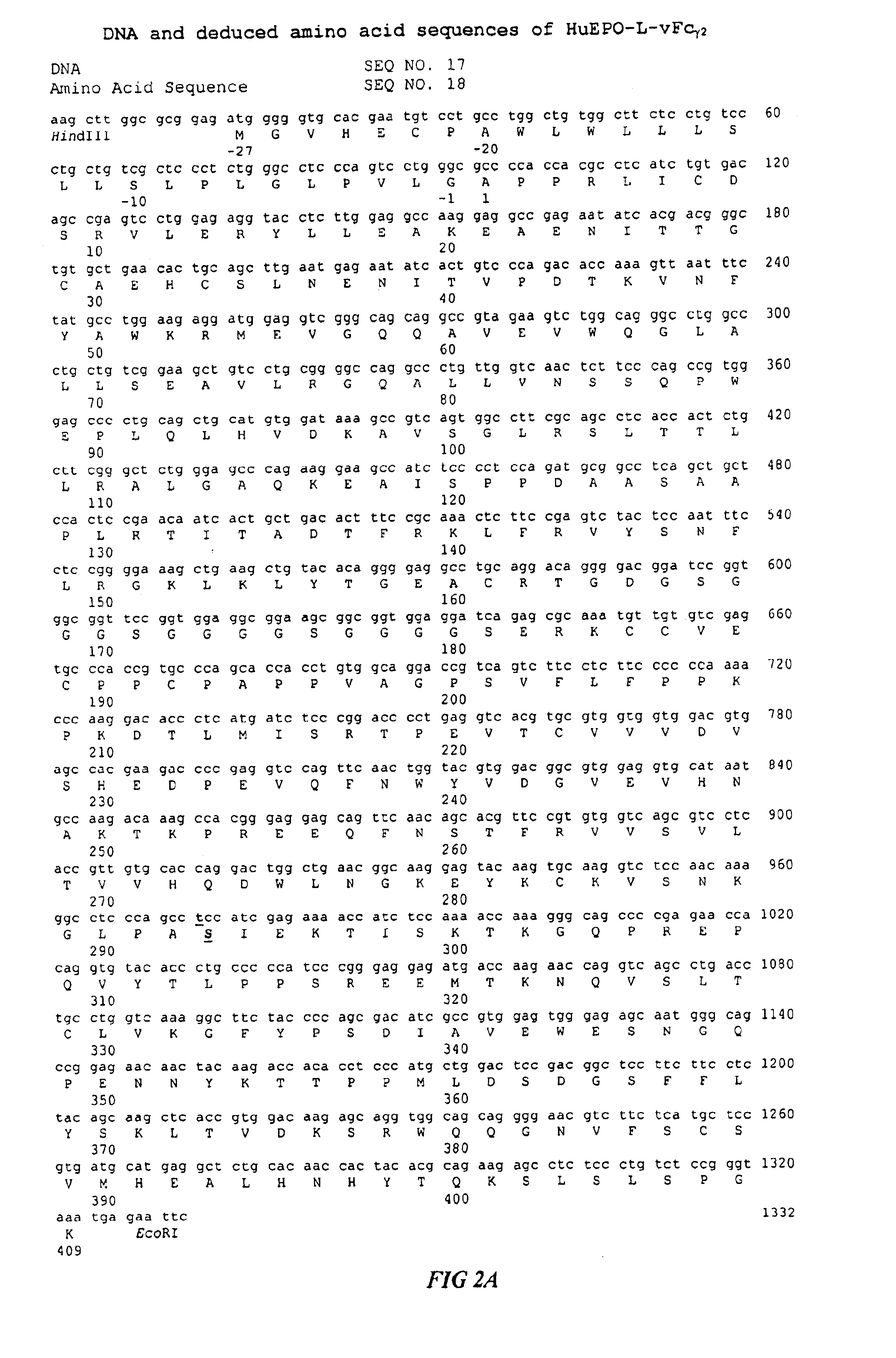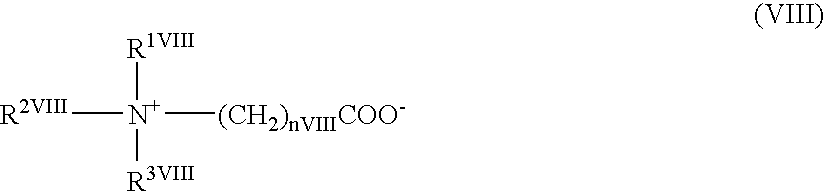Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
795 results about "Erythroid cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Erythroid Cells. Erythroid Cells are also called erythrocytes. These are the most common type of blood cell and the vertebrate organism's principal means of delivering oxygen to the body tissues via blood flow through the circulatory system.
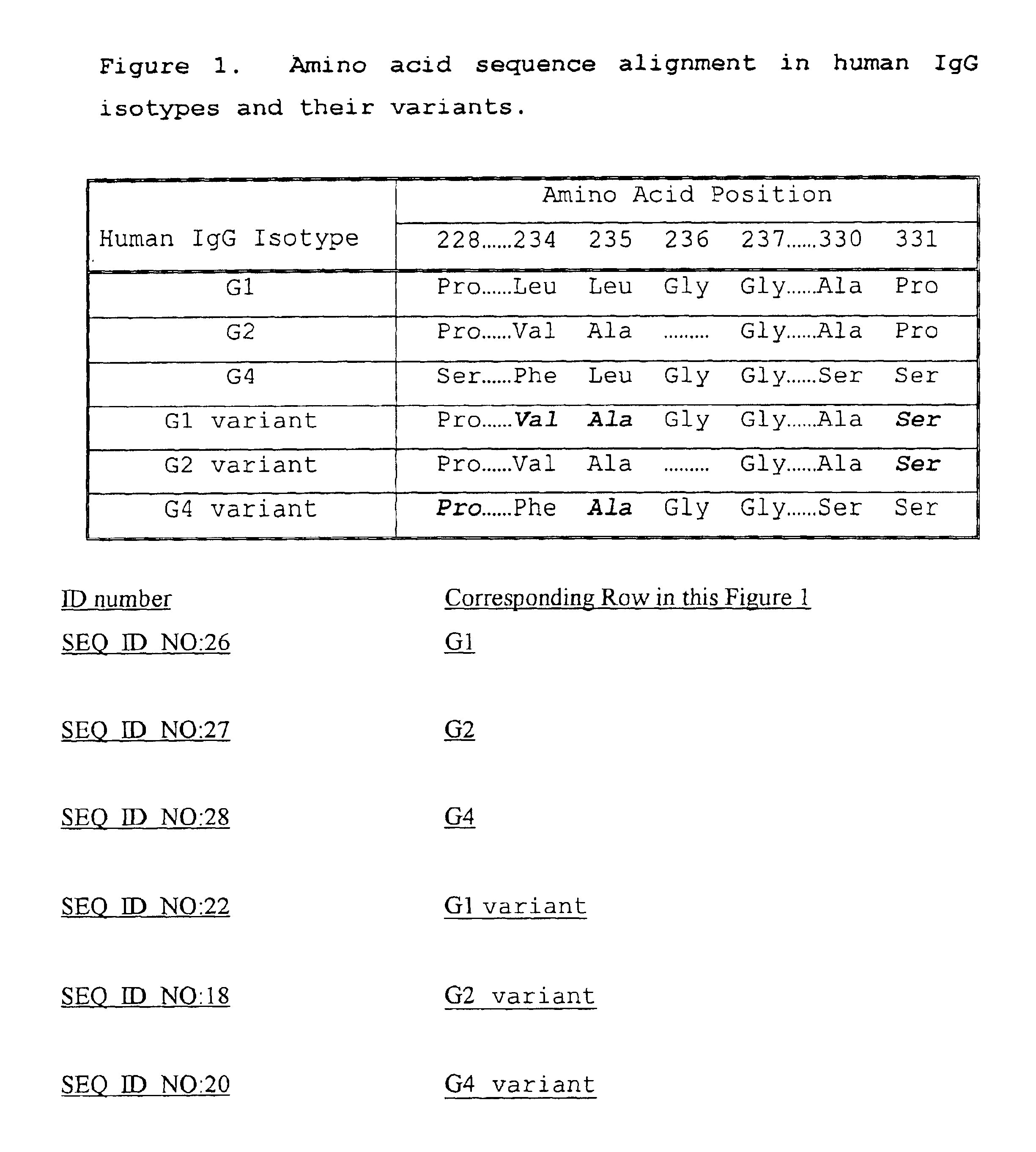
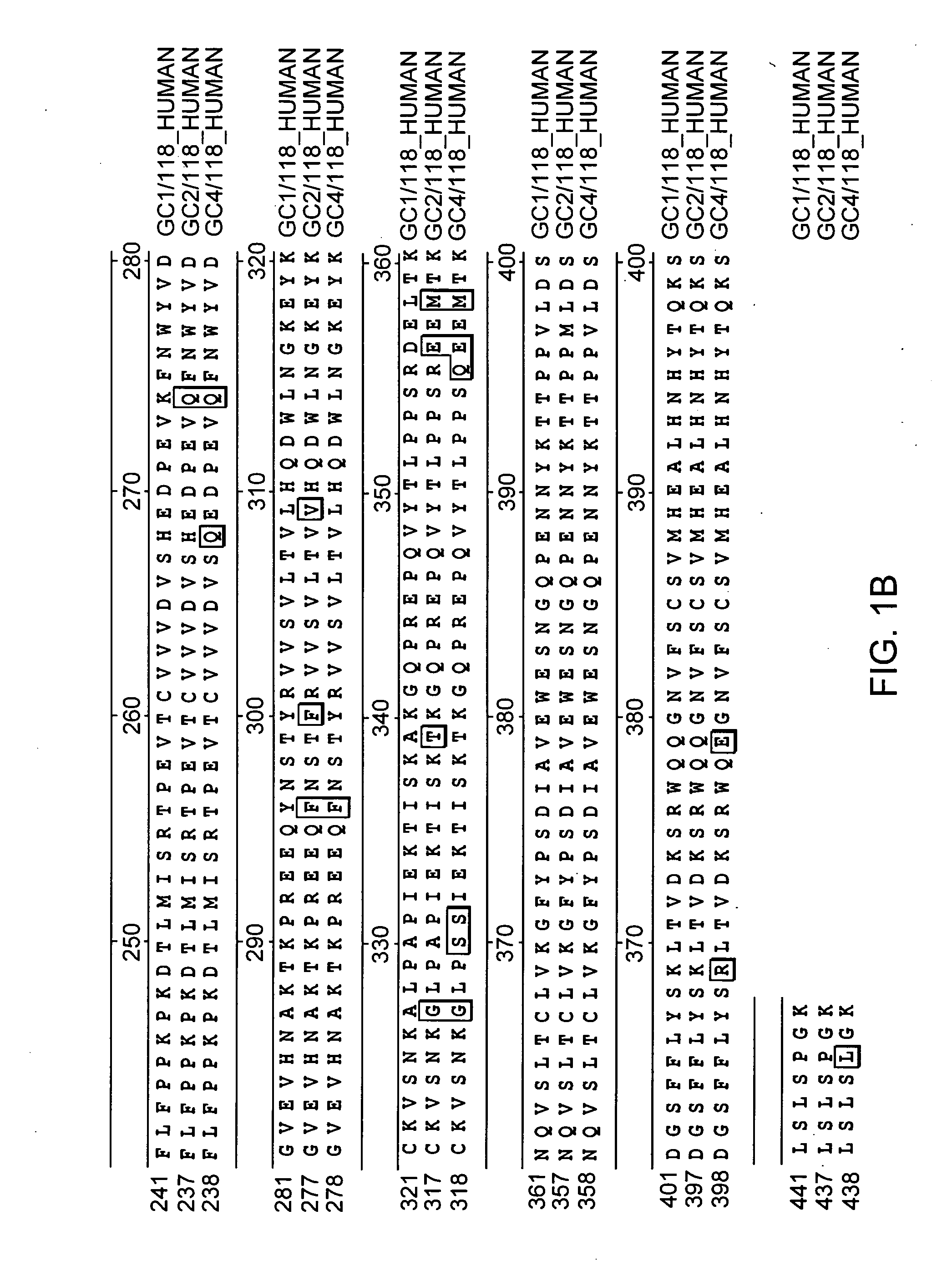
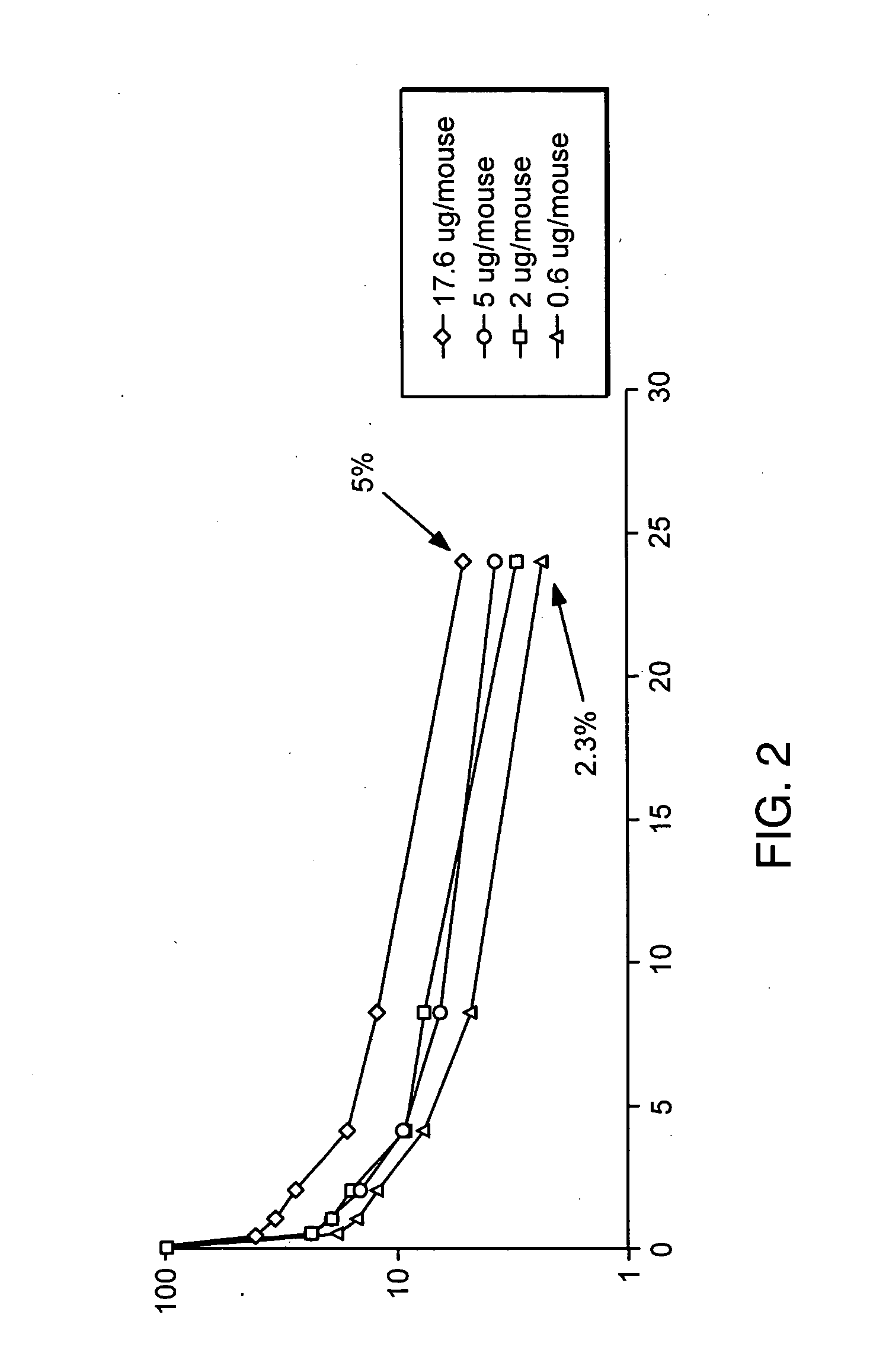
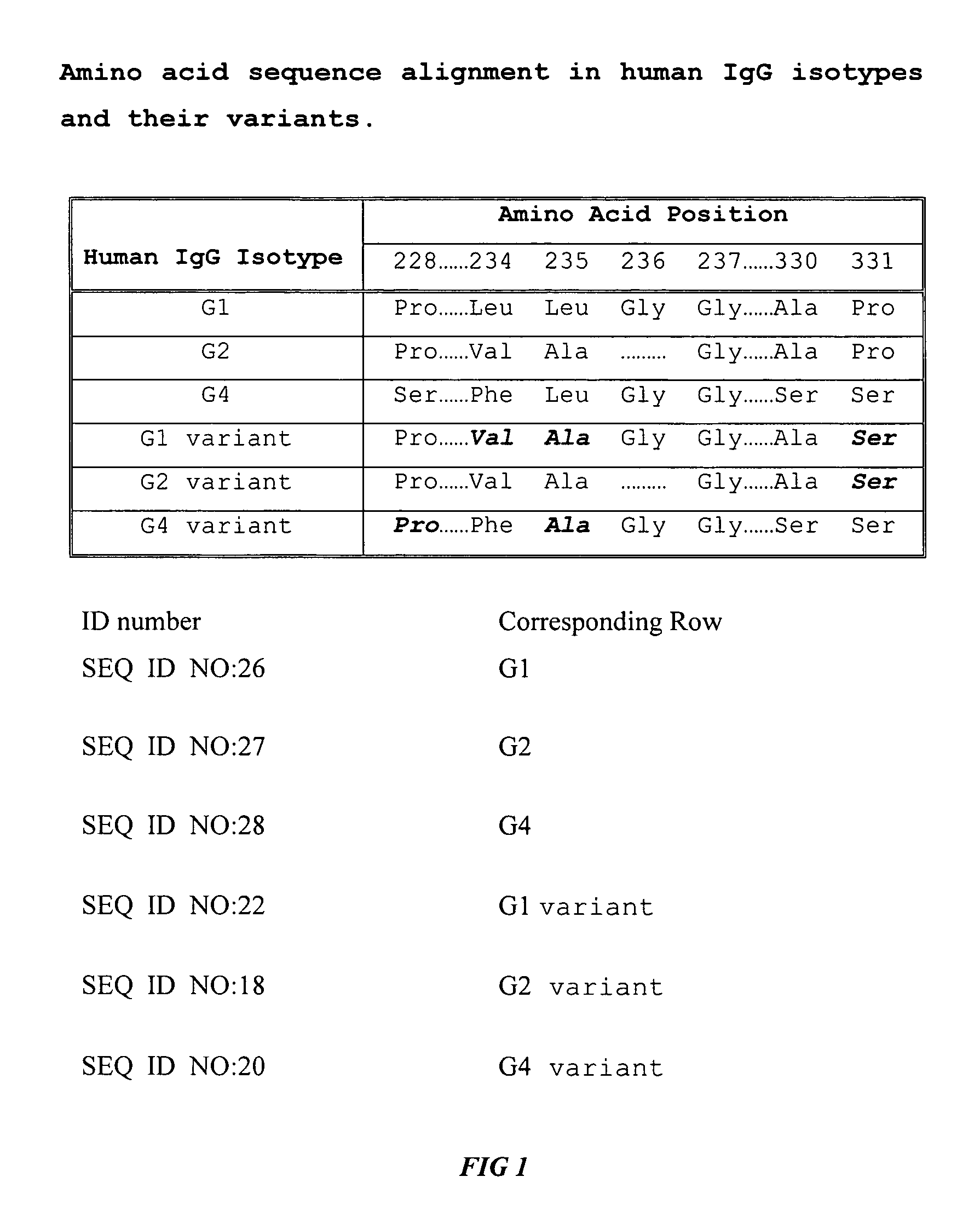
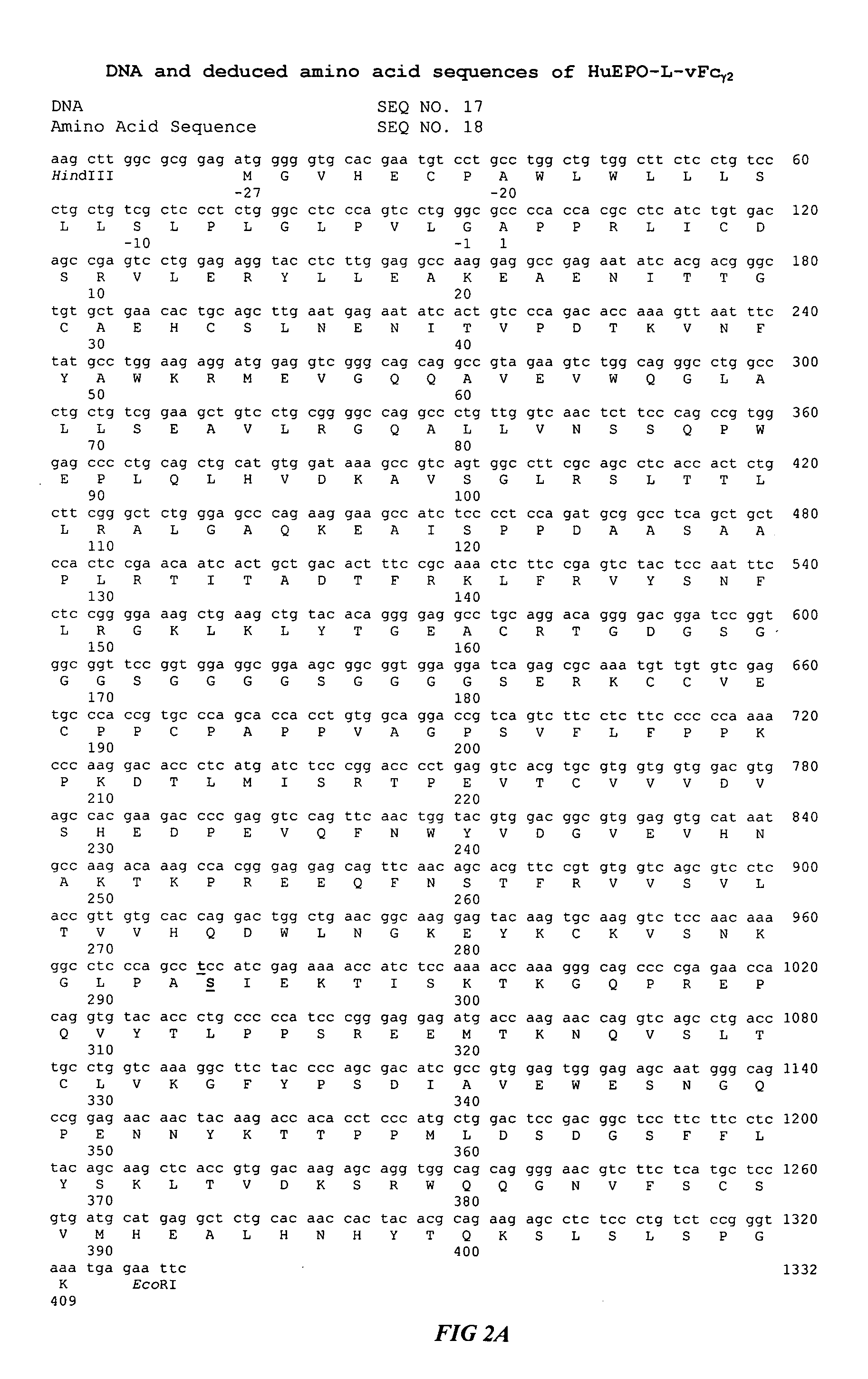
Fc fusion proteins of human erythropoietin with increased biological activities
InactiveUS6900292B2Improve biological activityExtended serumPeptide/protein ingredientsAntibody mimetics/scaffoldsSide effectHalf-life
Fc fusion proteins of human EPO with increased biological activities relative to rHuEPO on a molar basis are disclosed. The HuEPO-L-vFc fusion protein comprises HuEPO, a flexible peptide linker of about 20 or fewer amino acids, and a human IgG Fc variant. The Fc variant is of a non-lytic nature and shows minimal undesirable Fc-mediated side effects. A method is also disclosed to make or produce such fusion proteins at high expression levels. Such HuEPO-L-vFc fusion proteins exhibit extended serum half-life and increased biological activities, leading to improved pharmacokinetics and pharmacodynamics, thus fewer injections will be needed within a period of time.
