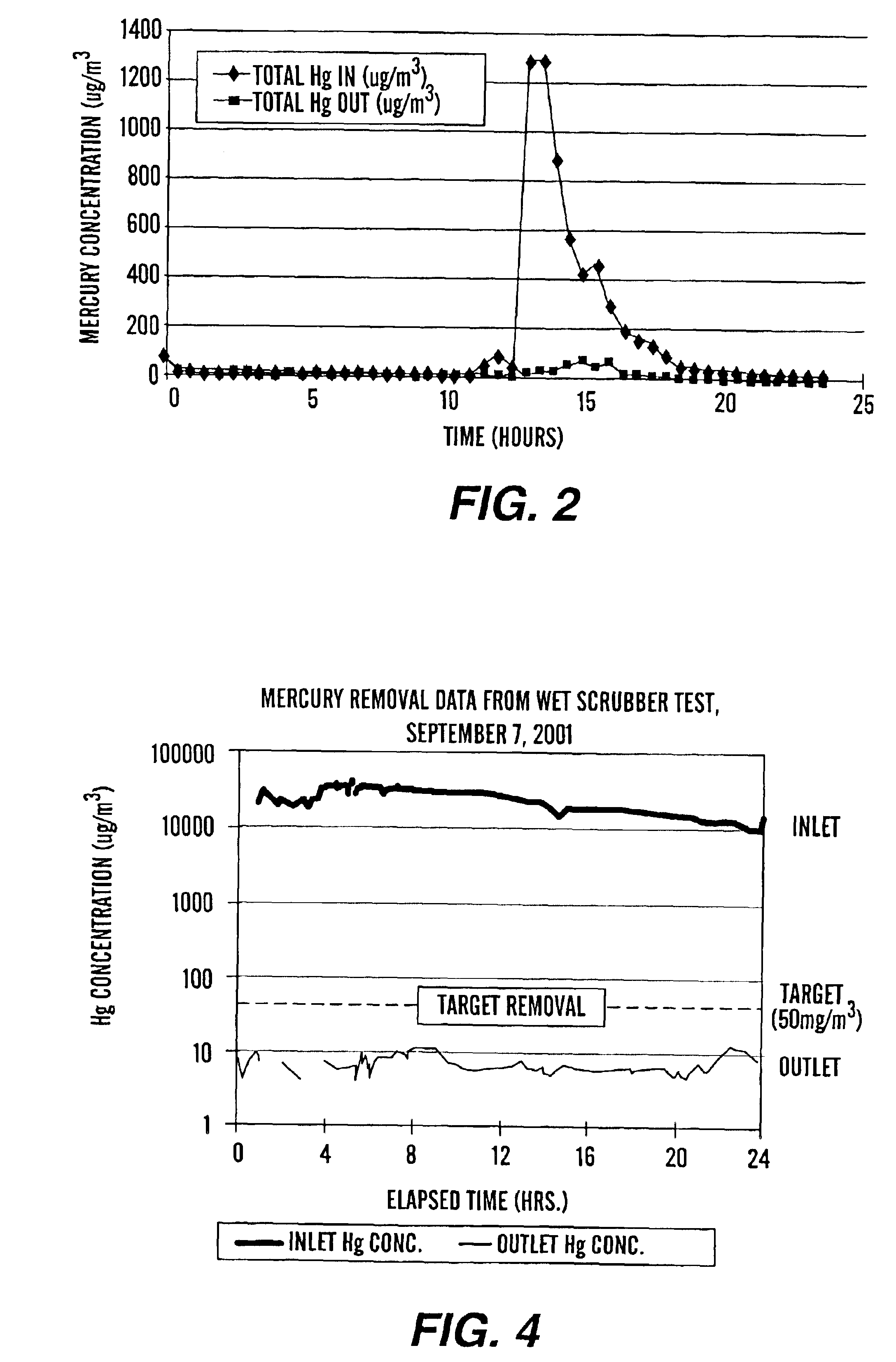
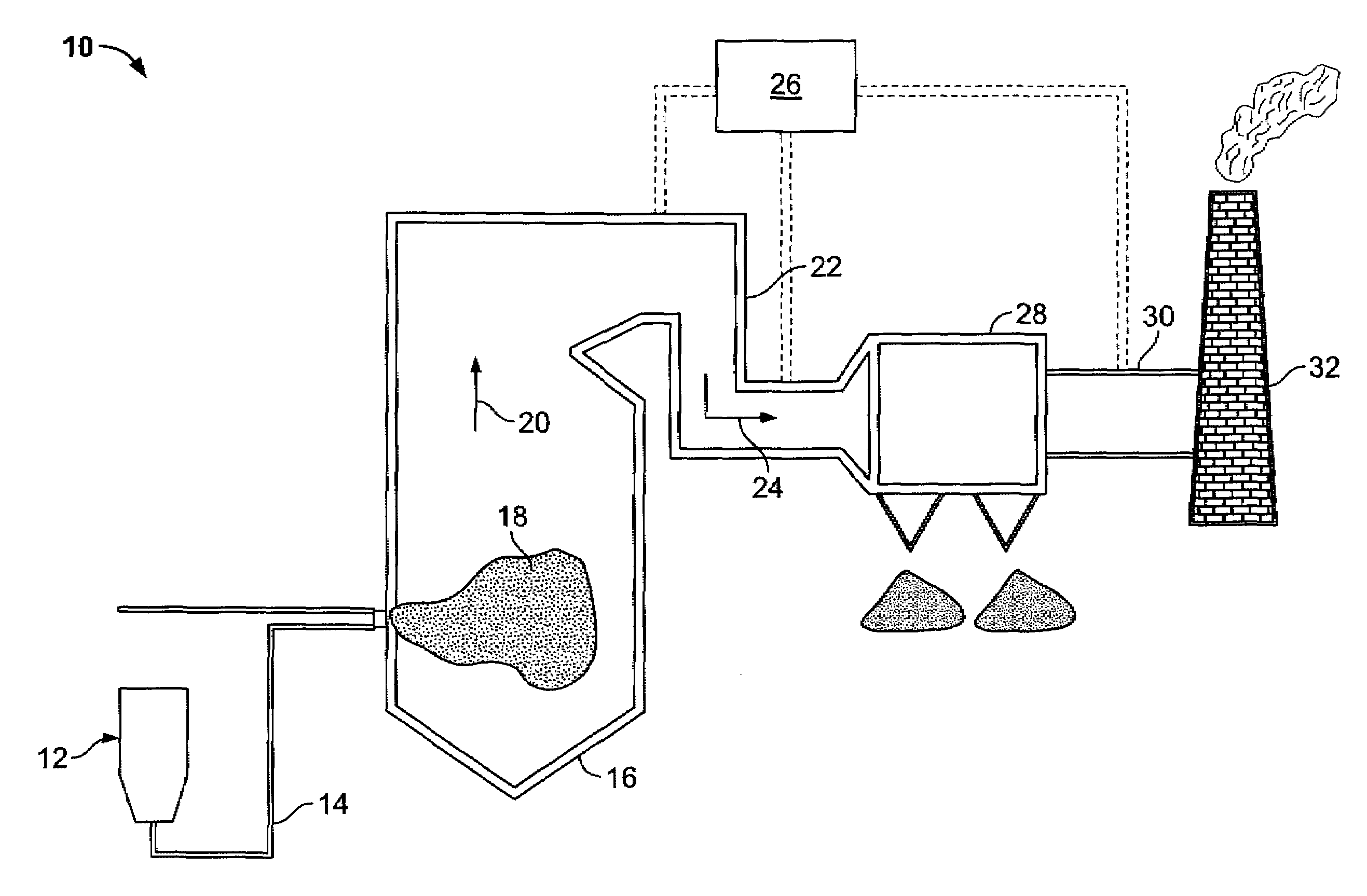
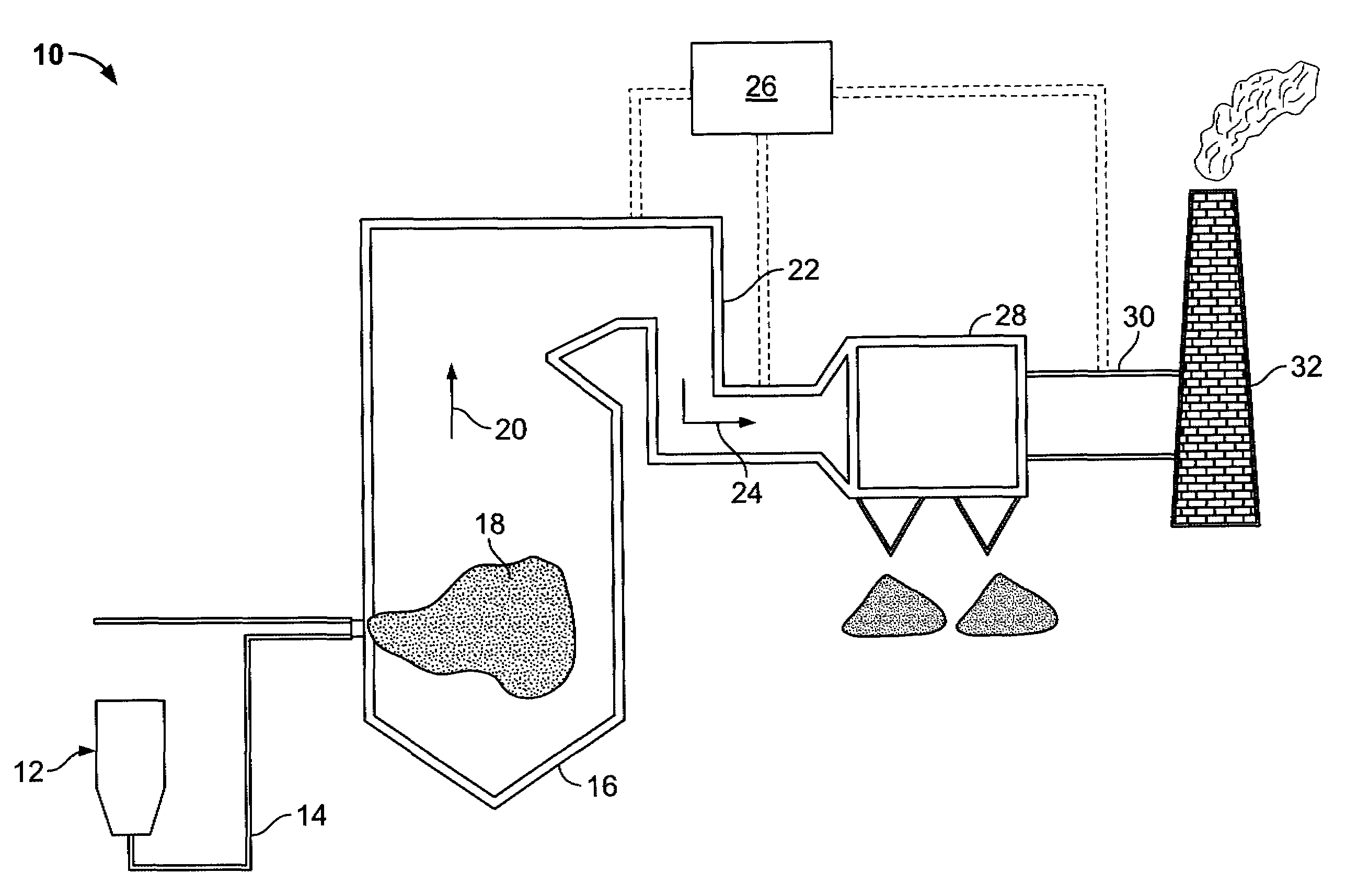
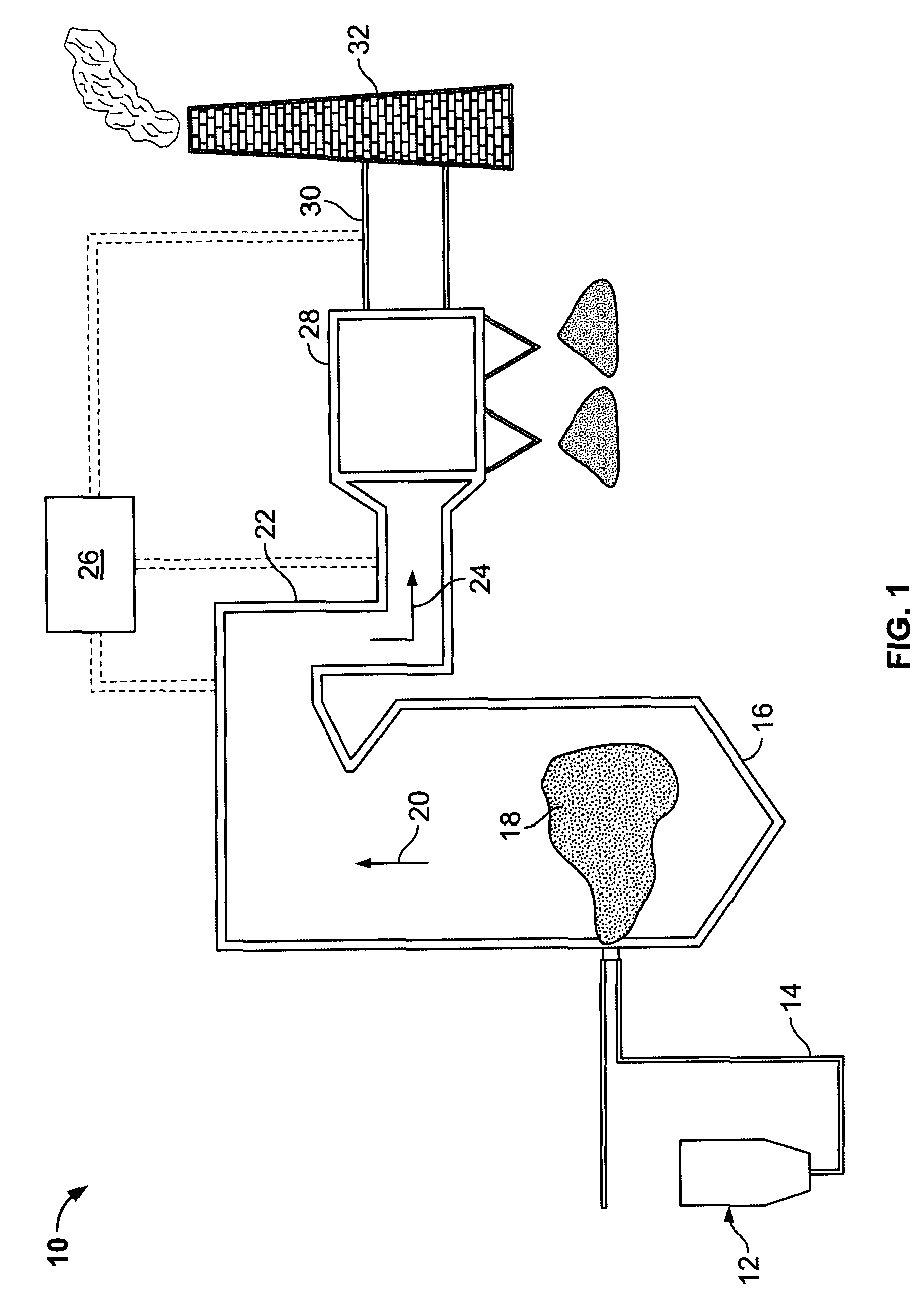
Method of mercury removal in a wet flue gas desulfurization system
Controlling the reductive capacity of an aqueous alkaline slurry (23) in a wet scrubber makes it possible to accurately control the mercury emission from the scrubber to a desired value. One method of controlling the reductive capacity of the slurry is to measure the reduction-oxidation potential (“redox potential”) of the aqueous alkaline slurry (23) and to add or remove substances that affect the redox potential and thus the reductive capacity of the slurry. In wet scrubbers in which limestone is used for absorption of acid gases and where a gypsum slurry is circulated, it has been found to be an attractive solution to control the amount of oxidation air blown into the scrubber in order to control the redox potential and thereby the mercury emissions.
