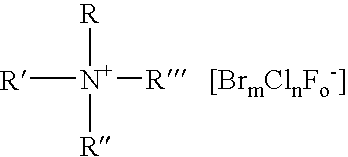Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
42 results about "CHLORITE ION" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
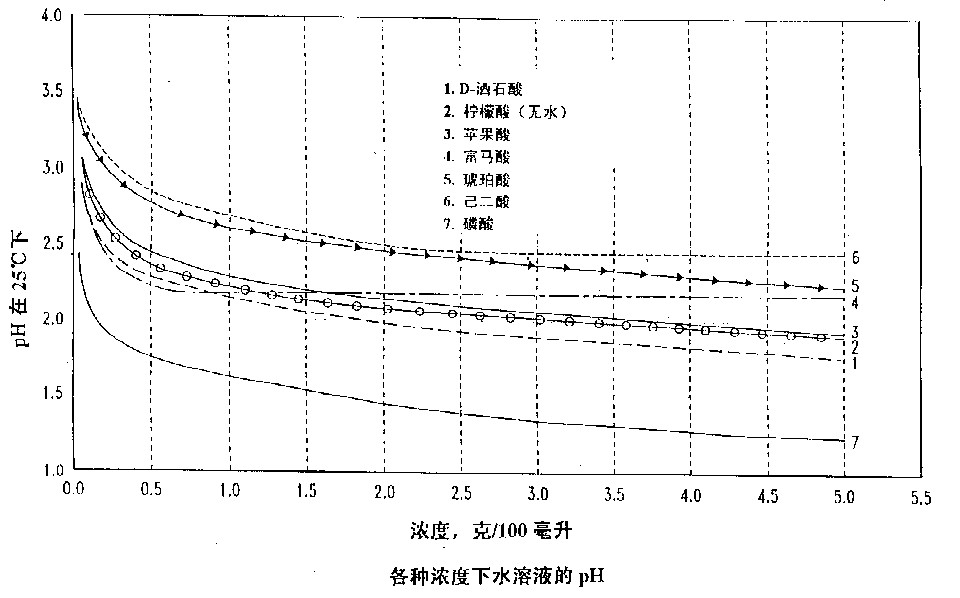
The chlorite ion, or chlorine dioxide anion, is ClO − 2.A chlorite (compound) is a compound that contains this group, with chlorine in oxidation state +3. Chlorites are also known as salts of chlorous acid
Promoting whole body health
InactiveUS6846478B1Promote and enhance whole body healthGood for healthCosmetic preparationsToilet preparationsDiseaseWhole body
Owner:THE PROCTER & GAMBLE COMPANY
Composition for the production of chlorine dioxide using non-iodo interhalides or polyhalides and methods of making and using the same
ActiveUS7087190B2Reduce microbial countQuick buildBiocideLiquid degasificationChlorine dioxideCHLORITE ION
A composition for the generation of chlorine dioxide including at least one non-iodo interhalide, polyhalide or salt thereof having the formulaBrmClnFoXpwherein m=0–3, n=0–4, o=0–3, p=0–2, X is a cationic moiety and with the provisos that m+n+o cannot be zero; if m+n+p<2, or mixtures thereof, and at least one source of chlorite ions.
Owner:ECOLAB USA INC
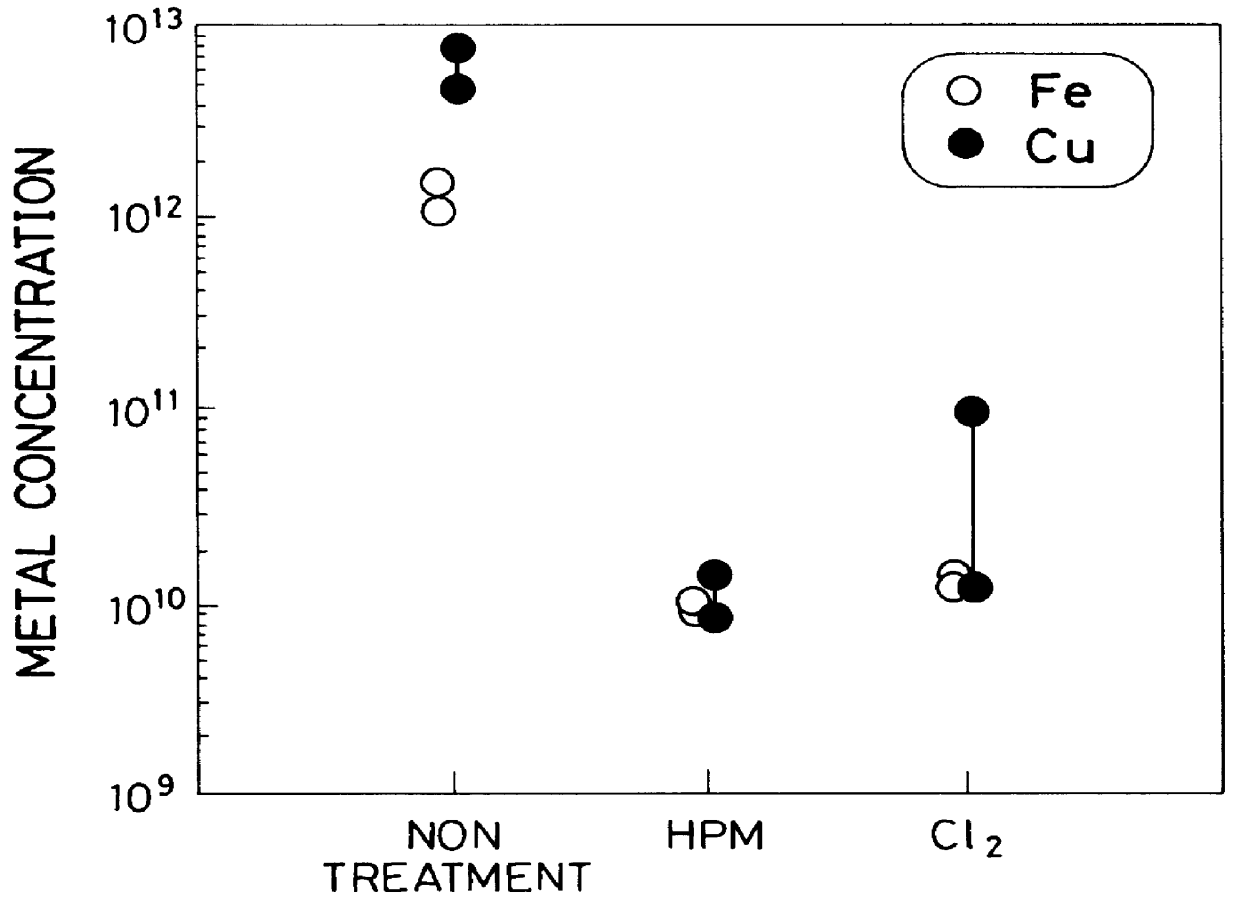
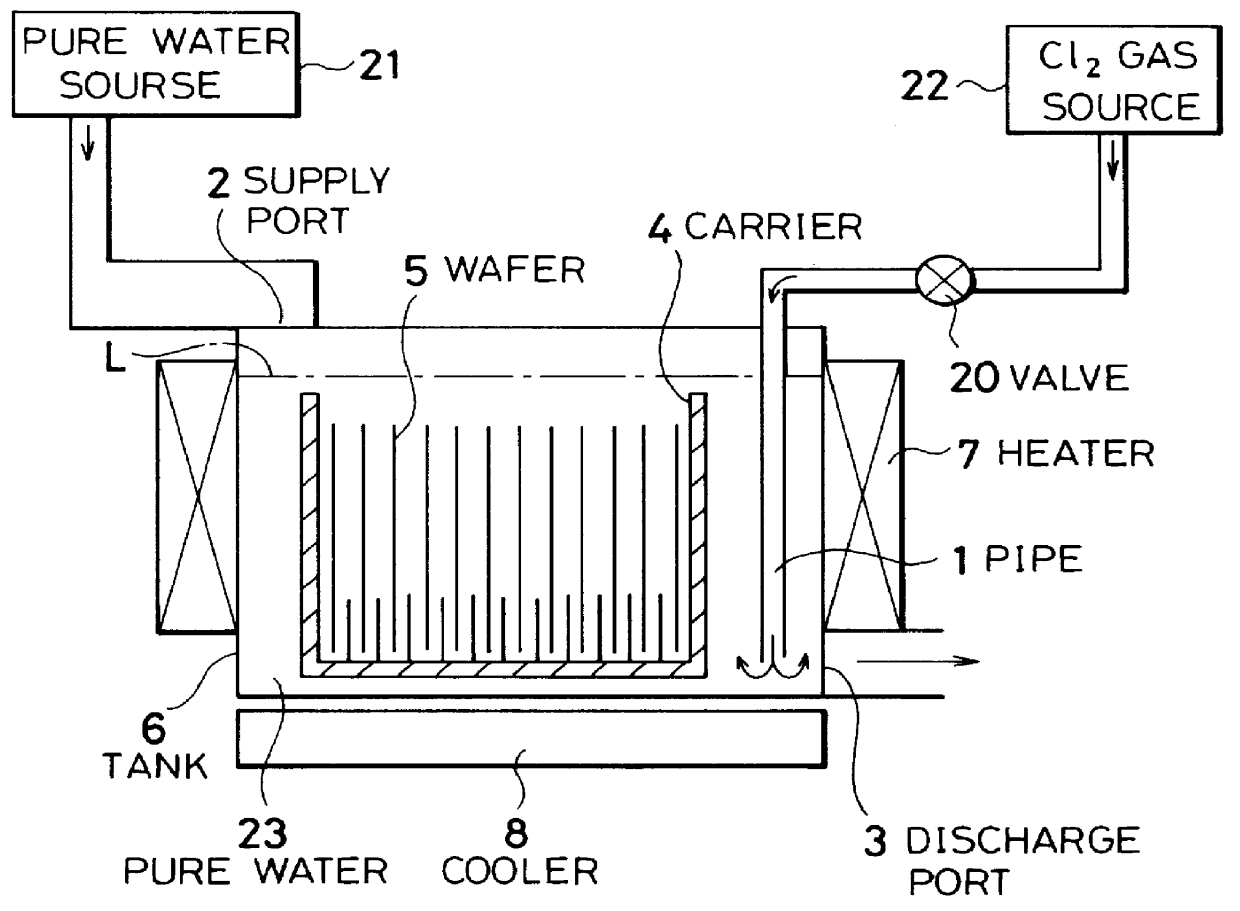