Owner:LONGBIO PHARM (SUZHOU) CO LTD
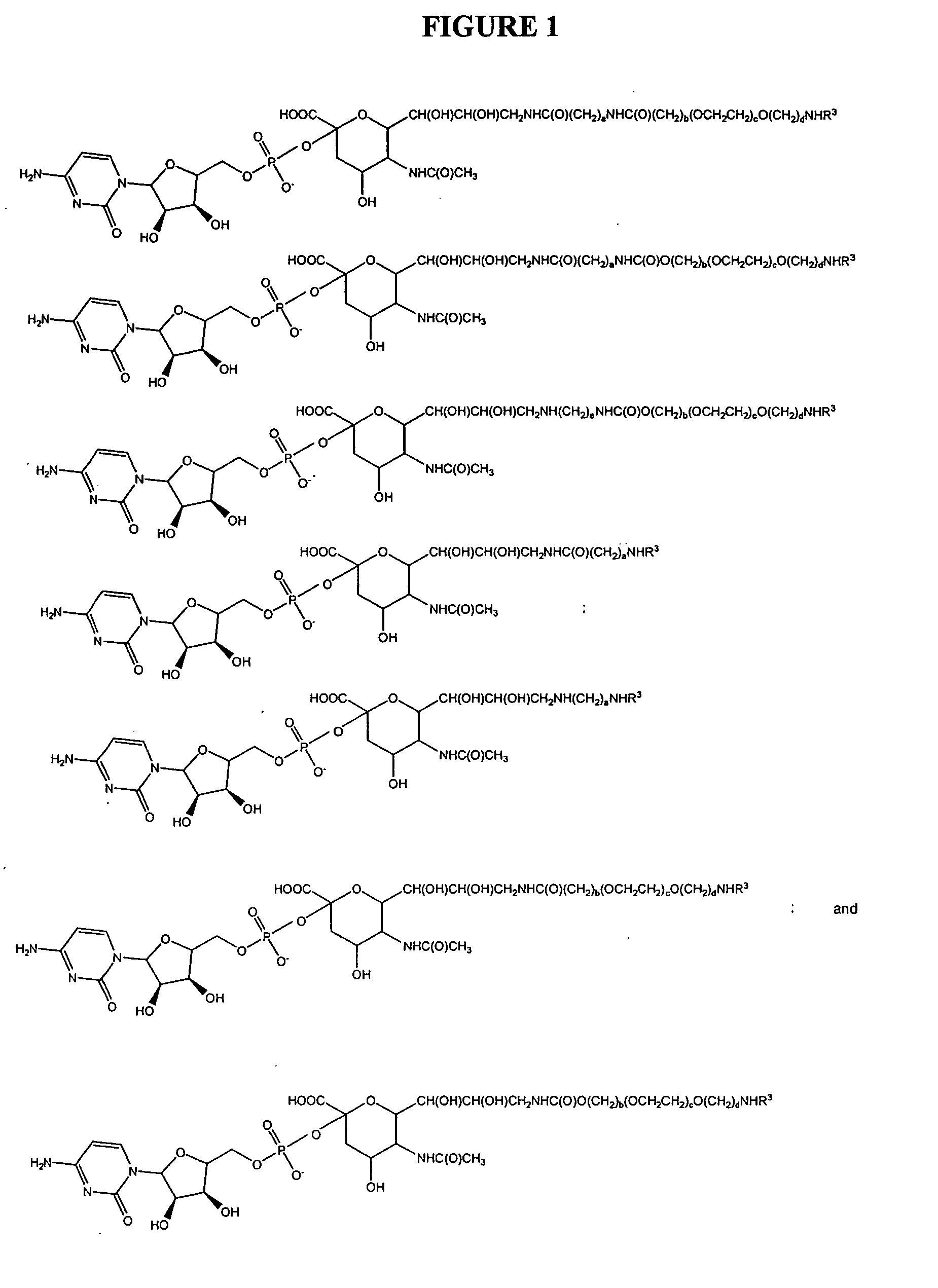
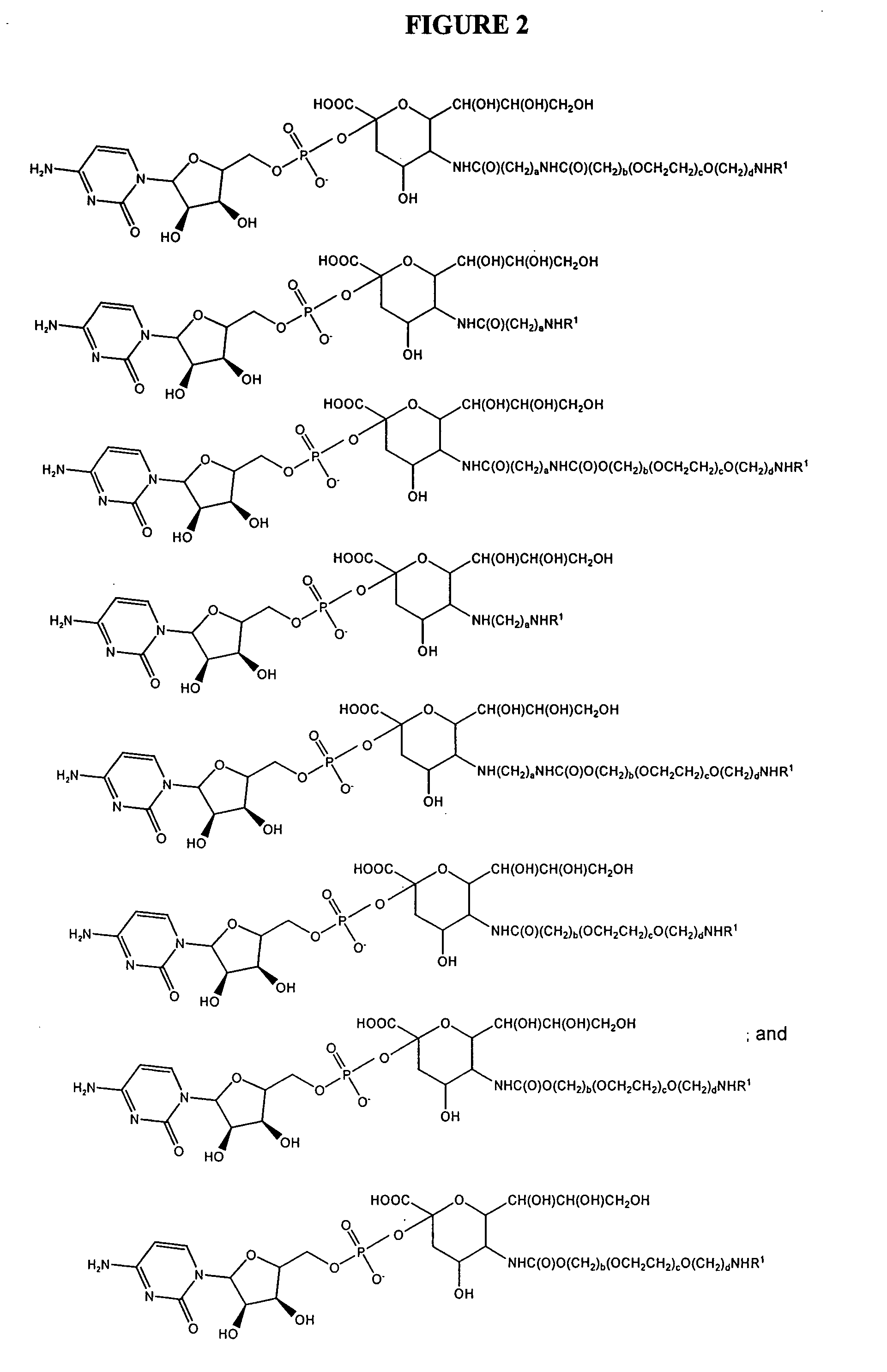
Glycopegylated erythropoietin
InactiveUS20050143292A1Improved pharmacokinetic propertiesCost-effectiveSugar derivativesPeptide/protein ingredientsDiseaseSugar moiety
The present invention provides conjugates between erythropoietin and PEG moieties. The conjugates are linked via an intact glycosyl linking group interposed between and covalently attached to the peptide and the modifying group. The conjugates are formed from glycosylated peptides by the action of a glycosyltransferase. The glycosyltransferase ligates a modified sugar moiety onto a glycosyl residue on the peptide. Also provided are methods for preparing the conjugates, methods for treating various disease conditions with the conjugates, and pharmaceutical formulations including the conjugates.
Owner:NOVO NORDISK AS
Plasma concentrate apparatus and method
ActiveUS6905612B2Effective absorptionPromote absorptionMixing methodsDead animal preservationRed blood cellAbsorption of water
A plasma concentrator for producing plasma concentrate from a plasma from which erythrocytes have been substantially removed. The device comprises a concentrating chamber having an inlet port and an concentrate outlet, the concentrating chamber containing hydrogel beads and at least one inert agitator; and a concentrate chamber having an inlet communicating with the concentrator outlet through a filter, and having an plasma concentrate outlet port. A process for producing plasma concentrate from plasma from which erythrocytes have been substantially removed, comprising the steps of a) moving the plasma into a concentrating chamber containing hydrogel beads and an agitator to form a hydrogel bead-plasma mixture; b) causing the agitator to stir the hydrogel bead-plasma mixture, facilitating absorption of water by the beads from the plasma, until a hydrogel bead-plasma concentrate is formed; and c) separating the plasma concentrate from the hydrogel beads by passing the plasma concentrate through a filter. The concentrator can be one or more syringe devices coupled for multiple concentrations.
Owner:HANUMAN
Glycosylation analogs of erythropoietin
ActiveUS7217689B1Increase the number ofHigh sialic acid contentPeptide/protein ingredientsTissue culturePlasmidDNA
Erythropoietin analogs having at least one additional site for glycosylation, or a rearrangement of at least one site for glycosylation are disclosed. The invention also relates to DNA sequences encoding said erythropoietin analogs, and recombinant plasmids and host cells for analog expression.
Owner:AMGEN INC
Fc-erythropoietin fusion protein with improved pharmacokinetics
InactiveUS20050192211A1Improve pharmacokineticsSimplify erythropoietin therapyPeptide/protein ingredientsAntibody mimetics/scaffoldsErythropoietinNucleic acid
The present invention provides Fc-erythropoietin (“Fc-EPO”) fusion proteins with improved pharmacokinetics. Nucleic acids, cells, and methods relating to the production and practice of the invention are also provided.
Owner:MERCK PATENT GMBH
Fc fusion proteins of human erythropoietin with increased biological activities
