Oxidation method and compositions therefor
a technology of oxidation method and composition, which is applied in the field of improved two-part oxidizing system, can solve the problems of affecting the efficiency of ph treatment, so as to achieve less water hardness ions, and less sodium acid sulfate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Impact of Ferric Sulfate Addition on COD
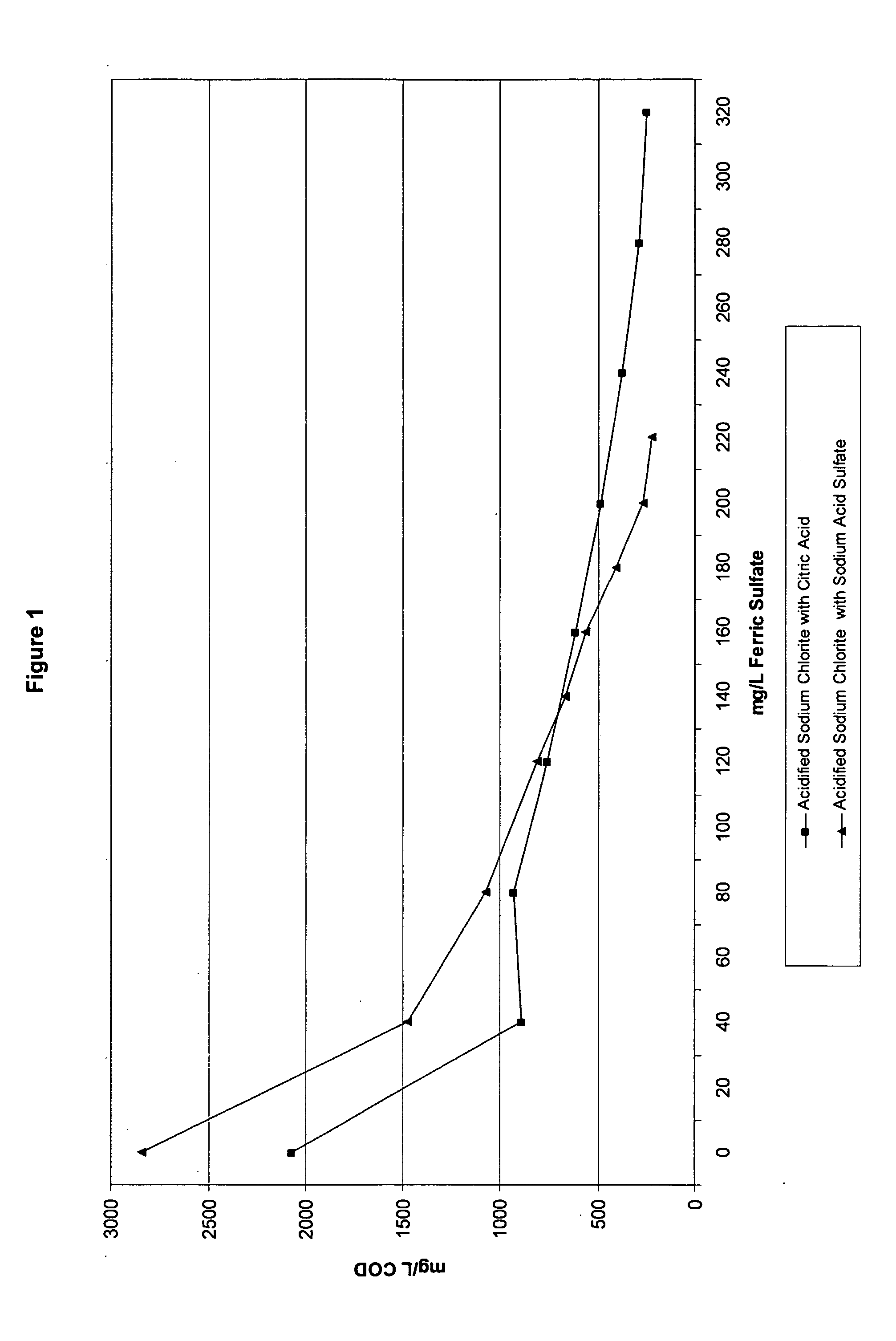
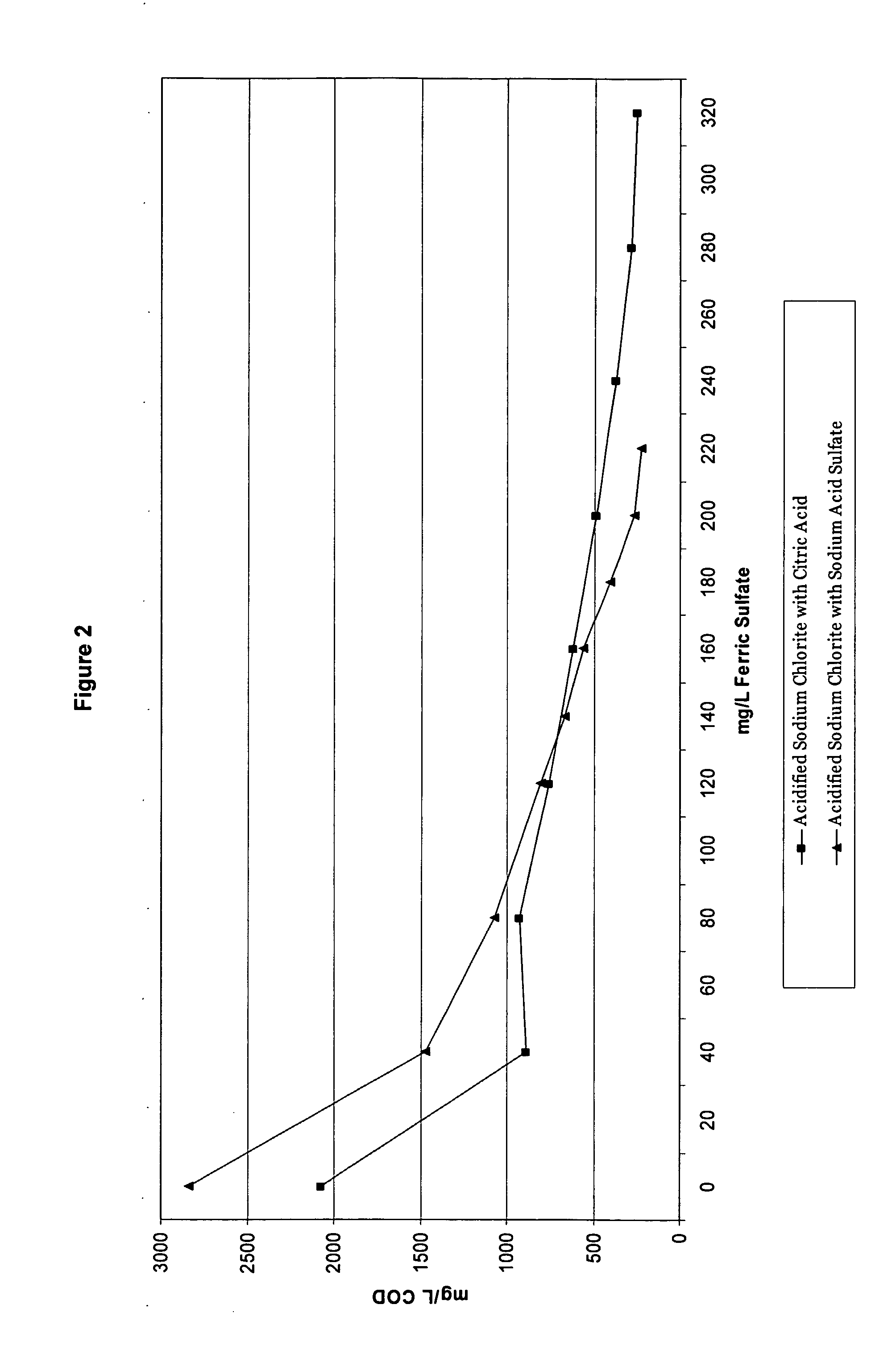
[0127] As previously discussed, coagulants are often used in wastewater treatment to remove charges from particles in solution and make them more likely to form larger particles that can float to the top and be skimmed off and removed. However, when acidified sodium chlorite solutions are present in the wastewater, the type of acid used to make the acidified sodium chlorite solution can impact the wastewater treatment process. Example 1 tested the impact of a known coagulant, ferric sulfate, on the COD (chemical oxygen demand) when the acidified sodium chlorite solutions of Table 2 are present. For this example, the wastewater treatment test method was used. After wastewater samples were pulled, various levels of ferric sulfate were added to the samples. The samples were then subjected to the Jar Test Profile and the mg / L COD was recorded.
[0128]FIGS. 1 and 2 show the impact of acidified sodium chlorite compositions made with citric acid and ...
