Recombinant adeno-associated virus vector as well as preparation method and application thereof
A viral vector, virus technology, applied in the field of biomedicine, to achieve broad market prospects, excellent infection effect, and the effect of reducing intraocular pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
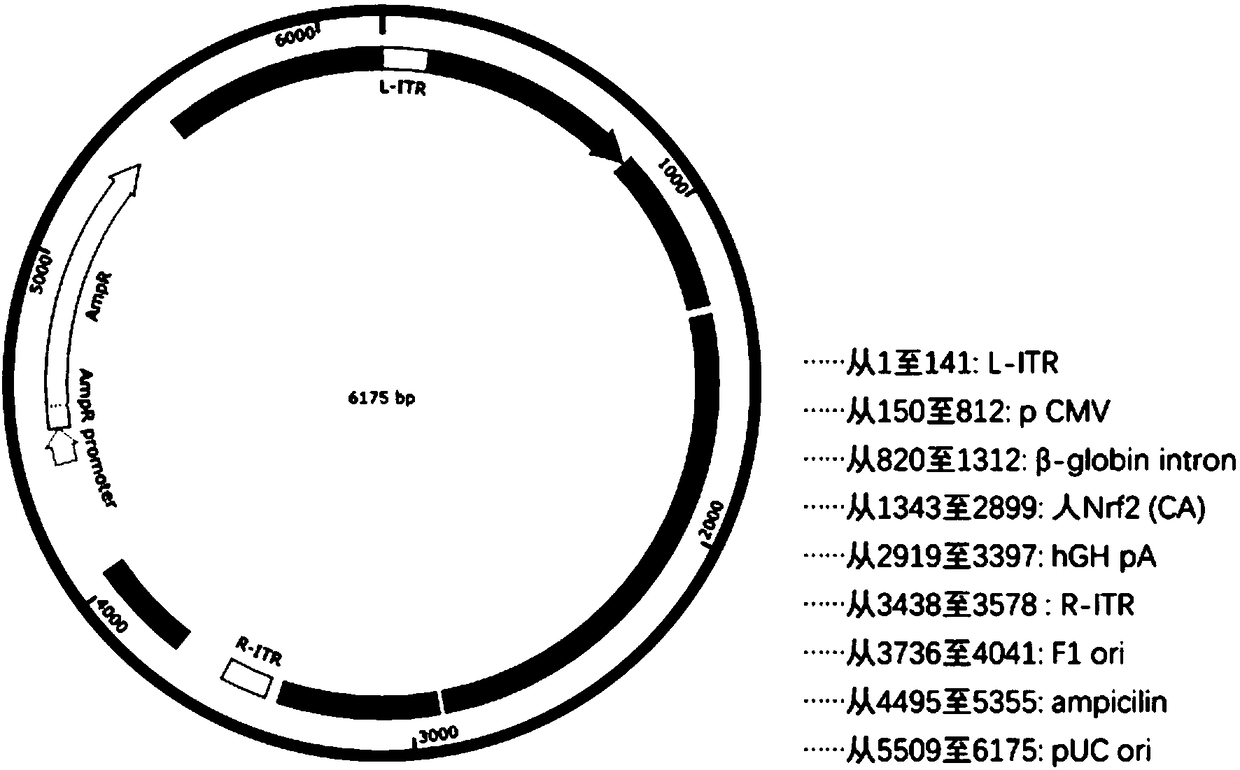
[0060] Example 1 Construction and separation and purification of recombinant adeno-associated virus vector
[0061] gene sequence
[0062] Shown in SEQ ID NO:1 is the Nrf2(CA) sequence after splicing,
[0063] Shown in SEQ ID NO: 2 is the amino acid sequence of human Nrf2 (CA) after splicing,
[0064] Shown in SEQ ID NO:3 is the Left ITR sequence,
[0065] Shown in SEQ ID NO:4 is the CMV sequence,
[0066] Shown in SEQ ID NO:5 is the humanβ-globin intron sequence,
[0067] Shown in SEQ ID NO:6 is the MCS sequence,
[0068] Shown in SEQ ID NO:7 is hGHpA sequence,
[0069] Shown in SEQ ID NO:8 is the Right ITR sequence,
[0070] Shown in SEQ ID NO: 9 is the Ampicillin Resistance sequence,
[0071] Shown in SEQ ID NO:10 is the full nucleotide sequence,
[0072] Shown in SEQ ID NO:11 is the f1ori sequence,
[0073] Shown in SEQ ID NO:12 is the recombinant sequence,
[0074] Shown in SEQ ID NO: 13 is the Nrf2(CA)' sequence.
[0075] The construction of plasmid AAV_Nrf2(C...
Embodiment 2
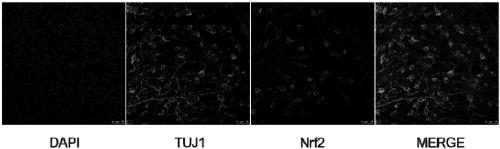
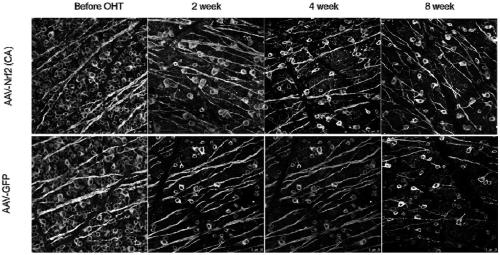
[0077] Embodiment 2 The treatment of recombinant adeno-associated virus vector to glaucoma model mice
[0078] 1 Experimental materials
[0079] 1.1 Experimental animals
[0080] Normal C57BL / 6J mice were purchased from Shanghai Slack Experimental Animal Co., Ltd., and the light cycle was 12h light-12h dark, with free access to food and water, and all animal research was strictly in accordance with the "Regulations on the Management of Experimental Animals" issued by the National Science and Technology Committee. "conduct.
[0081] 1.2 Experimental reagents and consumables
[0082] Normal saline (Zhejiang Tianrui Pharmaceutical Co., Ltd.), disposable injection needle (Becton Dickinson and Company, USA), Nrf2 antibody (Abcam), TUJ1 (Abcam), secondary antibody goat anti-rabbit IgG (JacksonImmuno), goat serum (Sigma), Polyethylene glycol octylphenyl ether (Triton X-100) (Sigma), Paraformaldehyde (Sigma).
[0083] 1.3 Experimental Instruments
[0084] Ophthalmology Experiment...
Embodiment 3
[0102] Example 3 Treatment of recombinant adeno-associated virus vectors to glaucoma model rats
[0103] 1 experimental drug
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com