InactiveUS20050124045A1Improve biological activityExtended serumPeptide/protein ingredientsAntibody mimetics/scaffoldsSide effectHalf-life
Fc fusion proteins of human EPO with increased biological activities relative to rHuEPO on a molar basis are disclosed. The HuEPO-L-vFc fusion protein comprises HuEPO, a flexible peptide linker of about 20 or fewer amino acids, and a human IgG Fc variant. The Fc variant is of a non-lytic nature and shows minimal undesirable Fc-mediated side effects. A method is also disclosed to make or produce such fusion proteins at high expression levels. Such HuEPO-L-vFc fusion proteins exhibit extended serum half-life and increased biological activities, leading to improved pharmacokinetics and pharmacodynamics, thus fewer injections will be needed within a period of time.
Owner:SUN LEE HWEI K +2
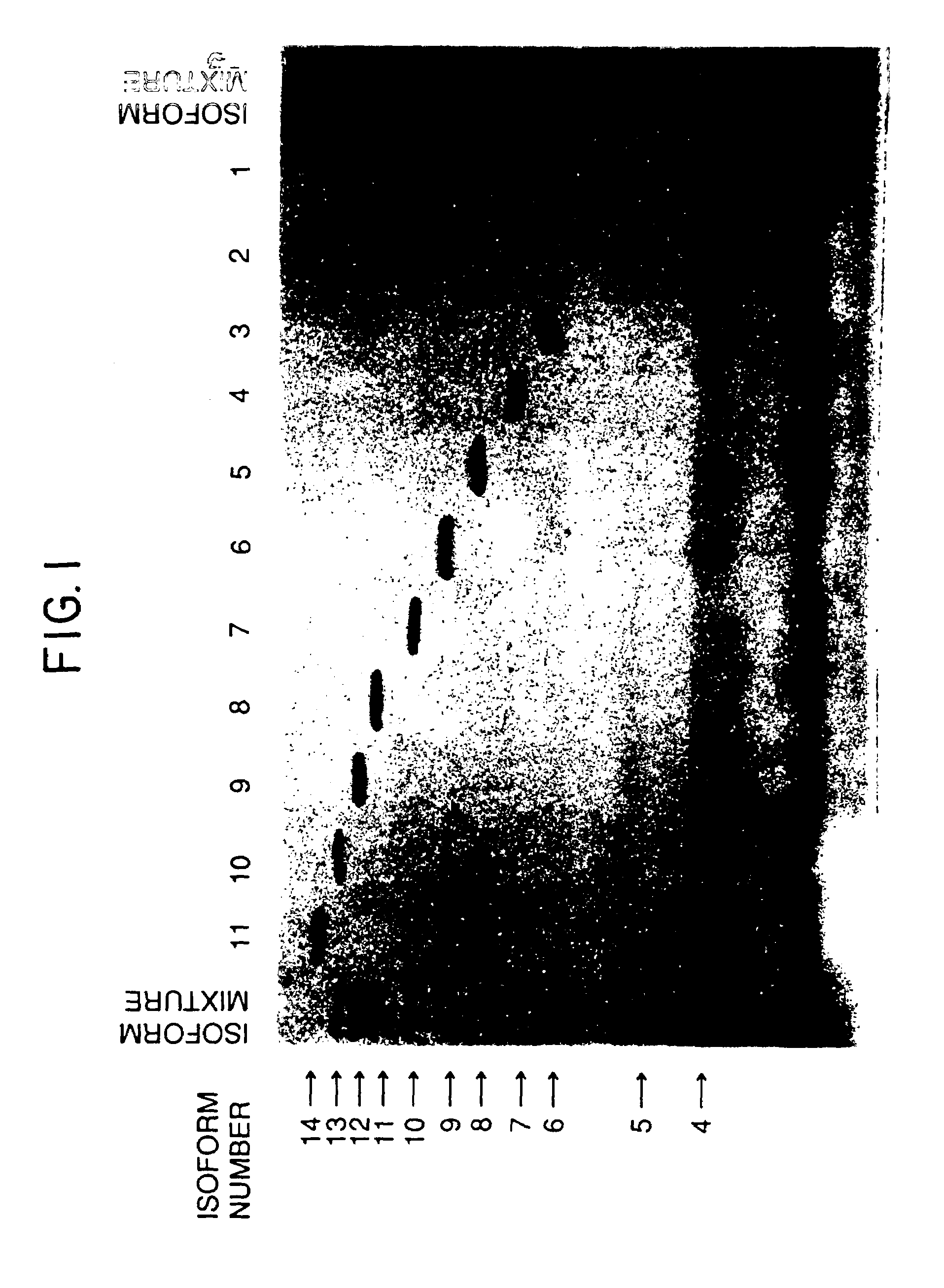
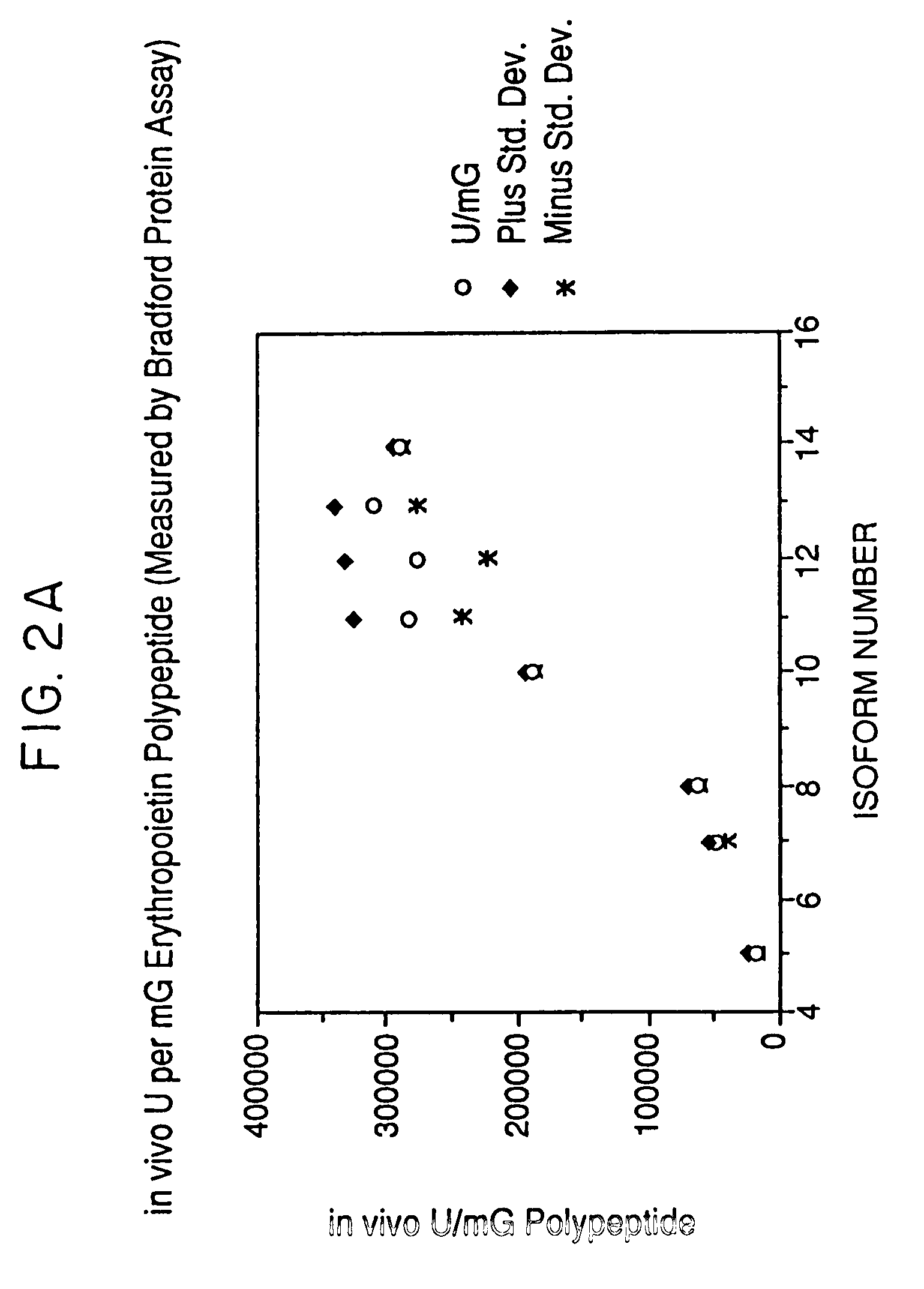
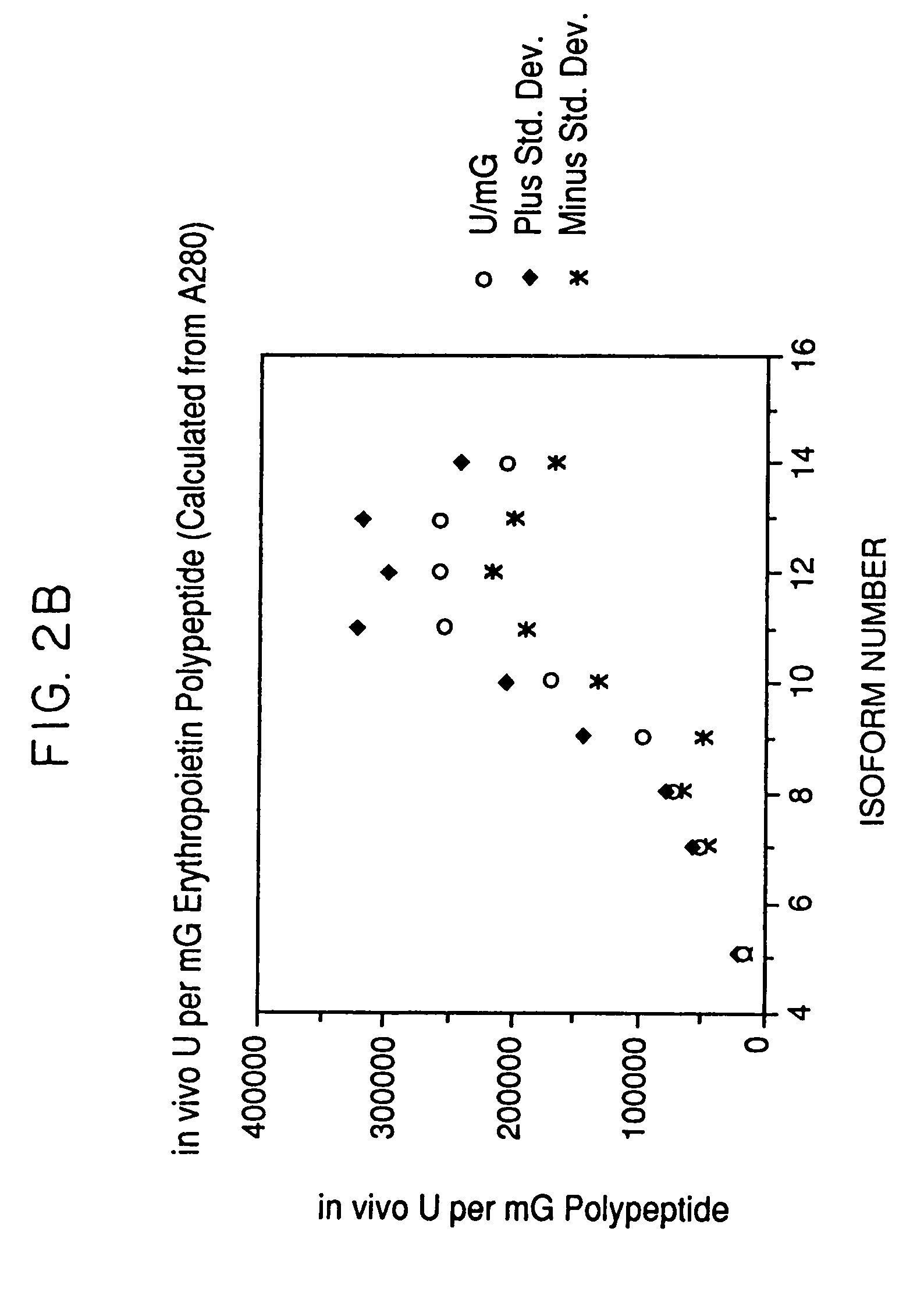
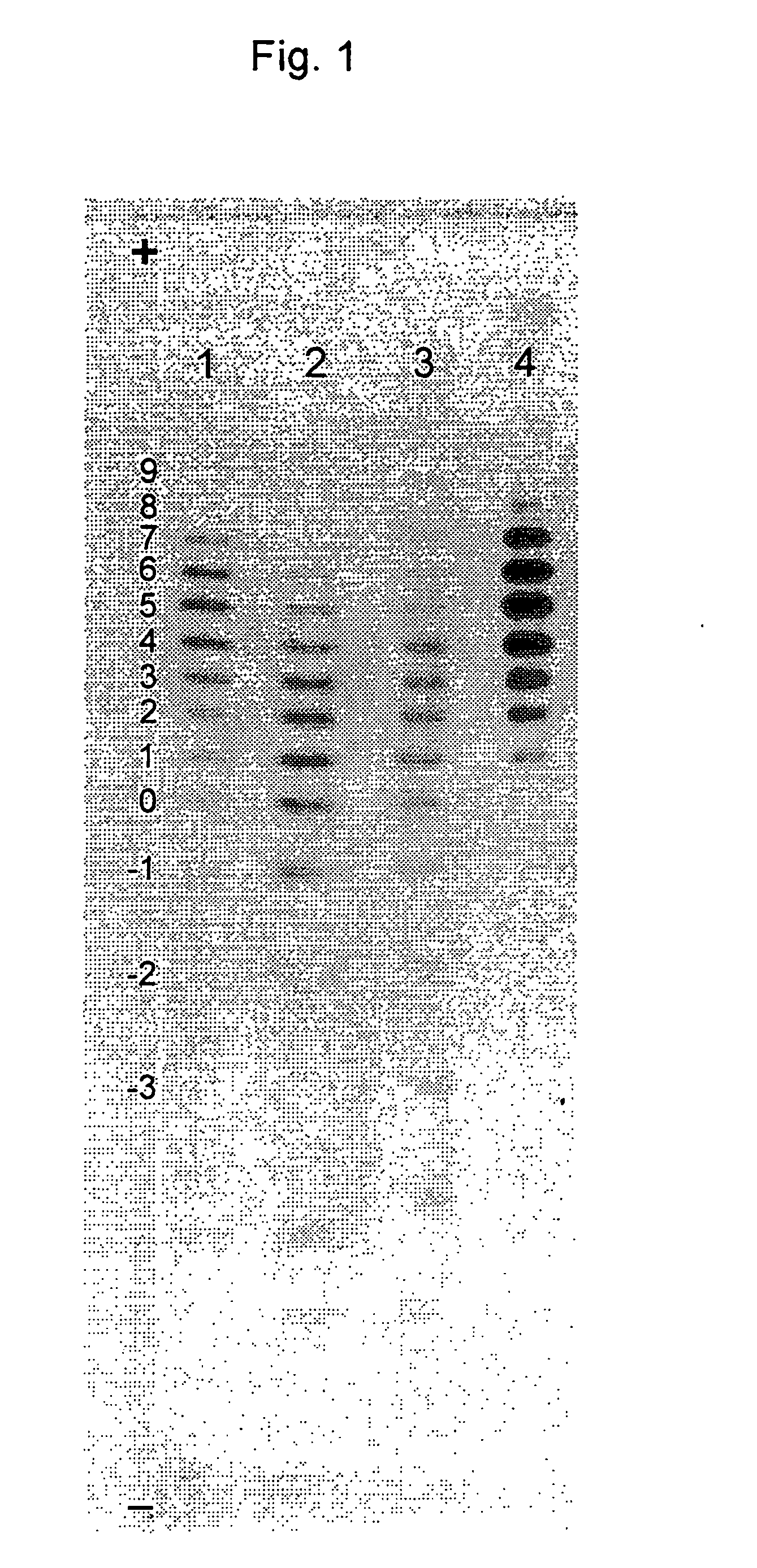
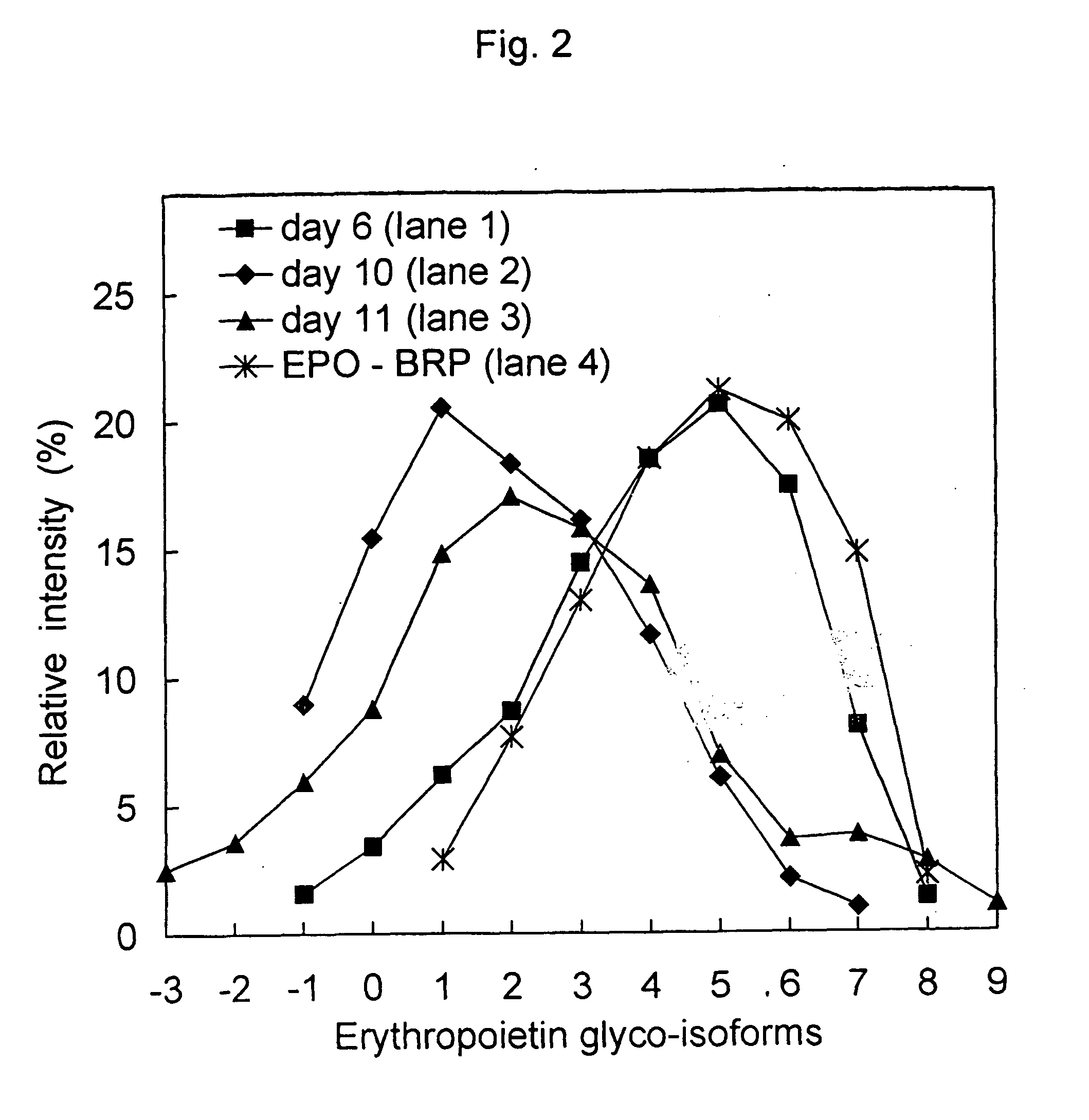
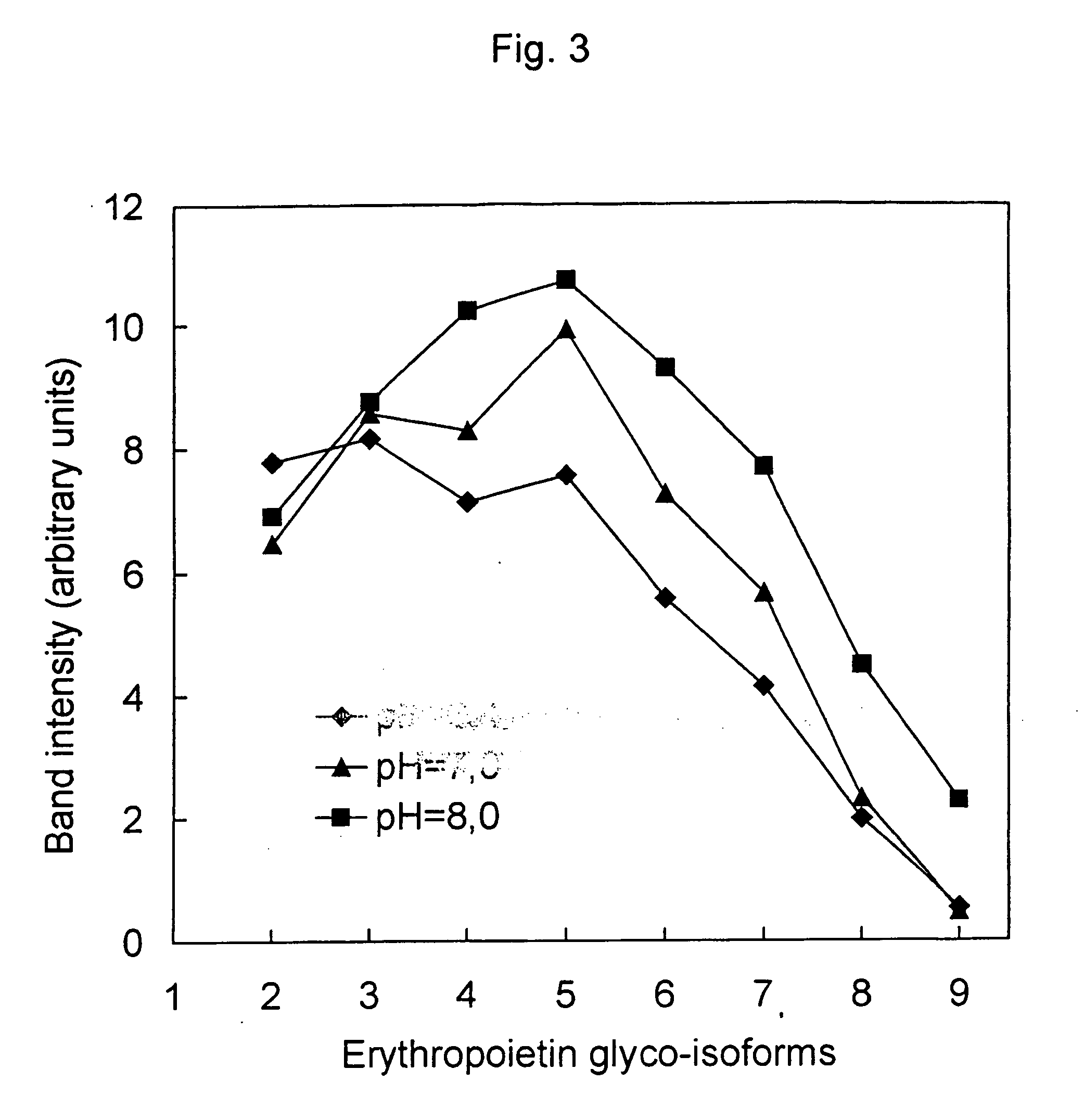
Process for the preparation of a desired erythropoietin glyco-isoform profile
InactiveUS20050153879A1High and uniform product specificityImprove product qualityPeptide/protein ingredientsComponent separationFiltrationRed blood cell
The present invention provides a process for the production of erythropoietin (EPO) with high purity and with a desired profile of EPO glycol-isoforms by using a combination of specific chromatographic steps in such a manner that the starting EPO glycol-isoform profile is changed or modified. The applied chromatographic steps includes at least (a) dye affinity chromatography, and (b) hydrophobic chromatography and / or (c) anion-exchange chromatography. In a preferred embodiment, the process further includes (d) gel filtration chromatography. The present invention also provides a process for the determination of erythropoietin (EPO) glycol-isoform profile in an EPO containing composition.
Owner:SVETINA MONICA +4
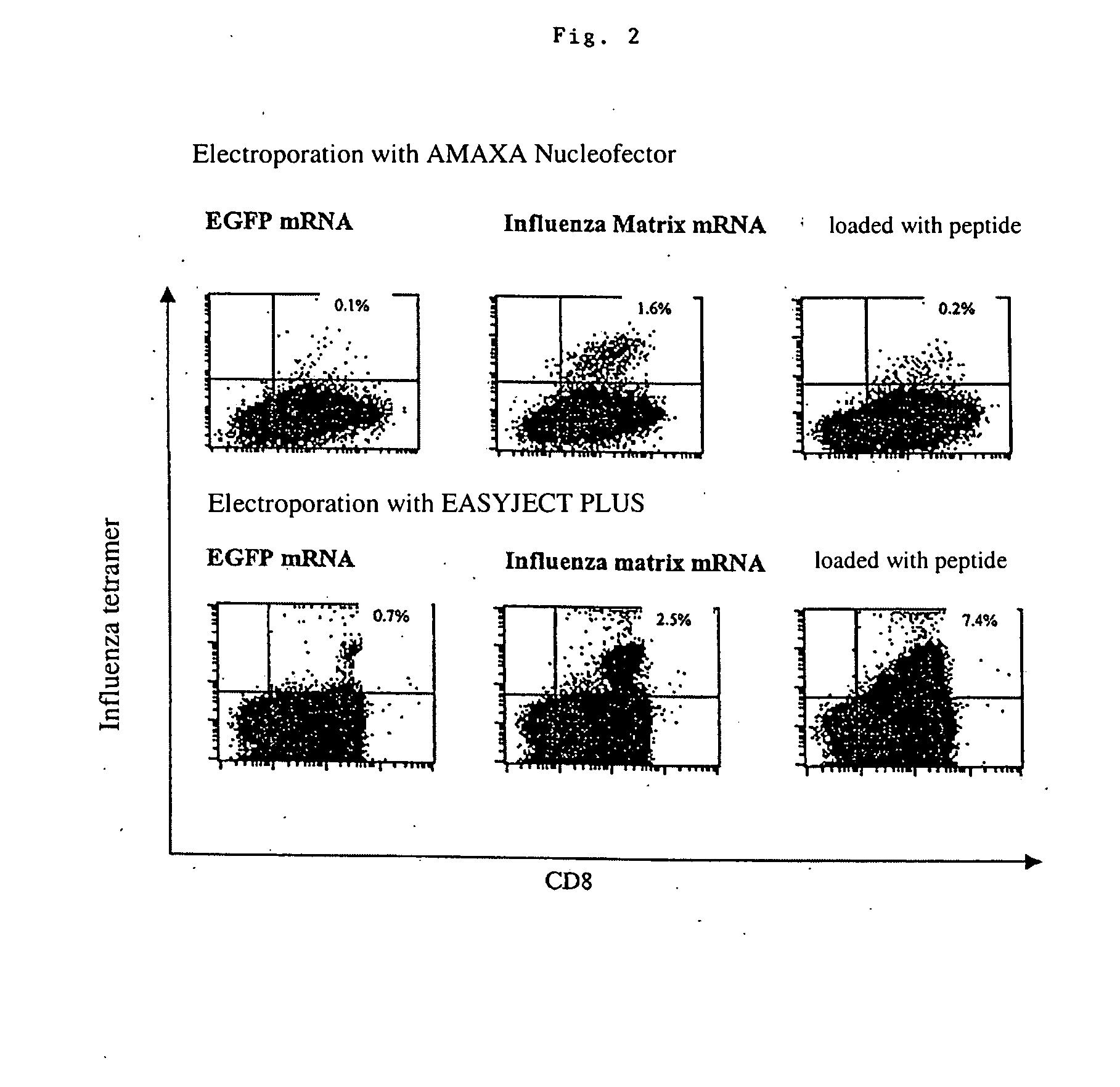
Transfection of blood cells with mRNA for immune stimulation and gene therapy
InactiveUS20060188490A1Improve stabilityIncrease transfectionSsRNA viruses negative-senseBiocideAntigenCancer prevention
The present invention relates to a pharmaceutical composition containing blood cells or haemopoietic cells, e.g. red blood cells (erythrocytes), granulocytes, mononuclear cells (PBMCs) and / or blood platelets, in combination with a pharmaceutically acceptable excipient and / or vehicle, wherein the cells are transfected with at least one mRNA comprising at least one region coding for at least one antigen. The invention further discloses a method of preparing the aforesaid pharmaceutical composition and the use of blood cells transfected in this way for the preparation of drugs or pharmaceutical compositions for immune stimulation against the antigens encoded by the mRNA. The subjects according to the invention are used especially for the therapy and / or prophylaxis of carcinoses or infectious diseases and can also be employed in gene therapy.