example 2
Impact of Ferric Sulfate Addition of Phosphorous Removal
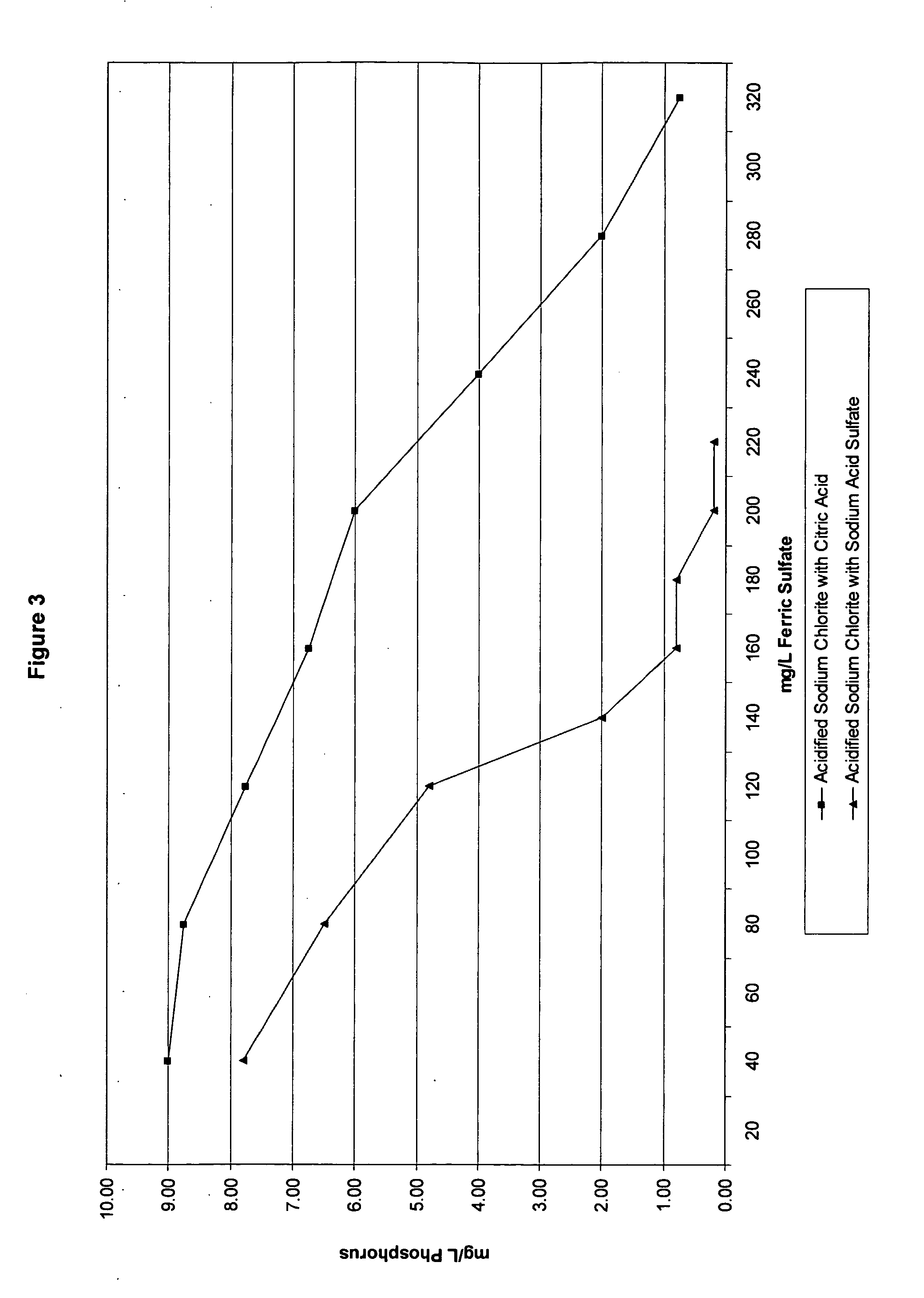
[0129] Again, ferric sulfate is a known coagulant used in wastewater treatment that can react with acidified sodium chlorite solutions in the waste water. As previously discussed, phosphorous is one of many species present in the wastewater that must be removed. Example 2 tested the impact of ferric sulfate concentration on phosphorous level left in the wastewater when the acidified sodium chlorite solutions of Table 2 are present. For this example, the wastewater treatment test method was used. After wastewater samples were pulled, various levels of ferric sulfate were added to the samples. The samples were then subjected to the Jar Test Profile and the mg / L phosphorous was recorded.
[0130]FIG. 3 shows the impact of acidified sodium chlorite compositions made with citric acid and sodium acid sulfate on the phosphorous level in the wastewater. The sodium acid sulfate formula always has a lower level of phosphorous in the waste...
example 3
Impact of Ferric Sulfate on Turbidity
[0131] As previously discussed, high turbidity or a high solid concentration in wastewater is undesirable for several reasons. High turbidity creates places for bacteria to grow. High turbidity also increases COD which is undesirable for the reasons previously discussed in Example 1. Finally, high turbidity or high solid is aesthetically undesirable, particularly in drinking water. Example 3 tested the impact of ferric sulfate concentration on turbidity in the wastewater when the acidified sodium chlorite solutions of Table 2 are present. For this example, the wastewater treatment test method was used. After wastewater samples were pulled, various levels of ferric sulfate were added to the samples. The samples were then subjected to the Jar Test Profile and the turbidity was recorded.
[0132]FIG. 4 shows the impact of acidified sodium chlorite compositions made with citric acid and sodium acid sulfate on the turbidity of the wastewater. Initially...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com