Owner:GENERAL ELECTRIC TECH GMBH
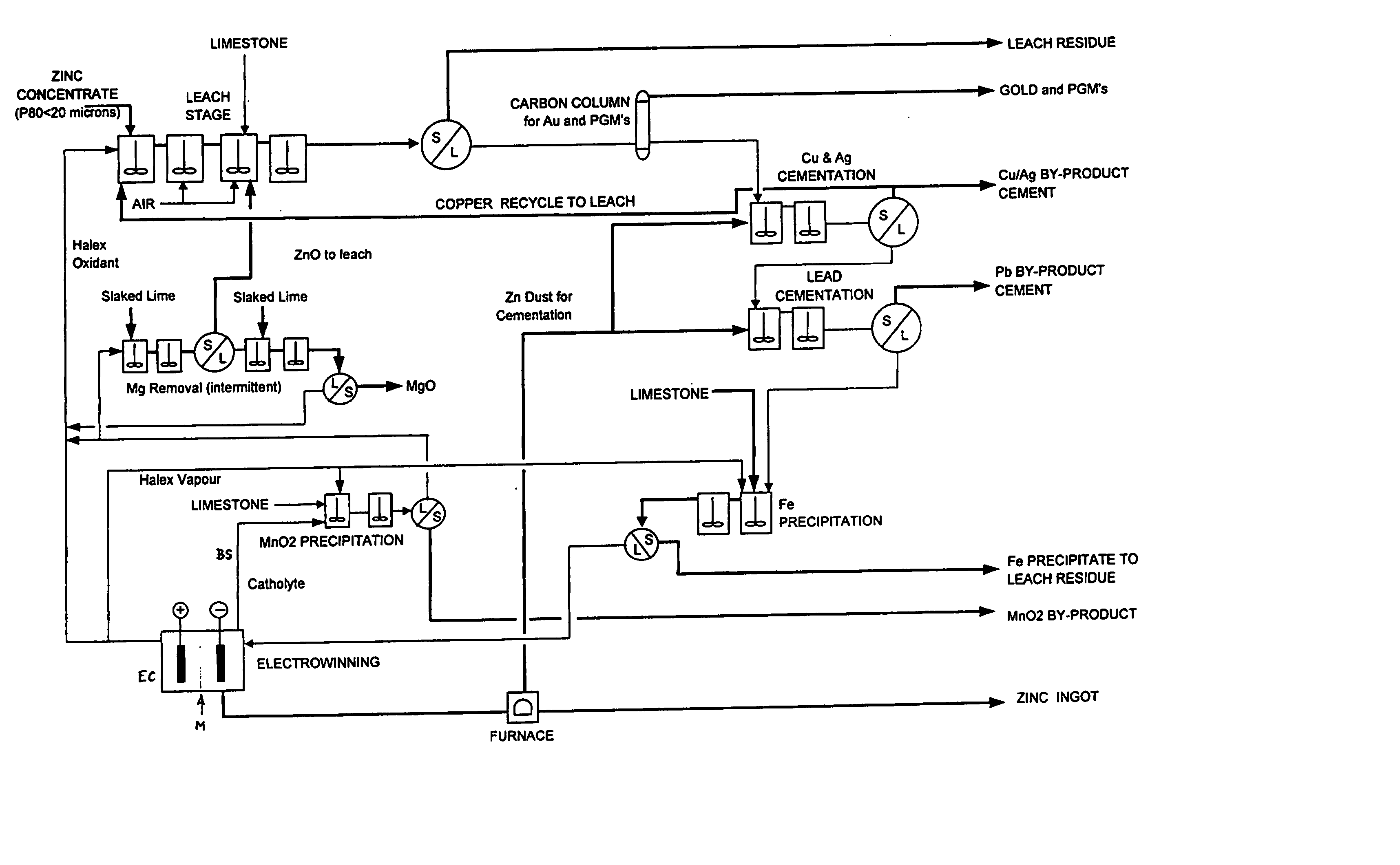
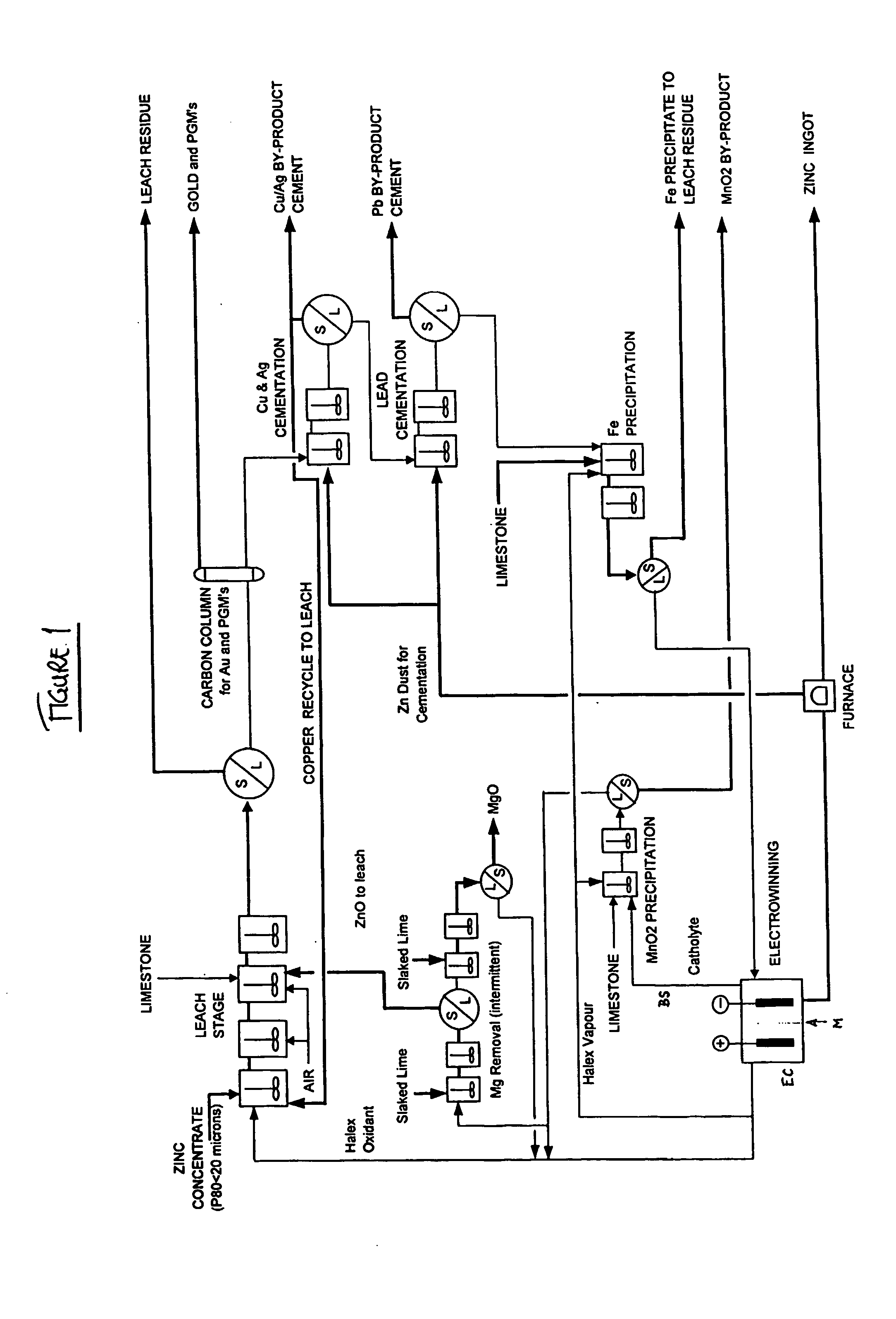
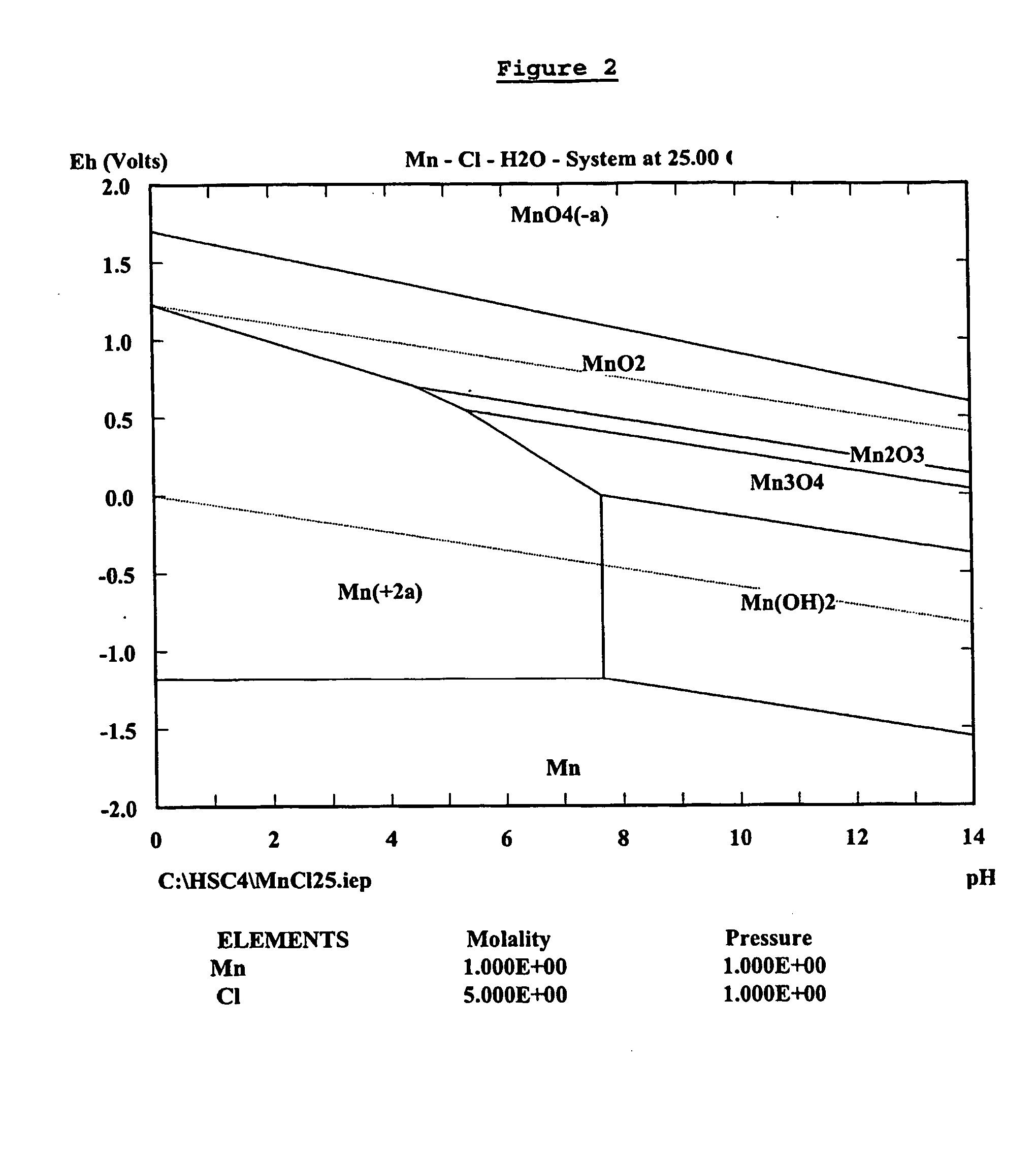
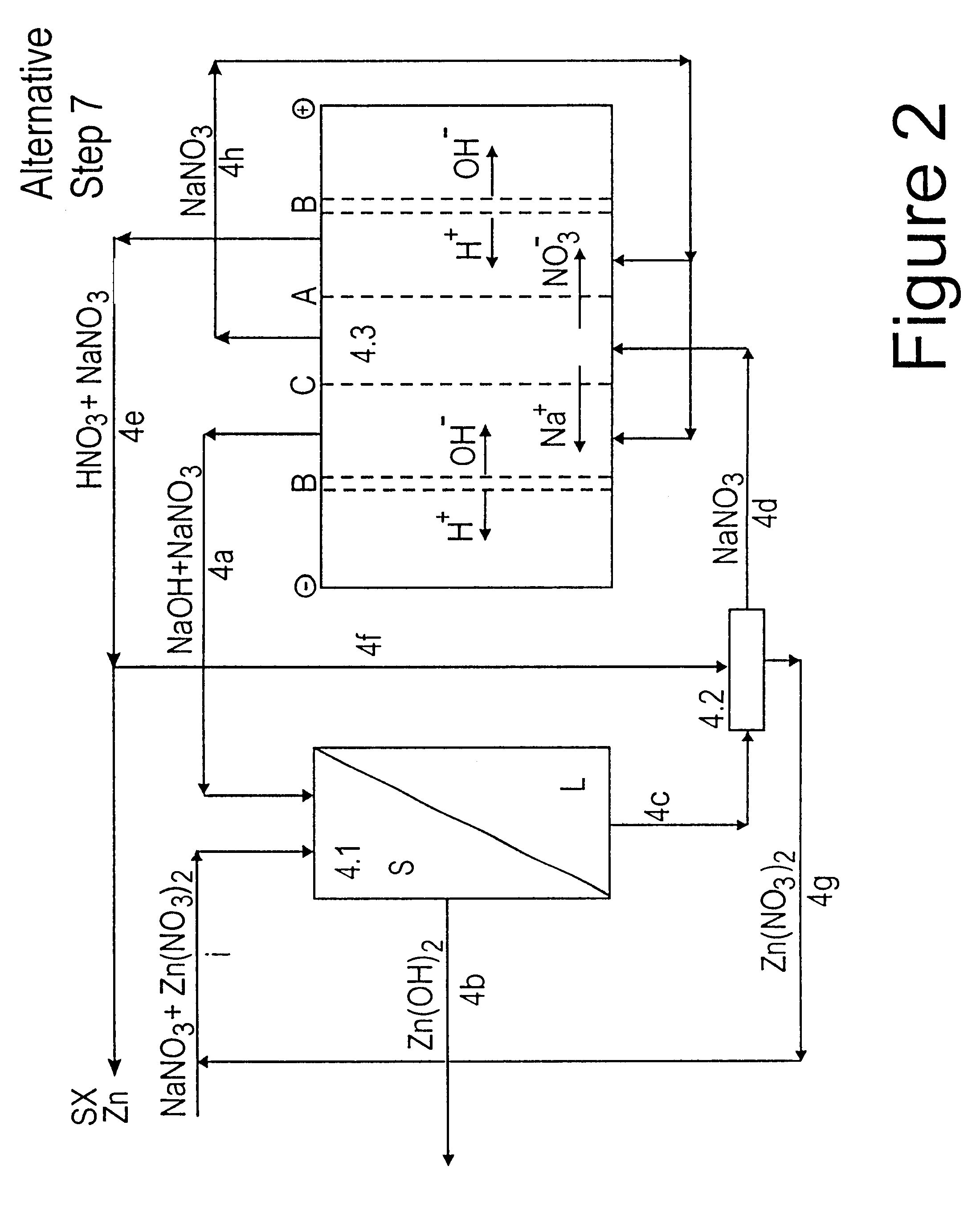
Zinc recovery process
InactiveUS20040237720A1Improve filtering effectEh is decreasedPhotography auxillary processesSolvent extractionZinc metalManganese
A process for the recovery of zinc metal from a zinc mineral includes the steps of leaching the zinc mineral in a solution including a halide species formed from two or more different halides, to leach the zinc into the solution. The zinc-bearing solution is then electrolysed to yield zinc metal and to generate the halide species. The electrolysed solution including the halide species is then returned to the leaching step. A portion of the electrolysed solution can be removed as a bleed stream from a cathode compartment of an electrolytic cell of the electrolysis process and processed to remove manganese as manganese dioxide precipitate by adding thereto limestone, and the halide species from an anode compartment of the electrolysis process. In this regard, the pH and Eh of the solution can regulated in a manner that favours the formation of the manganese dioxide precipitate over the formation of a precipitate of zinc.
Owner:INTEC INT PROJECTS
Sorbents and sorbent composition for mercury removal
A system for removing mercury from combustion gas. The system includes a combustion device, a stack, and a duct system that couples the combustion device to the stack. The system further comprises an injection system that is coupled to the duct system. The injection system injects sorbents including alkali-based sorbents and carbon-based sorbents into the duct system.
Owner:GENERAL ELECTRIC CO
Mercury reduction system and method in combustion flue gas using coal blending
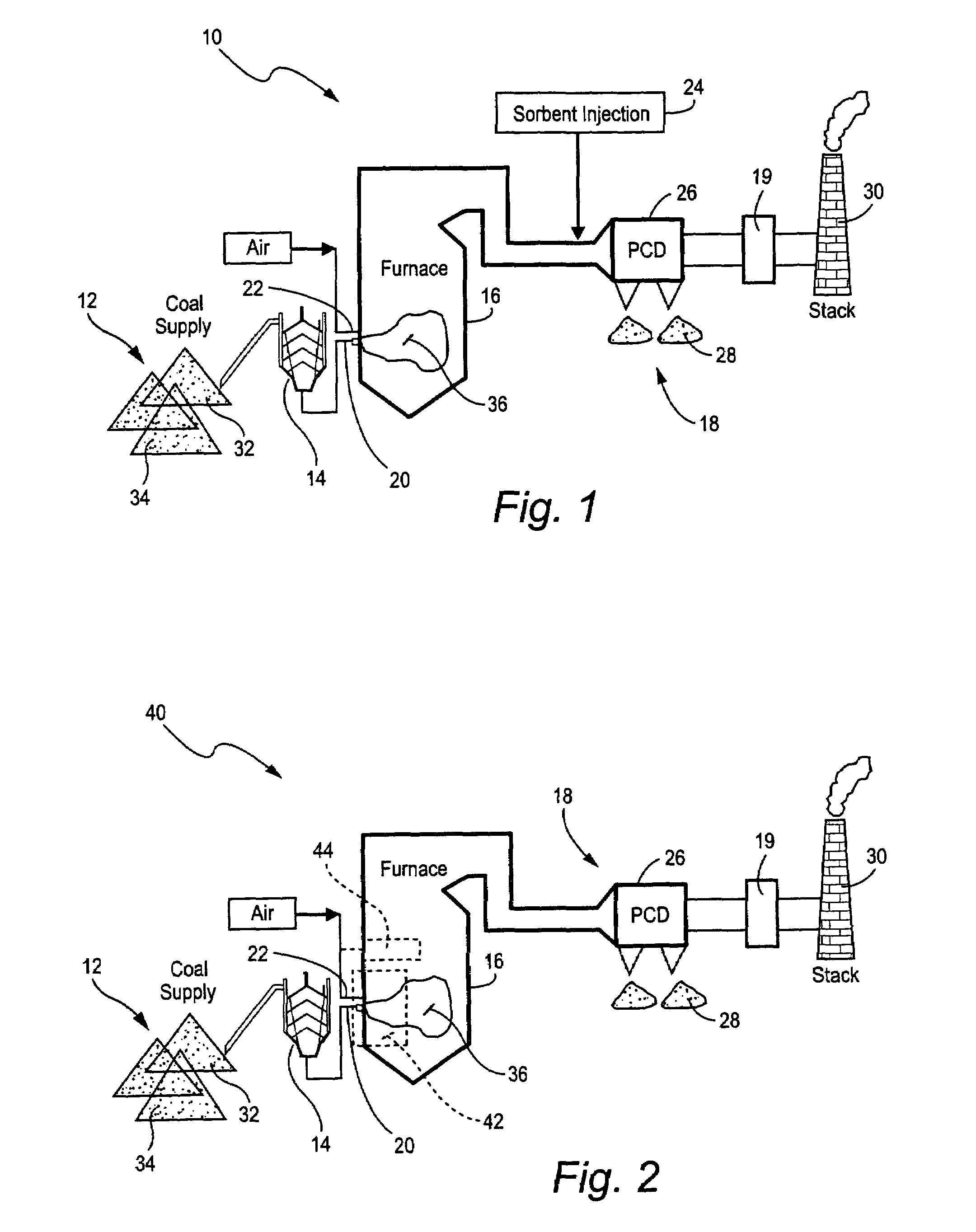
ActiveUS7381387B2Reduce gas emissionsGas treatmentUsing liquid separation agentCombustion systemCombustion