Owner:CUREVAC AG
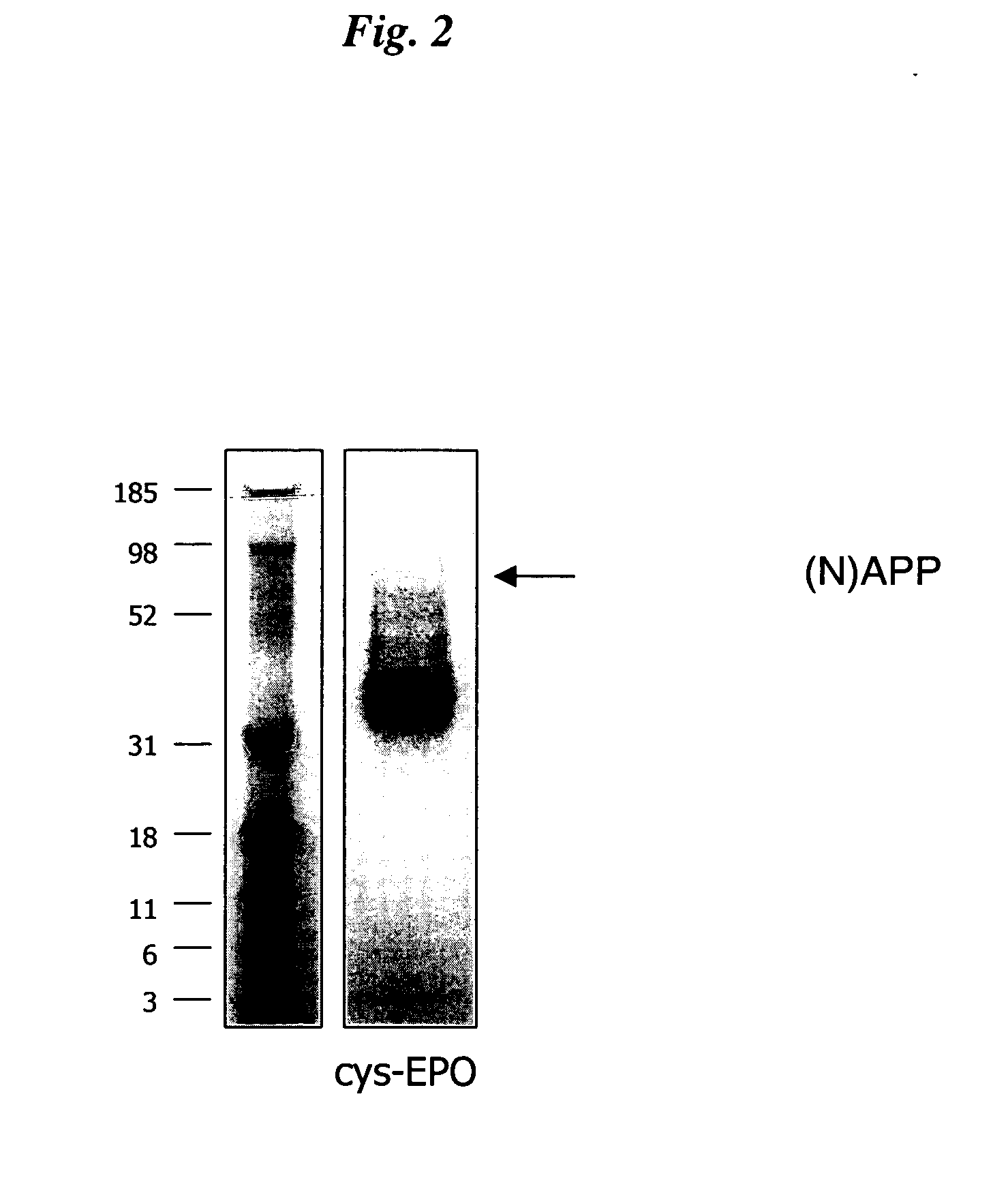
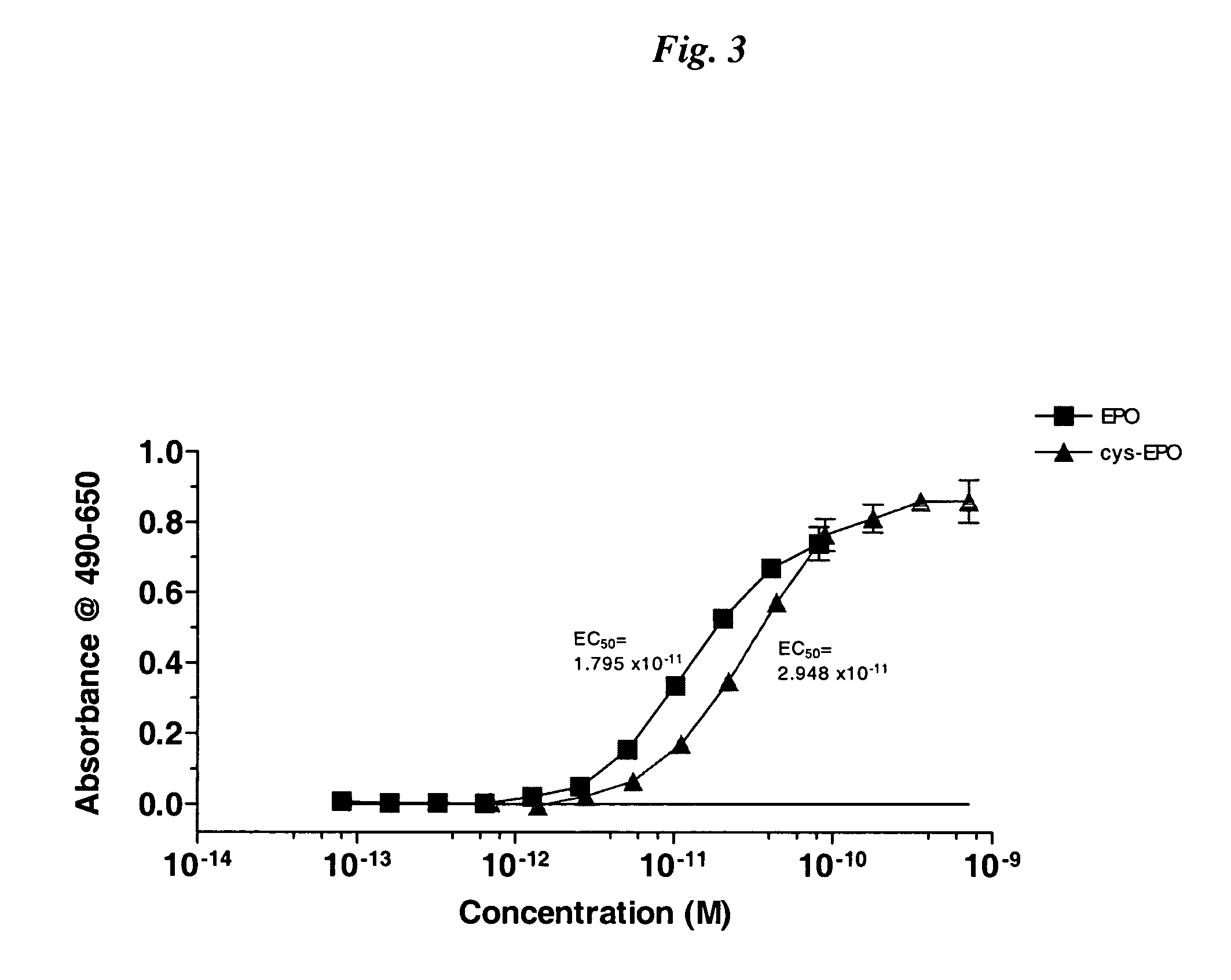
Novel recombinant proteins with N-terminal free thiol
InactiveUS20050170457A1Extended half-lifeIncreases circulating serum half-lifePeptide/protein ingredientsTissue cultureCysteine thiolateHalf-life
The present invention relates to novel modified proteins having N-terminal free thiols that can be produced by recombinant methods and are ready for further chemical derivatization. In particular, the invention relates to erythropoietin conjugate compounds having altered biochemical, physiochemical and pharmacokinetic properties. More particularly, one embodiment of the invention relates to erythropoietin conjugate compounds of the formula: (M)n-X-A-cys-EPO (I) where EPO is an erythropoeitin moiety selected from erythropoietin or an erythropoietin variant having at least one amino acid different from the wild-type human EPO, or any pharmaceutical acceptable derivatives thereof having biological properties of causing bone marrow cells to increase production of red blood cells; cys represents the amino acid cysteine and occurs at position −1 relative to the amino acid sequence of the erythropoietin moiety; A indicates the structure of the residual moiety used to chemically attach X to the thiol group of −1Cys; X is a water soluble polymer such as a polyalkylene glycol or other polymer; M is an organic molecule (including peptides and proteins) that increases the circulating half-life of the construct; and N is an integer from 0 to 15.
Owner:CENTOCOR
Methods and compositions for the treatment and management of hemoglobinopathy and anemia
InactiveUS20050143420A1Good effectRelieve symptomsBiocidePeptide/protein ingredientsRed blood cellThalassemia
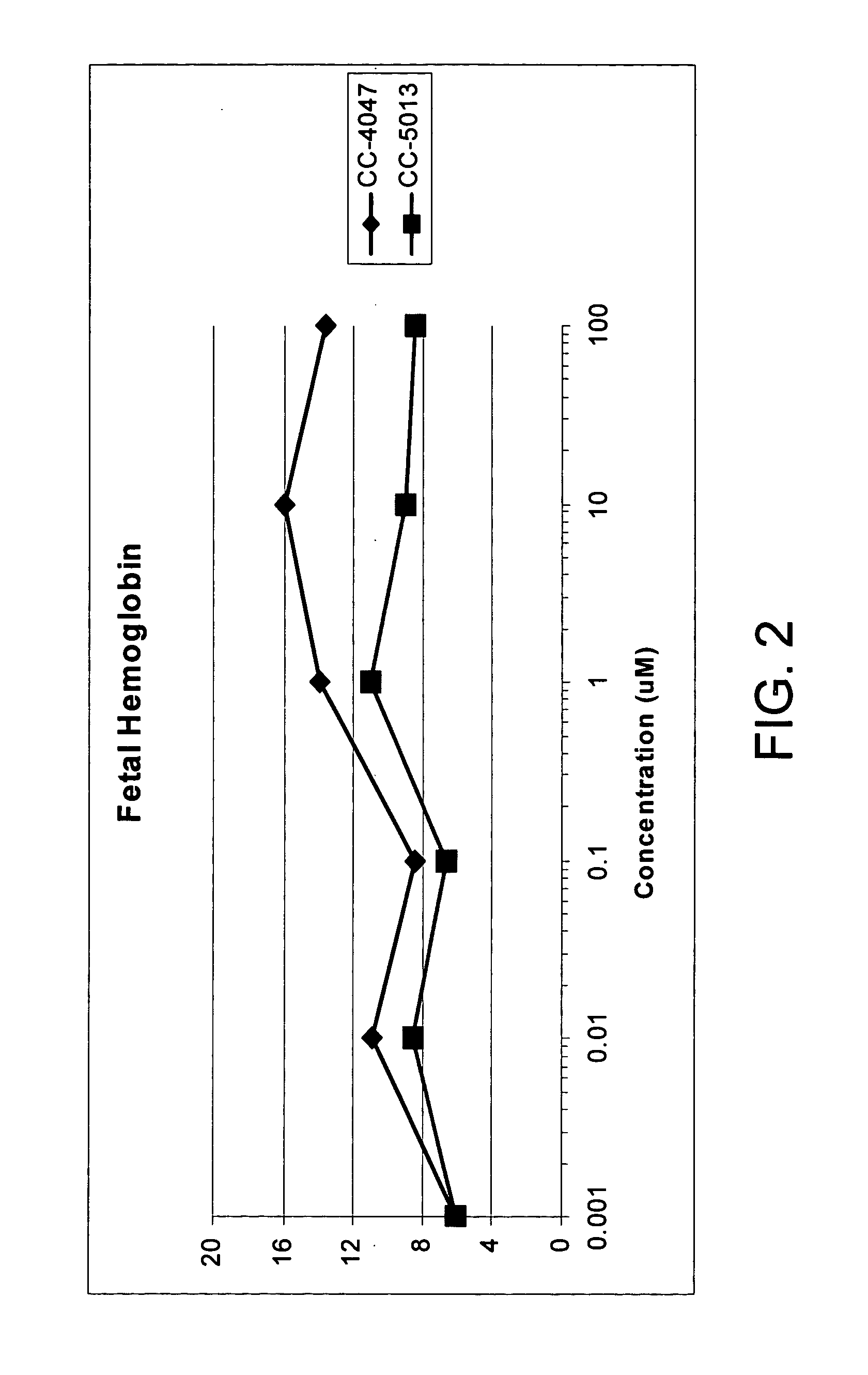
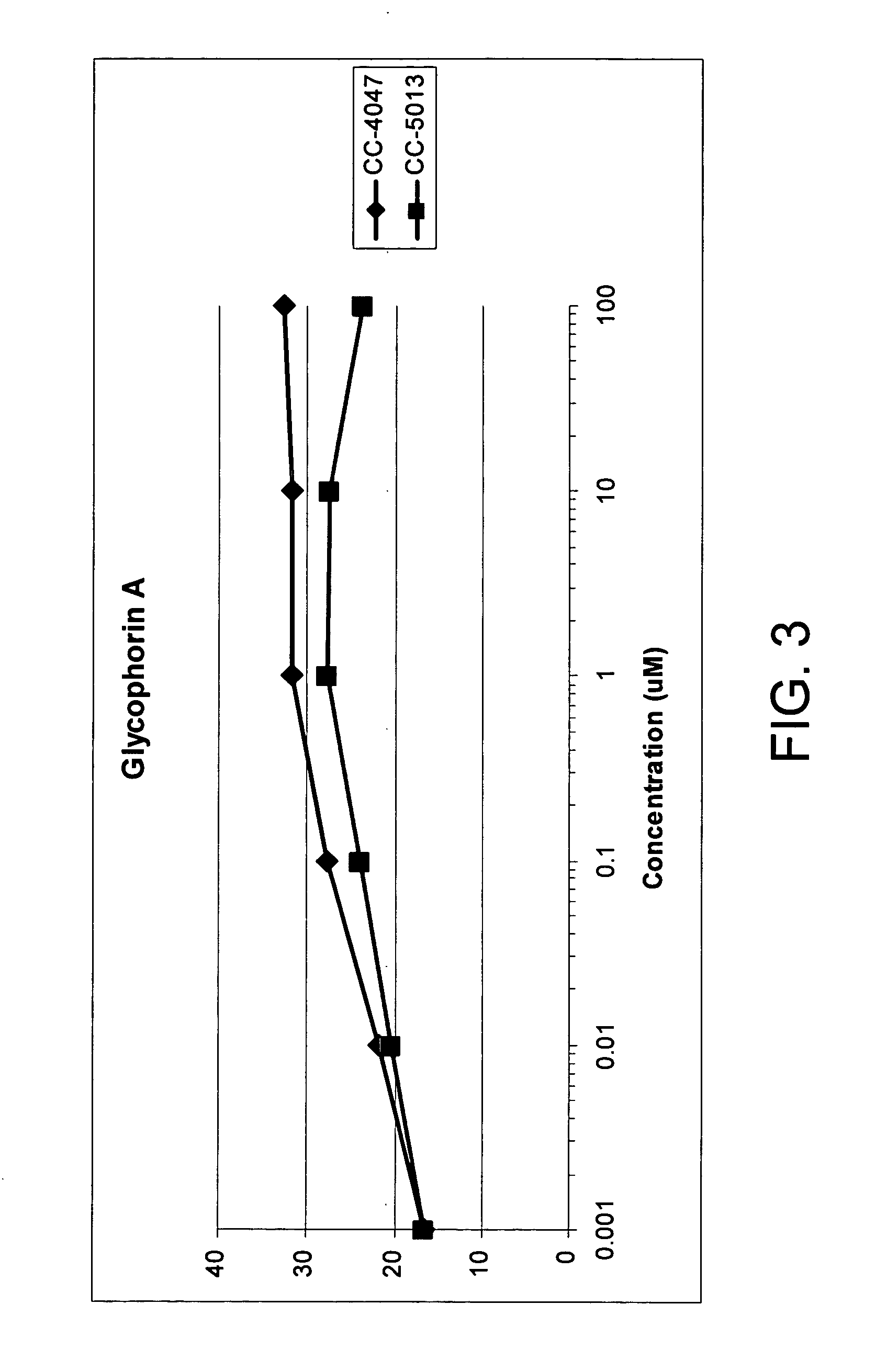
The present invention is directed to the use of immunomodulatory compounds, particularly members of the class of compounds known as IMiDs™, and more specifically the compounds 4-(Amino)-2-(2,6-dioxo(3-piperidyl))-isoindoline-1,3-dione and 3-(4-amino-1-oxo-1,3-dihydroisoindol-2-yl)-piperidine-2,6-dione, to induce the expression of fetal hemoglobin genes, genes essential for erythropoiesis, and genes encoding alpha hemoglobin stabilizing protein, within a population of CD34+ cells. These compounds are used to treat hemoglobinopathies such as sickle cell anemia or β-thalassemia, or anemias caused by disease, surgery, accident, or the introduction or ingestion of toxins, poisons or drugs.
Owner:SIGNAL PHARMA LLC
Transfection of blood cells with mRNA for immune stimulation and gene therapy
InactiveUS20120009221A1Improve stabilityIncrease transfectionSsRNA viruses negative-senseViral antigen ingredientsAntigenPharmaceutical medicine
The present invention relates to a pharmaceutical composition containing blood cells or haemopoietic cells, e.g. red blood cells (erythrocytes), granulocytes, mononuclear cells (PBMCs) and / or blood platelets, in combination with a pharmaceutically acceptable excipient and / or vehicle, wherein the cells are transfected with at least one mRNA comprising at least one region coding for at least one antigen. The invention further discloses a method of preparing the aforesaid pharmaceutical composition and the use of blood cells transfected in this way for the preparation of drugs or pharmaceutical compositions for immune stimulation against the antigens encoded by the mRNA. The subjects according to the invention are used especially for the therapy and / or prophylaxis of carcinoses or infectious diseases and can also be employed in gene therapy.