Cleaning method and system of semiconductor substrate and production method of cleaning solution
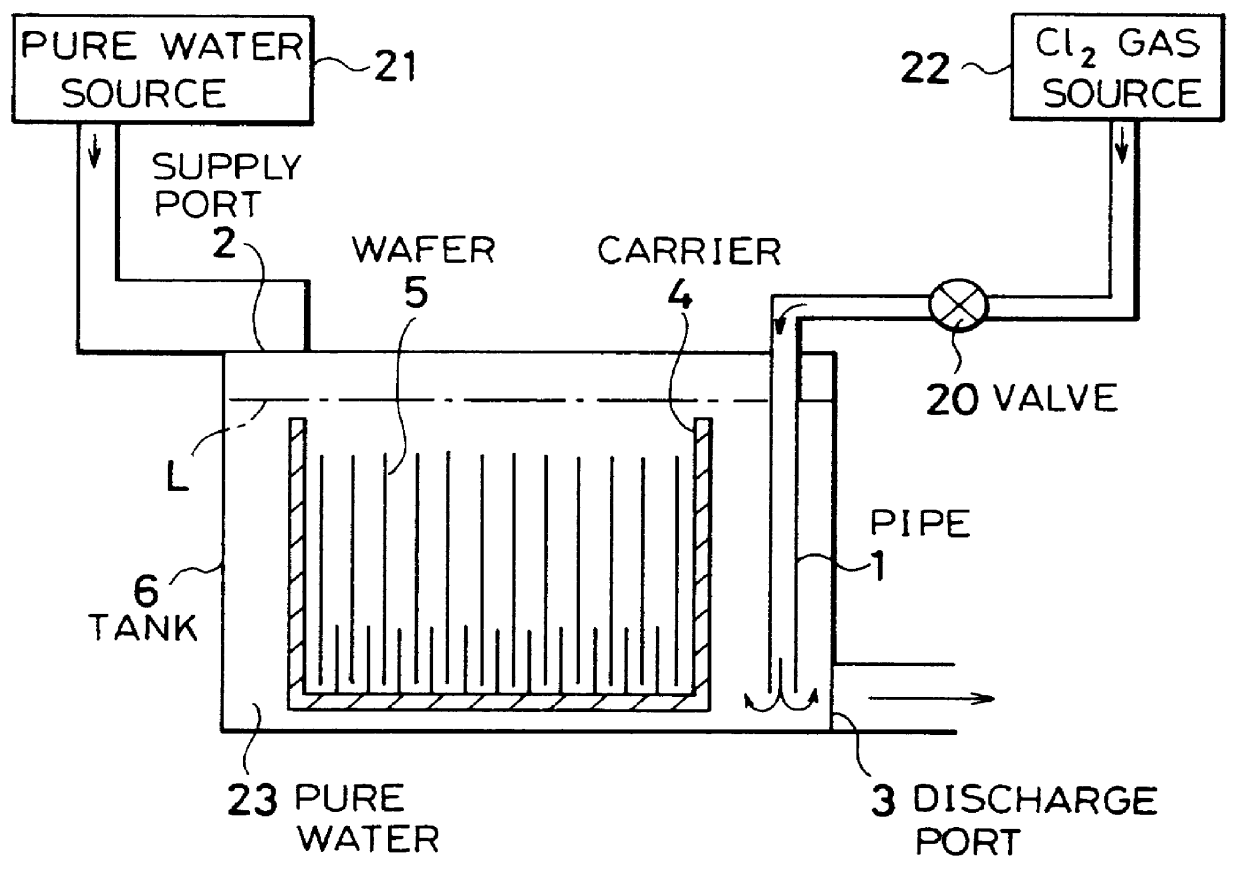
InactiveUS6116254AMinimize power consumptionReduce adverse effectsNon-surface-active detergent compositionsSemiconductor/solid-state device manufacturingChlorate ionHypochlorite
A cleaning method for a semiconductor substrate is provided. After pure water is supplied to a cleaning tank, a chlorine gas is supplied to the pure water to thereby generate chloride ions, hypochlorite ions, chlorite ions, and chlorate ions in the pure water. Then, a semiconductor substrate is immersed into the pure water containing the chloride ions, hypochlorite ions, chlorite ions, and chlorate ions. The fabrication cost of a semiconductor device and adverse effects on the earth environment can be reduced. The concentration of the dissolved chlorine gas in the pure water is preferably in the range from 0.003 to 0.3% by weight.
Owner:RENESAS ELECTRONICS CORP
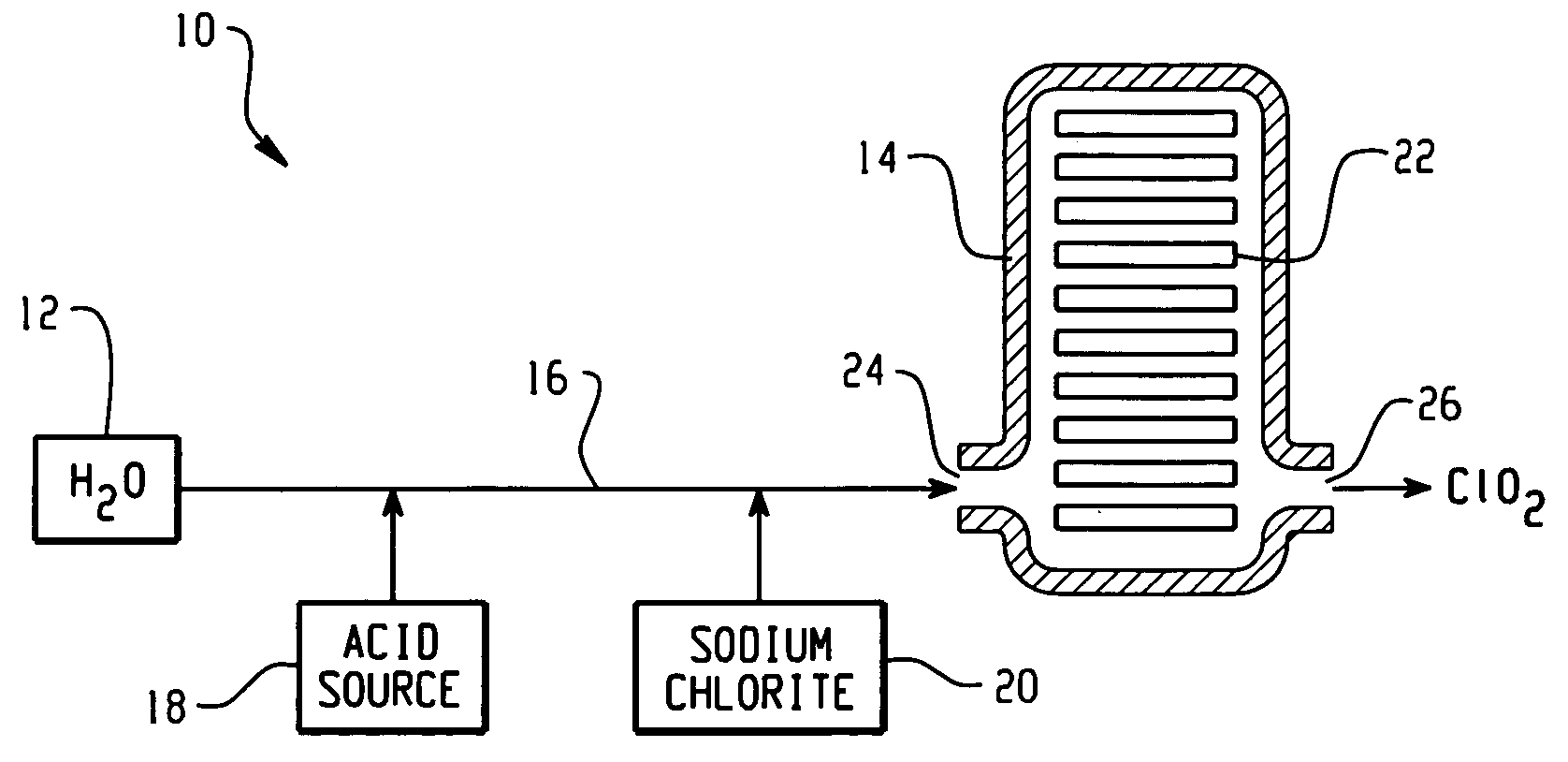
Systems and methods for generating chlorine dioxide
Processes for generating chlorine dioxide generally include acidifying an alkali metal chlorite solution; and contacting the acidified alkali metal chlorite solution with a solid phase chlorine-containing material to