A method to reduce mercury in gas emissions from the combustion of low rank coal in a combustion system including: combusting coal having a low chlorine content in the combustion system, wherein elemental mercury (Hg0) is released in the flue gas produced by the combustion of the low rank coal; releasing chlorine into the flue gas by combusting a coal having a high chlorine in the combustion system; reacting the elemental mercury and released chlorine in the flue gas to oxidize the mercury; adsorbing at least a portion of the oxidized mercury generated by the combustion of the coal with an adsorbent in the flue gas, and collecting the adsorbent with the oxidized mercury in a combustion waste treatment system.
Owner:GENERAL ELECTRIC CO
Electrode active material for secondary battery and method for preparing the same
ActiveUS20110311875A1Minimizing dead volumeHigh-capacity batteryMaterial nanotechnologyGallium/indium/thallium compoundsParticulatesLithium oxide
The disclosure relates to an electrode active material including: (a) first particulate of a metal (or metalloid) oxide alloyable with lithium; and (b) second particulate of an oxide containing lithium and the same metal (or metalloid) as that of the metal (or metalloid) oxide, and to a secondary battery including the electrode active material. When the electrode active material is used as an anode active material, reduced amounts of an irreversible phase such as a lithium oxide or a lithium metal oxide are produced during initial charge-discharge of a battery since lithium is already contained in the second particulate before the initial charge-discharge, and thus a dead volume on the side of the cathode can be minimized and a high-capacity battery can be fabricated.
Owner:LG ENERGY SOLUTION LTD
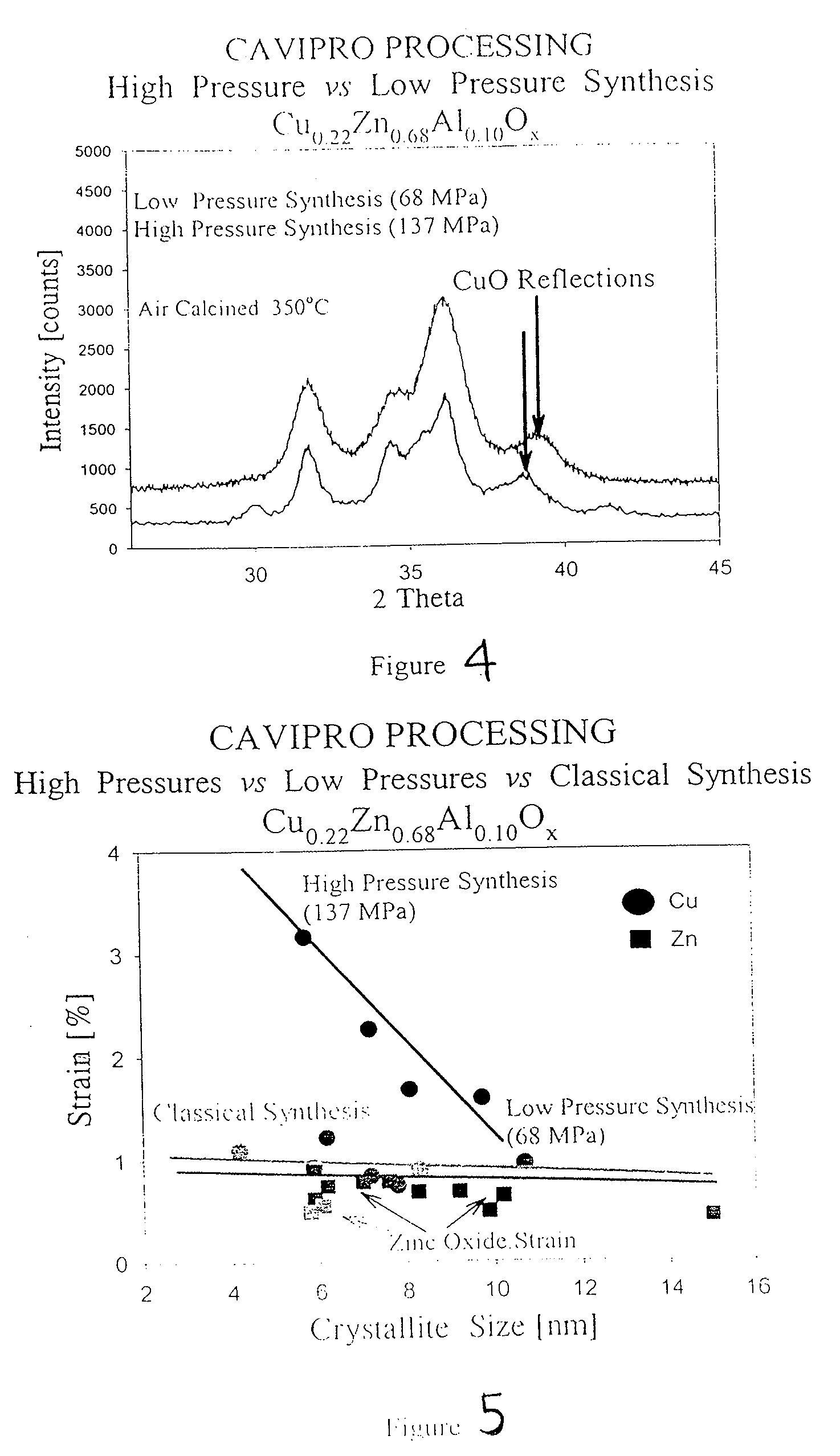
Method of preparing compounds using cavitation and compounds formed therefrom
InactiveUS20070066480A1Efficient modificationEasy to adjustMaterial nanotechnologyTransportation and packagingCompound (substance)Nanostructured materials
Nanostructured materials and processes for the preparation of these nanostructured materials in high phase purities using cavitation is disclosed. The method preferably comprises mixing a metal containing solution with a precipitating agent and passing the mixture into a cavitation chamber. The chamber consists of a first element to produce cavitation bubbles, and a second element that creates a pressure zone sufficient to collapse the bubbles. The process is useful for the preparation of catalysts and materials for piezoelectrics and superconductors.