Owner:CUREVAC AG
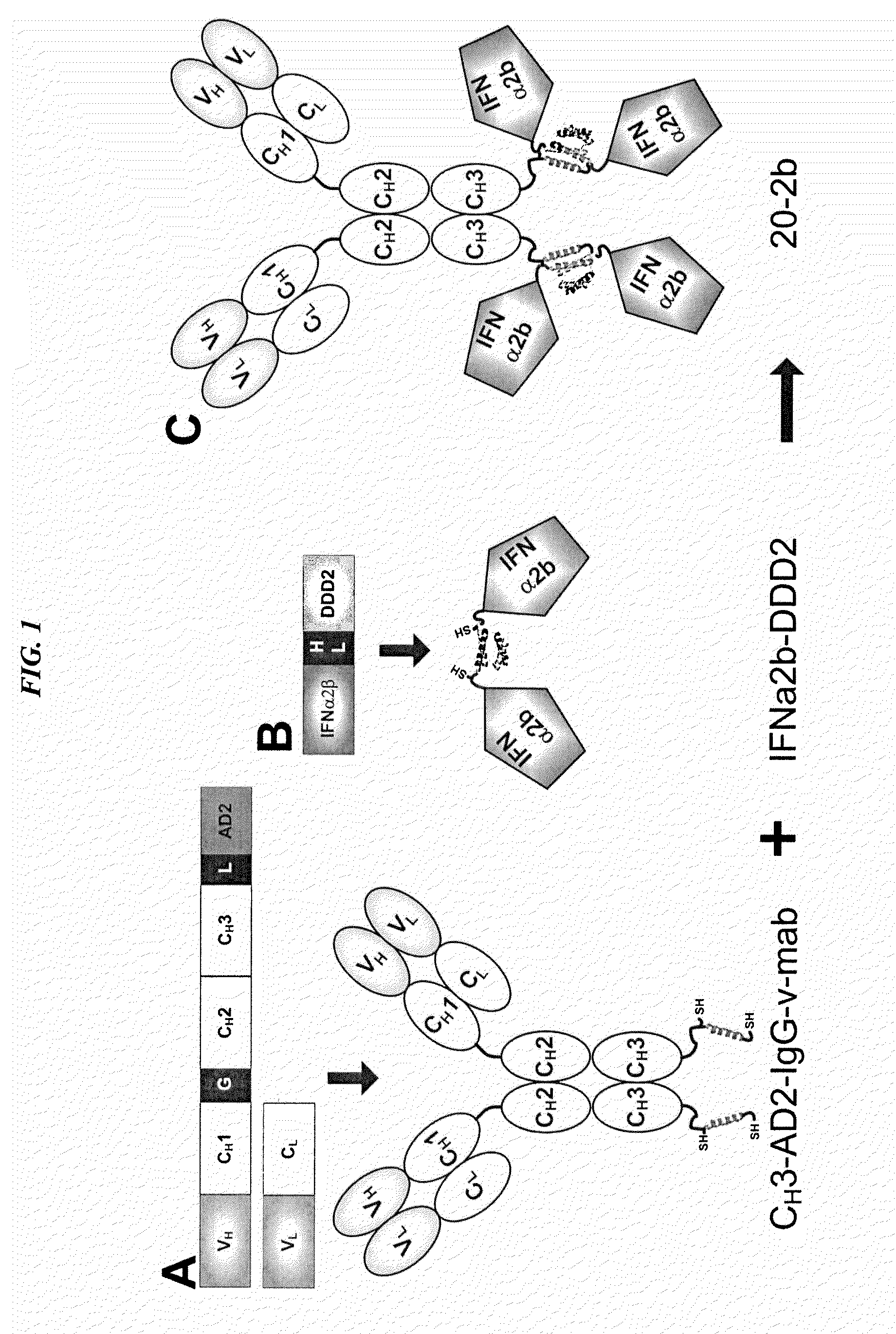
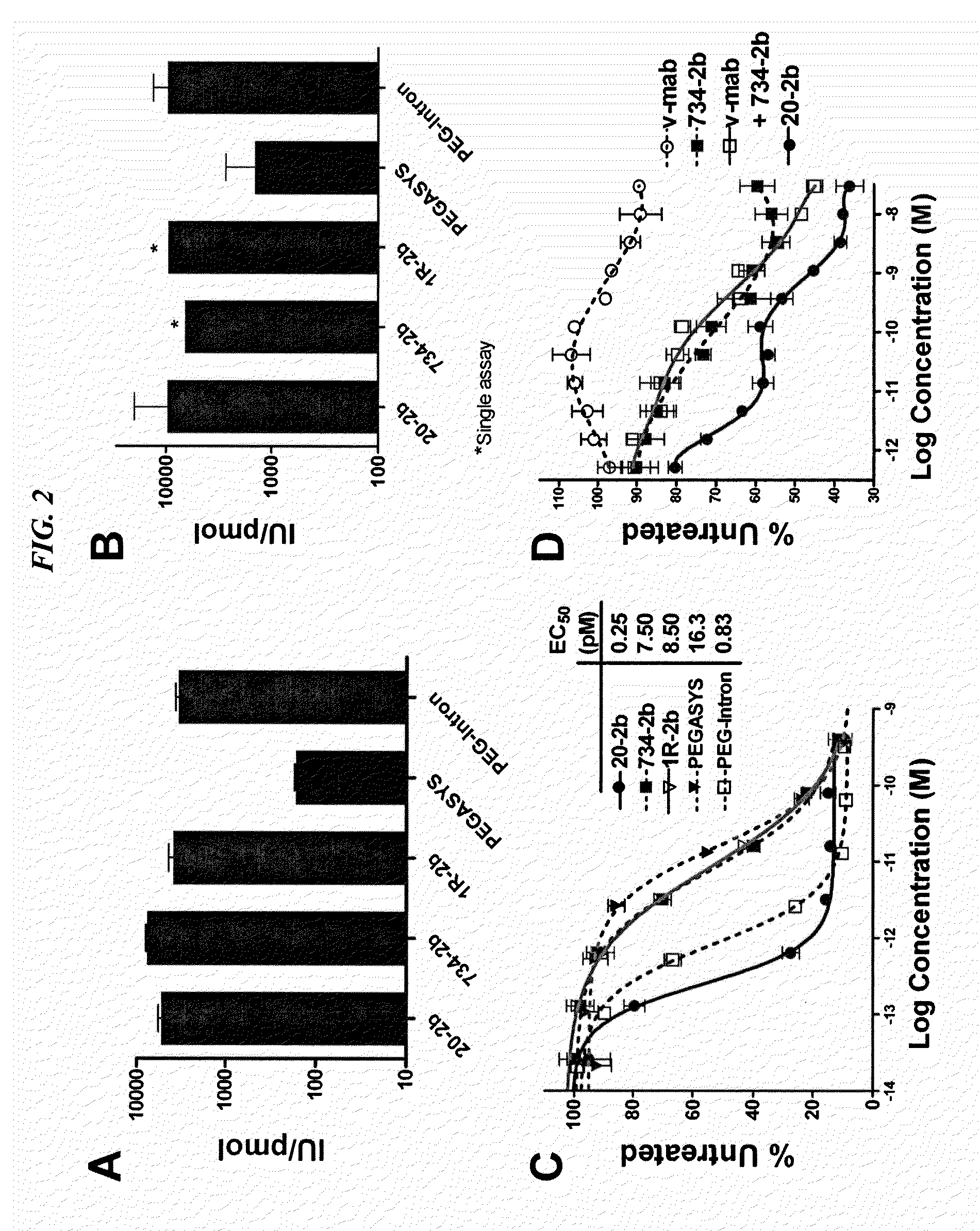
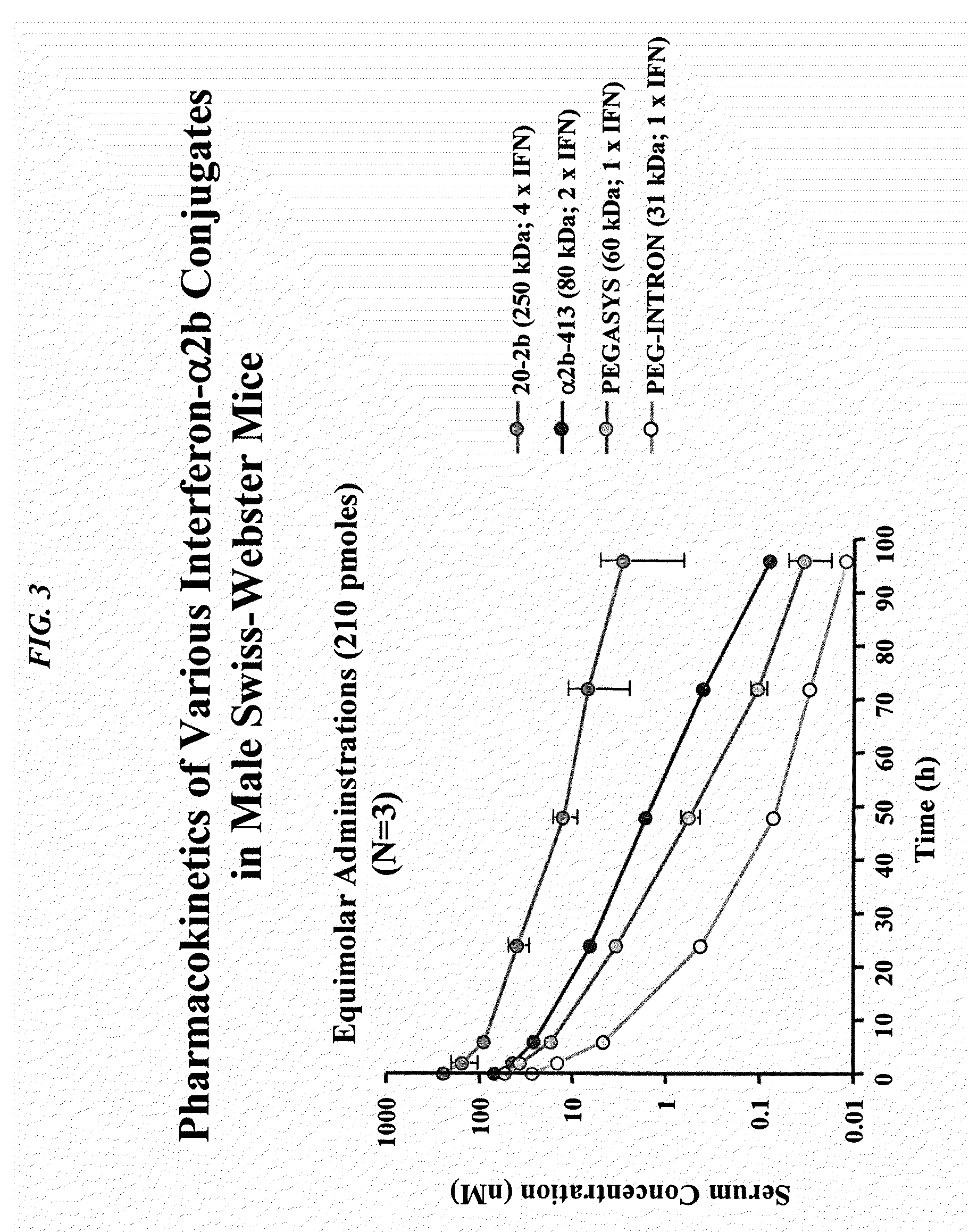
Tetrameric cytokines with improved biological activity
ActiveUS8034352B2Keep for a long timeLow toxicityMaterial nanotechnologyPeptide/protein ingredientsSerum igeHalf-life
The present invention concerns methods and compositions for forming cytokine-antibody complexes using dock-and-lock technology. In preferred embodiments, the cytokine-MAb DNL complex comprises an IgG antibody attached to two AD (anchor domain) moieties and four cytokines, each attached to a DDD (docking and dimerization domain) moiety. The DDD moieties form dimers that bind to the AD moieties, resulting in a 2:1 ratio of DDD to AD. The cytokine-MAb complex exhibits improved pharmacokinetics, with a significantly longer serum half-life than either naked cytokine or PEGylated cytokine. The cytokine-MAb complex also exhibits significantly improved in vitro and in vivo efficacy compared to cytokine alone, antibody alone, unconjugated cytokine plus antibody or cytokine-MAb DNL complexes incorporating an irrelevant antibody. In more preferred embodiment the cytokine is G-CSF, erythropoietin or INF-α2b.
Owner:IBC PHARMACEUTICALS INC
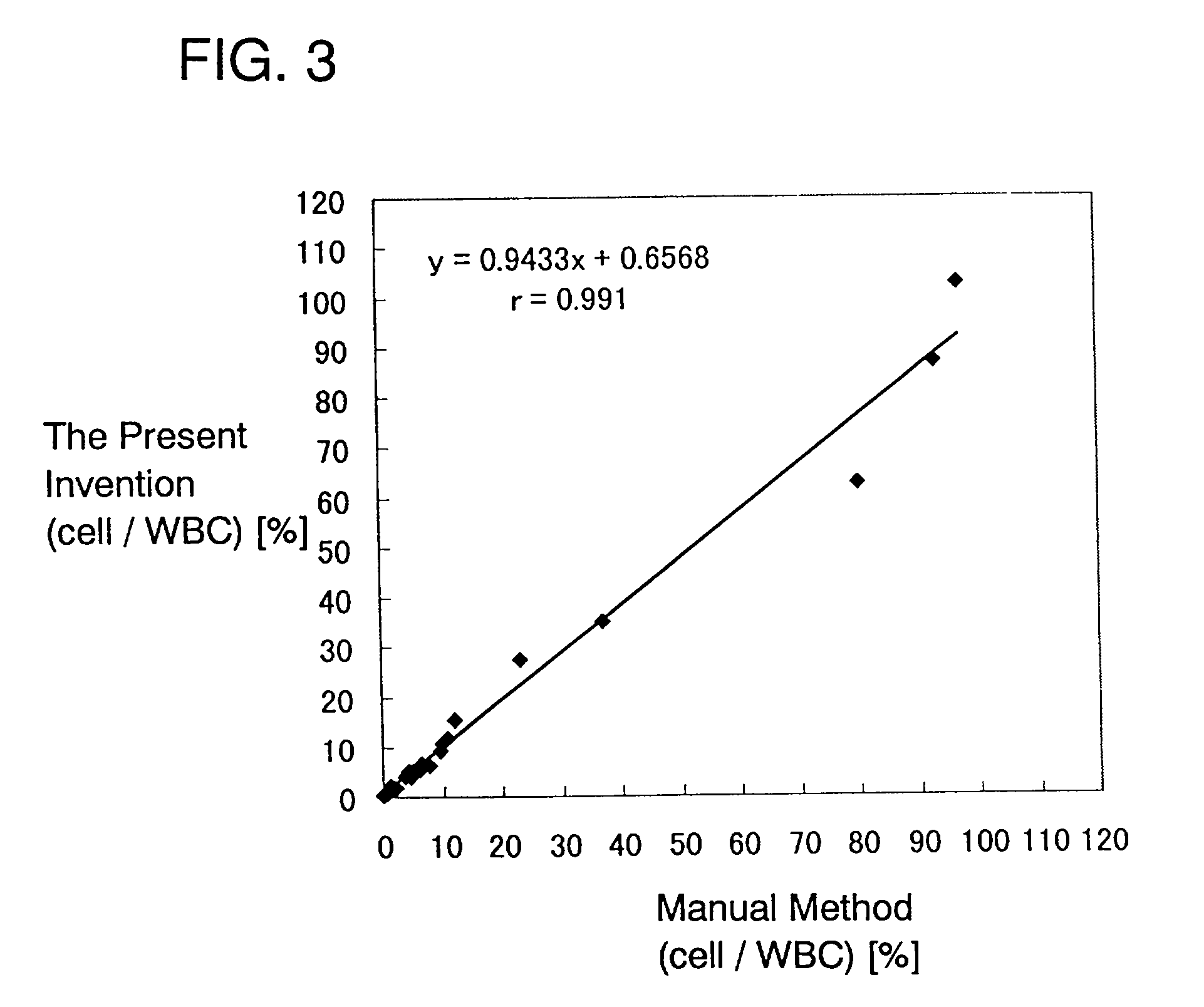
Method for automatically analyzing nucleated bone marrow cells
InactiveUS20030219850A1Material analysis by observing effect on chemical indicatorPreparing sample for investigationStainingLipid particle
An automatic method for analyzing nucleated bone marrow cells comprising partitioning one sample of bone marrow fluid with two samples, one sample being treated with a first lysing agent and a first staining solution and the other sample being treated with a second lysing agent and a second staining solution; and measuring each samples in a flow cytometer using a scattered light and fluoresence which enables to classify and count leukocytes, erythroid cells and lipid particles, as well as mature myeloid cells, lymphoid cells and immature myeloid cells, and to calculate the number of myeloid cells as well as the ratio of myeloid cells and erythroid cells.
Owner:SYSMEX CORP
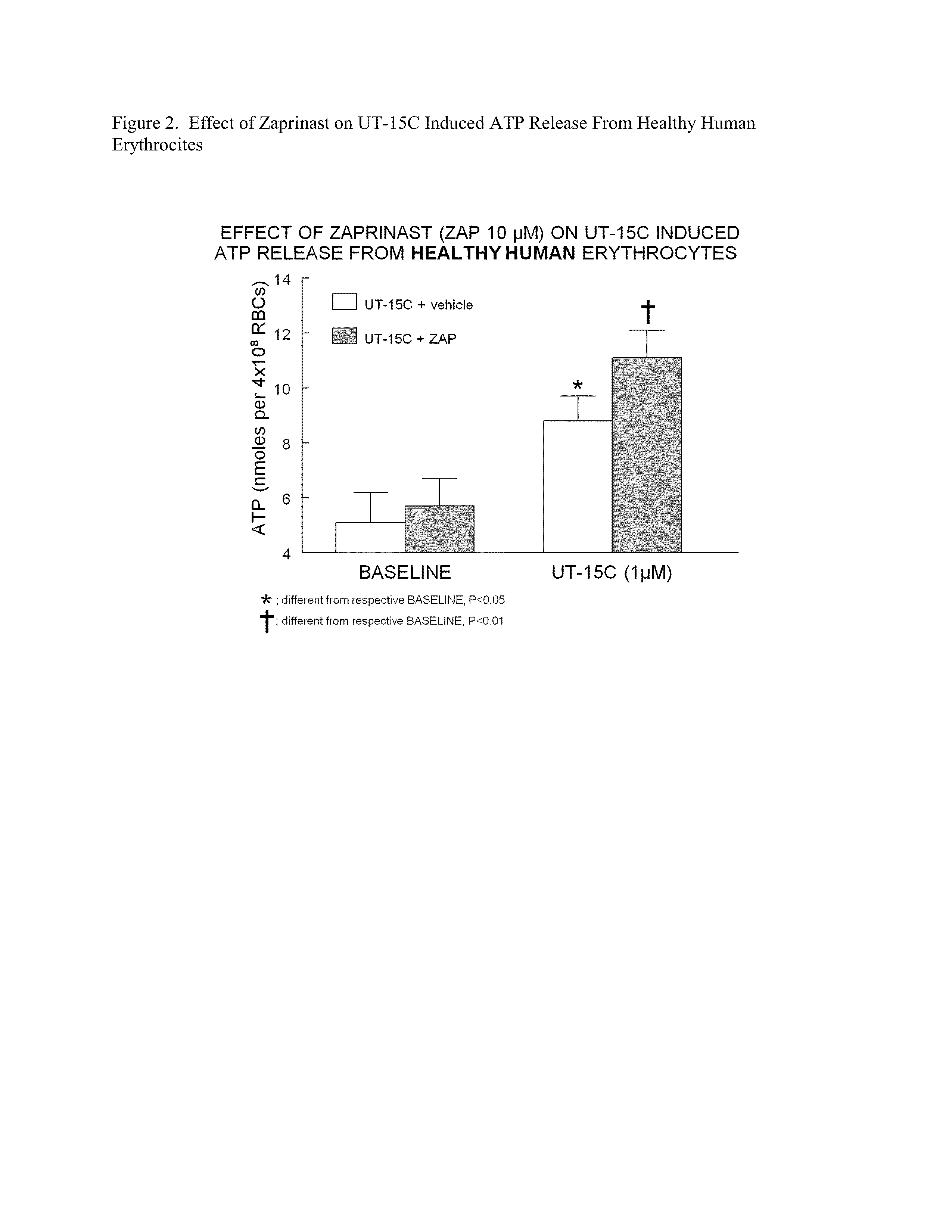
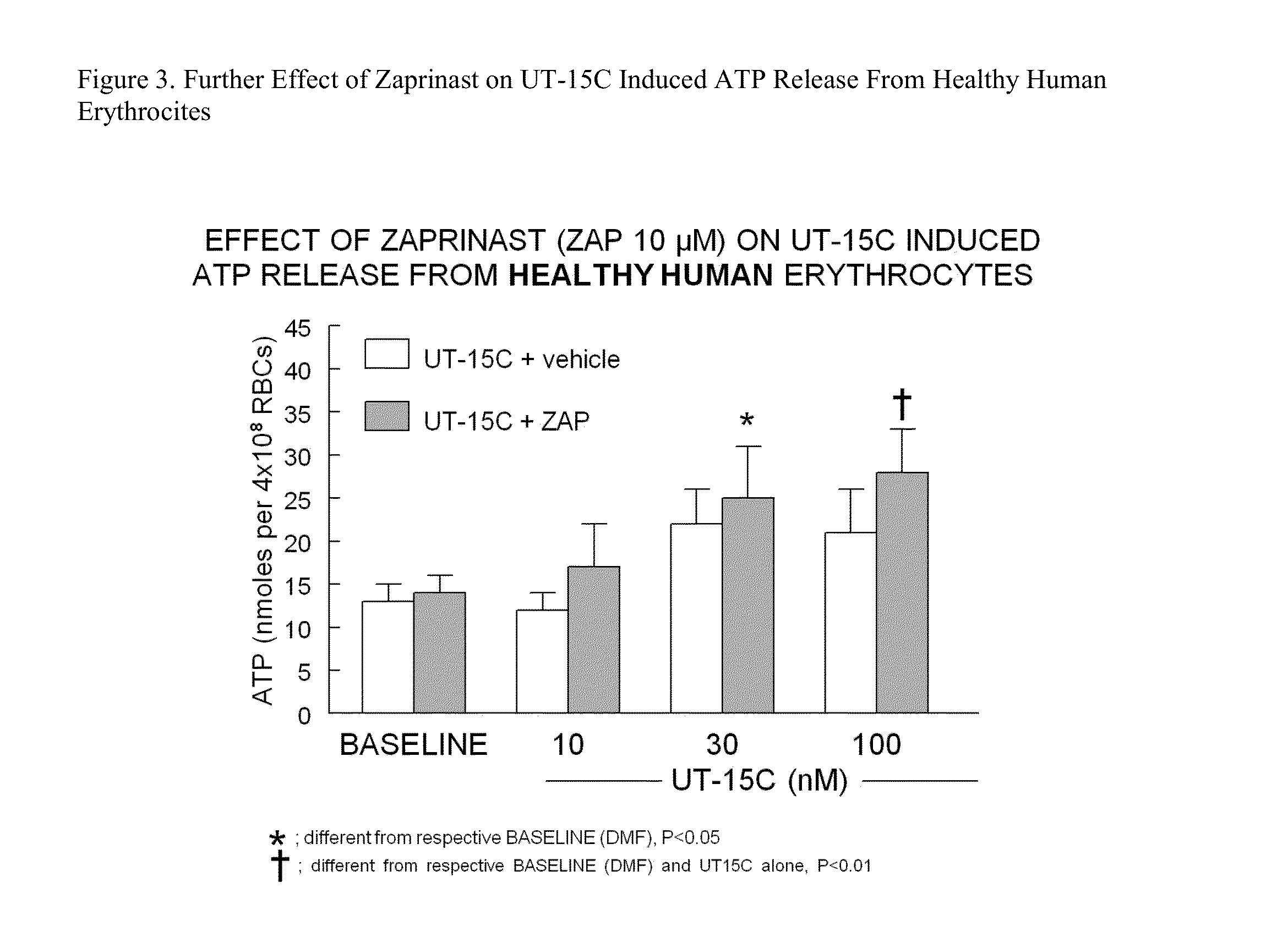
Method of identifying therapies for pulmonary hypertension
ActiveUS20130184295A1High activityBiocideOrganic active ingredientsPhosphodiesterase 5 inhibitorPde5 inhibition
The present invention is directed to a method of screening for a therapeutic agent useful for treating pulmonary hypertension comprising: contacting an erythrocyte with a candidate therapeutic agent; and detecting a presence or absence of erythrocyte-derived adenosine triphosphate, wherein a greater erythrocyte-derived adenosine triphosphate level indicates the candidate therapeutic agent has greater activity in treating pulmonary hypertension. Additionally, the present invention is directed to methods of treating pulmonary arterial hypertension by stimulating ATP release from erythrocytes through co-administration to a subject in need thereof an amount of a PDE5 inhibitor compound, and an amount of a prostacyclin compound.
Owner:UNITED THERAPEUTICS CORP
Treatment of mitochondrial diseases with an erythropoietin mimetic
InactiveUS20090291092A1Stimulating erythropoiesisNervous disorderPeptide/protein ingredientsDiseaseRed blood cell
Methods of treating mitochondrial disorders that are not respiratory chain disorders using compositions comprising EPO mimetic compounds or compounds capable of increasing endogenous EPO levels or stimulating erythropoiesis are disclosed. Methods of treating Friedreich's ataxia, Leigh's syndrome, or other disorders by increasing the expression of frataxin with an EPO mimetic compound or a compound capable of increasing endogenous EPO levels or stimulating erythropoiesis are also disclosed.
Owner:EDISON PHARMA
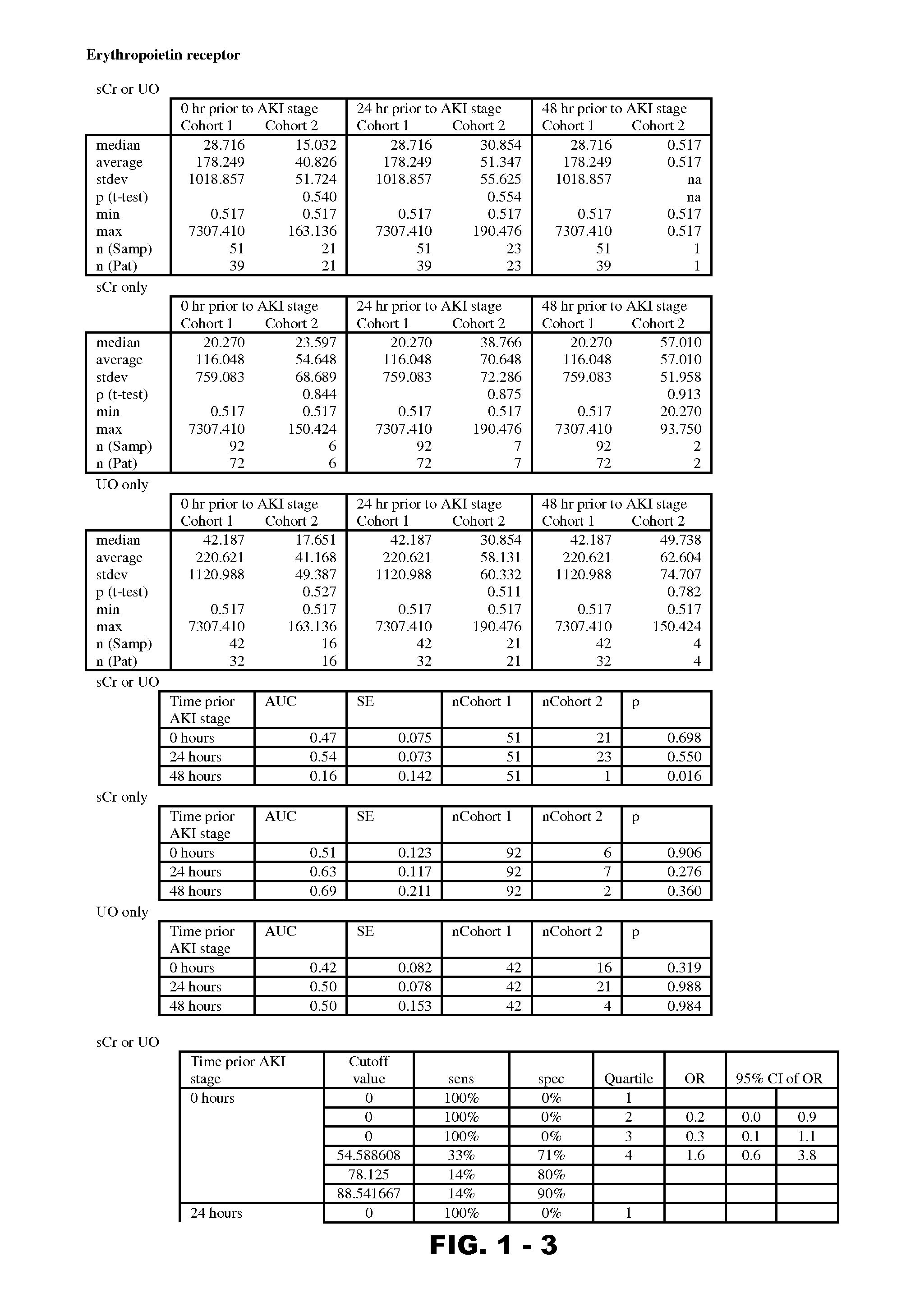
Antibodies which activate an erythropoietin receptor
Antibodies and fragments thereof which activate an erythropoietin receptor and stimulate erythropoiesis are described. Also described are hybridoma cell lines which produce the antibodies and methods and compositions for the treatment of anemia.