produce chlorine dioxide. An exemplary system for generating chlorine dioxide generally includes a water source in fluid communication with a conduit that is fluidly connected to a vessel, wherein the vessel comprises a housing, an inlet in fluid communication with the housing and the conduit, an outlet, and a solid phase chlorine-containing material disposed within the housing; an acid source downstream from the water source in fluid communication with the conduit; and a chlorite ion source in fluid communication with the conduit downstream from the acid source. Various means are provided for the acid source.
Owner:EASTERN PLASTICS +1
Biocidal fibrous and film materials comprising silver and chlorite ions
ActiveUS20070237810A1Reduces and prevents discolorationBiocideDead animal preservationWound dressingHydrophilic polymers
Disclosed are compositions comprising a hydrophilic polymer, a hydrophobic polymer, a silver ion, and a chlorite anion, wherein the silver ion associates with at least one hydrophilic or hydrophobic polymer, and wherein the chlorite ion associates with the hydrophilic polymer. Also disclosed are methods of making a photo-stable silver ion and chlorite ion containing wound dressing and methods of reducing or preventing infection of a wound by using the compositions and wound dressings of the present invention.
Owner:SOUTHWEST RES INST
Systems and methods for generating chlorine dioxide
Processes for generating chlorine dioxide generally include acidifying an alkali metal chlorite solution; and contacting the acidified alkali metal chlorite solution with a solid phase chlorine containing material to produce chlorine dioxide. An exemplary system for generating chlorine dioxide generally includes a water source in fluid communication with a conduit that is fluidly connected to a vessel, wherein the vessel comprises a housing, an inlet in fluid communication with the housing and the conduit, an outlet, and a solid phase chlorine containing material disposed within the housing; an acid source downstream from the water source in fluid communication with the conduit; and a chlorite ion source in fluid communication with the conduit downstream from the acid source. Various means are provided for the acid source.
Owner:EASTERN PLASTICS +1
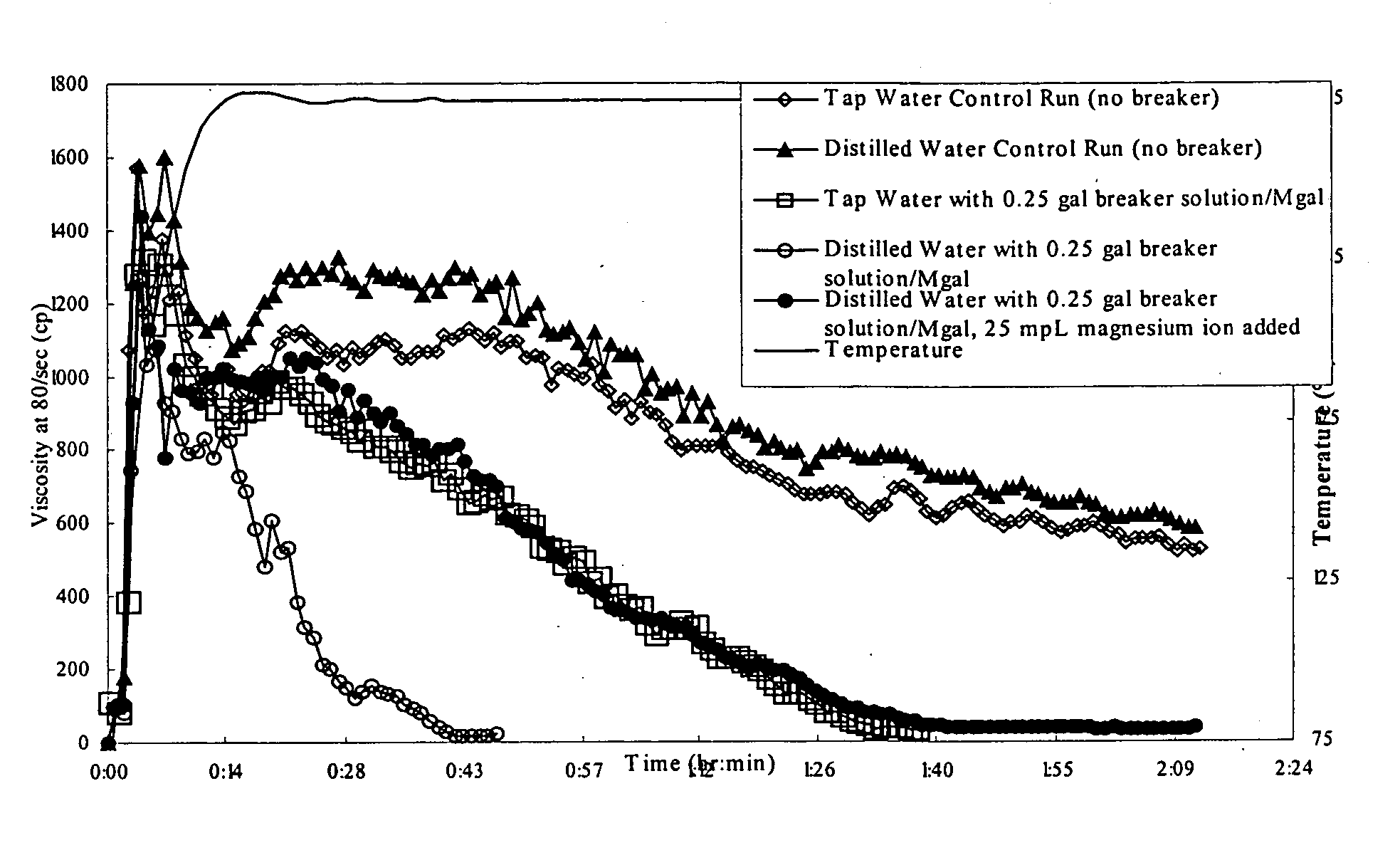
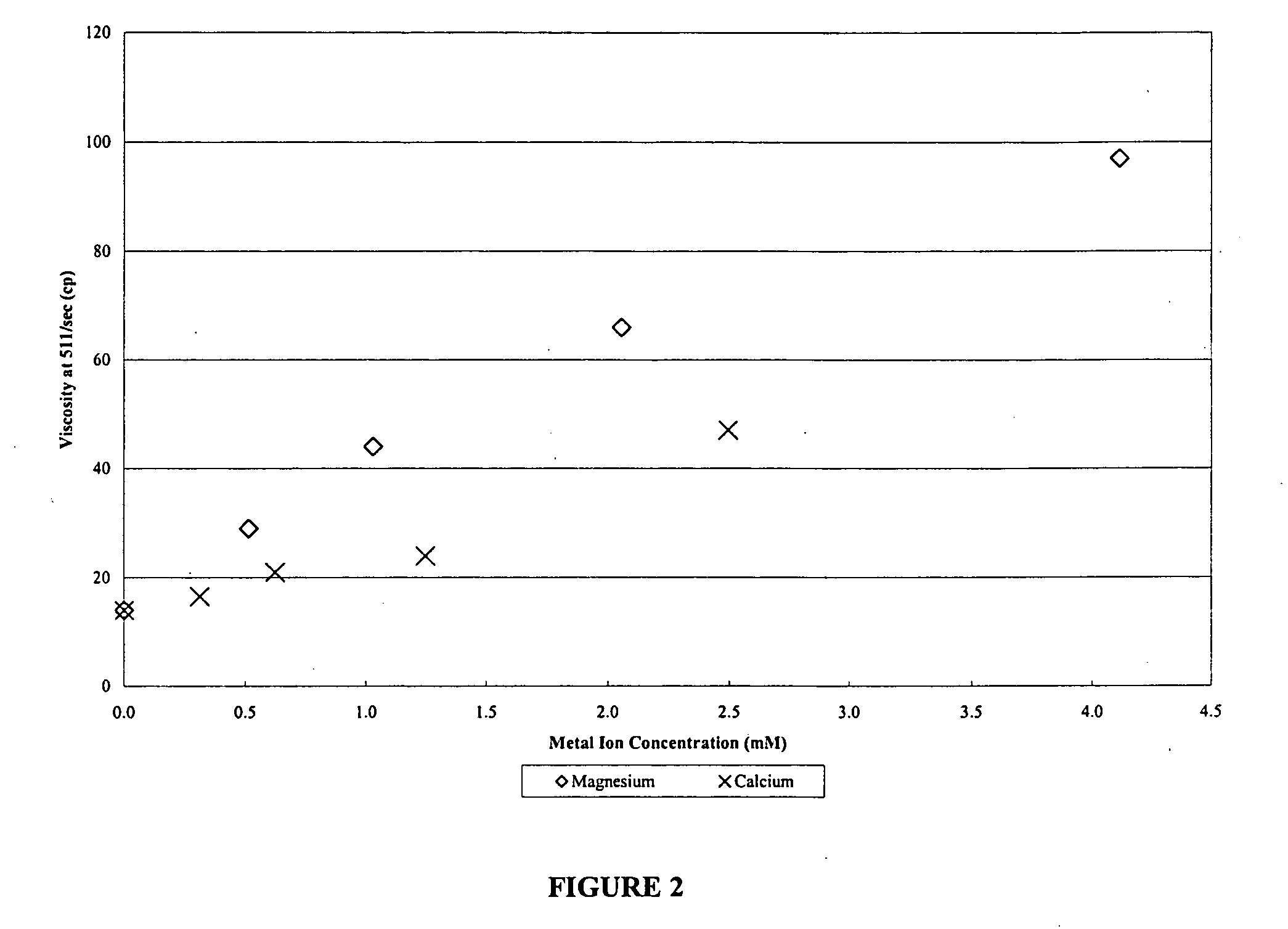
Controlling chlorite or hypochlorite break rate of well treatment fluids using magnesium or calcium ions
ActiveUS20050205259A1High viscosityBreak viscosityFluid removalFlushingHypochloriteWater soluble polysaccharides
The present invention provides methods and compositions for treating a subterranean formation penetrated by a well. The method comprising the steps of: (a) forming a treatment fluid, and (b) introducing the treatment fluid into the well and into contact with the formation. According to one aspect, the treatment fluid comprises: (1) water; (2) a water-soluble polysaccharide capable of increasing the viscosity of the water and present in a sufficient concentration to increase the viscosity of the water or a water-soluble polysaccharide and a crosslinking agent for the water-soluble polysaccharide, which are present in a sufficient concentration to effect crosslinking of the of the polysaccharide and increase the viscosity of the water; (3) a breaker comprising at least one member selected from the group consisting of a source of chlorite ions and a source of hypochlorite ions, wherein the breaker is present in a sufficient concentration to break the treatment fluid after introduction of the fluid into the subterranean formation; and (4) a breaker moderator comprising at least one member selected from the group consisting of a source of magnesium ions and a source of calcium ions, wherein the breaker moderator is present in a sufficient concentration to control the break rate of the fluid.