Owner:WORCESTER POLYTECHNIC INSTITUTE +1
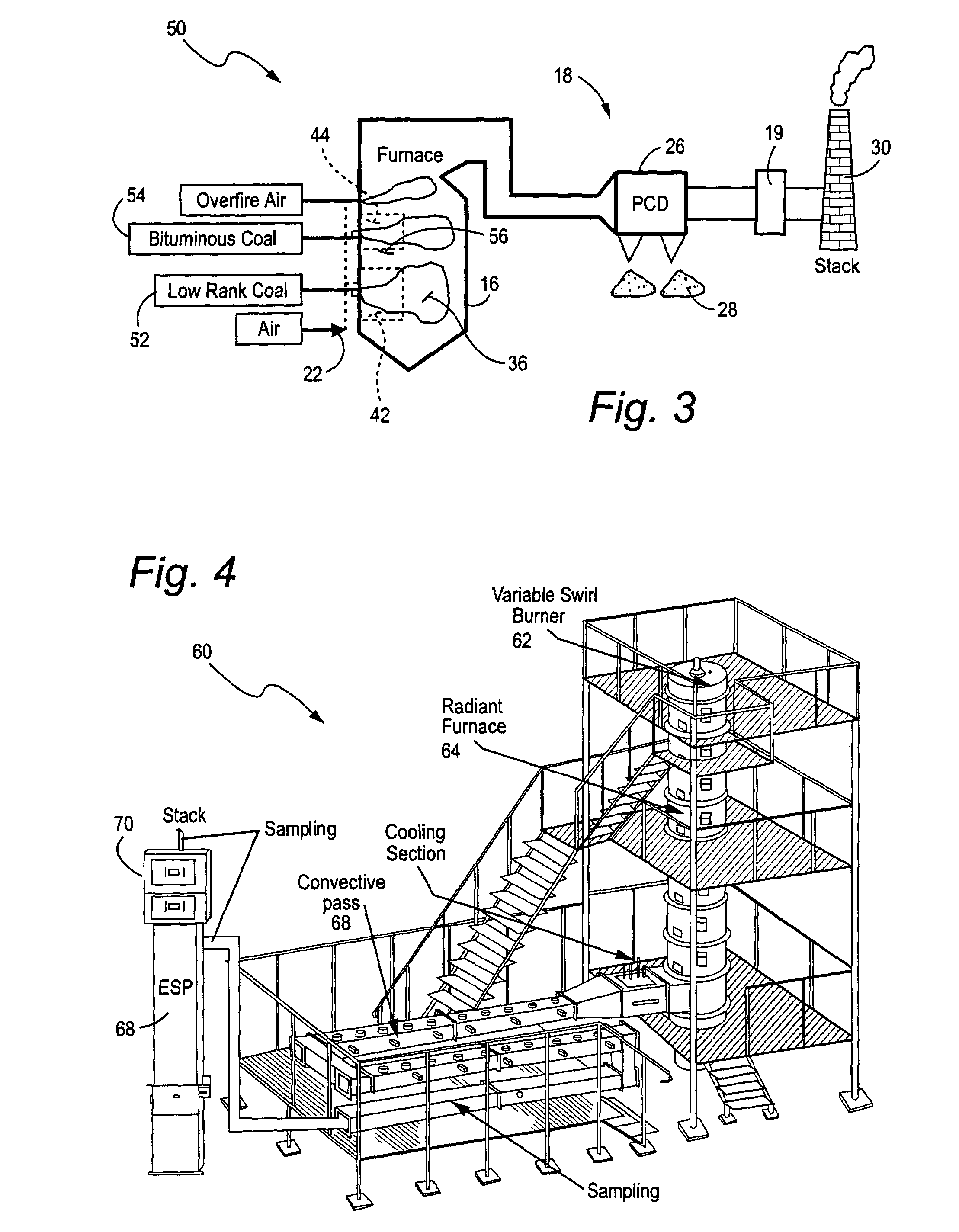
Method and apparatus for removing mercury from combustion exhaust gas
A method for reducing mercury emissions in combustion flue gas is provided. The method includes combusting coal such that a flue gas flow is created. The flue gas flow includes at least mercury and carbon-containing fly ash. The method further includes cooling the flue gas flow within a duct and creating turbulence in the flue gas flow. The mercury is removed from the flue gas flow.
Owner:GENERAL ELECTRIC CO
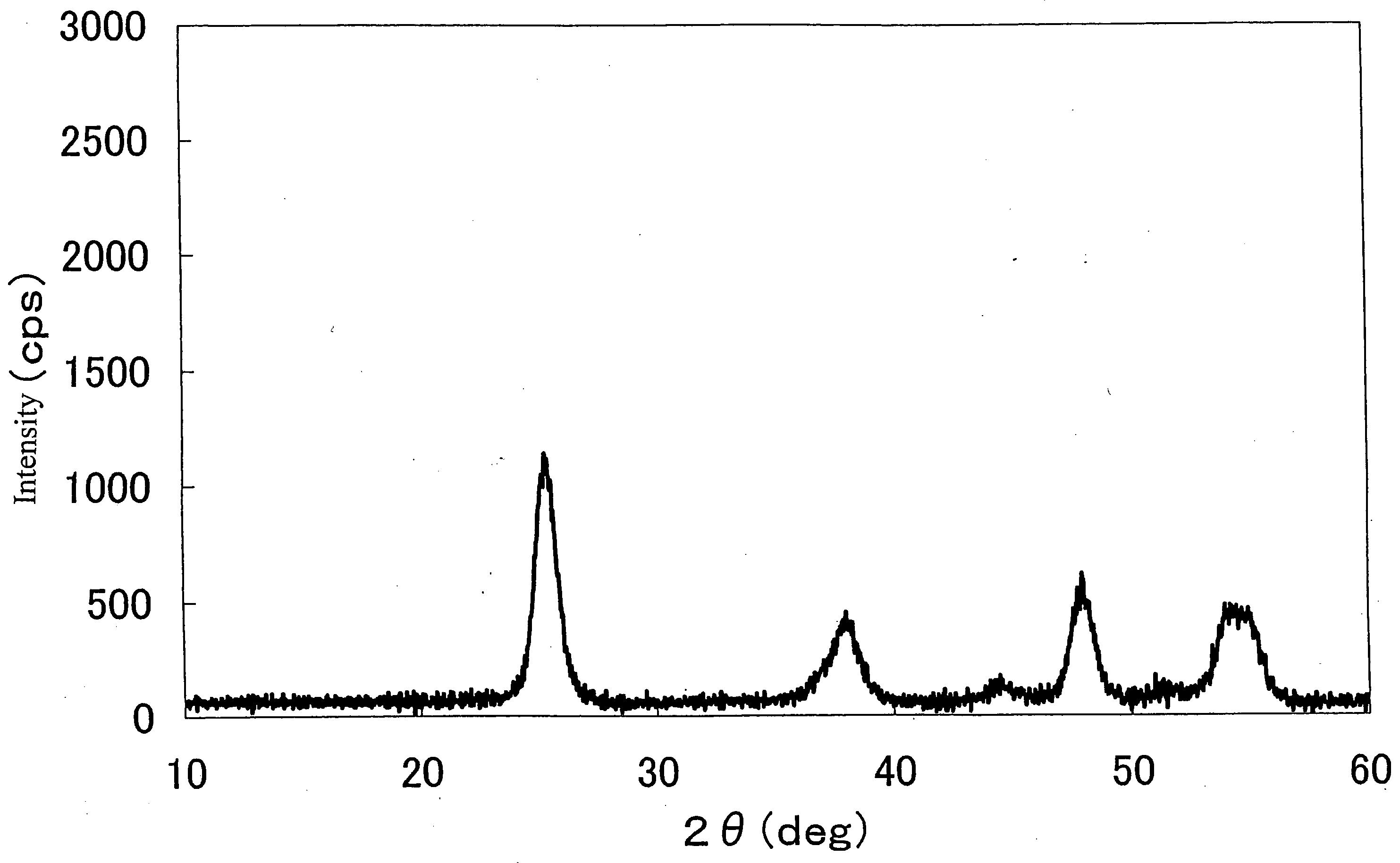
Method of preparing nano hydroxyl zinc stannate and nano zinc stannate
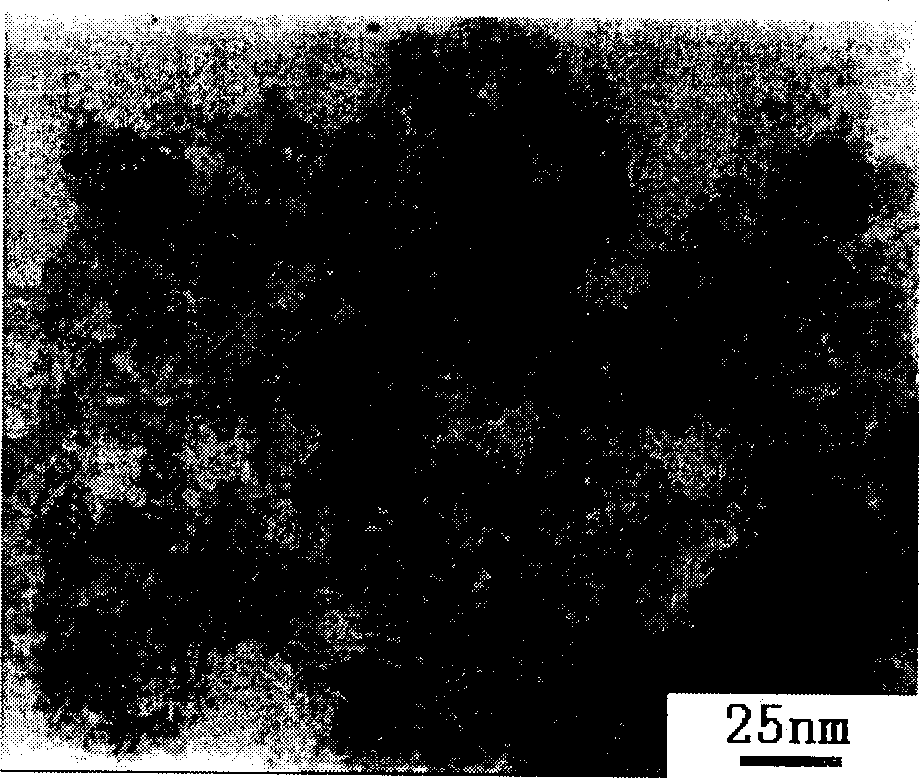
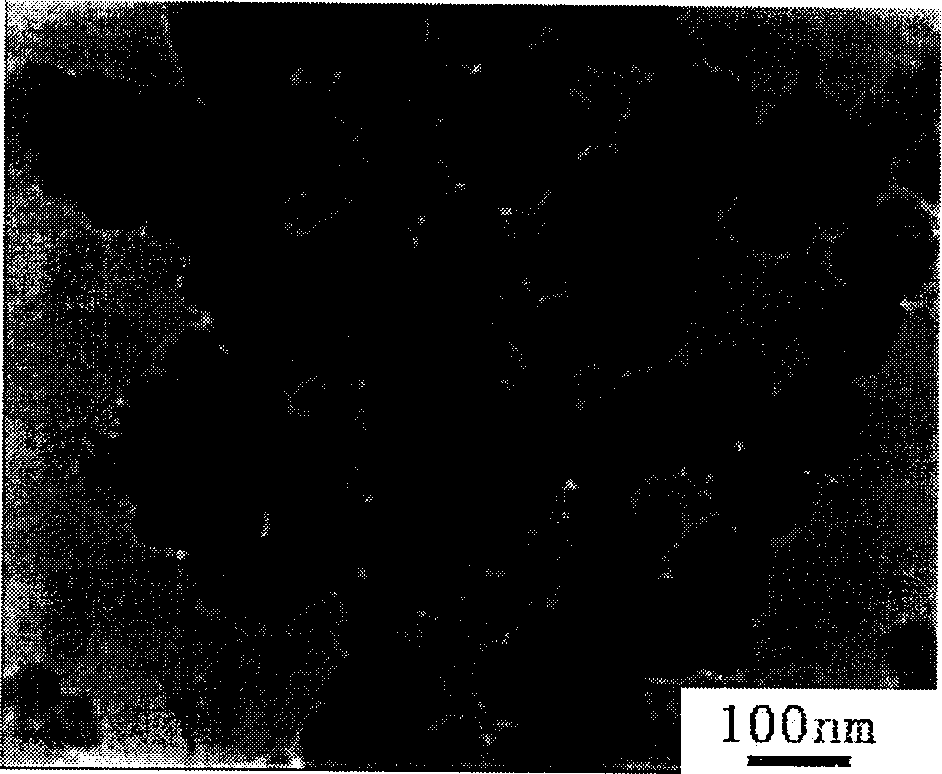
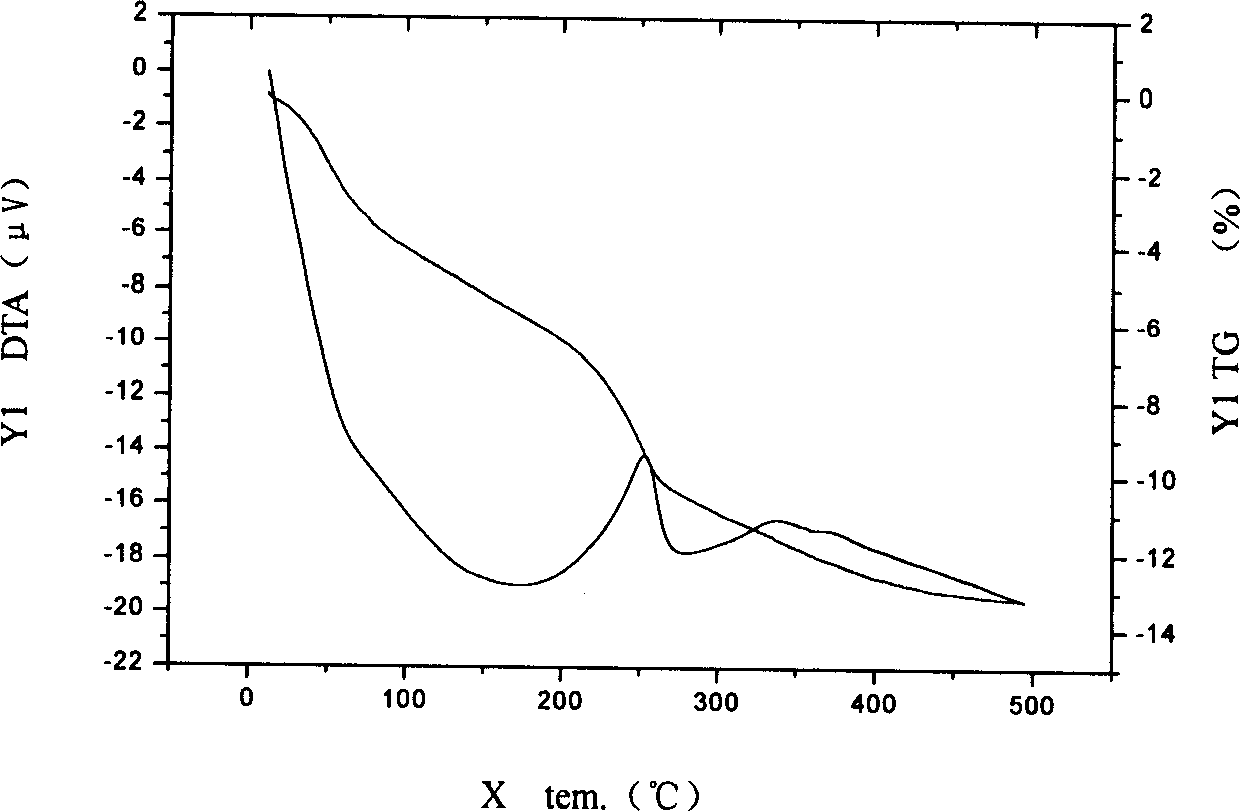
The invention provides a preparation method for nanometer zinc hydroxystannate and nanometer zinc stannate. Said method uses tin compounds as raw materials to prepare zinc hydroxystannate with particle size of 2-100 nm. Nanometer zinc stannate can be obtained by sintering the zinc hydroxystannate at high temperature. Zinc hydroxystannate (xZnO*ySnO*zH2O) has a molar ratio of x : y : z at 1 : 0.8-1.4 : 1-5. They can be used alone or mixed for polymer materials, and has a good effect on flame retardancy and smoke suppression. Nanometer zinc hydroxystannate can merely be used in the material operating less than 180DEG C, while nanometer zinc stannate can be used for various polymer materials. In addition, both can be also used for gas-sensitive materials and lubricant additives.