Owner:AMGEN INC
Tissue protective peptides and uses thereof
ActiveUS20090221482A1Avoid damageFacilitate optimal expressionSenses disorderPeptide/protein ingredientsDiseaseTissue protection
The present invention is directed to novel tissue protective peptides. The tissue protective peptides of the invention may bind to a tissue protective receptor complex. In particular, the present invention is drawn to tissue protective peptides derived from or sharing consensus sequences with portions of cytokine receptor ligands, including Erythropoietin (EPO), that are not involved in the binding of the ligand to the receptor complex, e.g., to the EPO receptor homodimer. Accordingly, the tissue protective peptides of the invention are derived from the amino acid sequences of regions of cytokine receptor ligands that are generally located on or within the region of the ligand protein that is opposite of the receptor complex, i.e., are generally derived from amino acid sequences of regions of the ligand protein that face away from the receptor complex while the ligand is bound to the receptor. The invention is further directed to the consensus sequences for use in engineering a synthetic tissue protective peptide. These tissue protective peptides also include fragments, chimeras, as well as peptides designed to mimic the spatial localization of key amino acid residues within the tissue protective receptor ligands, e.g., EPO. The invention further encompasses methods for treating or preventing a disease or disorder using tissue protective peptides of the current invention. The invention also encompasses methods for enhancing excitable tissue function using tissue protective peptides of the current invention.
Owner:ARAIM PHARMA INC
Treatment of disturbances of iron distribution
ActiveUS7459436B2Minimizing and suppressingBeneficial effect on disturbances of iron distributionPeptide/protein ingredientsDepsipeptidesPhysiologyHeart disease
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
InactiveUS20120190051A1Eliminate needPeptide/protein ingredientsTransferrinsErythropoietin receptorFactor VIII vWF
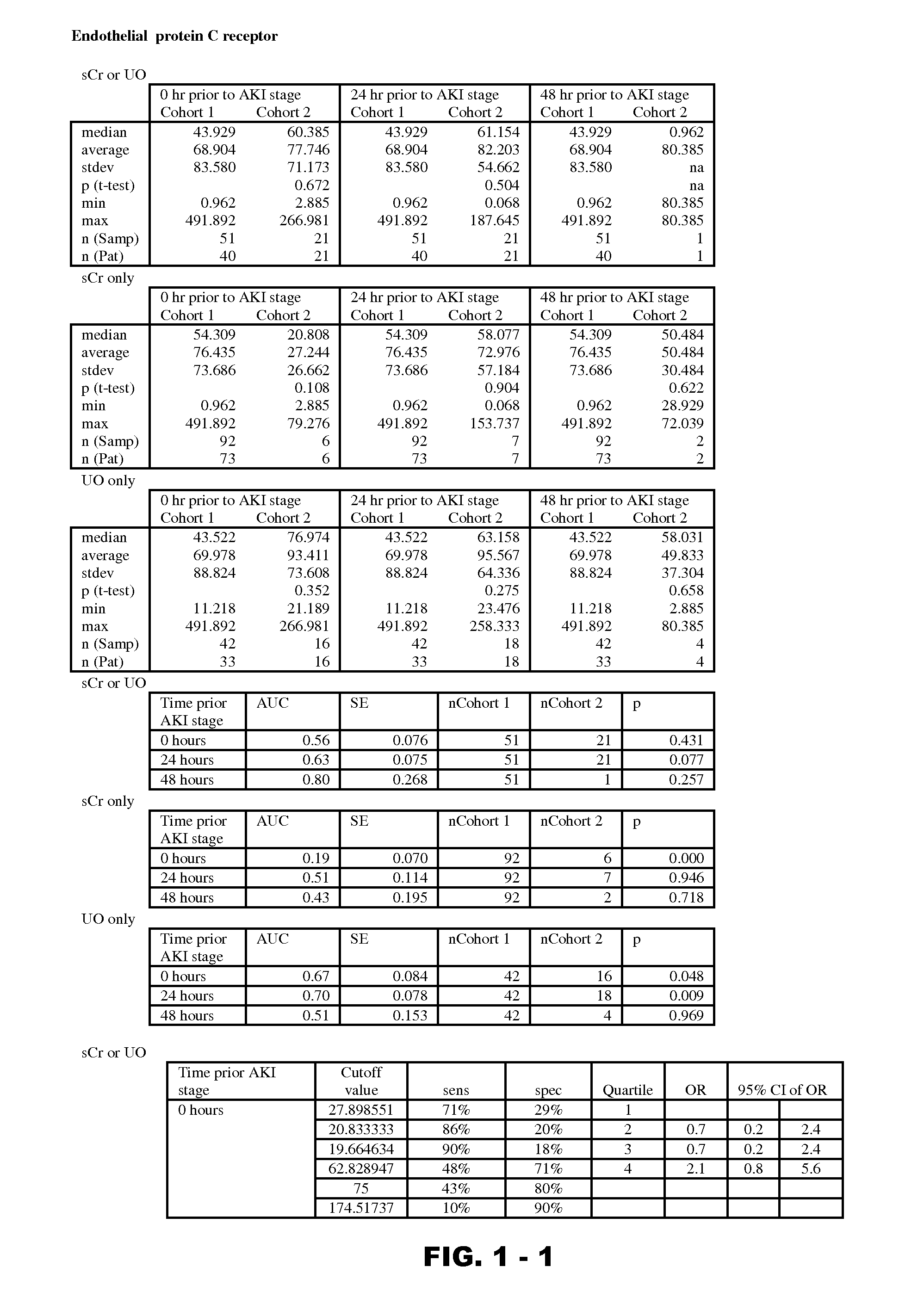
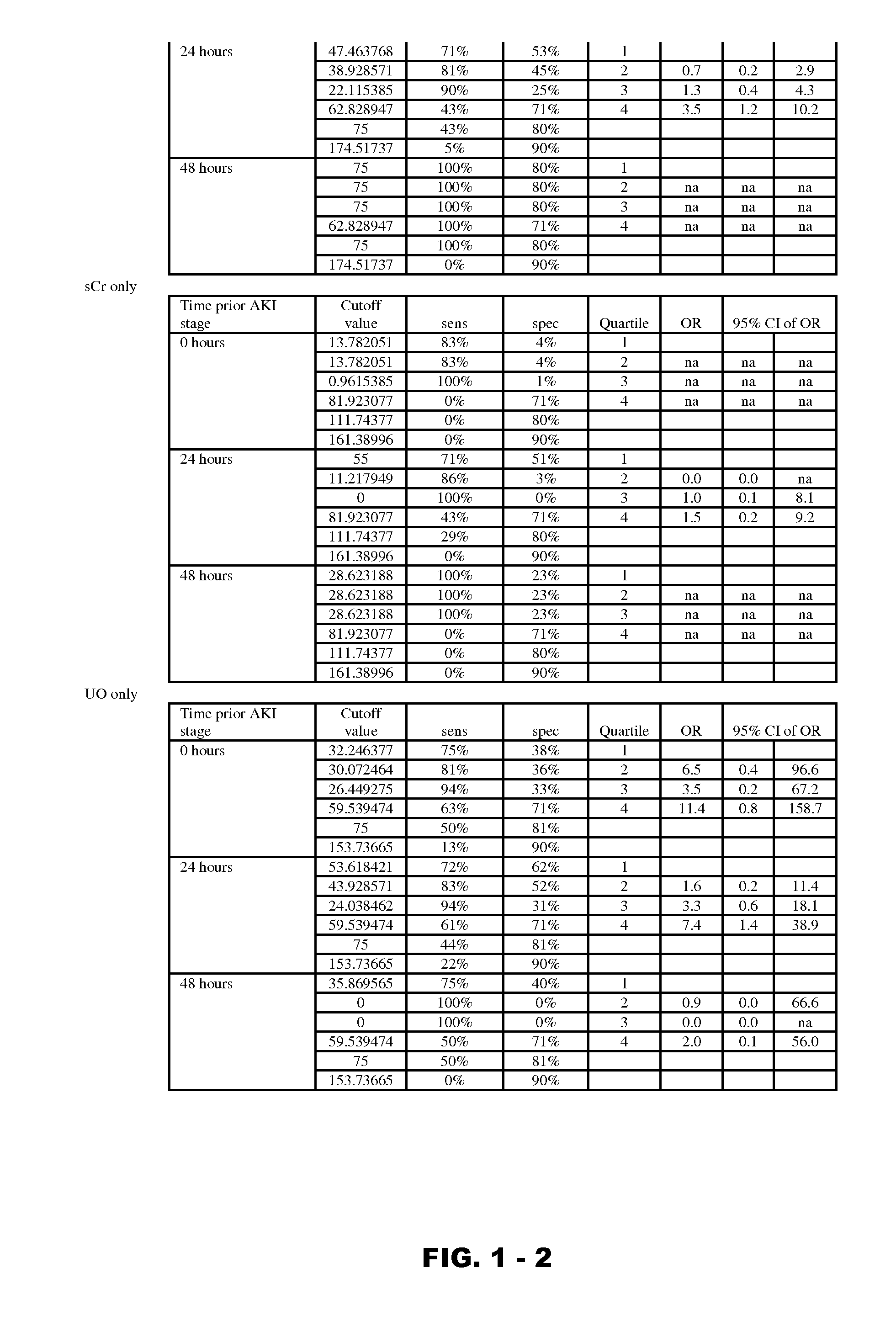
Disclosed are methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, disclosed are assays that detect one or more markers selected from the group consisting of Prostatic acid phosphatase, Lactotransfenin, Soluble erythropoietin receptor, Von Willebrand factor, Soluble endothelial protein C receptor, and Beta-2-glycoprotein 1 as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
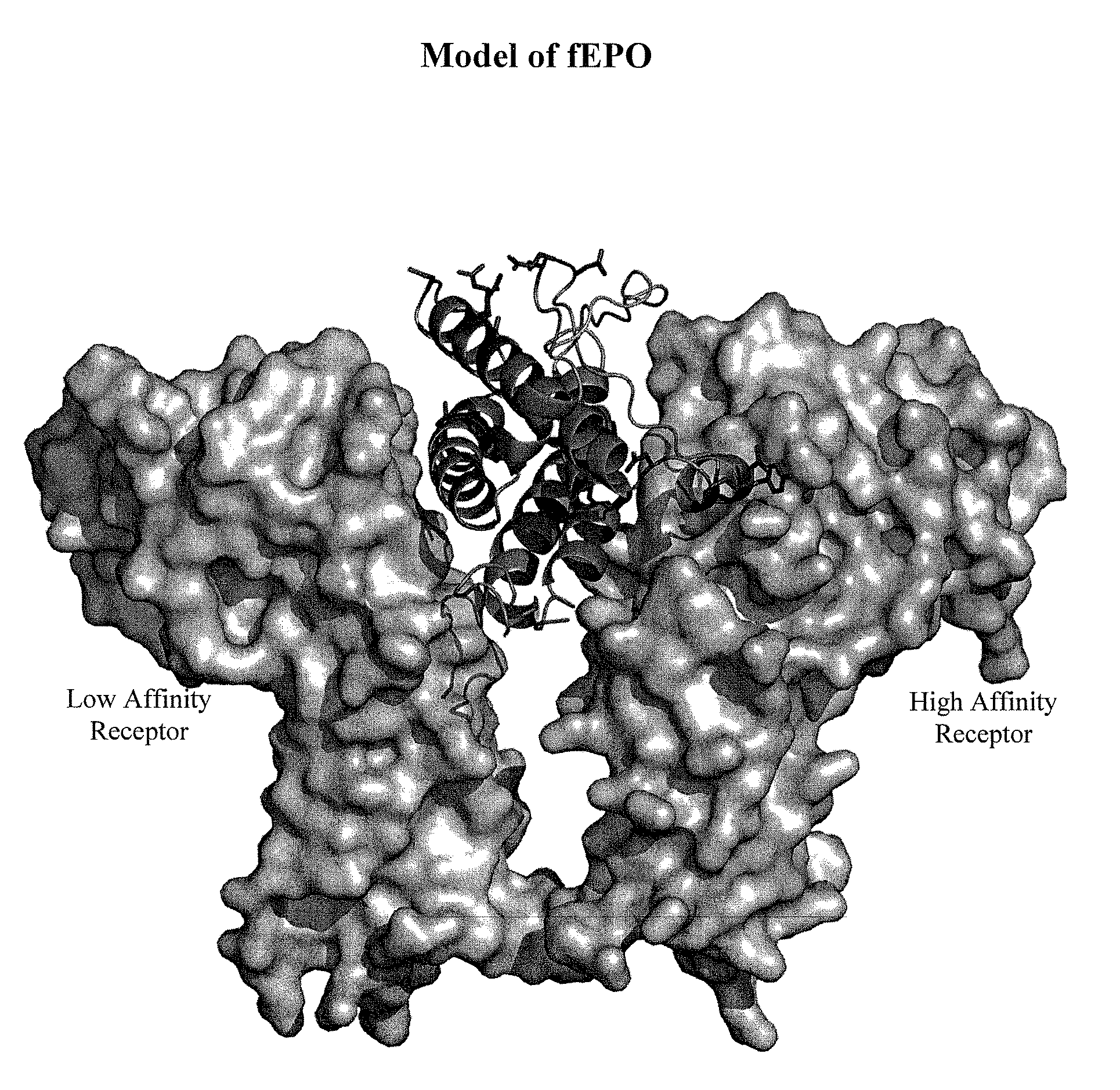