Owner:HALLIBURTON ENERGY SERVICES INC
Chlorine dioxide generator and associated methods
InactiveUS20060034750A1Easy maintenanceSmall footprintPhysical/chemical process catalystsFluidised-bed furnacesTemperature controlChlorine dioxide
An apparatus and method for producing chlorine dioxide includes a reactor for reacting an aqueous reaction solution including an aqueous acid solution and a solution of an alkali metal salt of a chlorite ion to form a product solution. The reactor includes a substantially cylindrical inner column for receiving the aqueous reaction solution and a substantially cylindrical outer column positioned in coaxial surrounding relation to at least a portion of the inner column. The outer column is for containing a temperature-controlled fluid for maintaining solution flowing through the inner column at a predetermined temperature. A stripper is in fluid communication with an outlet of the inner column for stripping chlorine dioxide from the product solution into a gas to provide a product gas and a stripped product solution. An absorber is provided for absorbing chlorine dioxide from the product gas to provide a substantially byproduct-free aqueous chlorine dioxide solution.
Owner:WATERTECH
Method for optimizing the efficacy of chlorous acid disinfecting sprays for poultry and other meats
InactiveCN1279584AEnhanced effectiveness in destroying microbes on surfacesExtended Commercial Shelf LifeMeat/fish preservation using liquidsMeat/fish preservation using chemicalsChlorous acidCHLORITE ION
A method for disinfecting a meat carcass by spray application of an aqueous solution containing from about 0.05-0.12% of a metal chlorite and a sufficient quantity of an acid having a pKa of from about 2.0-4.4 to adjust the pH of the aqueous solution to about 2.2-4.5 and to maintain the chlorite ion concentration in the form of chlorous acid to not more than about 35% by weight of the aqueous solution, the molar ratio of the acid to metal chlorite being at least equal to the first pKa of the acid multiplied by the grams / liter concentration of metal chlorite in the aqueous solution. In one embodiment, the meat carcass is poultry.
Owner:ALCIDE CORP
Method for reducing pathogens in the gastrointestinal tract of poultry and other food animals
ActiveUS20060024405A1Reduce decreaseReduce pathogensAntibacterial agentsBiocideMicroorganismChlorous acid
A method for reducing pathogens in the gastrointestinal tract of a live animal comprising orally administering to the animal an effective amount of an aqueous antimicrobial solution, wherein the aqueous antimicrobial solution comprises from about 0.01% to about 0.1% by weight of a metal chlorite and a sufficient quantity of an acid having a first pKa of from about 2.0 to about 4.4 to adjust the pH of the aqueous antimicrobial solution to about 2.2 to about 4.5 and to maintain the chlorite ion concentration in the form of chlorous acid to not more than about 35% by weight of the total chlorite ion present in the aqueous antimicrobial solution, is disclosed.
Owner:ECOLAB USA INC
Method for suppressing or preventing fibrous adhesion formation using a multicomponent aqueous oxychlorine composition prepared on-site
InactiveUS20100330203A1Reduce the possibilityMinimize alternative reactive pathwaysBiocidePeroxide active ingredientsChlorine dioxideHypochlorite
A composition and method are described for suppressing or preventing fibrous adhesion formation using a multicomponent aqueous oxychlorine composition. Fibrous adhesions typically form during healing of tissue, for example following a surgical procedure. A multicomponent oxychlorine composition is provided for irrigating the tissue which minimizes post-surgical adhesion formation, the composition containing both chlorine dioxide and chlorite ion, and a complex ion thereof. The chlorine dioxide level generally is in an effective range of ClO2 concentration from about 10 ppm to a maximum of about 110 ppm. In a preferred embodiment, a physiological composition is provided in a thickened form to increase retention in the area being treated. The composition is preferably based on a standard saline solution converted to the oxychlorine composition just prior to use by sequential addition of aqueous concentrates of a chlorite salt, a hypochlorite salt combined with a physiological buffer-producing salt of a multibasic acid, and an acidifying agent, optionally including a thickening agent.
Owner:KROSS LINK LABS LLC
Biocidal fibrous and film materials utilizing silver ion
ActiveUS20060057191A1Reduces and prevents discolorationBiocideInorganic active ingredientsWound dressingMedicine
Disclosed are compositions comprising a hydrophilic polymer, a hydrophobic polymer, and a silver ion, wherein the silver ion associates with at least one hydrophilic or hydrophobic polymer, and wherein the composition does not comprise an effective amount of a chlorite ion. Also disclosed are methods of making a photo-stable silver ion containing wound dressing and methods of reducing or preventing infection of a wound by using the compositions and wound dressings of the present invention.
Owner:SOUTHWEST RES INST
Biocidal fibrous and film materials comprising silver and chlorite ions
ActiveUS8900610B2Reduces and prevents discolorationBiocideDead animal preservationWound dressingHydrophilic polymers
Disclosed are compositions comprising a hydrophilic polymer, a hydrophobic polymer, a silver ion, and a chlorite anion, wherein the silver ion associates with at least one hydrophilic or hydrophobic polymer, and wherein the chlorite ion associates with the hydrophilic polymer. Also disclosed are methods of making a photo-stable silver ion and chlorite ion containing wound dressing and methods of reducing or preventing infection of a wound by using the compositions and wound dressings of the present invention.