Owner:HENAN UNIVERSITY
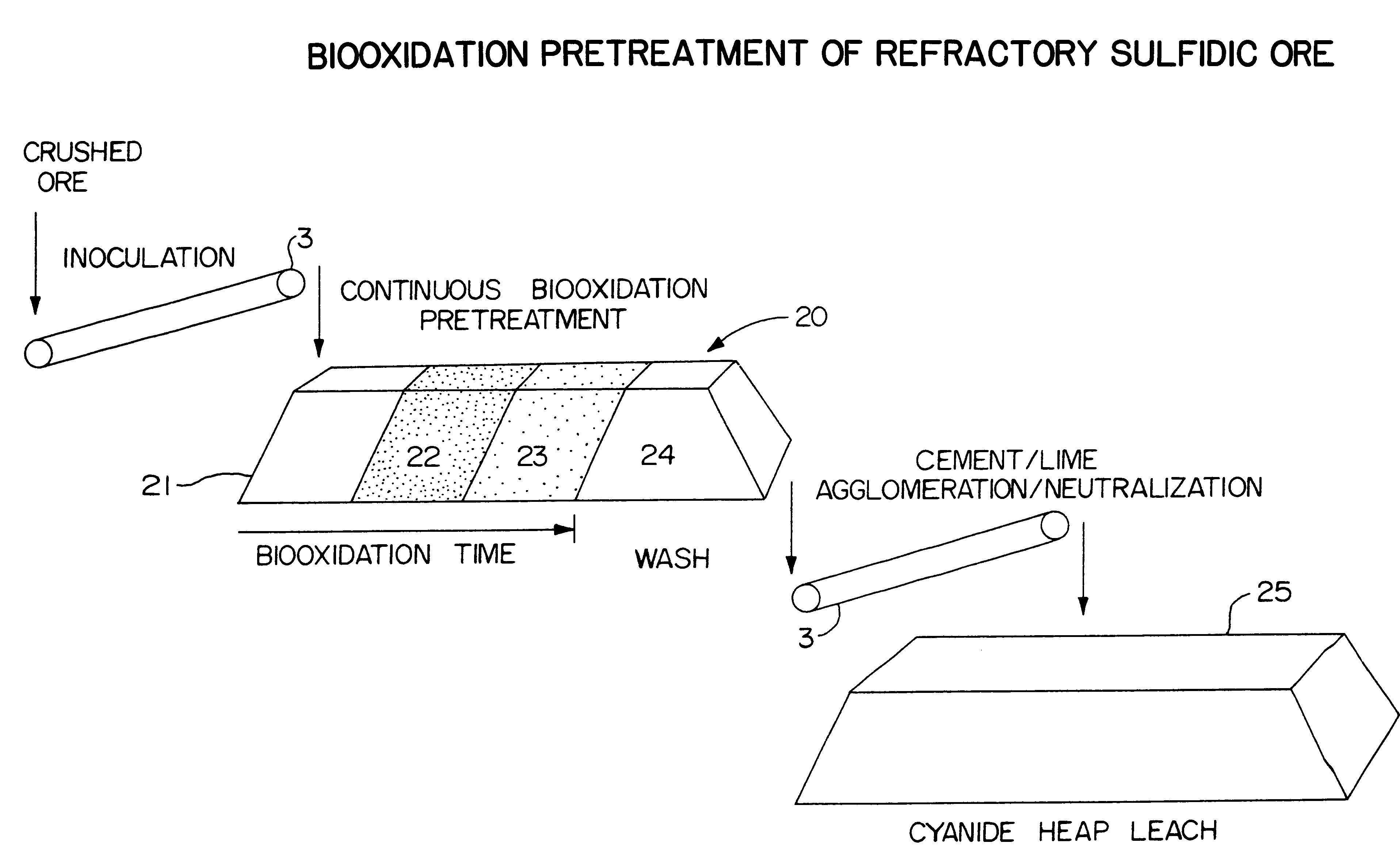
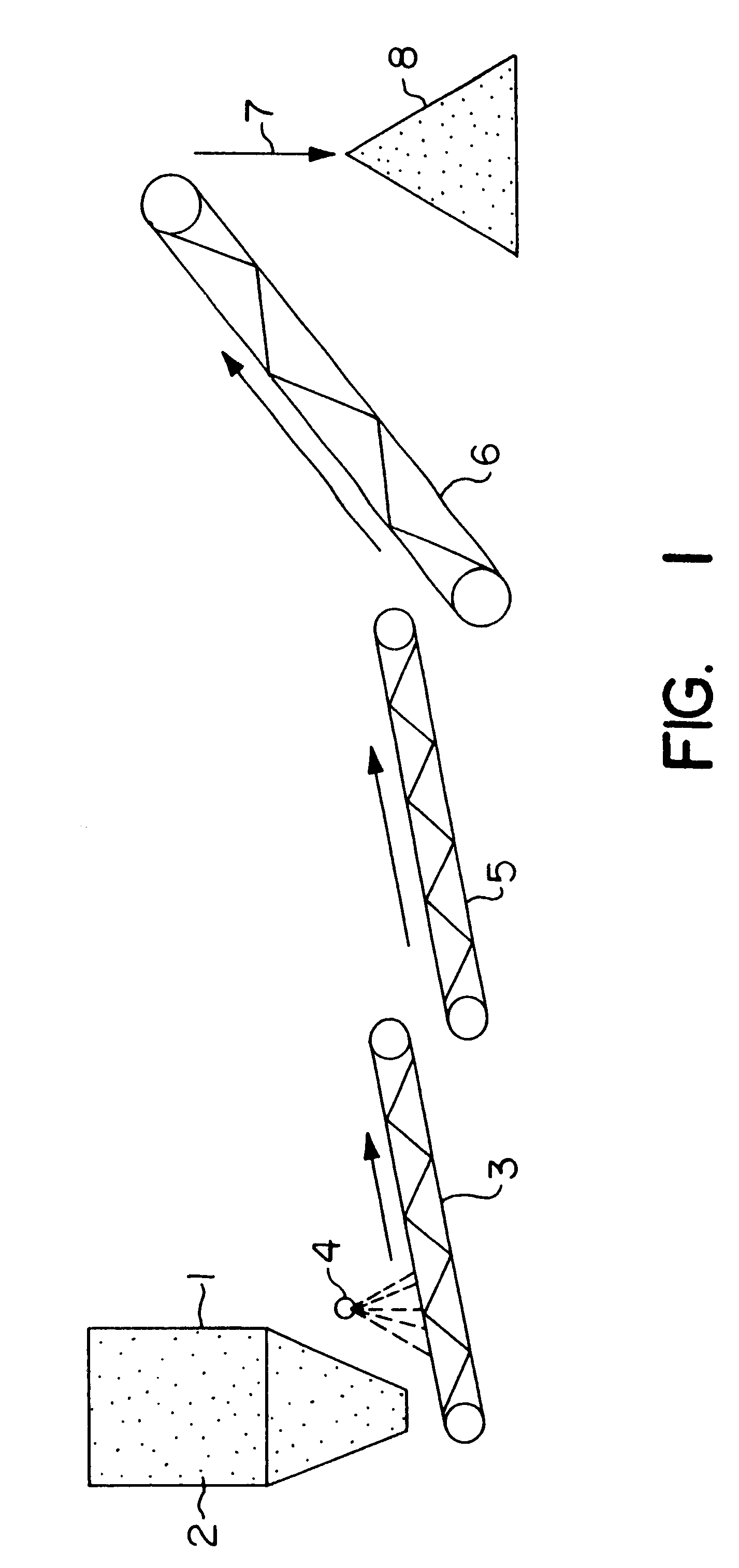
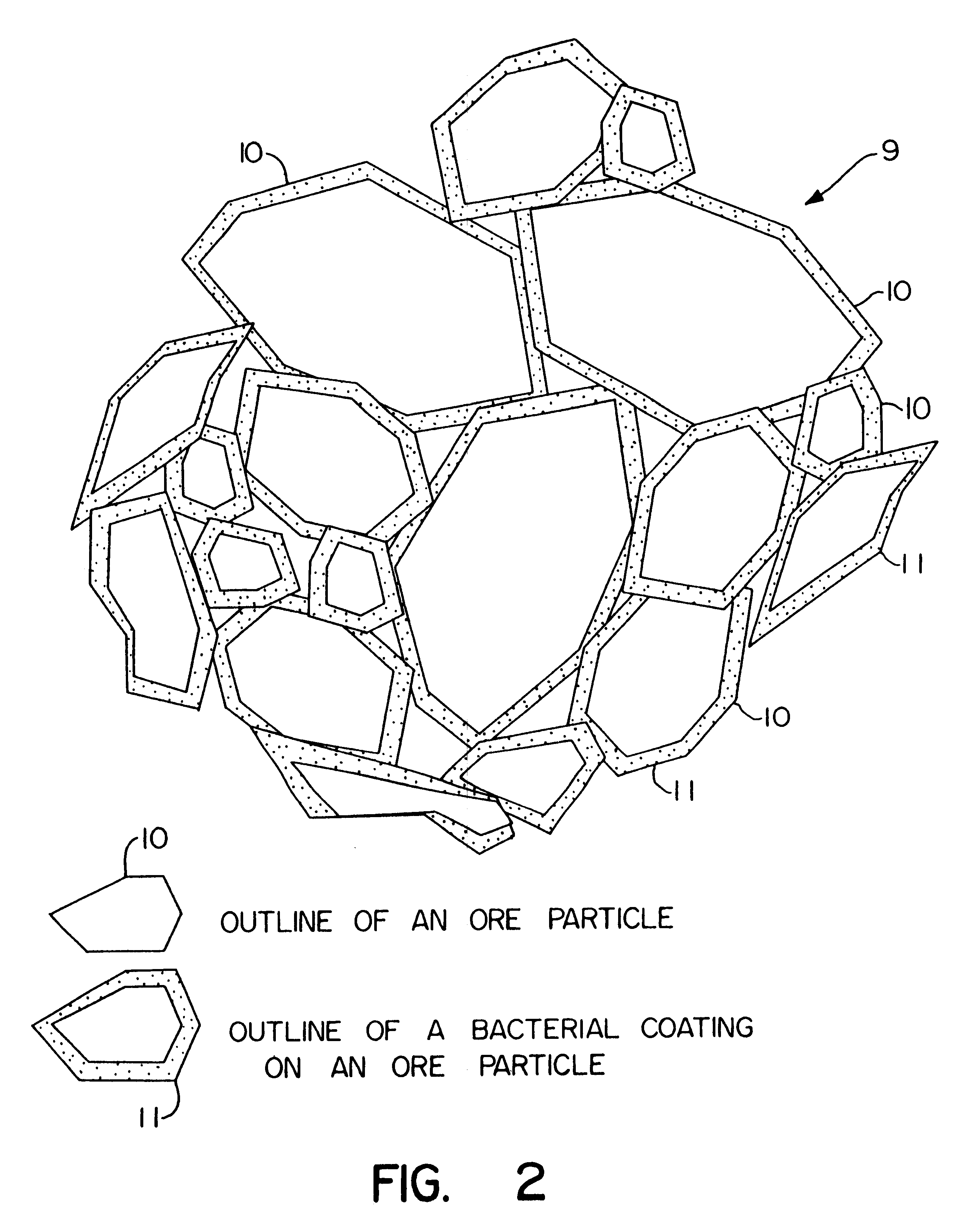
Biooxidation process for recovery of metal values from sulfur-containing ore materials
InactiveUS6383458B1Increase ratingsIncrease attractivenessTransuranic element compoundsSolvent extractionParticulatesPartial oxidation
A process for the recovery of one or more metal values from a metal ore material comprising those of one or more values and a matrix material having a sulfur content wherein the sulfur is present in an oxidation-reduction state of zero or less comprisinga. forming particulates from particles of said ore and an inoculate comprising bacteria capable of at least partially oxidizing the sulfur content;b. forming a heap of said particulates;c. biooxidizing the sulfur content andd. recovering those one or more metal values.