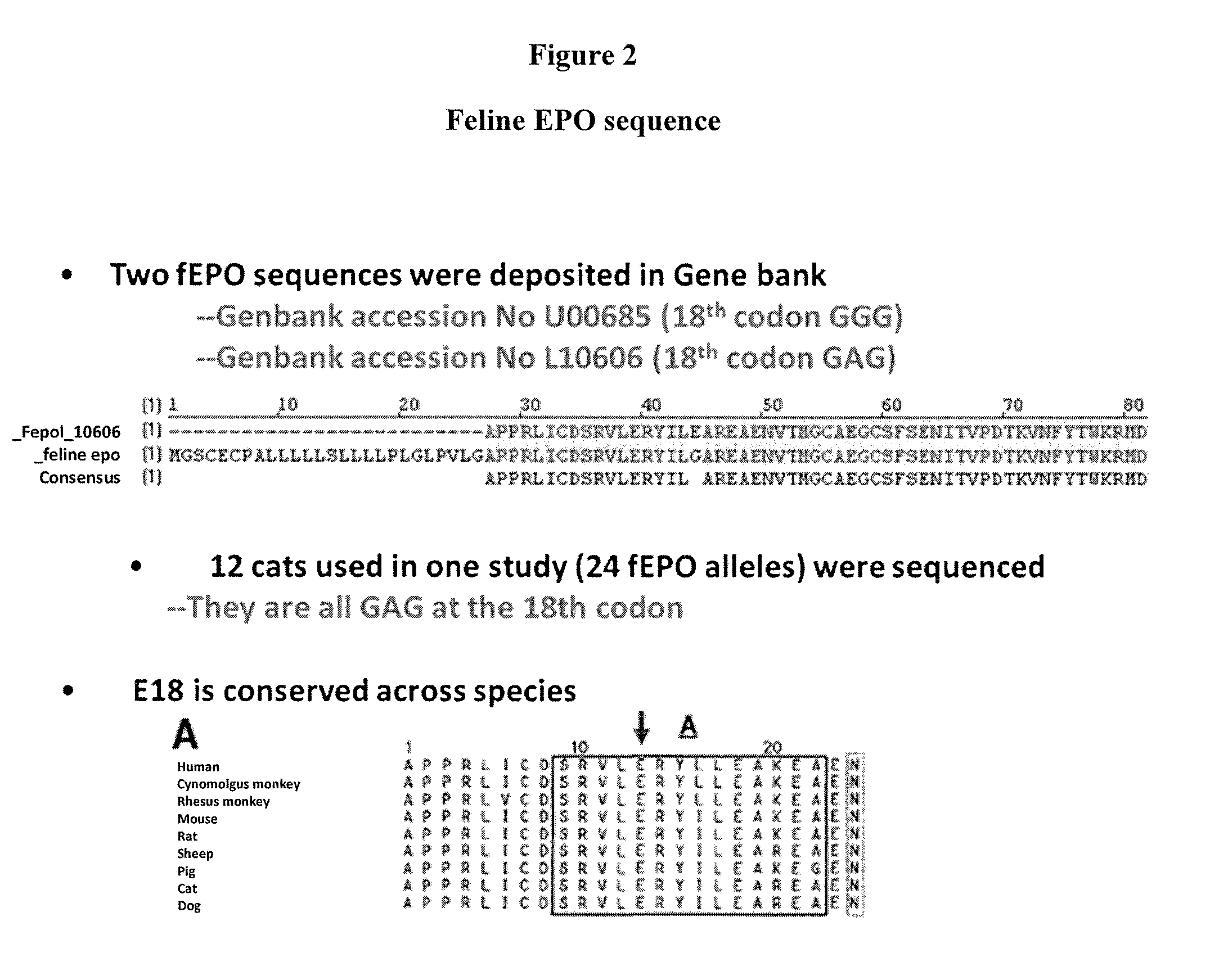
Modified animal erythropoietin polypeptides and their uses
InactiveUS20100093608A1Increasing therapeutic half-lifeIncreased serum half-lifePeptide/protein ingredientsAntibody mimetics/scaffoldsRed CellIndividual animal
Owner:ELANCO US INC +1
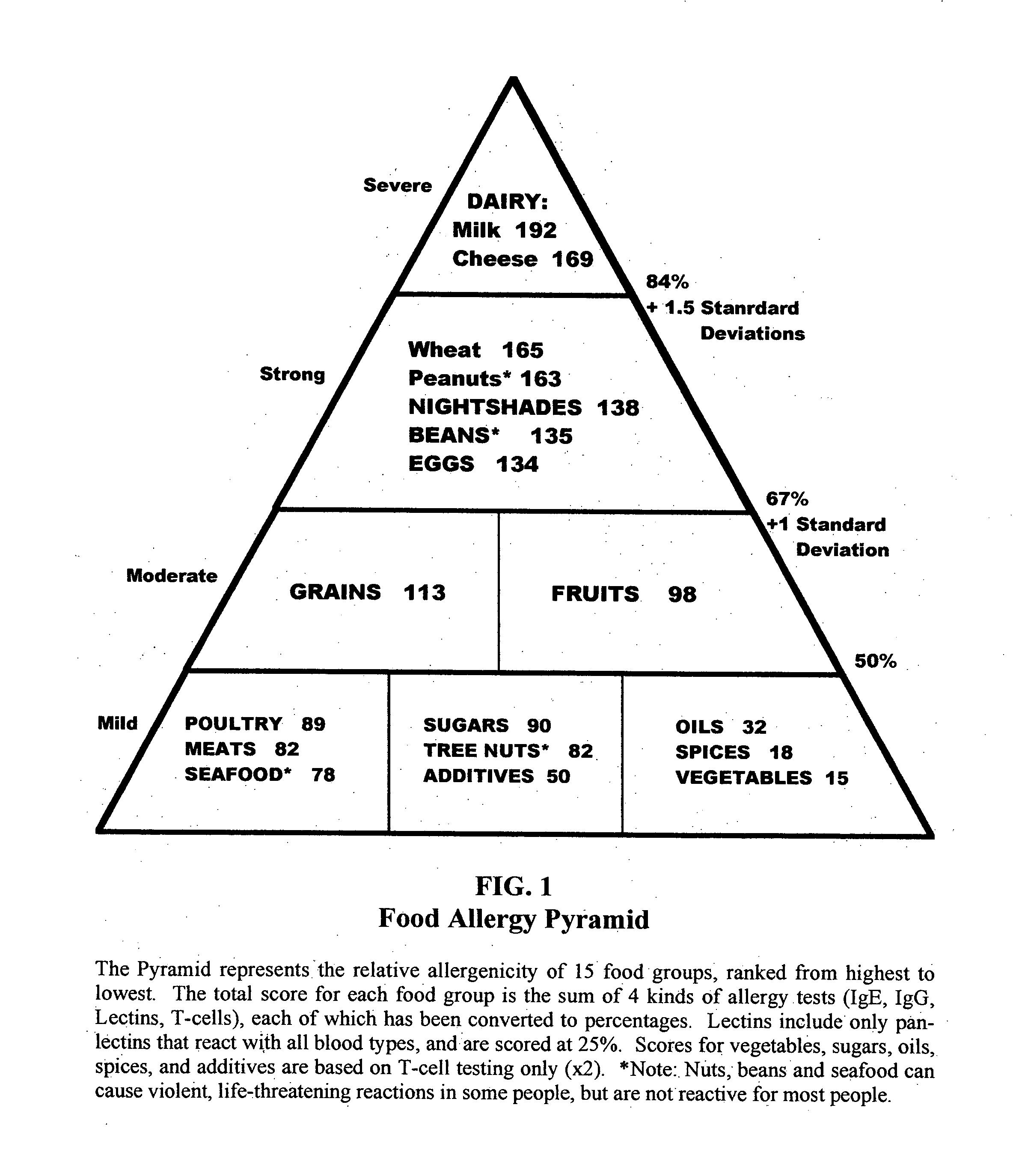
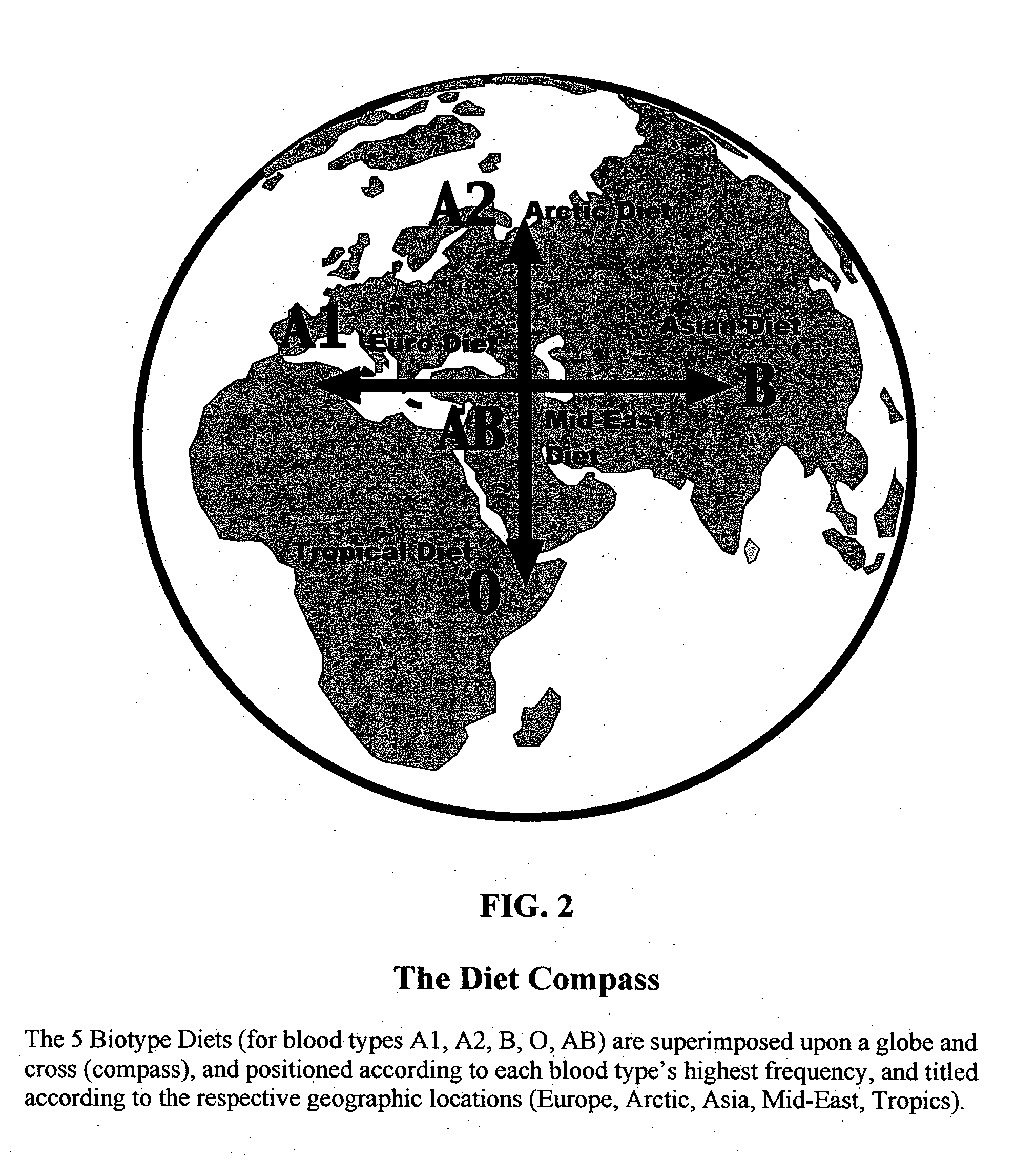
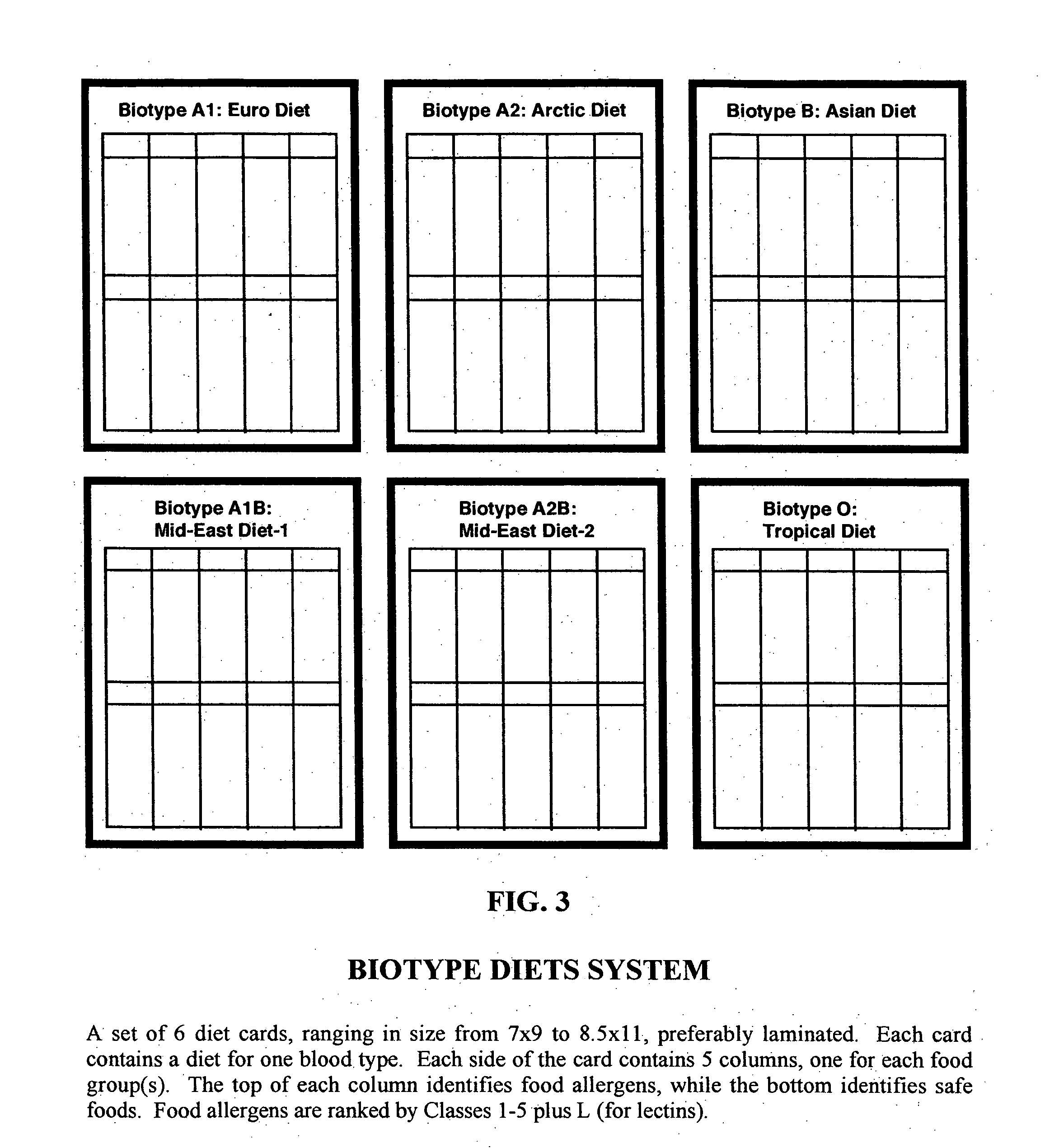
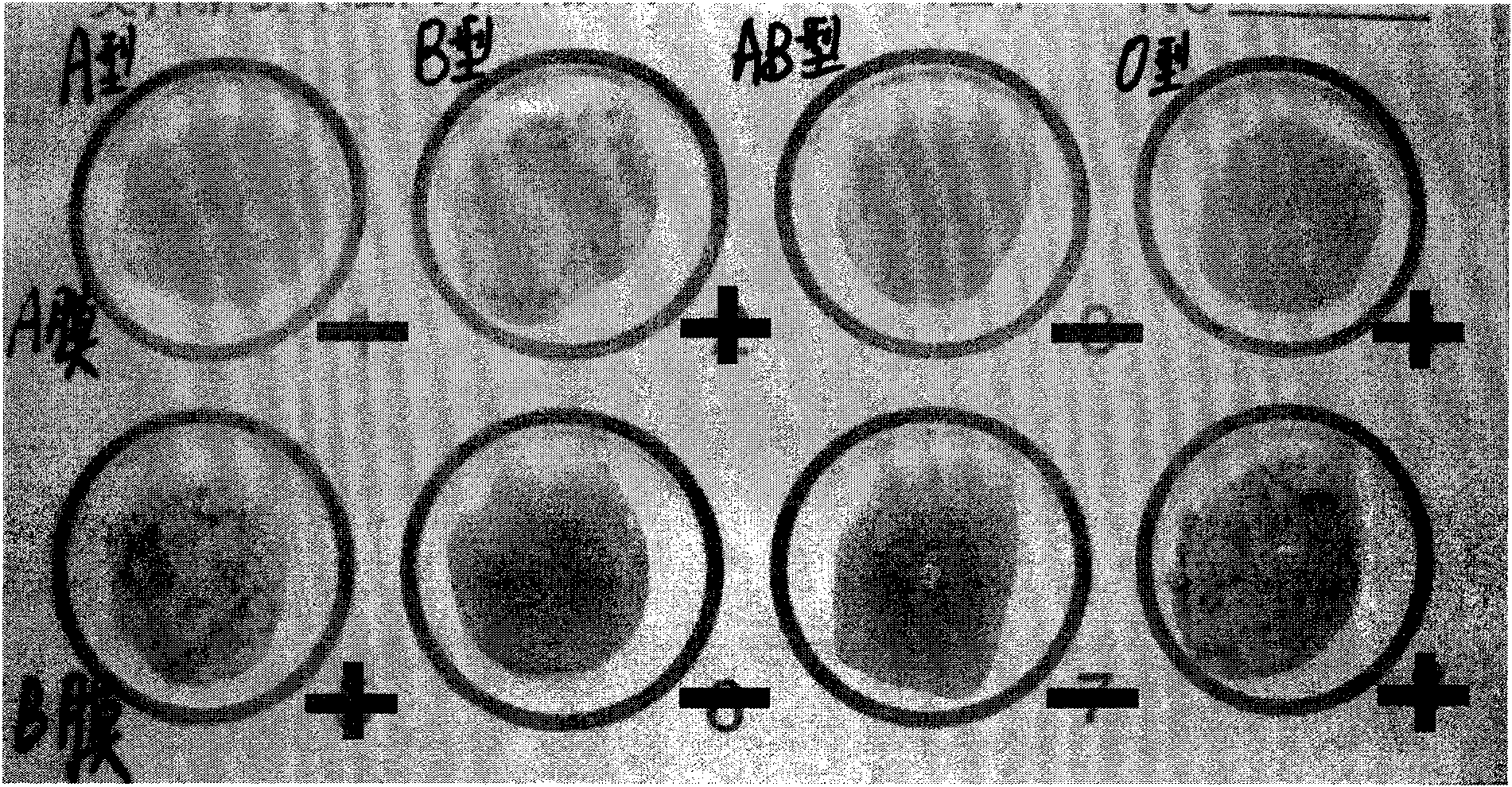
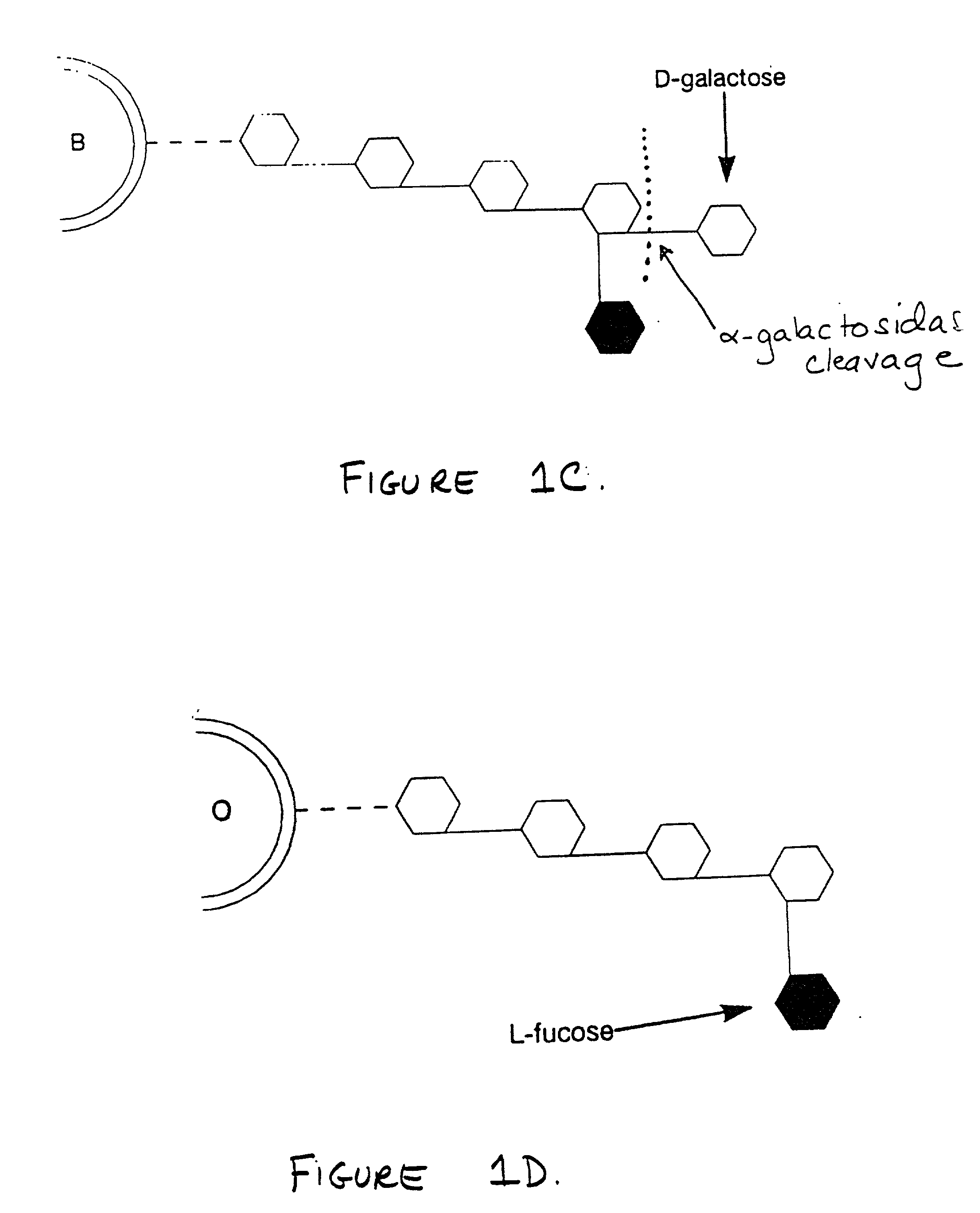
Biotype diets system: predicting food allergies by blood type
InactiveUS20060013773A1Ultrasonic/sonic/infrasonic diagnosticsAnalysis using chemical indicatorsGroup A - bloodAntigen
The invention is a diet-typing system for humans, including novel methods for diagnosis and treatment of food allergies and hypersensitivities. The diagnostic method correlates blood types (immunologically reactive antigens on RBC, skin and membranes) to four kinds of food allergies / hypersensitivities (IgE antibodies, IgG antibodies, T-cells, and Lectins). The results are used to identify and predict food allergies and hypersensitivities for six biological types (blood types A1, A2, B, O, A1B, and A2B), plus diet modifications for three subtypes (blood type Rh-negative, males and females). The treatment method uses the results to make food recommendations (to eat, limit, or avoid), based on the strength or classification of allergy scores, to mitigate the risk of food allergies and hypersensitivities in future persons. The diet-typing system presents the results on six diet cards, one for each blood type. The methods and resulting diets are unique, and differ substantially from prior inventions.
Owner:POWER LAURA W
Treatment of disturbances of iron distribution
ActiveUS7459435B2Beneficial effect disturbanceDisturbances of iron distributionPeptide/protein ingredientsMetabolism disorderDiabetes mellitusErythropoietin
A method of, and pharmaceutical composition for, treating disturbances of iron distribution in diabetes using erythropoietin are disclosed.
Owner:F HOFFMANN LA ROCHE & CO AG
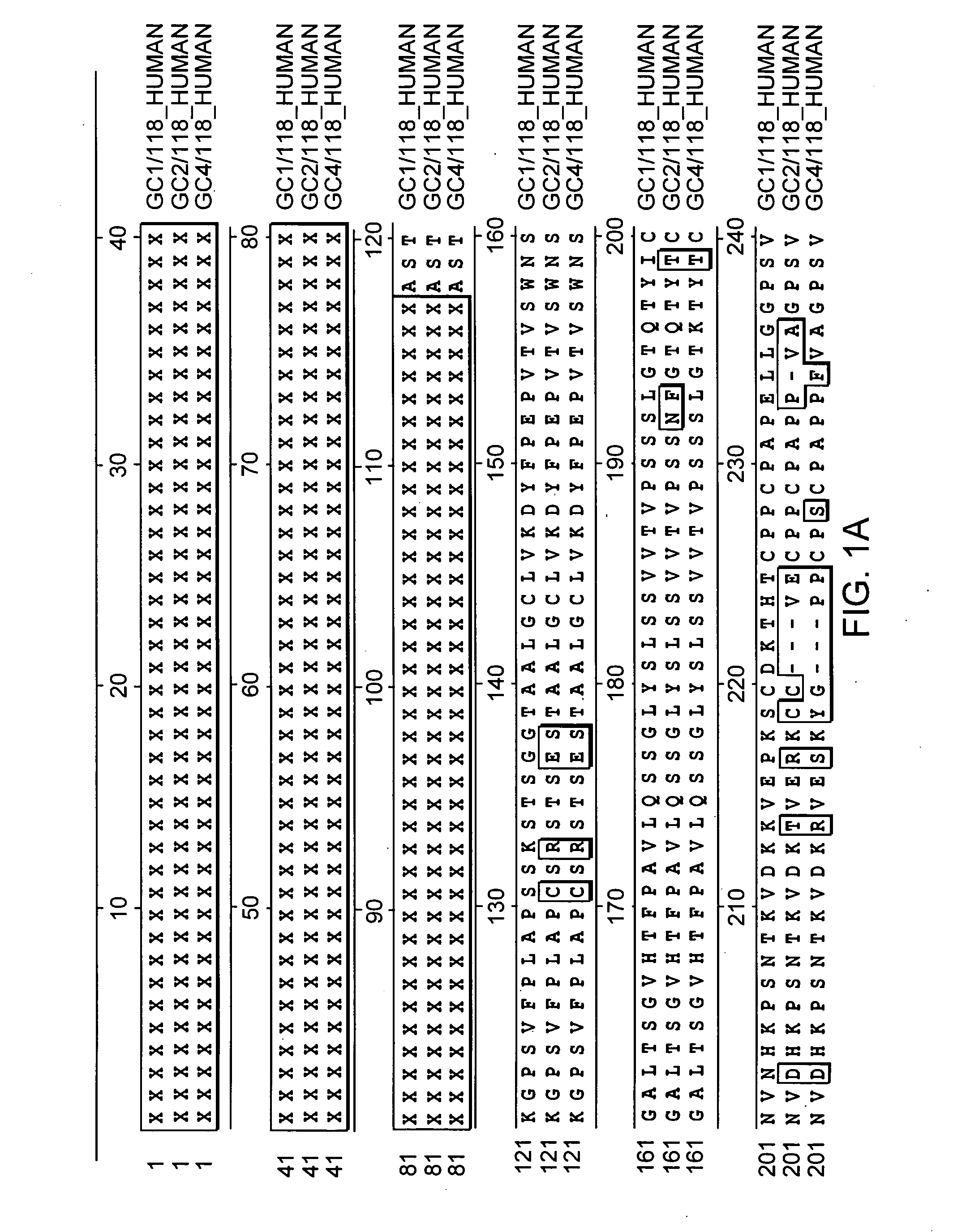
Polynucleotides and polypeptides of the erythropoietin gene
InactiveUS20030050269A1Increase productionNervous disorderPeptide/protein ingredientsErythroid cellErythropoietin Gene
The present invention relates to new polynucleotides deriving from the nucleotide sequence of the EPO gene and comprising new SNPs, new polypeptides derived from the natural EPO protein and comprising at least one mutation caused by the SNPs of the invention as well as their therapeutic uses.