Owner:SOUTHWEST RES INST
Regenerated solution of alkaline copper etching solution and method for increasing etching speed thereof
The invention provides a regenerated solution of alkaline copper etching solution and a method for increasing the etching speed thereof. The regenerated solution of alkaline copper etching solution contains a first accelerating agent and a second accelerating agent, wherein the first accelerating agent contains an organic thio-acid compound with a NH2-CS-NH-group, and the second accelerating agent is selected from at least one of a chlorition compound and a perboric acid ion compound. According to the method for increasing the etching speed of the regenerated solution of alkaline copper etching solution, an effective amount of second accelerating agent is added to the regenerated solution of alkaline copper etching solution, wherein the second accelerating agent is selected from at least one of the chlorition compound and the perboric acid ion compound. The regenerated solution of alkaline copper etching solution provided by the invention can effectively improve the etching speed of the alkaline etching solution, and can keep the etching speed stable, thereby prolonging the service life of the etching regenerated solution.
Owner:SHENZHEN JECH TECH
Methods and compositions for on-demand release of ClO2 gas from UV-activated chlorite ion
Compositions and methods for generating ClO2 gas are disclosed. A composition that includes a chlorite salt is activated by exposure to ultraviolet light. After an optional storage period, the composition is then exposed to moisture, resulting in the generation of ClO2 gas. Exemplary compositions include polymers in which the chlorite salt is dispersed. The polymers may be used to form films thatcan be used to package, e.g., food products, pharmaceutical products, medical devices, and / or laboratory devices. Upon exposure to ultraviolet light and moisture, the packaging releases controlled quantities of ClO2 gas, which may disinfect and / or deodorize the packaged device or product.
Owner:WISCONSIN ALUMNI RES FOUND +1
Promoting whole body health
The present invention relates to promoting whole body health in humans and animals by using topical oral compositions comprising a safe and effective amount of chlorite ion in admixture with a pharmaceutically acceptable carrier, said compositions being effective in controlling bacterial-mediated diseases and conditions present in the oral cavity and inhibiting the spread into the bloodstream of oral pathogenic bacteria and associated bacterial toxins and resultant inflammatory cytokines and mediators. The present invention also encompasses methods of use of these compositions by topically applying to the oral cavity, a safe and effective amount of chlorite ion to promote and / or enhance whole body health in humans and other animals.
Owner:PROCTER & GAMBLE CO
Biocidal fibrous and film materials utilizing silver ion
ActiveUS9364579B2Reduces and prevents discolorationBiocideInorganic active ingredientsWound dressingHydrophobic polymer
Disclosed are compositions comprising a hydrophilic polymer, a hydrophobic polymer, and a silver ion, wherein the silver ion associates with at least one hydrophilic or hydrophobic polymer, and wherein the composition does not comprise an effective amount of a chlorite ion. Also disclosed are methods of making a photo-stable silver ion containing wound dressing and methods of reducing or preventing infection of a wound by using the compositions and wound dressings of the present invention.
Owner:SOUTHWEST RES INST
Method for chlorite removal
InactiveUS20070080116A1Reaction is slowSolve reductionSpecific water treatment objectivesWater contaminantsCHLORITE IONCompound (substance)
The invention is directed to a process for removing chlorite ion from a body of water containing unacceptably high levels of chlorite comprising adding to said body of water a chlorite removal chemical selected from the group comprising sodium dichloroisocyanurate dihydrate, sodium dichloroisocyanurate, trichloroisocyanurate, polyaluminum chloride, sodium permanganate, potassium permanganate, and catalase enzyme.
Owner:EVOQUA WATER TECH LLC
Systems and methods for generating chlorine dioxide
Owner:EASTERN PLASTICS +1
Controlling chlorite or hypochlorite break rate of well treatment fluids using magnesium or calcium ions
ActiveUS7093659B2High viscosityBreak viscosityFluid removalDrilling compositionHypochloriteWater soluble polysaccharides
Methods and compositions are provided for treating a subterranean formation penetrated by a well. The method includes the steps of: (a) forming a treatment fluid, and (b) introducing the treatment fluid into the well and into contact with the formation. The treatment fluid includes: (1) water; (2) a water-soluble polysaccoharide capable of increasing the viscosity of the water or a water-soluble polysaccharide and a crosslinking agent for the water-soluble polysaccharide; (3) a breaker comprising at least one member selected from the group consisting of a source of chlorite ions and a source of hypochlorite ions; and (4) a breaker moderator comprising at least one member selected from the group consisting of a source of magnesium ions and a source of calcium ions.
Owner:HALLIBURTON ENERGY SERVICES INC
Method for reducing pathogens in the gastrointestinal tract of poultry and other food animals
Owner:ECOLAB USA INC
Oxychlorine oral rinse composition having enhanced oral-tissue compatibiilty for the destruction of malodorants, their putrefactive microbial sources and gum-disease pathogens anda method for the preparation thereof
ActiveUS20140341819A1Improve the immunityProtect the interiorAntibacterial agentsCosmetic preparationsChlorine dioxideTissue Compatibility
An antimicrobial oxychlorine oral rinse composition for preventing and treating oral malodor and diseases, tooth decay with improved palatability and tissue compatibility, includes an aqueous chlorine dioxide and chlorite ion solution with the chlorite ion predominating in the multiple oxychlorine rinse composition. A method for preparing such antimicrobial oxychlorine oral rinse composition comprising 3 ppm to about 200 ppm of chlorine dioxide, by first preparing an aqueous solution with about 7 to 400 ppm of chlorite ion; then an aqueous acidifying agent concentrate which, upon introduction to the chlorite solution, reduces its pH to about 4.5 to 6.0; then a second aqueous buffer-producing oxidant combination concentrate. The first acidifying agent concentrate is added to the chlorite ion solution and the second aqueous buffer-producing oxidant combination concentrate is added to the acidified chlorite ion solution, producing an antimicrobial oxychlorine oral rinse composition at about 5.0 to 6.8 pH.