Owner:NEWMONT USA LTD
Recovery of the transition metal component of catalyst used in heavy feed hydroconversion
The invention relates to a process for recovering the transition metal component of catalysts used in the hydroconversion of heavy hydrocarbonaceous materials. In accordance with the invention, a slurry of a transition metal catalyst and hydrocarbon is catalytically desulfurized resulting in a desulfurized product and a solid residue containing the transition metal. The transition metal may be recovered by coking the residue and then dividing the coker residue into two portions are combusted with the flue dust from the first combustion zone being conducted to the second combustion zone. The flue dust from the second combustion zone is treated with ammonia and ammonium carbonate in order to obtain ammonium molybdate.
Owner:EXXON RES & ENG CO
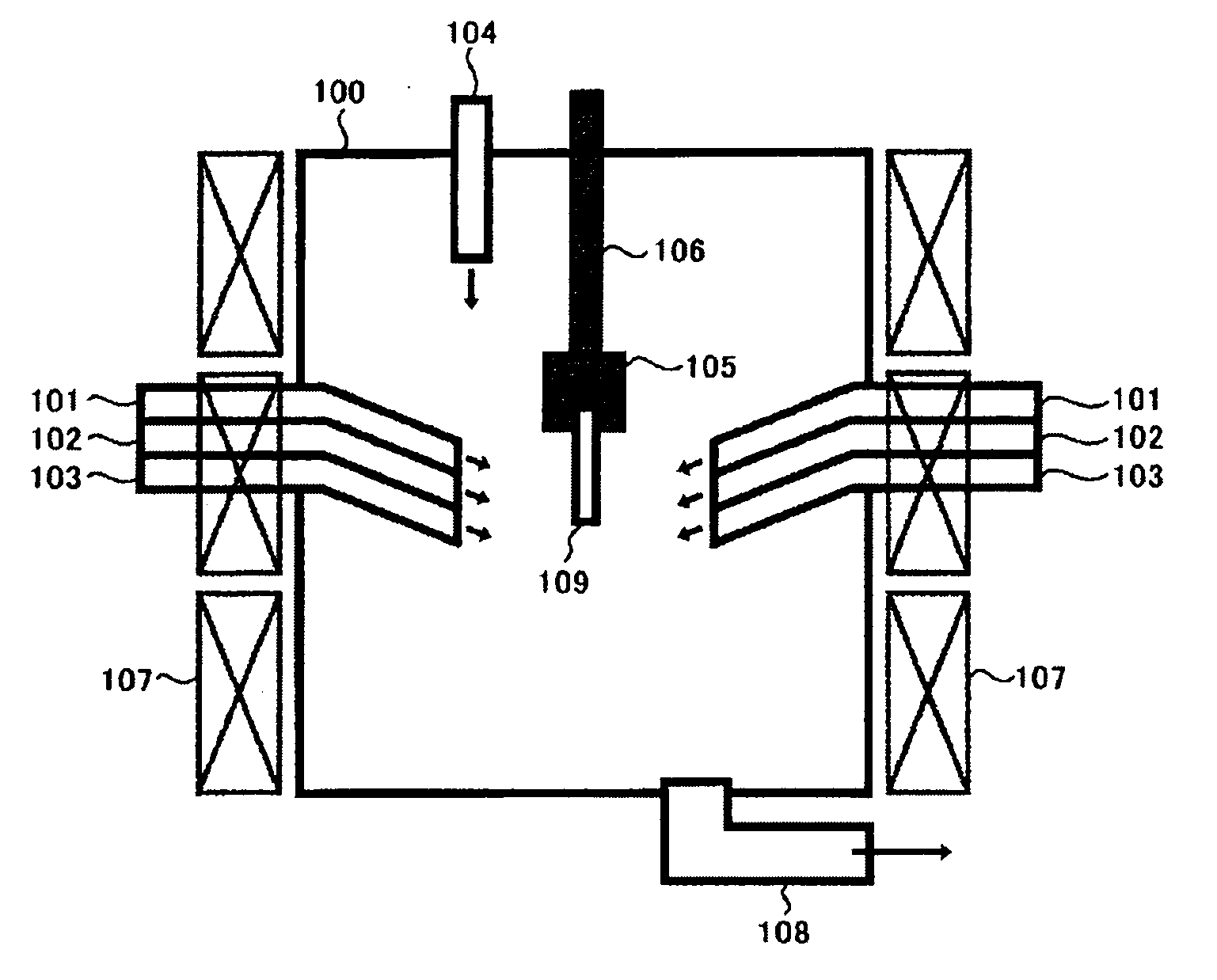
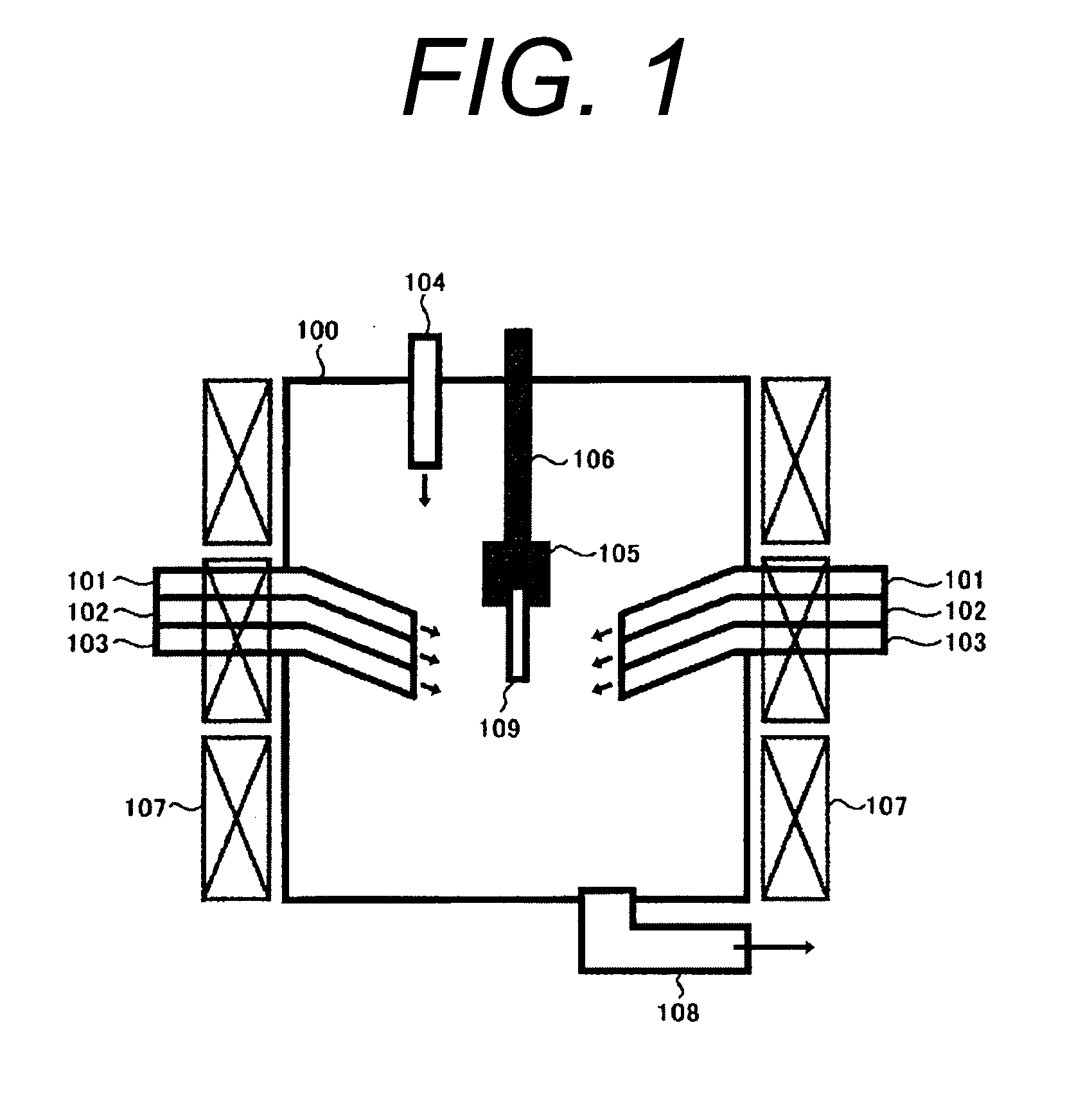
Method for treating exhaust gas
An object of the present invention is to provide a new method for treating an exhaust gas, which can effectively treat an exhaust gas containing a nitrogen oxide and metal mercury over a long term, and also can be applied to treatment of a large volume of an exhaust gas. As a means of achieving this object, a method according to the present invention for treating an exhaust gas comprises performing a reaction of changing metal mercury into mercury halide in the presence of a halogen compound and treatment of a nitrogen oxide, using a Ti—V-containing catalyst, upon treatment of an exhaust gas containing a nitrogen oxide and metal mercury.
Owner:NIPPON SHOKUBAI CO LTD
Emission Control System
InactiveUS20080241030A1Reduce mercury emissionEmission reductionCombination devicesNitrogen compoundsChlorine dioxideControl system
Methods and apparatus utilizing chlorine dioxide and hydrogen peroxide are useful to reduce emissions of NOx, SOx, and heavy metals, e.g., mercury, emissions from combustion flue gas streams.