Owner:GENODYSSEE SA
Triazolopyridine compound, and action thereof as prolyl hydroxylase inhibitor or erythropoietin production-inducing agent
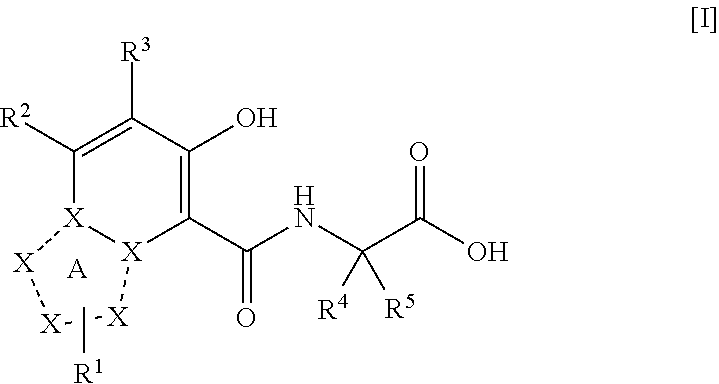
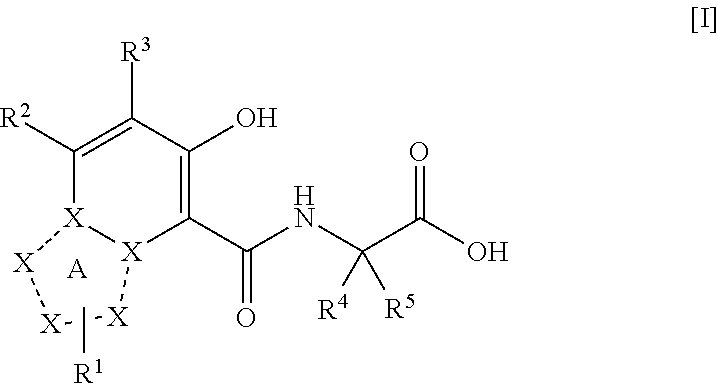
ActiveUS20110077267A1Easy to produceReduce productionBiocideGroup 5/15 element organic compoundsDiseaseMedicine
The present invention provides a triazolopyridine compound having a prolyl hydroxylase inhibitory action and an erythropoietin production-inducing ability. The present invention relates to a compound represented by the following formula [I]:wherein each symbol is as defined in the specification, or a pharmaceutically acceptable salt thereof, or a solvate thereof, as well as a prolyl hydroxylase inhibitor or erythropoietin production-inducing agent containing the compound. The compound of the present invention shows a prolyl hydroxylase inhibitory action and an erythropoietin production-inducing ability and is useful as a prophylactic or therapeutic agent for various diseases and pathologies (disorders) caused by decreased production of erythropoietin.
Owner:JAPAN TOBACCO INC
Human erythrocyte membrane antigen coated microsphere and application thereof
InactiveCN101957366AMaintain and preserve antigenic activityLong validity periodBiological testingAntiendomysial antibodiesMicrosphere
The invention provides a method for preserving the activity of a human cell membrane blood group antigen, which comprises the steps of: preparing an erythrocyte membrane blood group antigen extract, and then coating the prepared erythrocyte membrane blood group antigen on a solid microsphere so as to replace a fresh erythrocyte to be used for detecting a blood group antibody in a sample.
Owner:INTEC PROD INC
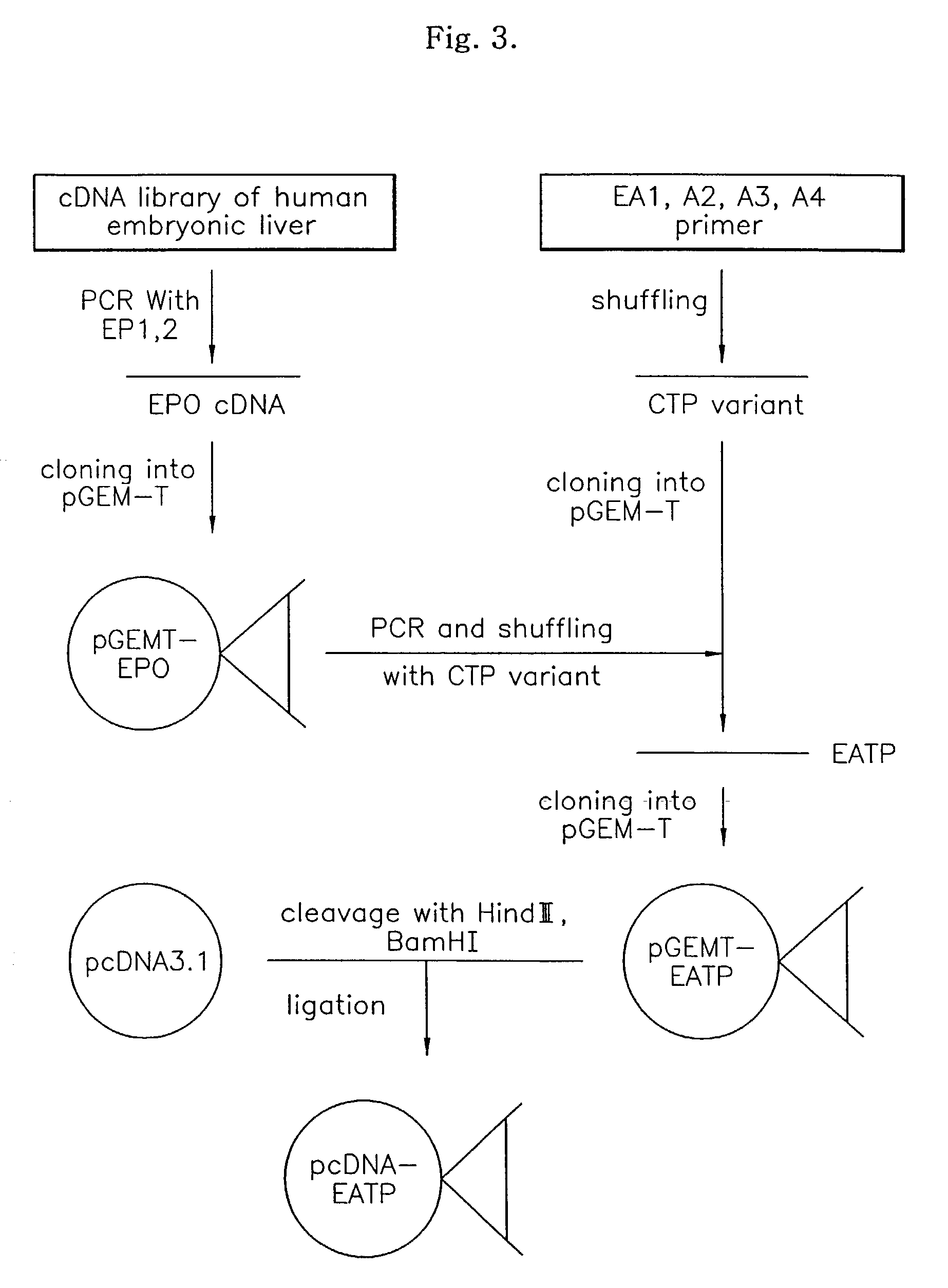
Fusion protein having enhanced in vivo erythropoietin activity
ActiveUS7091326B2Enhanced human EPO activityProlong half-life in vivoPeptide/protein ingredientsAntibody mimetics/scaffoldsRed blood cellHalf-life
Provided is a fusion protein comprising, at its carboxy terminal of human erythropoietin (EPO), a mutant having one to four amino acid substitutions in the carboxy terminal peptide (CTP) fragment of a human chorionic gonadotropin (HCG) β subunit, for increasing an in vivo half-life activity of EPO. The in vivo half-life can be greatly elongated while retaining the intrinsic activity of the EPO, without increasing the sugar chain content.
Owner:CJ HEALTHCARE CORP
Method for conversion of blood type
InactiveUS20010006772A1Mammal material medical ingredientsDead animal preservationPolyethylene glycolGroup A - blood
The present invention relates to an improved method for enzymatically removing blood type-specific antigens from erythrocytes, comprising titrating the pH of the erythrocytes first to a pH suitable for enzyme activity and then, once the desired extent of antigen removal has been achieved, to a pH appropriate for storage and / or transfusion. The buffers used for titration have pH values significantly above or below the target pHs for erythrocyte conversion or storage / transfusion. The invention is based, at least in part, on the discovery that the structural integrity of the erythrocytes is not substantially disrupted by titration. The present invention further relates to methods wherein the addition of polyethylene glycol improves the efficiency of enzymatic removal of erythrocyte antigens.
Owner:GOLDSTEIN JACK +2
Process for discriminating and counting erythroblasts
InactiveUS20020006631A1Microbiological testing/measurementChemiluminescene/bioluminescenceRed blood cellWhite blood cell
A method for discriminating and counting erythroblasts comprises the steps of: (i) staining leukocytes in a hematologic sample by adding a fluorescent labeled antibody capable of binding specifically with leukocytes to the hematologic sample; (ii) raising the permeability only of cell membranes of erythroblasts in the hematologic sample to a nucleotide fluorescent dye which does not permeate a cell membrane usually, the nucleotide fluorescent dye having a fluorescent spectrum capable of being distinguished from that of a fluorescent labeling compound of the fluorescent labeled antibody in step (i); (iii) staining nuclei of the erythroblasts in the hematologic sample with the nucleotide fluorescent dye; (iv) subjecting the hematologic sample to flowcytometry to detect at least two fluorescent signals from each cell; and (v) discriminating and counting the erythroblasts from difference in intensity between the at least two fluorescent signals.
Owner:SYSMEX CORP
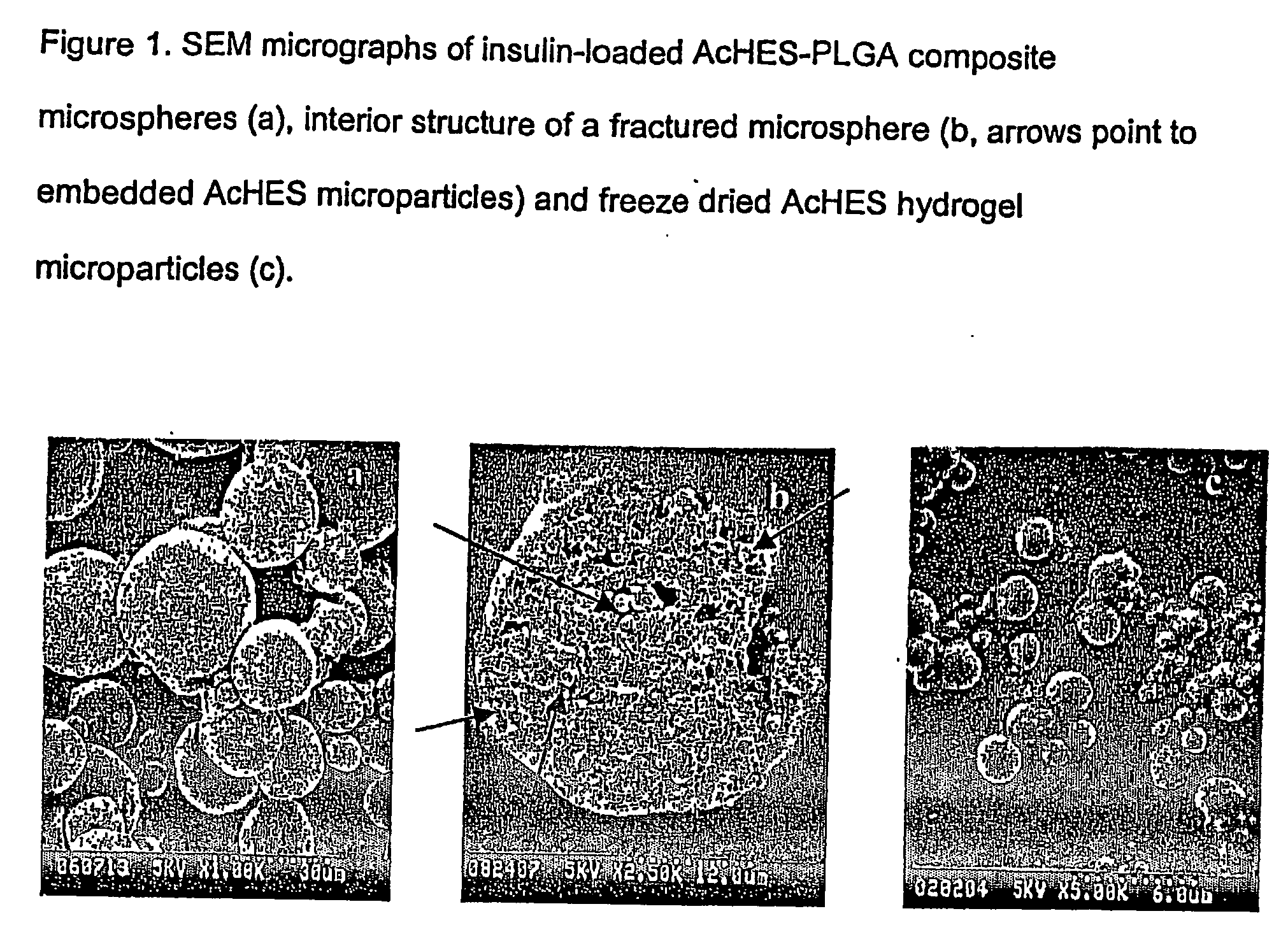
(Poly(acryloyl-hydroxyethyl starch)-plga composition microspheres
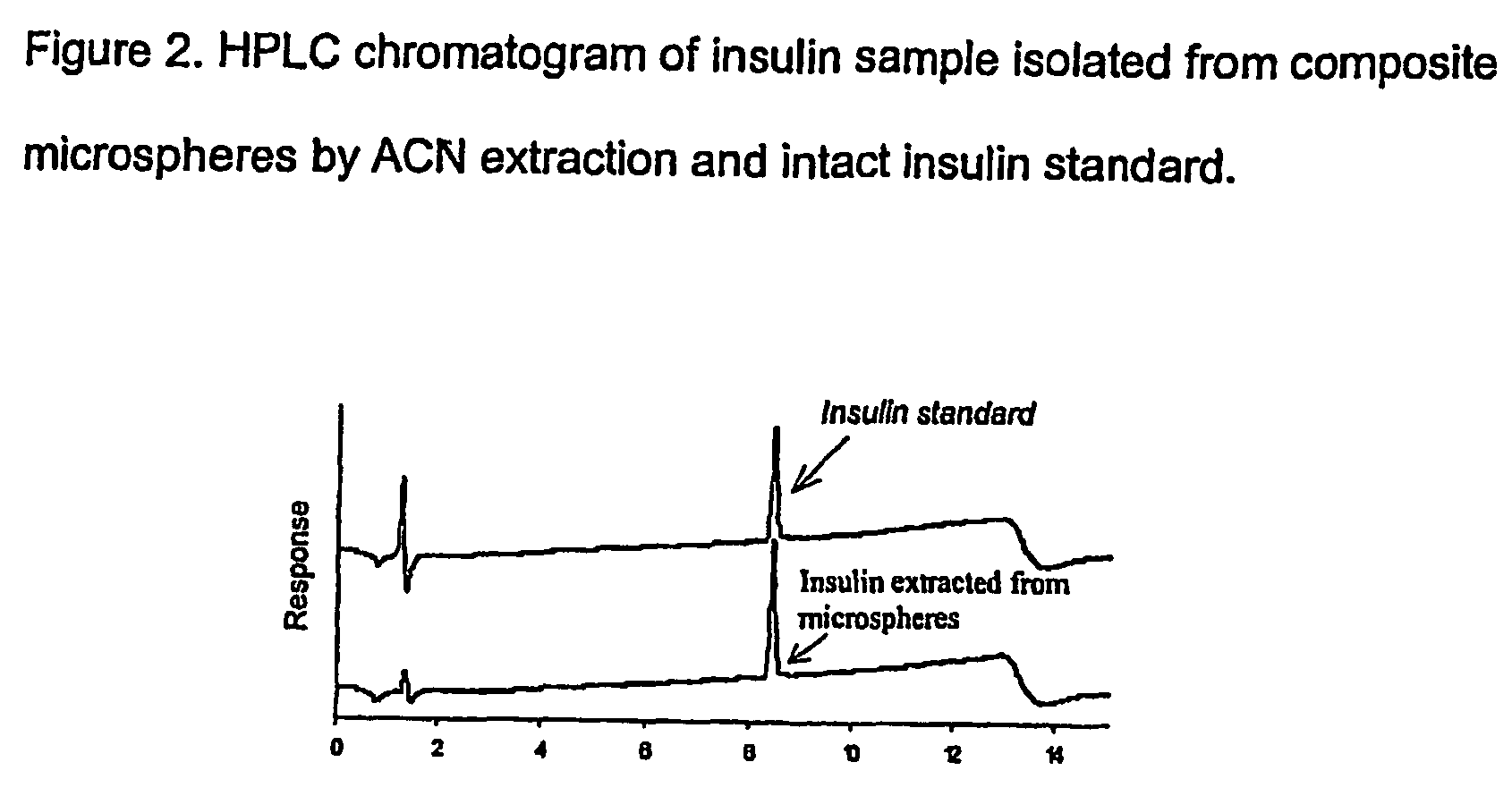
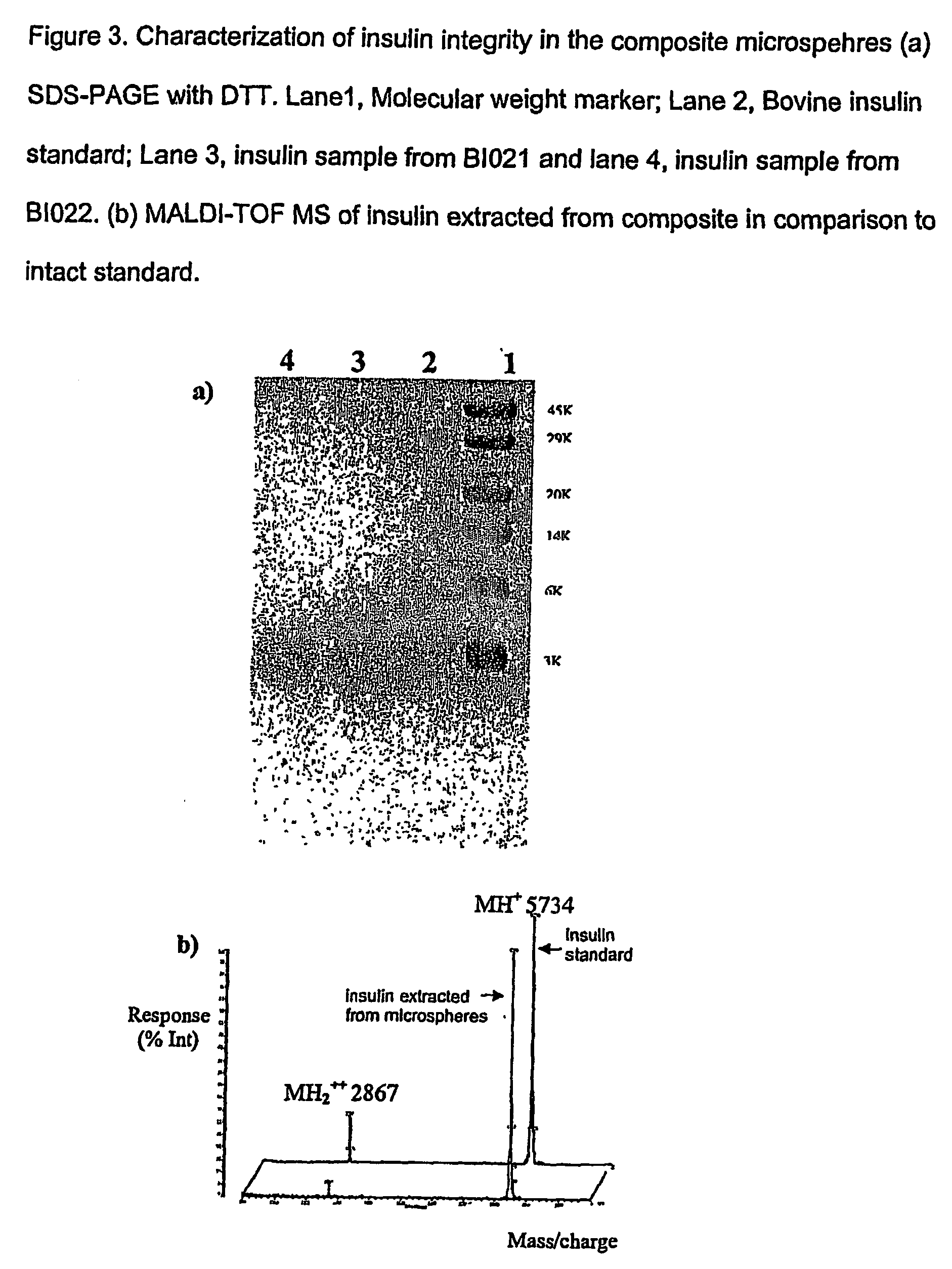
The present invention relates to a composite microsphere system comprising poly(D,L-lactide-co-glycolide) (PLGA), poly(acryloyl hydroxyethyl starch) (AcHES), and a pharmaceutically effective amount of a biologically active compound. The active compound may be, for example, an insulin, an interferon, a luteinizing hormone-releasing hormone (LHRH) analog, a somatostatin and / or derivatives thereof, a calicitonin, a parathyroid hormone (PTH), a bone morphogenic protein (BMP), an erythropoietin (EPO), an epidermal growth factor (EGF) or a growth hormone. This invention also relates to methods of using the composite microspheres, and methods of preparing same.
Owner:UNIV OF KENTUCKY RES FOUND
Reagent and method for classifying leucocyte
ActiveCN101078721AGuaranteed accuracyLow costIndividual particle analysisBiological testingWhite blood cellActive agent
The invention discloses a reagent for four-classification of leukocyte. The reagent comprises a. at least a surfactant which can dissolve erythrocyte and destroy leukocyte membrane; b. at least an organic compound with anionic group which can combine with cationic in leukocyte and make leukocytes generate configuration difference; c. buffering agent which can adjust PH value in 2-8. The invention also discloses the method for four-classification by said reagent. When four-classification of leukocyte is carried out by using the reagent and the method in invention, reaction can be carried out under the condition from acidity to weak alkaline PH and temperature and class of main component surfactant all have very wide selecting range so that the application range of invention is developed greatly. Compared with prior technology it has a great improvement.
Owner:SHENZHEN MINDRAY BIO MEDICAL ELECTRONICS CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com