Owner:PROFRESH PROPERTIES
Systems and methods for generating chlorine dioxide
Processes for generating chlorine dioxide generally include acidifying an alkali metal chlorite solution; and contacting the acidified alkali metal chlorite solution with a solid phase chlorine-containing material to produce chlorine dioxide. An exemplary system for generating chlorine dioxide generally includes a water source in fluid communication with a conduit that is fluidly connected to a vessel, wherein the vessel comprises a housing, an inlet in fluid communication with the housing and the conduit, an outlet, and a solid phase chlorine-containing material disposed within the housing; an acid source downstream from the water source in fluid communication with the conduit; and a chlorite ion source in fluid communication with the conduit downstream from the acid source. Various means are provided for the acid source.
Owner:EASTERN PLASTICS +1
Disinfecting oral rinse compositions and process for using the same
InactiveUS20040131557A1Enhance and prolongMinimize formationCosmetic preparationsBiocideChlorous acidCHLORITE ION
This invention relates generally to compositions and methods useful for oral hygiene rinses, and more specifically to oral rinses in which the antimicrobial activity of chlorous acid is supplemented by that of lactic acid as one of a combination of antimicrobial acids, preferably acids which serve to partially convert chlorite ion to chlorous acid.
Owner:ALL USA DIRECT
Release of CIO2 gas from produce packaging film
The invention relates to a multilayer produce packaging film. The multilayer produce packaging film includes a first layer and a chlorine dioxide-producing layer. The chlorine dioxide-producing layerincludes a polymer composition and a plurality of chlorite ions. The chlorine dioxide-producing layer is substantially free of an energy-activated catalyst and is substantially free of an acid-releasing compound. However, the film is capable of generating chlorine dioxide when exposed to UV light and moisture.
Owner:WISCONSIN ALUMNI RES FOUND +1
Method for chlorite removal
InactiveUS7384565B2Solve reductionSpecific water treatment objectivesWater contaminantsCHLORITE IONTrichloroisocyanuric acid
The invention is directed to a process for removing chlorite ion from a body of water containing unacceptably high levels of chlorite comprising adding to said body of water a chlorite removal chemical selected from the group comprising sodium dichloroisocyanurate dihydrate, sodium dichloroisocyanurate, trichloroisocyanurate, polyaluminum chloride, sodium permanganate, potassium permanganate, and catalase enzyme.
Owner:EVOQUA WATER TECH LLC
RELEASE OF ClO2 GAS FROM MEDICAL DEVICE PACKAGING FILM
InactiveCN108349223AWon't happenControl releaseWrappersAdhesive articlesChlorine dioxideAcid release
A multilayer medical packaging film includes a first layer and a chlorine dioxide-producing layer. The chlorine dioxide-producing layer includes a polymer composition and a plurality of chlorite ions.The chlorine dioxide-producing layer is substantially free of an energy-activated catalyst and is substantially free of an acid-releasing compound. However, the film is capable of generating chlorinedioxide when exposed to UV light and moisture.
Owner:WISCONSIN ALUMNI RES FOUND +1
Manufacturing method for obtaining novel chlorine oxide composition from degraded hypochlorite
PendingUS20210206636A1Poor storabilityChlorine odorBiocideSedimentation separationHypochloriteChlorine dioxide
The purpose of the present invention is to provide a method for manufacturing a new disinfectant from sodium hypochlorite that has degraded in quality during storage. A method for manufacturing a novel disinfectant from a solution containing hypochlorite ions, chlorate ions, and chloride ions, wherein the method includes: a first reaction step for adding sulfuric acid to the solution and generating chlorine gas; a step in which, in a recovery liquid A, the generated chlorine gas is caused to react with sodium hydroxide or calcium hydroxide and recovered as hypochlorite ions; a second reaction step for adding, to a reaction mother liquid after the first reaction step, sulfuric acid having a higher concentration than that in the first reaction step, and generating chlorine dioxide gas; a step in which, in a recovery liquid B, the generated chlorine dioxide gas is caused to react with sodium hydroxide and hydrogen peroxide and recovered as chlorite ions; and a step for mixing the recovery liquid A and the recovery liquid B and obtaining a novel disinfectant.
Owner:HONBU SANKEI
Reagent, device and method for purification
InactiveCN111547683AControl concentrationLong-term stable preparation and releaseBiocideSpecific water treatment objectivesChlorine dioxidePtru catalyst
The invention relates to a reagent, a device and a method for purification, and in particular, relates to a reagent, a device and a method for purification by generating chlorine dioxide. The purifying agent comprises a first component and a second component which exist independently, the first component comprises chlorite or a chlorite aqueous solution, and the second component comprises an active agent, a catalyst and an activity inhibitor; the molar ratio of chlorite ions in the chlorite or chlorite aqueous solution to hydrogen ions in the active agent to iodide ions in the catalyst to hydroxide radicals generated by the activity inhibitor is 1:(0.3-3):(0.04-0.4):(0.05-0.3). The purifying agent can be rapidly prepared under the condition of ensuring controllable safe exposure concentration of a human body and stably release chlorine dioxide gas for a long time within a certain period, so that the chlorine dioxide gas can be prepared and released instantly and stably for a long time.
Owner:白金制药(西安)有限公司
Method and composition for preventing and healing osteonecrosis of the jaw
ActiveUS8697141B2Facilitate prevention and healingPromote healingBiocideSkeletal disorderCHLORITE IONBody area
A method and composition for preventing and treating all forms of osteonecrosis of the jaw are disclosed. The composition is comprised of 0.005%-2.0% weight / volume (w / v) chlorine dioxide source, such as sodium chlorite, chlorite ion, stabilized chlorine dioxide or similar and may take the form of a paste, gel, rinse, spray, powder, varnish or similar. The method for treatment and prevention includes the application of the composition in the oral cavity and other body areas affected by osteonecrosis of the jaw.
Owner:MICROPURE INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com