Owner:NASA
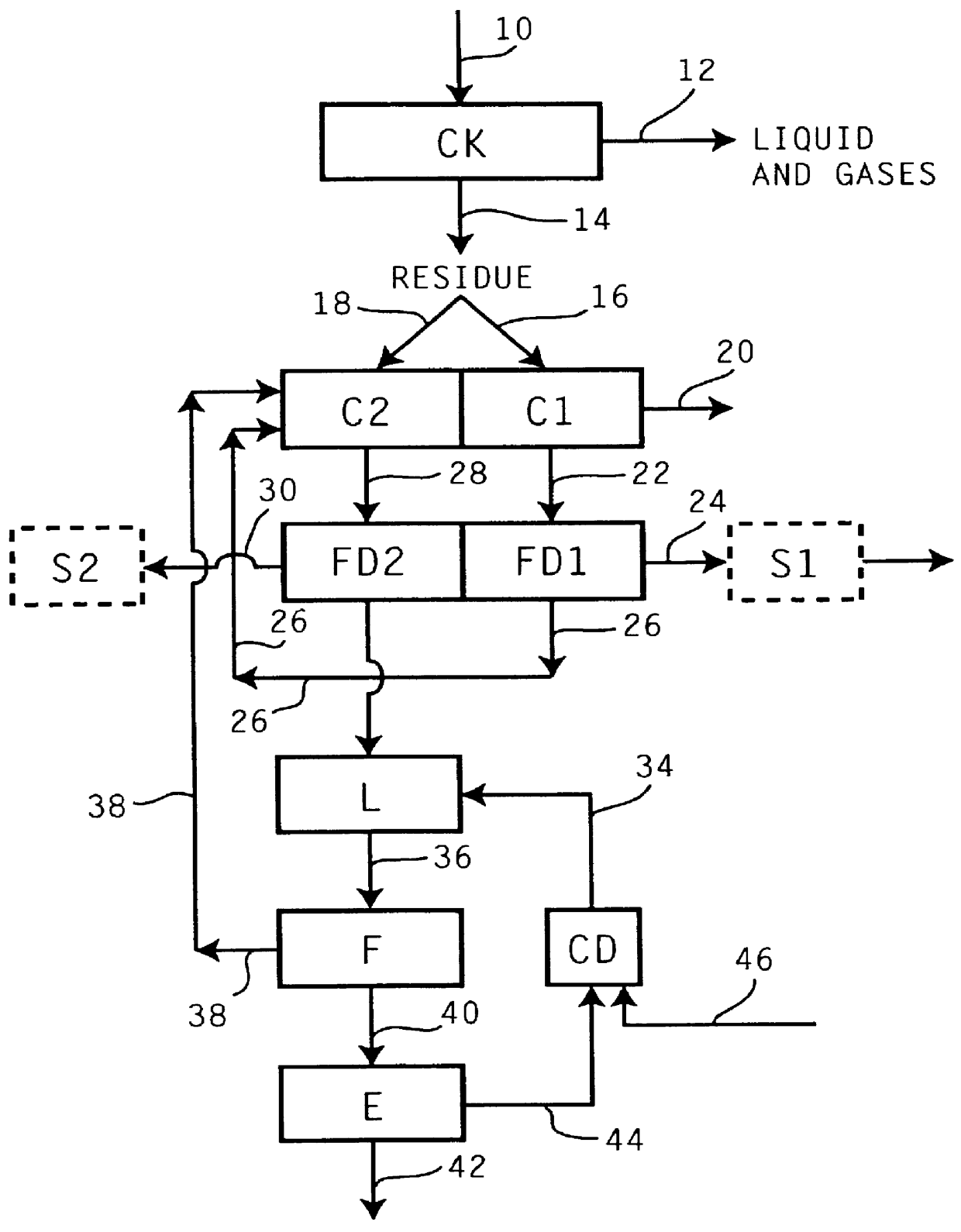
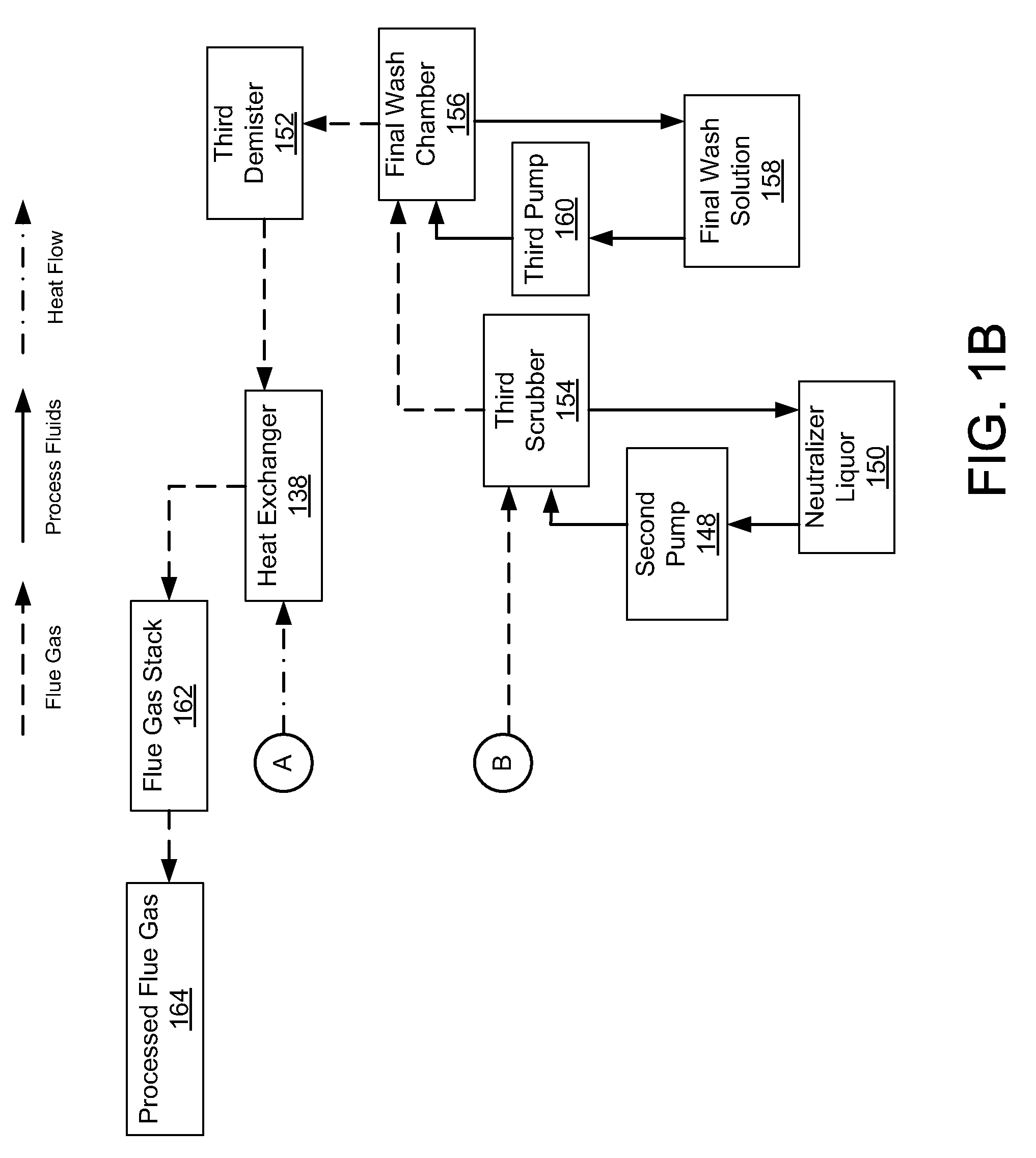
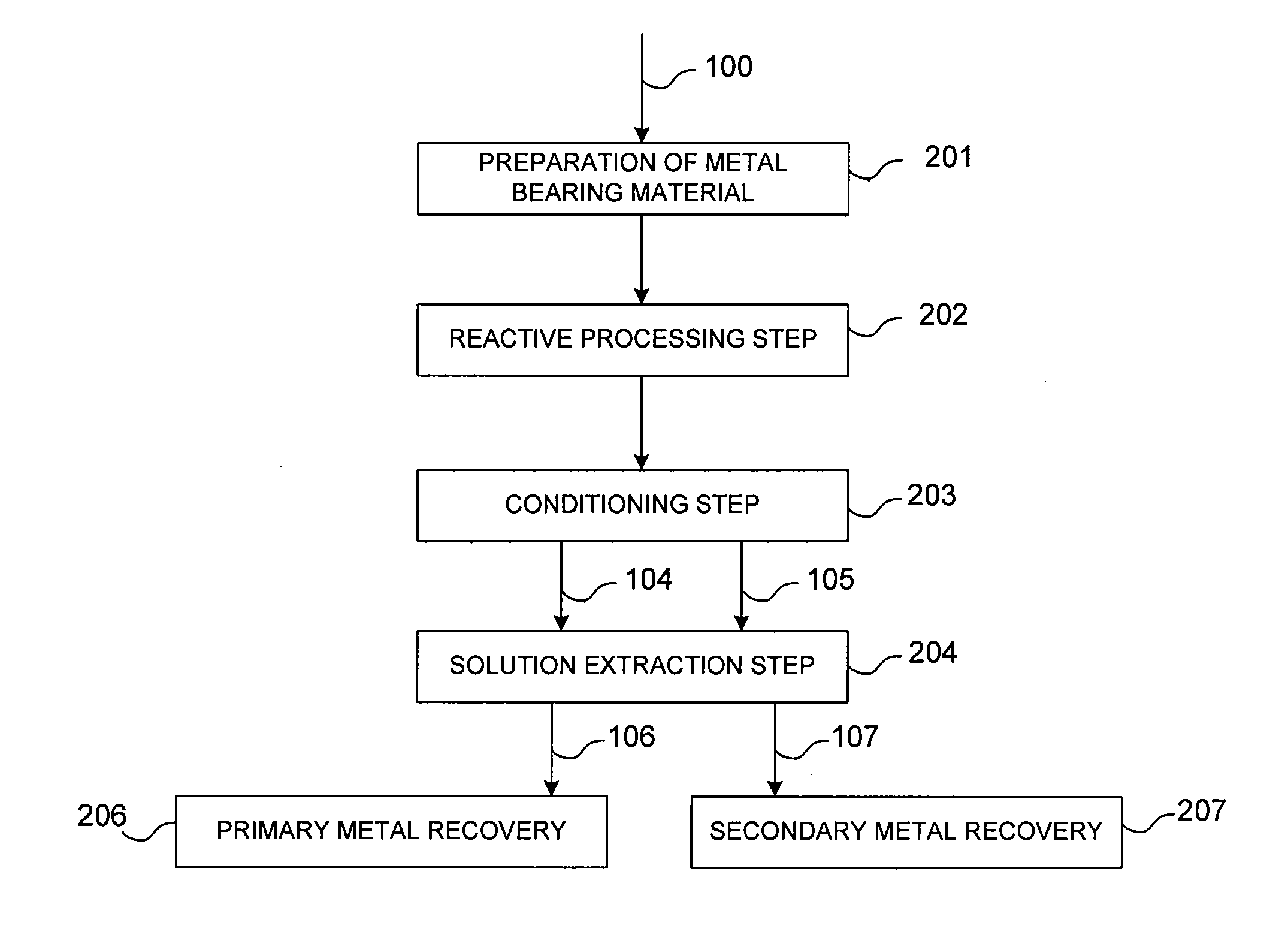
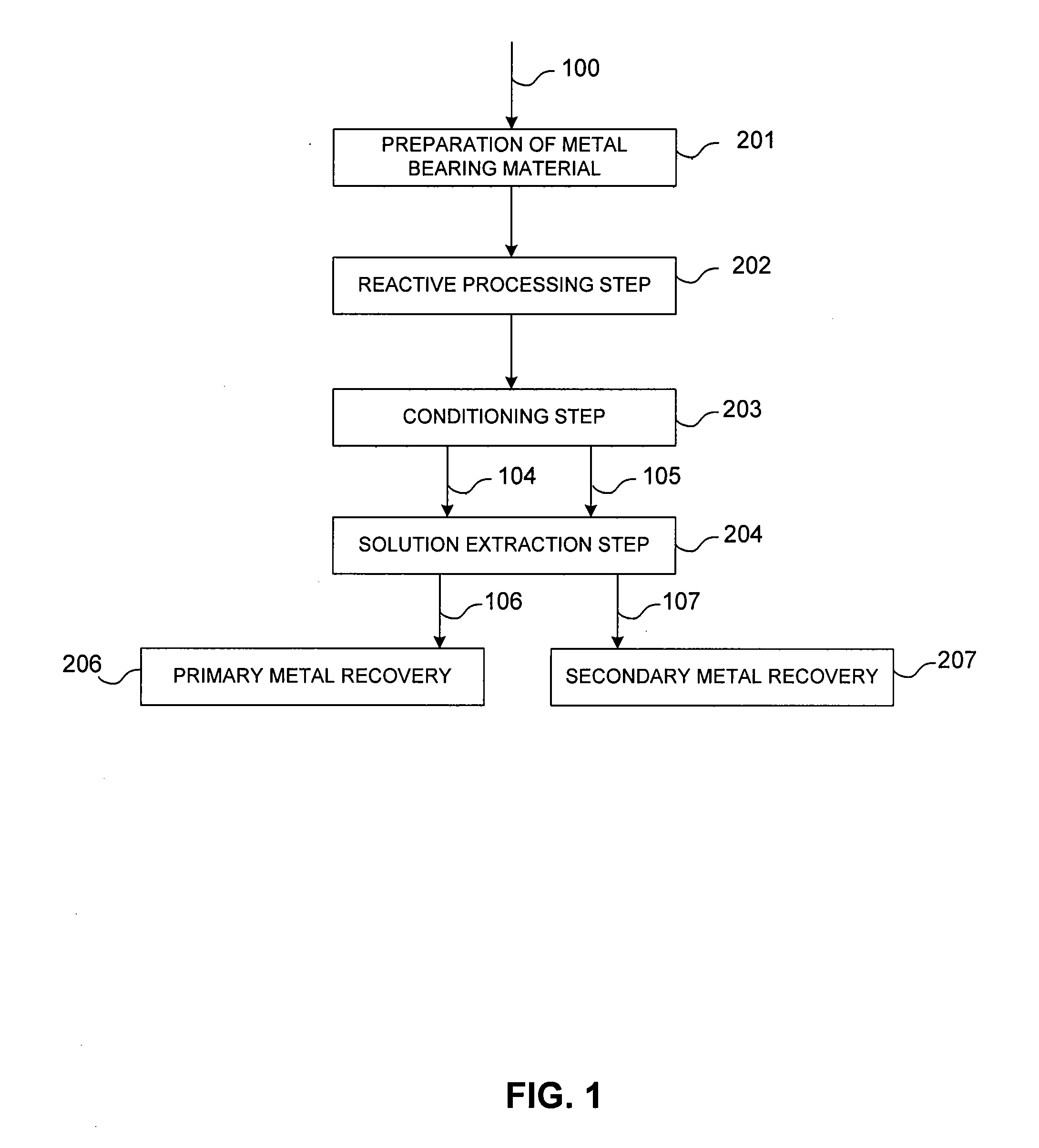
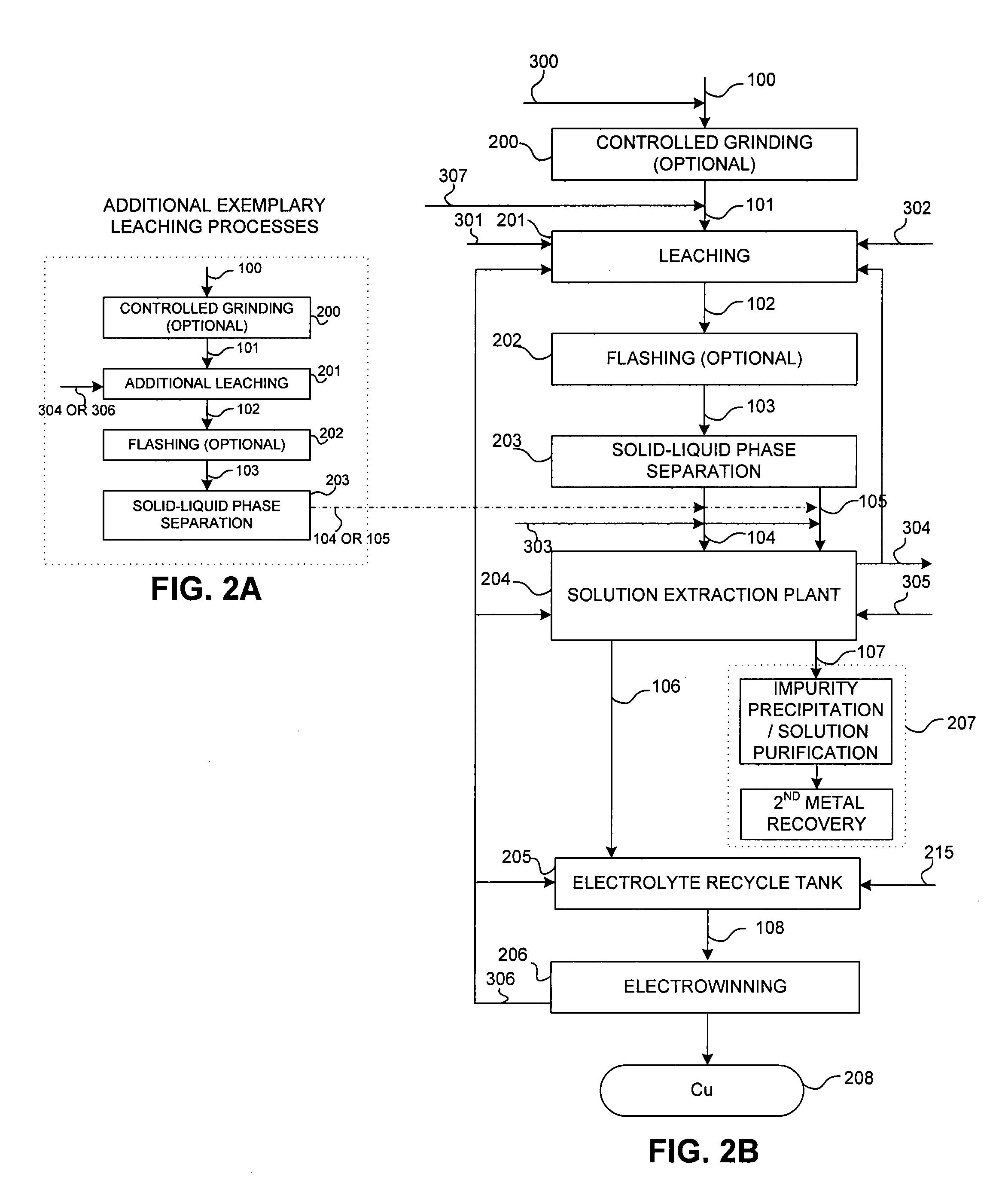
Controlled copper leach recovery circuit
ActiveUS20090074639A1Effective recoveryLow constantPhotography auxillary processesTransuranic element compoundsPregnant leach solutionLower grade
The present invention relates generally to a process for controlled leaching and sequential recovery of two or more metals from metal-bearing materials. In one exemplary embodiment, recovery of metals from a leached metal-bearing material is controlled and improved by providing a high grade pregnant leach solution (“HGPLS”) and a low grade pregnant leach solution (“LGPLS”) to a single solution extraction plant comprising at least two solution extractor units, at least two stripping units, and, optionally, at least one wash stage.
Owner:FREEPORT MCMORAN COPPER & GOLD INC
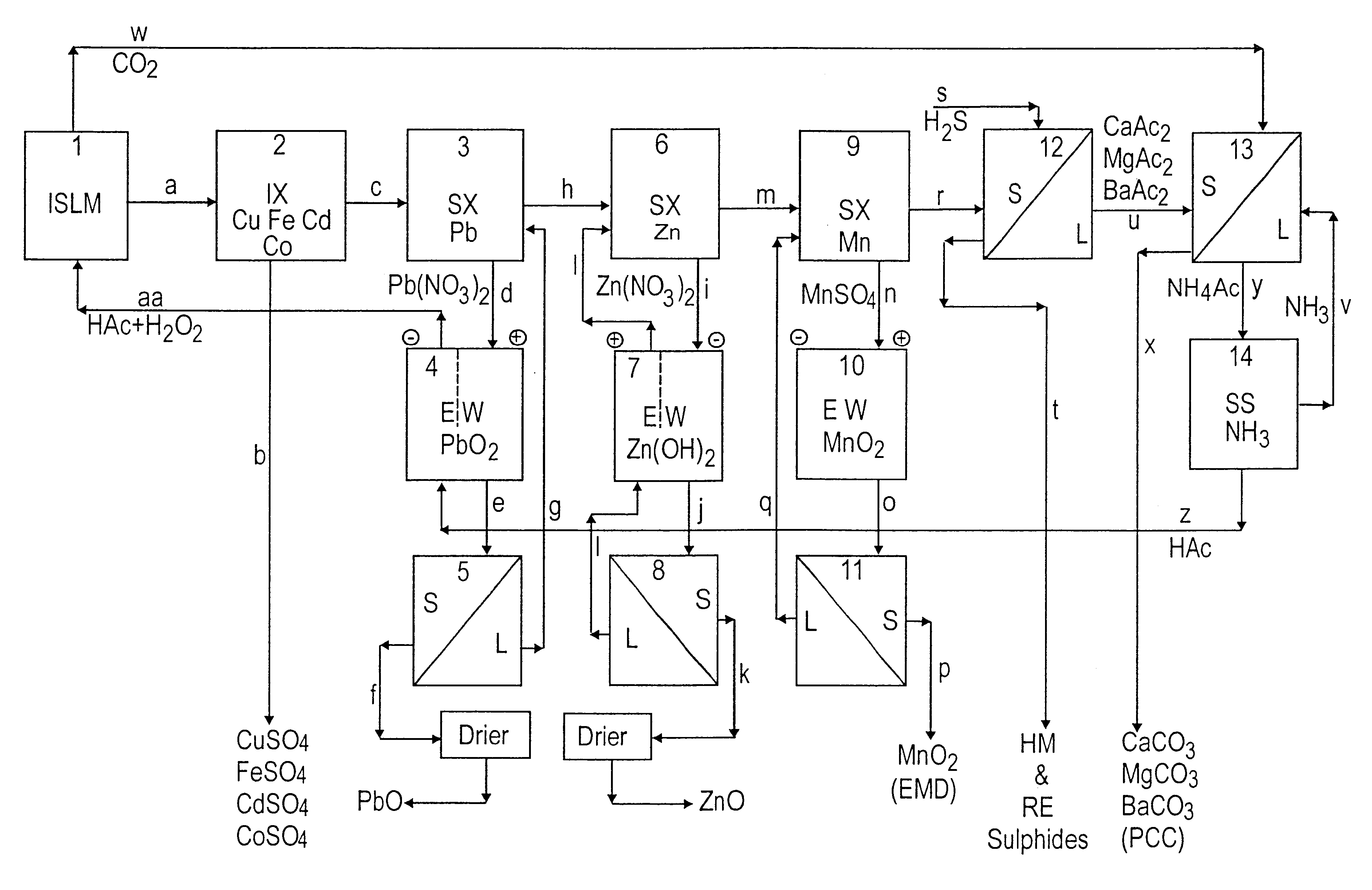
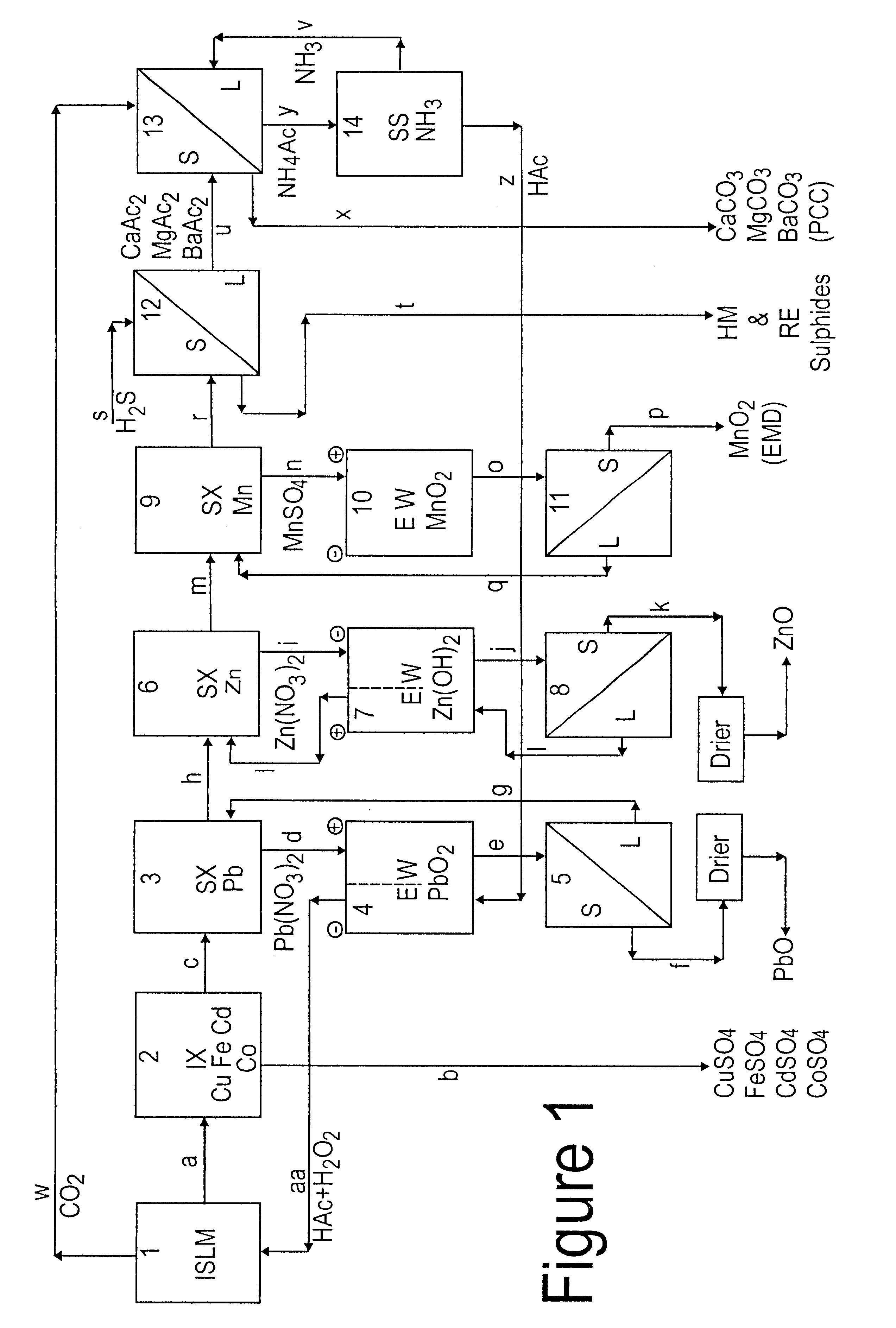
Lead, zinc and manganese recovery from aqueous solutions
InactiveUS6517701B1Low costShorten the timePhotography auxillary processesElectrolysis componentsIon exchangeManganese
Aqueous solutions containing lead, zinc and manganese are treated to recover these metals by sequential solvent extraction steps. Solvent extractants are selected to extract preferentially lead, then zinc and then manganese in that order. Any interfering metals are removed (as by ion exchange) before extraction. The loaded extractant phases are stripped with selected acids and lead, zinc and manganese each recovered from the strip solutions. Optionally calcium can be recovered when present. A preferred type of extractant (for lead especially) is substituted monothiophosphinic acids. A closed loop system is described which is advantageous with leachate from sulphide and carbonate ores.
Owner:CENTAUR MINING EXPLORATION
Method for effecting solvent extraction of metal ions using hydrocarbon soluble aminomethylene phosphonic acid compounds
Solvent extraction of one or more metal ions from an aqueous solution in the presence of hydrocarbon-soluble aminomethylenephosphonic acid derivatives.
Owner:BASF AG +1
Semiconductor nanocrystal, method of manufacture thereof and articles including the same
ActiveUS20110315954A1Improve stabilitySmall sizeMaterial nanotechnologyLight-sensitive devicesSemiconductor nanocrystalsSemiconductor
A semiconductor nanocrystal including a core including ZnSe, ZnTe, ZnS, ZnO, or a combination comprising at least one of the foregoing, wherein the core has a diameter of about 2 nanometers to about 5 nanometers and an emitted light wavelength of about 405 nanometers to about 530 nanometers; and a first layer disposed on the core, the first layer including a Group III-V semiconductor, wherein the semiconductor nanocrystal has a full width at half maximum of an emitted light wavelength of less than or equal to about 60 nanometers.
Owner:SAMSUNG ELECTRONICS CO LTD
ZnIn2S4 nano materials and their synthesis method and application
InactiveCN1884090AImprove photoelectric performanceSimple methodGallium/indium/thallium compoundsZinc compoundsOrganic sulfide compoundIndium
The invention discloses a Znln2S4 nanometer material and synthesizing method and application, which is characterized by the following: adopting bivalent zinc salt, trivalent indium salt and organic sulfide as raw material; proceeding solvent heating or water heating reaction at 100-220 deg.c in the sealing reactor; setting surface activist as addictive; synthesizing Znln2S4 nanometer pipe, nanometer band and nanometer line selectively through controlling solvent kind, reacting temperature and reacting time. The invention belongs to layer-shaped hexagonal crystal phase with kind's direction of specific property, which is fit for relative domains of solar battery, optical catalyst, high-energy battery, and heat-conversion.
Owner:NANKAI UNIV
Method of removing mercury from mercury contaminated materials
InactiveUS7214254B2The process is compact and efficientThe equipment is compact and efficientZinc compoundsProcess efficiency improvementMercury levelMicrowave irradiation
A method of reducing mercury levels in a mercury contaminated material using microwave energy. The method comprises the steps of (a) placing the mercury contaminated material in a microwave reactor; (b) providing a stream of gas in the microwave reactor, the stream causing agitation of the mercury contaminated material; and (c) exposing the mercury contaminated material to microwave radiation so as to raise the temperature to at least 357° C., producing a vapour phase which contains mercury and a treated material. The method also allows for a simultaneous reduction of mercury and carbon levels in the material to be treated as well as the use of a carbon-free material in the reactor.
Owner:HENDRIX HLDG CO INC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com